Abstract
Background
The aim of this study was to investigate the effects of oxygen and cholinesterase inhibitor (donepezil) therapy on dementia in patients with age-exacerbated chronic obstructive pulmonary disease (COPD) in China’s northwestern high-altitude area.
Material/Methods
A total of 145 patients with acute exacerbation of COPD admitted to the Gerontology Department of the First People’s Hospital of Xining City were initially retrospectively screened. From among these 145 patients, we selected 33 cases with dementia and 33 patients without dementia through use of the Mini-Mental State Examination (MMSE), the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), and Activities of Daily Living (ADL) Scale evaluated before, 7 days after, and at the end of the treatment after 3 months. Both patient groups received oxygen therapy for 7 days, but patients with dementia in the intervention group were medicated additionally with donepezil (5 mg/day for 1 week, followed by 10 mg/day for another 12 weeks).
Results
Mild dementia was found in 35 of the 145 COPD patients. ADL, MMSE, and ADAS-Cog scores were all significantly lower in the intervention group before treatment, improved after the first 7 days, and continued to improve significantly until week 12 in the intervention group, but were still significantly lower than in the control group.
Conclusions
Dementia in elderly COPD patients was mainly manifested as decreased executive function, attention, language, and delayed recall, while oxygen and donepezil therapy had beneficial effects on the symptoms.
MeSH Keywords: Acetylcholinesterase; Mild Cognitive Impairment; Pulmonary Disease, Chronic Obstructive
Background
Chronic obstructive pulmonary disease (COPD) is characterized by continuous, incomplete, reversible airflow limitation and is associated with a chronic inflammatory response to harmful substances in the airway and lungs. Recent studies have shown that COPD is not only associated with lung and airway inflammation, but also with significant systemic reaction, especially in the central nervous system [1], which may be responsible for the occurrence of pulmonary encephalopathy [2]. In addition, various studies have found cognitive impairment in COPD patients [3–5], with an estimated incidence of 10% to 61% [6]. For example, Antonelli Incalzi et al. performed a comparative study of COPD patients with or without hypoxia and Alzheimer disease (AD) patients, reporting a correlation between anterior cerebral hypoperfusion and neuropsychological dysfunctions in hypoxemic COPD patients [7], and there is evidence that COPD can cause hypoxemia, which can be exacerbated by concomitant sleep-disordered breathing [8]. Another study has found an association between COPD and a decrease in cognitive performance at high altitudes [9]. In addition, the COPD mortality rate rose by 1/100 000 for each 95-meter altitude increase and was 3–4/10 000 greater at altitudes above 1000 meters compared to 100 meters [10].
Donepezil hydrochloride is the second FDA-approved acetylcholinesterase inhibitor with relative specificity, which plays a therapeutic role by enhancing the function of cholinergic nerves and is used as a cognition-enhancing medication. Several studies showed that acetylcholinesterase inhibitors can reverse the effects of hypoxia on cognitive functions [11–13]. Although there is currently no optimal therapy for cognitive impairment, early screening, prophylaxis, and treatment have been reported to be of some value [14]. Therefore, we retrospectively enrolled COPD patients admitted to the Gerontology Department of our hospital, which is located at an altitude of 2275 meters above sea level, between April 2014 and December 2016 (Figure 1). COPD patients with or without dementia were selected and we analyzed and compared the risk factors and the effects of oxygen uptake and drug (donepezil) therapy on cognitive impairment between the 2 groups. We hypothesized that donepezil would affect dementia in COPD patients.
Figure 1.
Location of the Xining area from which the COPD patients were recruited.
Material and Methods
This study was approved by the Ethics Committee of our hospital. According to the diagnostic criteria of COPD formulated by the Chinese Thoracic Society Chronic Obstructive Pulmonary Disease group [15], 145 COPD patients admitted to the Gerontology Department of our hospital between April 2014 and December 2016 were screened. The following data were collected using a questionnaire: gender, age, level of education, occupation, marital status, fertility status, body mass index (BMI), newspaper and telephone use, using the internet, going outside alone, hypertension, diabetes, length of stay, diet, history of serious mental illness or surgery, history of stroke or malignancy, paralysis, smoking history, course of diseases, family history, medication history, preferred foods, exercise status, presence and duration of memory complaints, and whether the information was provided by the patient or a caregiver.
Cognitive impairment was diagnosed according to the criteria by Albert et al. (2011) [16]. Patients with anxiety and depression according to the Hamilton Anxiety and Depression Scale were excluded. Finally, 33 cases with cognitive impairment and 33 patients without cognitive impairment were selected and evaluated using the Mini-Mental State Examination (MMSE), ADAS-Cog, and Activities of Daily Living (ADL) scales (Figure 2).
Figure 2.
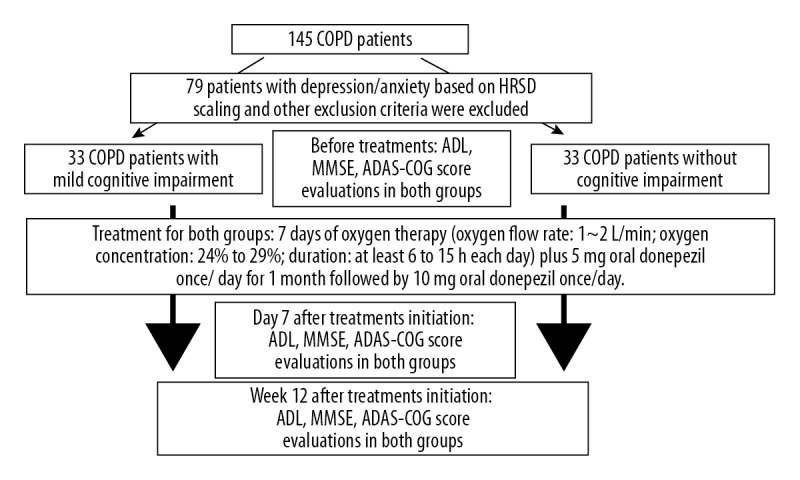
Flow chart of the present study. HRSD – Hamilton Rating Scale for Depression; COPD – chronic obstructive pulmonary disease; ADL – activities of daily living; MMSE – mini-mental state examination; ASAS-COG – Alzheimer’s Disease Assessment Scale-Cognitive Subscale.
Inclusion criteria
We included patients with acute exacerbation of COPD, complete clinical data, without mental and psychological diseases, without serious lesions of major organs, and without drug dependence. Data on blood gas analysis, pulmonary CT, electrocardiogram, and lung function were recorded before the treatments.
Exclusion criteria
We excluded patients who were unable to think clearly or express themselves, or who had: 1) severe dementia; 2) history of cerebrovascular disease; 3) central nervous system injury or brain damage caused by other diseases such as brain tumors or intracranial infection, or 4) history of CO poisoning or demyelinating disease of the central nervous system.
Arterial blood gas analysis method and pulmonary function test
For arterial blood gas analysis, we used the PHOX automatic blood gas analyzer (NOVA Biomedical, Waltham, MA USA). Radial arterial blood of patients was drawn to measure oxygen (PaO2), carbon dioxide (PaCO2) pressures, and oxygen saturation (SaO2%). Pulmonary function testing was performed with a spirometer (SensorMedics, Los Angeles, CA, USA). We used the flow-volume curve method to determine the maximum forced expiratory volume in the first second (FEV1) and Forced Vital Capacity (FVC) tested 3 times in parallel. The tests should have a deviation of less than 5%. The data in the table indicate FVC values/normal values ×100% and FEV1/FVC x 100%. Normal ranges for PaO2 (1.5–13.5 kPa), PaCO2 (4.5–6 kPa) and SaO2 (95–97%) are 20% lower in Xining.
Intervention methods and evaluation of therapeutic effect
In addition to anti-inflammatory and cough-suppressant drug treatment, patients in the 2 groups were given 7 days of oxygen therapy (general oxygen flow rate: 1–2 L/min; oxygen concentration: 24–29%; duration: at least 6–15 h each day). Intervention patients with dementia also received oral donepezil 5 mg/day for 7 days, which was increased to 10 mg/d for another 12 weeks. Therapeutic effects were evaluated using the MMSE, ADL, and ADAS-Cog scales before, 7 days after treatment initiation, and after another 12 weeks of treatment.
Assessment scales
The Hamilton Rating Scale for Depression (HRSD), first compiled by Hamilton in 1960, is the most widely used rating scale for depression. We used the 24-item version, in which a score of ≥20 means that the patient “may have depression (mild or moderate)”, while a score of <8 refers to normal. According to the data provided by the China Scale Collaboration Group, a score of ≥29, ≥21, ≥14, ≥7, and <7 points refer to possible serious anxiety, obvious anxiety, anxiety, possible anxiety, and no anxiety, respectively.
We used the Chinese version of the 20-item Activities of Daily Living scale (ADL) by Lawton and Brody [17], which has 2 parts: One is the physical Self-Maintenance Scale, which includes using the toilet, feeding, dressing, grooming, physical ambulation, and bathing. The other is the Instrumental Activities of Daily Living Scale, which includes using the telephone, shopping, preparing food, doing housework, washing laundry, using transportation, taking medicine, and handling finances. For each item, 1, 2, 3, and 4 points are given for being able to do it alone without difficulty, with some difficulty, with help, and not being able to do it, respectively. A total score of 20 points means ‘completely normal,’ while a score of >23 points refers to various levels of functional decline.
The Mini-Mental State Examination (MMSE) scale, first published by Folstein in 1975 [18], was used in the evaluation of space and time orientation, attention, computing power, memory, and language ability, with a maximum full score of 30 points, and a higher score indicating better cognitive function. The MMSE is the most widely used tool for cognitive evaluation, with advantages of simplicity and good sensitivity, which can be used in epidemiological surveys or for determining the severity of cognitive function decline. The scores for cognitive impairments were severe (less than 9), moderate (10–18), mild (19–23) and no impairment (24 and above) [19].
A modified Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) was used to evaluate cognitive impairments. The maximum score is 85, with higher scores indicating greater dysfunction, with a score of 26–30 indicating mild cognitive impairment, >30–35 indicating mild AD, 35–39 indicating moderate AD, and 40–45 indicating severe AD [20].
Statistical analysis
All analyses were performed with SPSS Statistics for Windows (Version 17.0. Chicago; SPSS Inc.). The chi-square test was used for comparison of numerical data. The data are displayed as χ̄±SD and the independent-samples t test was used for their comparison. A paired t test was used for comparing the data collected 7 days after admission with data collected 12 weeks after discharge. A result of P<0.05 was considered to be statistically significant.
Sample size calculation
We anticipated that the score change of MMSE from baseline is 5.5±3.0 after 12 weeks of intervention, and the score change of MMSE from baseline is 3±3.0 in the control group. With a P-value of 0.05, a test power of 90% and anticipating losses of 10% in follow-ups, we established a target sample size of 34 participants in each group.
Results
Baseline characteristics
Cognitive impairment was found in 35 of the 145 enrolled patients, and 33 COPD patients without cognitive impairment were selected as the control group. During the trial, 1 patient withdrew from the study and 1 patient died in the intervention group, so a total of 66 patients were followed up (Figure 2).
There were 24 males and 9 females in the control group, with an average age of 70.6 years, while there were 17 males and 16 females in the intervention group, with an average age of 78.1 years. There was no significant difference in gender, age, course of disease, and level of education between the 2 groups (Table 1).
Table 1.
Baseline characteristics of COPD patients in the 2 groups.
| Control group (33 cases) | Intervention group (33 cases) | t/χ2 | P-value | ||
|---|---|---|---|---|---|
| Gender | Male | 24 | 17 | 3.879 | 0.076 |
| Female | 9 | 16 | |||
| Age | ≤ 75 | 15 | 12 | 2.182 | 0.140 |
| > 75 | 18 | 21 | |||
| Level of education | Illiteracy | 9 | 13 | 0.253 | 0.553 |
| Primary | 10 | 6 | |||
| Middle/high school | 10 | 11 | |||
| College degree or above | 4 | 3 | |||
| Course of disease | ~10 years | 12 | 12 | 0.766 | 0.945 |
| ~15 years | 18 | 17 | |||
| More than 20 years | 3 | 4 |
Data about arterial blood gas and pulmonary function were derived at baseline as well as 1 week and 2 weeks after treatment initiation during the hospital stay (Table 2). All parameters other than FEV1% were comparable between the 2 groups at baseline.
Table 2.
Arterial blood gas and pulmonary function comparisons of the control and intervention groups (mean ±SD).
| Control group | Intervention group | P-value | |
|---|---|---|---|
| Baseline | |||
| PaO2 (kPa) | 5.27±2.41 | 5.41±2.80 | 0.8284 |
| PaCO2 (kPa) | 7.66±2.90 | 7.60 ±2.82 | 0.9324 |
| After 1 week of treatment | |||
| PaO2 (kPa) | 5.93±2.87 | 5.87±2.63 | 0.9297 |
| PaCO2 (kPa) | 7.55 ±2.61 | 7.48±3.12 | 0.9216 |
| After 2 weeks of treatment | |||
| PaO2 (kPa) | 6.88±2.32** | 6.79±3.11 | 0.8944 |
| PaCO2 (kPa) | 7.44 ±3.17 | 7.41 ±2.96 | 0.9684 |
| Baseline | |||
| FVC% | 43.3±11.6 | 47.4±13.1 | 0.1830 |
| FEV1% | 51.94±3.56 | 54.2±3.68 | 0.0137 |
| After 1 week of treatment | |||
| FVC% | 46.4±11.6 | 47.76±12.7 | 0.6512 |
| FEV1% | 54.28±2.39** | 55.16±4.03 | 0.2847 |
| After 2 weeks of treatment | |||
| FVC% | 46.56±12.7 | 48.8±11.4 | 0.4536 |
| FEV1% | 54.89±2.74 *** | 55.99±4.03 | 0.1994 |
| Baseline | |||
| SaO2 (%) | 84.52±3.77 | 85.23±3.28 | 0.4174 |
| After 1 week of treatment | |||
| SaO2 (%) | 86.15±4.12 | 87.09±4.34 | 0.3702 |
| After 2 weeks of treatment | |||
| SaO2 (%) | 90.12±5.67 *** | 91.22±5.02 *** | 0.4071 |
FVC%=FVC values/normal values ×100%; FEV1%=FEV1/FVC ×100%. PaO2 – alveolar oxygen partial pressure; PaCO2 – arterial partial pressure of carbon dioxide; FVC – forced vital capacity; FEV1 – forced expiratory volume in one second; SaO2: arterial oxygen saturation;
P<0.01 compared to the baseline;
P<0.001 compared to the baseline.
MMSE, ADAS-Cog scale, and ADL scores for COPD patients in the 2 groups
Significant differences were found between the 2 groups in all items of the ADL scale (P<0.001), except for taking medicine (P=0.354), putting on and taking off clothes (P=0.507), combing and brushing hair (P=0.265), walking on a flat interior floor (P=0.153), using the telephone (P=0.911) and handling finances (P=0.658) (Tables 3, 4).
Table 3.
Comparison of ADL scores between intervention and control groups before treatments (mean ±SD).
| n | ADL score | t | P-value | |
|---|---|---|---|---|
| Intervention group | 33 | 35.24±2.75 | 21.470 | <0.001 |
| Control group | 33 | 28.36±2.08 |
Table 4.
ADL assessment of patients in the 2 groups according to Lawton and Brody before treatments (mean ±SD).
| Items | Intervention group (n=33) | Control group (n=33) | t | P-value |
|---|---|---|---|---|
| Taking a bus | 3.03±0.78 | 2.03±0.68 | 5.551 | <0.001 |
| Ambulation (walking distance) | 3.98±0.30 | 3.32±0.34 | 8.362 | <0.001 |
| Cooking | 3.96±0.21 | 3.83±0.27 | 2.183 | 0.033 |
| Doing housework | 3.96±0.21 | 2.38±0.32 | 23.714 | <0.001 |
| Taking medicine | 3.29±0.72 | 3.12±0.76 | 0.933 | 0.354 |
| preparing food | 3.86±0.67 | 2.5±0.58 | 8.297 | 0.001 |
| Putting on and taking off clothes | 3.49±0.92 | 3.12±0.83 | 0.671 | 0.507 |
| Combing and brushing hair | 3.89±0.86 | 3.78±0.83 | 1.125 | 0.265 |
| Doing the laundry | 3.72±0.46 | 3.32±0.32 | 4.101 | 0.001 |
| Walking on flat interior floor | 3.96±0.73 | 3.68±0.84 | 1.445 | 0.153 |
| Walking up and down stairs | 3.69±0.21 | 2.32±0.41 | 17.085 | <0.001 |
| Getting in and out of bed, sitting down or standing up | 3.96±0.22 | 3.36±0.36 | 8.169 | <0.001 |
| Preparing water to cook and bathe | 3.49±0.78 | 1.42±0.64 | 11.786 | <0.001 |
| Bathing (water has been prepared) | 3.92±0.21 | 3.42±0.64 | 4.264 | <0.001 |
| Cutting toenails | 3.83±0.23 | 3.32±0.28 | 8.085 | <0.001 |
| Shopping | 3.89±0.22 | 3.54±0.35 | 4.861 | <0.001 |
| Going to the toilet at regular times | 3.88±0.26 | 2.36±0.36 | 19.663 | <0.001 |
| Using the telephone | 3.89±0.28 | 3.88±0.43 | 0.112 | 0.911 |
| Handling finances | 3.96±0.49 | 3.89±0.76 | 0.447 | 0.658 |
| Staying at home alone | 3.92±0.73 | 3.12±0.59 | 4.896 | <0.001 |
There were also significant differences in MMSE scores between the 2 groups (P<0.001), and cognitive impairment in the intervention group was mainly manifested as impairment of orientation (P<0.001), attention (P<0.001, computational power (P<0.001), recall (P<0.001), and language abilities (P<0.001) (Tables 5, 6).
Table 5.
Comparison of MMSE scores between intervention and control groups before treatments (mean ±SD).
| n | MMSE score | t | P-value | |
|---|---|---|---|---|
| Intervention group | 33 | 16.67±1.44 | 25.361 | <0.001 |
| Control group | 33 | 24.36±0.98 |
Table 6.
MMSE assessment for patients in the 2 groups before treatments (mean ±SD).
| Items | Intervention group (n=33) | Control group (n=33) | t | P-value |
|---|---|---|---|---|
| Orientation | 7.40±0.38 | 8.43±0.46 | 9.917 | <0.001 |
| Memory | 2.00±0.46 | 2.96±0.72 | 6.455 | <0.001 |
| Attention and computational power | 3.68±0.61 | 4.78±0.75 | 6.536 | <0.001 |
| Recall ability | 2.34±0.59 | 2.96±0.76 | 3.702 | <0.001 |
| Language ability | 6.36±0.63 | 8.76±0.58 | 16.100 | <0.001 |
ADAS-Cog scales also showed significant differences between the 2 groups (P<0.001) in all tested items (P<0.001) (Table 7).
Table 7.
Comparison of ADAS-Cog score between intervention and control groups before treatments (mean ±SD).
| ADAS-Cog score | P-value | ||
|---|---|---|---|
| Intervention group (n=33) | Control group (n=33) | ||
| Items | 33.03±5.75 | 17.76±4.64 | <0.001 |
| Word memory | 5.46±0.48 | 3.23±0.21 | <0.001 |
| Naming | 3.98±0.42 | 2.62±0.32 | <0.001 |
| Instructions | 2.98±0.46 | 2.48±0.64 | <0.001 |
| Structural practice | 2.69±0.57 | 1.98±0.41 | <0.001 |
| Intentionality | 2.84±0.86 | 1.52±0.72 | <0.001 |
| Orientation | 3.68±0.32 | 2.43±0.26 | <0.001 |
| Word recognition | 4.23±0.56 | 1.87±0.36 | <0.001 |
| Recall of testing instructions | 2.63±0.64 | 1.82±0.63 | <0.001 |
| Oral ability | 2.86±0.78 | 1.69±0.66 | <0.001 |
| Finding words | 2.82±0.36 | 1.76±0.47 | <0.001 |
| Language comprehension | 3.97±0.38 | 2.12±0.52 | <0.001 |
| Attention | 2.88±0.49 | 1.82±0.67 | <0.001 |
Assessment of therapeutic effect
After oxygen and drug (donepezil) therapy, ADL scores improved significantly in the intervention group between day 7 days and week 12 of the intervention, and there was no significant difference in ADL scores between the intervention and the control group at 12 weeks after treatment initiation (P=0.247). MMSE and ADAS-Cog scores significantly improved in the intervention group after 12 weeks, but were still inferior to the control group (P=0.003), (P=0.002) (Table 8).
Table 8.
Comparison of therapeutic effects between the 2 groups 7 days and 12 weeks after treatment initiation (mean ±SD).
| Group | N | Time points | P-value | |
|---|---|---|---|---|
| 7 days | 12 weeks | |||
| ADL | ||||
| Intervention group | 33 | 33.55±2.51 | 28.37±3.44 | <0.001 |
| Control group | 33 | 28.64±3.92 | 27.42±3.08 | 0.872 |
| P-value | <0.001 | 0.247 | ||
| MMSE | ||||
| Intervention group | 33 | 17.34±3.5 | 23.14±2.70 | <0.001 |
| Control group | 33 | 24.36±2.78 | 22.91±2.21 | 0.750 |
| P-value | <0.001 | 0.003 | ||
| ADAS-Cog | ||||
| Intervention group | 33 | 33.02+4.51 | 27.12+4.17 | <0.001 |
| Control group | 33 | 16.23+4.23 | 15.93+6.07 | 0.956 |
| P-value | <0.001 | 0.002 | ||
Adverse effects
Donepezil adverse effects were mild diarrhea in 4 patients, dizziness in 2 patients, and anorexia in 1 patient.
Discussion
A study of global disease burden predicted that by 2020, COPD would be the third leading cause of disease death in the world, and the World Bank and Health Organization forecast at a large epidemiological statistical meeting that by 2020, COPD would rank fifth in global disease burden [21,22].
Severe cognitive dysfunction often occurs in COPD patients, manifested by decreased alertness, delayed reaction time, and abnormal logical thinking [23]. In the present study, cognitive impairment was diagnosed in 24.2% of the initially screened COPD patients living in the Xining region with 25% reduced atmospheric O2 content. However, since 54.5% of the initially screened COPD cases were excluded in this study, the overall dementia incidence might have been underestimated due to the presence of more serious depression and/or anxiety symptoms in the excluded patients. It has been proposed that hypoxemia is a crucial factor for cognitive impairment in COPD patients [24,25]. Furthermore, some researchers have suggested that cognitive impairment is prone to occur in COPD patients with hypoxemia, while the risk of subclinical cognitive dysfunction would not be increased in COPD patients without hypoxemia, and the degree of cognitive impairment in COPD patients with hypoxia is closely related to the degree of hypoxia. This is supported by the clinical finding that cognitive function can be improved by long-term oxygen therapy in COPD patients [26,27]. A proposed reason for the correlation between hypoxia and cognitive impairment is the shortage of cholinergic transmitters, since hypoxemia affects oxygen-dependent enzymes for the synthesis of acetylcholine [28]. In our study, oxygen treatment for 1 week, in combination with low-dose donepezil, significantly improved ADL scores, and there was some improvement in ADAS-Cog scores in the intervention group, whereas the control group did not show significant changes. However, there were still significant differences between the groups regarding ADL, MMSE, and ADAS-Cog scores at day 7 after treatment initiation. After 12 weeks of treatment, ADL scores showed no difference between the groups, but MMSE and ADAS-Cog scores were still significantly inferior in the intervention group, although they were significantly improved compared to before treatment. These results indicate that high doses of the acetylcholinesterase inhibitor donepezil improve the cognitive impairment in COPD patients, even without oxygen therapy. The cognitive impairment screening used in the current study employed a variety of clinical neuropsychological scales, and could be used to assess the overall state of cognitive function, as well as specific cognitive domain status, identifying some cognitive impairments that would be harder to detect by routine examination [29]. A limitation of our study is the small sample size, and further large-scale studies are necessary to confirm the findings.
Conclusions
Dementia developed in a large percentage of COPD patients from China’s northwestern region, mainly manifested as symptoms of decreased performance on executive function, attention, language, and delayed recall. Regular cognitive impairment screening and intervention should be available to COPD patients, particularly those living in high-altitude areas. Oxygen and donepezil therapy had a distinct beneficial effect on cognitive impairment in COPD patients with dementia.
Footnotes
Source of support: Departmental sources
References
- 1.Cleutjens FA, Spruit MA, Beckervordersandforth J, et al. Presence of brain pathology in deceased subjects with and without chronic obstructive pulmonary disease. Chron Respir Dis. 2015;12(4):284–90. doi: 10.1177/1479972315588005. [DOI] [PubMed] [Google Scholar]
- 2.Spera K, Rubin D, Gupta T, et al. Clinical reasoning: An 87-year-old man with chronic obstructive pulmonary disease and acute encephalopathy. Neurology. 2016;87(13):e135–39. doi: 10.1212/WNL.0000000000003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleutjens F, Ponds R, Spruit MA, et al. The relationship between cerebral small vessel disease, hippocampal volume and cognitive functioning in patients with COPD: An MRI study. Front Aging Neurosci. 2017;9:88. doi: 10.3389/fnagi.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roncero C, Campuzano AI, Quintano JA, et al. Cognitive status among patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:543–51. doi: 10.2147/COPD.S100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yohannes AM, Chen W, Moga AM, et al. Cognitive impairment in chronic obstructive pulmonary disease and chronic heart failure: A systematic review and meta-analysis of observational studies. J Am Med Dir Assoc. 2017;18(5):451e1–11. doi: 10.1016/j.jamda.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Dodd JW. Lung disease as a determinant of cognitive decline and dementia. Alzheimers Res Ther. 2015;7(1):32. doi: 10.1186/s13195-015-0116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonelli Incalzi R, Marra C, Giordano A, et al. Cognitive impairment in chronic obstructive pulmonary disease – a neuropsychological and spect study. J Neurol. 2003;250(3):325–32. doi: 10.1007/s00415-003-1005-4. [DOI] [PubMed] [Google Scholar]
- 8.Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199–208. doi: 10.2147/COPD.S10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kourtidou-Papadeli C, Papadelis C, Koutsonikolas D, et al. High altitude cognitive performance and COPD interaction. Hippokratia. 2008;12(Suppl 1):84–90. [PMC free article] [PubMed] [Google Scholar]
- 10.Burtscher M. Effects of living at higher altitudes on mortality: A narrative review. Aging Dis. 2014;5(4):274–80. doi: 10.14336/AD.2014.0500274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muthuraju S, Maiti P, Solanki P, et al. Acetylcholinesterase inhibitors enhance cognitive functions in rats following hypobaric hypoxia. Behav Brain Res. 2009;203(1):1–14. doi: 10.1016/j.bbr.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 12.Muthuraju S, Maiti P, Solanki P, et al. Possible role of cholinesterase inhibitors on memory consolidation following hypobaric hypoxia of rats. Int J Neurosci. 2011;121(5):279–88. doi: 10.3109/00207454.2011.556279. [DOI] [PubMed] [Google Scholar]
- 13.Bekker A, Haile M, Gingrich K, et al. Physostigmine reverses cognitive dysfunction caused by moderate hypoxia in adult mice. Anesth Analg. 2007;105(3):739–43. doi: 10.1213/01.ane.0000265555.57472.49. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Li Y, Zha Y. [Cognitive impairment in patients with stable chronic obstructive pulmonary disease]. Journal of Clinical Internal Medicine. 2013;31(3):175–76. [in Chinese] [Google Scholar]
- 15.Society CT. Guidelines for the diagnosis and treatment of chronic obstructive pulmonary disease (2013 edition) Clin J Tuberc Respir Dis. 2013;36(4):255–64. [Google Scholar]
- 16.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–79. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969 Autumn;9(3):179–86. [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Mungas D. In-office mental status testing: A practical guide. Geriatrics. 1991;46(7):54–58. 63, 66. [PubMed] [Google Scholar]
- 20.Huang XX, He MC. [Research advance of Alzheimer’ s disease assessment scale in China]. Medical Recapitulate. 2017;23(16):3202–6. [in Chinese] [Google Scholar]
- 21.Jin Z, Wang G. [Interpretation of Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2014)]. Chinese Journal of the Frontiers of Medical Science (Electronic Version) 2014;6(2):94–97. [in Chinese] [Google Scholar]
- 22.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 23.Yuan J, Hu W. Chronic obstructive pulmonary disease and cognitive impairment. Int J Respir. 2013;33(23):1814–16. [Google Scholar]
- 24.Grant I, Heaton RK, McSweeny AJ, et al. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1982;142(8):1470–76. [PubMed] [Google Scholar]
- 25.Thakur N, Blanc PD, Julian LJ, et al. COPD and cognitive impairment: the role of hypoxemia and oxygen therapy. Int J Chron Obstruct Pulmon Dis. 2010;5:263–69. doi: 10.2147/copd.s10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Y. [Influence of long-term family oxygen therapy on life quality of patients with chronic obstructive pulmonary disease]. Chinese Journal of Practical Nursing. 2011;27(14):25–26. [in Chinese] [Google Scholar]
- 27.Hou X, Kong C. [Effect of long-term home oxygen therapy on patients with remission of COPD]. Journal of Taishan Medical College. 2009;19(4):949–50. [Google Scholar]
- 28.Heaton RK, Grant I, McSweeny AJ, et al. Psychologic effects of continuous and nocturnal oxygen therapy in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1983;143(10):1941–47. [PubMed] [Google Scholar]
- 29.Li Y. Expert consensus on prevention and treatment of cognitive dysfunction in China. Clin J Geriatr. 2006;25(7):485–96. [Google Scholar]


