Significance
The aleurone, storing proteins, lipids, vitamins, and minerals, is the most nutritious part of cereal grains. Genetic analyses were conducted to screen for mutants with thickened aleurone, and identified thick aleurone 2-1, which exhibits a multicell-layered aleurone and an improved nutritional profile. Map-based cloning showed that TA2 encodes a DNA demethylase. This study provides a strategy for enhancing the nutritional value of rice, and possibly of other cereals as well.
Keywords: rice, endosperm, thick aleurone, nutrition, DNA demethylation
Abstract
The rice endosperm, consisting of an outer single-cell layer aleurone and an inner starchy endosperm, is an important staple food for humans. While starchy endosperm stores mainly starch, the aleurone is rich in an array of proteins, vitamins, and minerals. To improve the nutritional value of rice, we screened for mutants with thickened aleurones using a half-seed assay and identified thick aleurone 2–1 (ta2-1), in which the aleurone has 4.8 ± 2.2 cell layers on average. Except for starch, the contents of all measured nutritional factors, including lipids, proteins, vitamins, minerals, and dietary fibers, were increased in ta2-1 grains. Map-based cloning showed that TA2 encodes the DNA demethylase OsROS1. A point mutation in the 14th intron of OsROS1 led to alternative splicing that generated an extra transcript, mOsROS1, with a 21-nt insertion from the intron. Genetic analyses showed that the ta2-1 phenotype is inherited with an unusual gametophytic maternal effect, which is caused not by imprinted gene expression but rather by the presence of the mOsROS1 transcript. Five additional ta2 alleles with the increased aleurone cell layer and different inheritance patterns were identified by TILLING. Genome-wide bisulfite sequencing revealed general increases in CG and CHG methylations in ta2-1 endosperms, along with hypermethylation and reduced expression in two putative aleurone differentiation-related transcription factors. This study thus suggests that OsROS1-mediated DNA demethylation restricts the number of aleurone cell layers in rice and provides a way to improve the nutrition of rice.
The triploid cereal endosperm, consisting of an outer aleurone and an inner starchy endosperm, contributes to >70% of the staple foods consumed by humans (1). Endosperm development commences with coenocytic nuclear division, followed by cytokinesis and differentiation of the aleurone and starchy endosperm (2, 3). Although these two tissues have the same developmental origin, they differ in morphology, cell fate, and storage product accumulation. Starchy endosperm, a dead tissue at maturity, accumulates mainly starch, whereas the aleurone, a living tissue, accumulates mainly storage proteins, lipids, vitamins, and minerals (3, 4).
Most cereal grains have a single-cell layered aleurone; the sole known exception is barley (Hordeum vulgare), in which the aleurone has two to three cell layers (5). In rice (Oryza sativa), the aleurone is mostly a single cell layer but consists of three or four cell layers in a small, thickened region near the dorsal vascular bundle (3). Several genes involved in aleurone differentiation have been identified in maize (Zea mays) and rice. In maize, mutations of DEFECTIVE KERNEL 1 (DEK1) or CRINKLY4 (CR4) lead to partial loss of the aleurone (6, 7). In rice, mutation of a DEK1 homolog, ADAXIALIZED LEAF1 (ADL1), or suppressed expression of OsCR4 also causes partial loss of the aleurone cell layer in endosperms (8, 9). In contrast, mutations of NAKED ENDOSPERM (NKD) transcription factors or a class E vacuolar sorting protein SUPERNUMERARY ALEURONE LAYER1 (SAL1) lead to the formation of endosperms with multiple aleurone cell layers (10, 11). Endosperms with multicell layered aleurone also have been observed in rice when the expression of transcriptional activators RISBZ1 and RPBF are suppressed (12). Since all these grains with multicell layered aleurone exhibit severe defects in grain filling, none has been adopted in crop breeding.
DNA methylation plays an essential role in many biological processes, including growth, development, and stress responses (13). In plants, DNA methylation commonly occurs at the cytosine (C) residue in both the symmetrical CG and CHG and the asymmetrical CHH sequence contexts (H = A, C, or T), and is regulated dynamically by balanced methylation and demethylation (13). Previous studies have identified several enzymes underlying active DNA demethylation in Arabidopsis. DEMETER (DME), expressed in the central cell of the female gametophyte and the early endosperm, is critical for establishing imprinted expression of MEDEA by allele-specific demethylation (14, 15). A paralog of DME, ROS1, is constitutively expressed and regulates gene expression by preventing the spread of DNA methylation and transcriptional silencing from transposons (16, 17). In rice, a knockout mutation of the ROS1 homolog, OsROS1, is defective in both male and female gametogenesis and thus produces no seeds (18).
To improve the nutritional value of rice, we screened for mutants with thickened aleurone using a forward genetic approach and identified the thick aleurone 2–1 (ta2-1) mutant, which exhibits enhanced contents of a large set of nutritional factors. The thick aleurone (ta) phenotype is inherited via an unusual gametophytic maternal effect. Molecular genetic analyses revealed that the phenotype is caused by the presence of a malfunctioning mOsROS1 transcript with a dominant negative effect.
Results
The ta2-1 Mutant Exhibits an Increased Number of Aleurone Cell Layers.
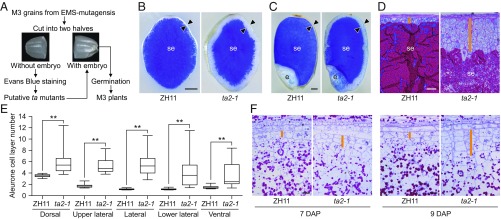
To identify mutants with the ta phenotype, we screened approximately 36,000 M3 grains from more than 6,000 ethyl methanesulfonate-mutagenized M2 rice plants (Oryza sativa, Geng var. Zhonghua 11, ZH11) using a half-seed assay (Fig. 1A). Dehusked mature grains without visible defects were selected, sectioned transversally with a razor blade into two halves, and placed on two parallel 96-well plates. The halves without embryos were stained with Evans blue, a dye that stains dead tissues by penetrating membranes with compromised integrity, which allowed us to distinguish the starchy endosperm, which was stained blue, from the aleurone, which was not stained (Fig. 1B). This screening, performed under a dissecting microscope, allowed us to identify 24 putative ta lines. The corresponding halves with embryos were then sterilized and germinated in vitro on 1/2 MS basal salt medium with 1% sucrose (Fig. 1A). Examination of the M4 grains thus produced allowed us to identify the ta2-1 mutant characterized in detail in this study.
Fig. 1.
Identification and characterization of the ta2-1 mutant. (A) Scheme of the genetic screen for ta mutants in rice using a half-seed assay. (B and C) Transversally (B) and longitudinally (C) sectioned ZH11 and ta2-1 dehusked mature grains, stained with the cell viability dye Evans blue. (Scale bar: 0.5 mm.) (D) Morphology of the aleurone at the lateral positions of ZH11 and ta2-1 endosperms. (Scale bar: 50 μm.) (E) Average numbers of aleurone cell layers, calculated in 20-cell file regions at the dorsal, upper lateral, lateral, lower lateral, and ventral positions of ZH11 and ta2-1 endosperms (n = 30). **Significant differences from ZH11 at the 0.01 level according to Student’s t test. (F) Aleurone at the lateral positions of ZH11 and ta2-1 endosperms at 7 and 9 DAP. (Scale bar: 20 μm.) Arrowheads in B and C and orange lines in D and F indicate the aleurone. e, embryo; se, starchy endosperm.
The ta phenotype of ta2-1 was recognizable in both transversally (Fig. 1B) and longitudinally (Fig. 1C) sectioned grains stained with Evans blue and was confirmed by counterstaining with Sudan red IV, which stains lipids, and Lugol’s iodine, which stains starch (SI Appendix, Fig. S1 A and B). Cytohistological analysis performed on semithin sectioned ta2-1 grains, stained with periodic acid-Schiff (PAS) reagent and Coomassie brilliant blue (CBB), showed that instead of having mostly a single-cell layered aleurone in ZH11, the aleurone of ta2-1 consisted of 2–12 cell layers, with an average of 4.8 ± 2.2 layers (Fig. 1 D and E and SI Appendix, Fig. S1 C and D). In contrast to the semitransparent grains of the wild type, some of the ta2-1 grains were opaque (SI Appendix, Fig. S1 E and F). The differences in aleurones between ZH11 and ta2-1 endosperms were recognizable at 7 d after pollination (DAP) and were more pronounced from 9 DAP onward (Fig. 1F and SI Appendix, Fig. S2).
We examined various agronomic traits after five backcrosses and found that most of these traits in ta2-1 exhibited no detectable defects (SI Appendix, Fig. S3 A–K). However, the 1,000-grain weight and seed setting rate of ta2-1 were reduced by 5.9% and 8.2%, respectively (SI Appendix, Fig. S3 L and M), which led to a 12.1% decrease in grain yield per plant and an 18.0% decrease in the yield per plot (SI Appendix, Fig. S3 N and O).
ta2-1 Grains Showed Improved Nutritional Content.
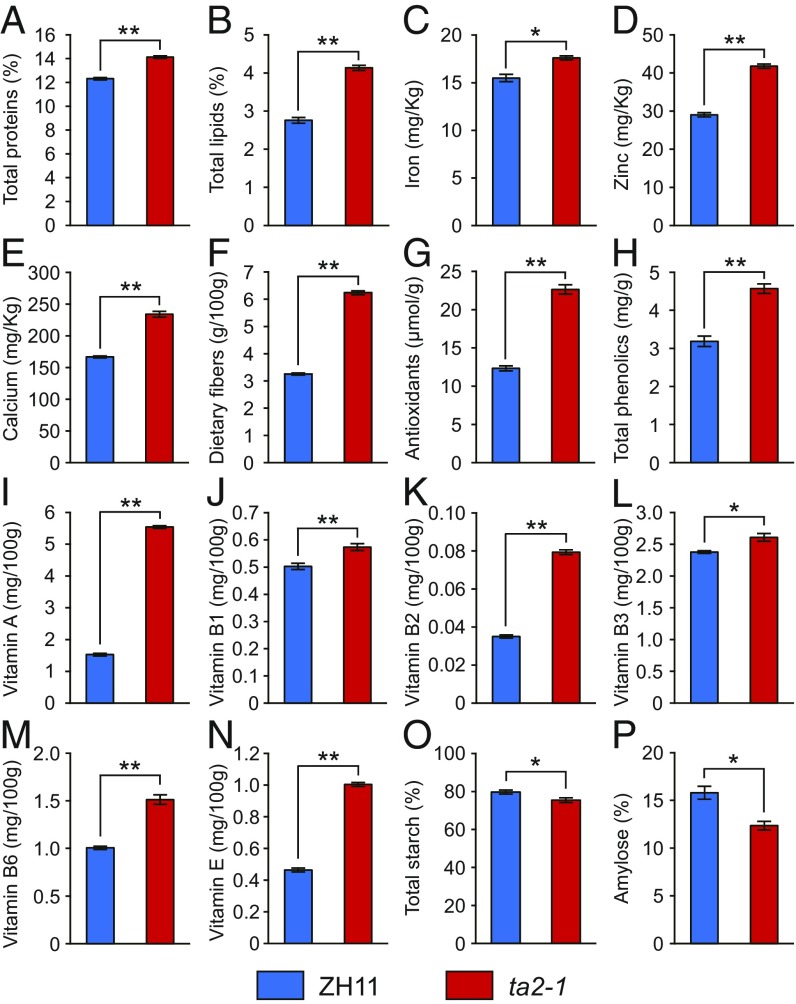
To examine whether the increased number of aleurone cell layers in ta2-1 led to enhanced nutritional value, we measured the contents of various nutritional factors in the dehusked mature grains of ZH11 and ta2-1 plants. As expected, the total protein, lipid, iron, zinc, calcium, dietary fiber, antioxidant, phenolic, and vitamin A, B1, B2, B3, B6, and E contents of ta2-1 grains were significantly higher compared with wild type (Fig. 2 A–N), whereas the total starch and amylose contents were slightly lower (Fig. 2 O and P).
Fig. 2.
Improved nutritional content of dehusked mature grains of ta2-1. (A) Total proteins. (B) Total lipids. (C) Iron. (D) Zinc. (E) Calcium. (F) Dietary fiber. (G) Antioxidants. (H) Total phenolics. (I) Vitamin A. (J) Vitamin B1. (K) Vitamin B2. (L) Vitamin B3. (M) Vitamin B6. (N) Vitamin E. (O) Total starch. (P) Amylose. Data are mean ± SD (n = 3). * and ** denote significant differences from ZH11 at the 0.05 and 0.01 levels, respectively, according to Student’s t test.
Genetic Analysis of ta2-1.
Genetic analysis revealed that 49.4% of grains produced by heterozygous plants carrying the ta2-1 mutation (TA2/ta2-1 hereinafter) exhibited the ta phenotype (Table 1). When ZH11 was pollinated by either ta2-1 or TA2-1/ta2-1, all grains produced exhibited the wild-type phenotype, whereas when ta2-1 was pollinated by ZH11 or TA2-1/ta2-1, all grains exhibited the ta phenotype (Table 1 and SI Appendix, Fig. S4). Moreover, when TA2/ta2-1 was pollinated by ZH11 or ta2-1, approximately 50% of grains exhibited the ta phenotype (Table 1). These results suggest that the inheritance of the ta2-1 trait has a gametophytic maternal effect.
Table 1.
Genetic analyses of ta2-1
| Cross combination | Endosperm phenotype, n | Grains with ta phenotype, % | P for 1:1 | |
| Wild type | ta | |||
| TA2/ta2-1 × TA2/ta2-1 | 321 | 313 | 49.4 | 0.7506 (NS) |
| ZH11 × ta2-1 | 197 | 0 | 0 | NA |
| ZH11 × TA2/ta2-1 | 171 | 0 | 0 | NA |
| ta2-1 × ZH11 | 0 | 589 | 100 | NA |
| ta2-1 × TA2/ta2-1 | 0 | 422 | 100 | NA |
| TA2/ta2-1 × ZH11 | 199 | 193 | 49.2 | 0.7618 (NS) |
| TA2/ta2-1 × ta2-1 | 212 | 214 | 50.2 | 0.9228 (NS) |
NA, not applicable; NS, not significant.
Molecular Cloning of TA2.
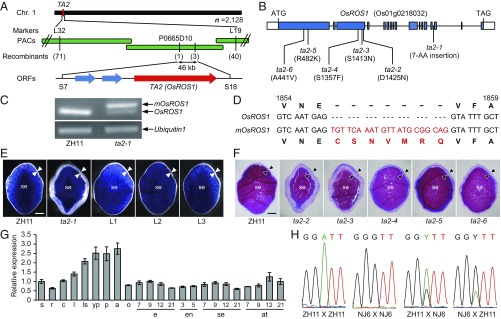
To identify the TA2 gene, map-based cloning was performed on the progeny of a cross between ta2-1 and a Xian rice variety, Nanjing 6 (NJ6). Using a population of >7,000 F2 individuals, TA2 was located to a 46-kb genomic region that contains three genes (Fig. 3A). Sequence analysis revealed a guanine (G)-to-adenine (A) mutation in the 14th intron of Os01g0218032 in ta2-1 (Fig. 3B). The protein encoded by this gene shared the highest level of sequence identity with ROS1 in Arabidopsis (SI Appendix, Fig. S5 and Table S1), and thus was designated OsROS1. OsROS1 contains all three conserved domains present in ROS1 and DME in Arabidopsis (SI Appendix, Figs. S5 and S6).
Fig. 3.
Molecular characterization of TA2. (A) TA2 was mapped to chromosome 1. Numbers of recombinants are indicated in parentheses. (B) The TA2/OsROS1 gene model and mutations in ta2-1 and TILLING alleles, with mutated amino acids indicated in parentheses. AA, amino acid. (C) Two types of transcripts are present in ta2-1: a normal OsROS1 and a slightly larger mOsROS1. Ubiquitin served as a control. (D) mOsROS1 had a 21-nt insertion from the 14th intron of OsROS1, resulting in an in-frame insertion of seven amino acids. (E) Transversally sectioned dehusked grains of ZH11, ta2-1, and three independent ta2-1 transgenic lines (L1, L2, and L3) carrying pCAMBIA1300-OsROS1, stained with Evans blue, to show complete complementation. (Scale bar: 0.5 mm.) (F) Semithin sections of the dehusked mature grains of ZH11 and ta2-2–ta2-6, stained with PAS and CBB. (Scale bar: 0.5 mm.) (G) Relative expression levels of OsROS1 in various tissues of ZH11: seedlings (s), roots (r), culms (c), young leaves (l), leaf sheaths (ls), young panicles (yp), mature pollen (p), mature anthers (a), ovaries before anthesis (o), embryos (e), endosperm (en), starchy endosperm (se), and mixed aleurone and testa samples (at) at different DAPs. Values are plotted relative to OsROS1 expression in seedlings, which was set to 1. Data are mean ± SD (n = 3). (H) Sequencing chromatographs of RT-PCR products, with an SNP (A or G) in the amplicon, to show the relative abundance of maternal and paternal OsROS1 transcripts in 2-DAP endosperms after reciprocal crosses between ZH11 and NJ6, with the female parent written first. Y represents the presence of both A and G. Arrowheads in E and F indicate the aleurone. se, starchy endosperm.
To examine whether the intronic mutation in ta2-1 altered the transcription, OsROS1 cDNA from ZH11 and ta2-1 endosperms was amplified by reverse transcription PCR (RT-PCR). In addition to the normal OsROS1 transcript detected in ZH11, another slightly larger one, designated as mOsROS1, was found in ta2-1 (Fig. 3C). Sequence analysis revealed the presence of an extra 21 nucleotides originating from the 14th intron of OsROS1 in mOsROS1, expected to cause an in-frame insertion of seven amino acid residues (CSNVMRQ) in the conserved C-terminal region of the encoded protein (Fig. 3D and SI Appendix, Fig. S5).
A 16.1-kb genomic sequence harboring the 4.7-kb 5′ upstream region and the 11.4-kb coding sequence of OsROS1 was amplified from ZH11 and introduced into ta2-1 by transformation. Complete complementation of the ta phenotype was observed in three independent lines (Fig. 3E and SI Appendix, Fig. S7), confirming that the ta2-1 phenotype was a result of a mutation in OsROS1.
Furthermore, using an in-house TILLING platform, we identified an additional 23 ta2 alleles with point mutations in OsROS1 (SI Appendix, Table S2). Among these, five (ta2-2–ta2-6) exhibited the ta phenotype (Fig. 3 B and F and SI Appendix, Fig. S5). The number of aleurone cell layers varied from two to six in ta2-4, ta2-5, and ta2-6, which had mutations in nonconserved regions, and from five to 11 in ta2-2 and ta2-3, which had mutations in the conserved glycosylase domain (SI Appendix, Fig. S8).
Genetic analysis in selected alleles showed that the ta2-3 phenotype was inherited in the same manner as ta2-1, while ta2-4, ta2-5, and ta2-6 had reduced proportions of grains with the ta phenotype (SI Appendix, Table S3). Analyses of agronomic traits revealed that while ta2-3 showed reductions in grain thickness, 1,000-grain weights, seed setting rate, and grain yield per plant, no detectable differences were observed in yield-related traits of ta2-4, ta2-5, and ta2-6 (SI Appendix, Fig. S9).
Expression of OsROS1.
Quantitative RT-PCR (qRT-PCR) analysis showed OsROS1 expression in all tissues examined, with higher expression levels in mature pollen, anthers, and ovaries (Fig. 3G). In situ hybridization revealed OsROS1 expression in the vascular tissue, pollen grain, pericarp, aleurone, and starchy endosperm (SI Appendix, Fig. S10). We sequenced the RT-PCR products prepared from endosperms collected at 2, 3, 4, 5, 6, and 8 DAP after reciprocal crosses between ZH11 and NJ6, which had a single nucleotide polymorphism (SNP) in the amplicon of OsROS1. Our results showed expression of both maternal and paternal OsROS1 in all stages examined, with the expected higher maternal expression due to the contribution of two maternal genomes and one paternal genome in the triploid endosperm (Fig. 3H and SI Appendix, Fig. S11), suggesting that OsROS1 is not an imprinted gene.
The mOsROS1 Transcript Caused the ta Phenotype in ta2-1.
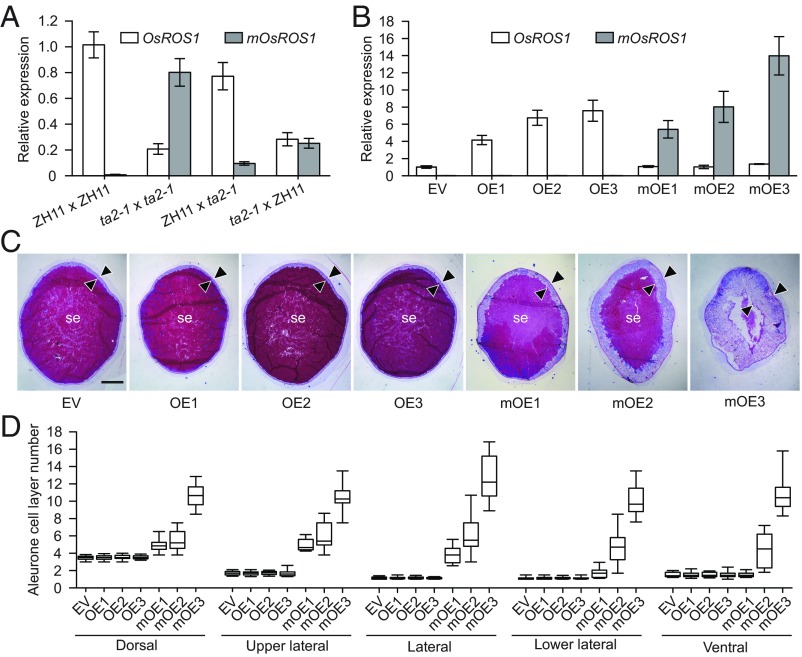
The qRT-PCR analysis using primers able to distinguish OsROS1 from mOsROS1 showed that the mOsROS1 transcript was approximately four times more abundant than OsROS1 in ta2-1 endosperm (Fig. 4A). However, in endosperm produced in ZH11 pollinated by ta2-1, the mOsROS1 transcript was only roughly one-eighth as abundant as OsROS1 (Fig. 4A). The two transcripts were approximately equally abundant in endosperms produced in ta2-1 plants pollinated by ZH11 (Fig. 4A). We thus propose that the ta phenotype is positively associated with the dosage of the mOsROS1 transcript, such that when ≥50% of the transcripts produced are mOsROS1, the ta phenotype is inevitable.
Fig. 4.
The presence of mOsROS1 led to the ta phenotype. (A) qRT-PCR showing the relative abundance of OsROS1 and mOsROS1 transcripts in 9-DAP endosperms produced in crosses between ZH11 and ta2-1, with the female parent written first. Values are plotted relative to the expression of OsROS1 in endosperms of ZH11 × ZH11, which was set to 1. Data are shown as mean ± SD (n = 3). (B) Relative OsROS1 and mOsROS1 transcript levels in 9-DAP endosperms in transgenic plants carrying an empty vector (EV), pUbi::OsROS1 (lines OE1–OE3), or pUbi::mOsROS1 (lines mOE1–mOE3). Values are plotted relative to the expression of OsROS1 in transgenic lines carrying an EV, which was set to 1. Data are shown as mean ± SD (n = 3). (C) Endosperm phenotypes of EV, OE, and mOE plants. Arrowheads indicate the aleurone. se, starchy endosperm. (Scale bar: 0.5 mm.) (D) Average numbers of aleurone cell layers, calculated in 20-cell file regions at different positions of endosperms in EV, OE, and mOE plants (n = 15).
To validate this hypothesis, we generated transgenic ZH11 plants carrying either pUbi::OsROS1 (OE) or pUbi::mOsROS1 (mOE), in which OsROS1 or mOsROS1, respectively, was overexpressed under control of the maize Ubiquitin 1 promoter (pUbi). While none of the OE lines exhibited the ta phenotype, three independent mOE lines showed the ta phenotype (Fig. 4 C and D). In mOE plants, the thickness of the aleurone was positively associated with the expression level of the mOsROS1 transcript (Fig. 4 B–D). These observations confirm that the gametophytic maternal effect observed in ta2-1 is caused by the dominant negative effect of mOsROS1.
The Role of OsROS1 in CG and CHG Demethylation in Endosperms.
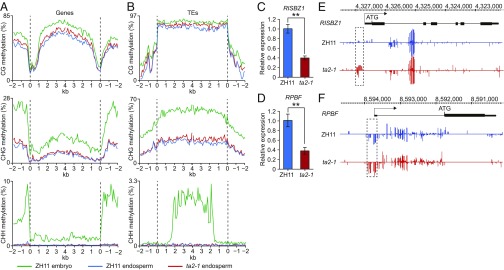
To investigate the function of OsROS1 in DNA demethylation in rice endosperm, we performed whole-genome bisulfite sequencing (WGBS) using DNA extracted from embryos and endosperms of ZH11 and ta2-1 grains, and then analyzed the distribution of DNA methylation along the gene bodies, transposable elements (TEs), and their flanking regions. In agreement with previously reported results (19, 20), in ZH11, higher CG, CHG, and CHH methylation levels were observed in the embryo than in the endosperm (Fig. 5 A and B). Evident higher levels of CG and CHG, but not of CHH, methylation were observed in the ta2-1 endosperm (Fig. 5 A and B), suggesting that OsROS1 is involved in CG and CHG demethylation in the endosperm.
Fig. 5.
Altered DNA methylation and gene expression in ta2-1 endosperms revealed by WGBS. (A and B) Distribution of DNA methylation levels along gene bodies (A) and TEs (B) in embryos and endosperms of ZH11 and ta2-1 at 9 DAP. (C and D) Reduced expression levels of RISBZ1 (C) and RPBF (D) in ta2-1 9-DAP endosperms revealed by qRT-PCR. Data are shown as mean ± SD (n = 3). **Significant differences from ZH11 at the 0.01 level according to Student’s t test. (E and F) Snapshots in the Integrated Genome Browser showing DNA methylation levels in the RISBZ1 (E) and RPBF (F) of ZH11 and ta2-1 endosperms at 9 DAP. Dashed frames indicate the hypermethylated regions in ta2-1.
We then analyzed differentially methylated regions (DMRs) in the ta2-1 endosperm collected at 9 DAP. A total of 18,399 DMRs were identified in ta2-1, which contained 15,147 hypermethylated DMRs (hyper-DMRs) with an average length of 257 bp (Dataset S1). The average methylation levels of CG and CHG hyper-DMRs in ta2-1 endosperms were significantly higher than those in ZH11 (SI Appendix, Fig. S12A), which is consistent with the role of OsROS1 in DNA demethylation. Among these hyper-DMRs, 43.2% (n = 6,548) were located in TE regions, 38.7% (n = 5,860) were located in intergenic regions, 13.5% (n = 2,042) were located in genic regions, and 4.6% (n = 697) were located in the TE and gene overlapping regions (SI Appendix, Fig. S12B), indicating that OsROS1, like DME and ROS1 in Arabidopsis, preferentially targets TEs and intergenic regions.
Hypermethylation and Reduced Expression of RISBZ1 and RPBF in ta2-1.
To identify the potential targets responsible for the ta phenotype, the DNA methylation and expression levels of genes known to be involved in aleurone differentiation were analyzed in endosperms at 9 DAP. In ta2-1 endosperms, the expression levels of OsNKD1 and OsDEK1 were not affected (SI Appendix, Fig. S13 A and B), whereas the expression levels of RISBZ1 and RPBF were significantly reduced (Fig. 5 C and D). By scanning the DNA methylation status of the RISBZ1 and RPBF promoters, we found that a 205-bp region at −68 to −273 bp of RISBZ1 (Fig. 5E) and a 219-bp region at 45 to −174 bp of RPBF (Fig. 5F) relative to the transcription start site were hypermethylated in the ta2-1 endosperms. As expected, no obvious difference in OsNKD1 and OsDEK1 was observed (SI Appendix, Fig. S13 C and D). These results suggest that DNA hypermethylation in the promoter regions of RISBZ1 and RPBF may compromise the expression of these genes.
Discussion
Nutritional improvement in food crops is an important goal in modern agriculture (21) that is achievable for individual nutritional factors by introducing a single gene, such as ferritin (22), or multiple genes, such as those in folic acid (23) and β-carotenoid biosynthesis (24). The results obtained in this study demonstrate the feasibility of improving the general nutritional profile of rice by screening for ta mutants. We showed that weak allele mutations of OsROS1 led to increased numbers of aleurone cell layers and improved the contents of a large set of nutritional factors.
Four putative 5-methylcytosine DNA demethylase genes (DME, ROS1, DML2, and DML3) are present in Arabidopsis, and six such genes (OsROS1, OsROS1b, OsROS1c, OsROS1d, OsDML3a, and OsDML3b) are present in rice. DME is preferentially expressed in the central cell of the female gametophyte and in early endosperms and activates the expression of MEDEA by allele-specific DNA demethylation (14, 15). Thus, mutations of DME lead to a seed abortion phenotype with a gametophytic maternal effect (14). In contrast, ROS1 in Arabidopsis is expressed in all tissues examined and is crucial for preventing transcriptional gene silencing by DNA demethylation (16, 17). In this study, we showed that ta2-1 carries a point mutation in the intronic region of OsROS1, leading to the production of an alternatively spliced mOsROS1 transcript. Expression analysis performed in reciprocal crosses between ZH11 and NJ6 showed that OsROS1 is not an imprinted gene. Together with the fact that three TILLING alleles (ta2-4, ta2-5, and ta2-6) of OsROS1 did not show gametophytic maternal effects, our findings suggest that OsROS1 is a functional analog of ROS1, not of DME.
A previous study showed that an OsROS1 knockout mutant exhibits severe defects in both male and female gametogenesis, and thus is not able to set seed (18). However, we have shown that ta2-1, a weak mutant allele of OsROS1, exhibits an increased number of aleurone cell layers and an improved nutritional profile. TILLING alleles of ta2-4, ta2-5, and ta2-6, with single amino acid substitutions in the nonconserved domain of OsROS1, do not have defects in yield-related traits. These findings suggest the critical importance of exploring the function and phenotype of a gene with multiple alleles.
Expression and phenotypic analyses of endosperms produced after the reciprocal crosses between ZH11 and ta2-1, and by transgenic overexpression of mOsROS1 in the wild type, indicated that the unusual inheritance pattern of ta2-1 is caused by the presence of the mOsROS1 transcript. We noted that the abundance of mOsROS1 in the endosperm produced in ZH11 plants pollinated by ta2-1 was lower than expected. Whether this is caused by differential splicing or by suppressed expression of mOsROS1 remains to be determined.
WGBS analysis revealed increased levels of CG and CHG methylations in the ta2-1 endosperms, approaching the levels seen in ZH11 embryos, suggesting that OsROS1 functions in CG and CHG demethylation. On examination of methylation levels of putative genes involved in aleurone differentiation (8, 10, 12), we observed that in ta2-1 endosperms, the promoter regions of two transcription factors, RISBZ1 and RPBF, were hypermethylated, and that the expression levels of these genes were substantially reduced, indicating that they are potential target genes causing the ta phenotype.
In summary, our results suggest that active DNA demethylation in rice endosperms, executed by OsROS1, restricts the number of aleurone cell layers. These results highlight the potential for manipulating OsROS1 activity to improve the nutritional value of rice and may have applications in other cereal crops as well.
Materials and Methods
Details on plant growth conditions, histological analyses, nutritional content measurements, agronomic trait analyses, map-based cloning, expression analyses, plant transformations, in situ hybridization, and WGBS are provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Chinese Cereal Quality Supervision and Monitoring Center, Ministry of Agriculture and Dr. Tony Bird at CSIRO Health and Biosecurity for their assistance with nutritional analyses. This work was supported by the Ministry of Science and Technology of China (Grant 2016YFD0100501/2014CB943401), the Chinese Academy of Sciences (CAS) Innovation Project “Molecular Modules for Breeding Design” (Grant XDA08010301), and the CAS-Commonwealth Scientific and Industrial Research Organization Bilateral Collaboration Project (GJHZ1406).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequencing data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo/ (accession no. GSE117187).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1806304115/-/DCSupplemental.
References
- 1.Food and Agriculture Organization Human Nutrition in the Developing World. Available at www.fao.org/docrep/W0073e/w0073e06.htm. Accessed July 10, 2018.
- 2.Olsen OA. Nuclear endosperm development in cereals and Arabidopsis thaliana. Plant Cell. 2004;16(Suppl):S214–S227. doi: 10.1105/tpc.017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X, Liu J, Li D, Liu CM. Rice caryopsis development II: Dynamic changes in the endosperm. J Integr Plant Biol. 2016;58:786–798. doi: 10.1111/jipb.12488. [DOI] [PubMed] [Google Scholar]
- 4.Becraft PW, Yi G. Regulation of aleurone development in cereal grains. J Exp Bot. 2011;62:1669–1675. doi: 10.1093/jxb/erq372. [DOI] [PubMed] [Google Scholar]
- 5.Jestin L, et al. Inheritance of the number and thickness of cell layers in barley aleurone tissue (Hordeum vulgare L.): An approach using F2-F3 progeny. Theor Appl Genet. 2008;116:991–1002. doi: 10.1007/s00122-008-0730-6. [DOI] [PubMed] [Google Scholar]
- 6.Lid SE, et al. The defective kernel 1 (dek1) gene required for aleurone cell development in the endosperm of maize grains encodes a membrane protein of the calpain gene superfamily. Proc Natl Acad Sci USA. 2002;99:5460–5465. doi: 10.1073/pnas.042098799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becraft PW, Stinard PS, McCarty DR. CRINKLY4: A TNFR-like receptor kinase involved in maize epidermal differentiation. Science. 1996;273:1406–1409. doi: 10.1126/science.273.5280.1406. [DOI] [PubMed] [Google Scholar]
- 8.Hibara K, et al. The ADAXIALIZED LEAF1 gene functions in leaf and embryonic pattern formation in rice. Dev Biol. 2009;334:345–354. doi: 10.1016/j.ydbio.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 9.Pu CX, et al. Crinkly4 receptor-like kinase is required to maintain the interlocking of the palea and lemma, and fertility in rice, by promoting epidermal cell differentiation. Plant J. 2012;70:940–953. doi: 10.1111/j.1365-313X.2012.04925.x. [DOI] [PubMed] [Google Scholar]
- 10.Yi G, Neelakandan AK, Gontarek BC, Vollbrecht E, Becraft PW. The naked endosperm genes encode duplicate INDETERMINATE domain transcription factors required for maize endosperm cell patterning and differentiation. Plant Physiol. 2015;167:443–456. doi: 10.1104/pp.114.251413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen B, et al. sal1 determines the number of aleurone cell layers in maize endosperm and encodes a class E vacuolar sorting protein. Proc Natl Acad Sci USA. 2003;100:6552–6557. doi: 10.1073/pnas.0732023100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawakatsu T, Yamamoto MP, Touno SM, Yasuda H, Takaiwa F. Compensation and interaction between RISBZ1 and RPBF during grain filling in rice. Plant J. 2009;59:908–920. doi: 10.1111/j.1365-313X.2009.03925.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Lang Z, Zhu JK. Dynamics and function of DNA methylation in plants. Nat Rev Mol Cell Biol. 2018;19:489–506. doi: 10.1038/s41580-018-0016-z. [DOI] [PubMed] [Google Scholar]
- 14.Choi Y, et al. DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in Arabidopsis. Cell. 2002;110:33–42. doi: 10.1016/s0092-8674(02)00807-3. [DOI] [PubMed] [Google Scholar]
- 15.Gehring M, et al. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell. 2006;124:495–506. doi: 10.1016/j.cell.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong Z, et al. ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell. 2002;111:803–814. doi: 10.1016/s0092-8674(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 17.Tang K, Lang Z, Zhang H, Zhu JK. The DNA demethylase ROS1 targets genomic regions with distinct chromatin modifications. Nat Plants. 2016;2:16169. doi: 10.1038/nplants.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono A, et al. A null mutation of ROS1a for DNA demethylation in rice is not transmittable to progeny. Plant J. 2012;71:564–574. doi: 10.1111/j.1365-313X.2012.05009.x. [DOI] [PubMed] [Google Scholar]
- 19.Zemach A, et al. Local DNA hypomethylation activates genes in rice endosperm. Proc Natl Acad Sci USA. 2010;107:18729–18734. doi: 10.1073/pnas.1009695107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh TF, et al. Genome-wide demethylation of Arabidopsis endosperm. Science. 2009;324:1451–1454. doi: 10.1126/science.1172417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeiffer WH, McClafferty B. HarvestPlus: Breeding crops for better nutrition. Crop Sci. 2007;47(Suppl_3):S88–S105. [Google Scholar]
- 22.Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F. Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol. 1999;17:282–286. doi: 10.1038/7029. [DOI] [PubMed] [Google Scholar]
- 23.Storozhenko S, et al. Folate fortification of rice by metabolic engineering. Nat Biotechnol. 2007;25:1277–1279. doi: 10.1038/nbt1351. [DOI] [PubMed] [Google Scholar]
- 24.Paine JA, et al. Improving the nutritional value of golden rice through increased pro-vitamin A content. Nat Biotechnol. 2005;23:482–487. doi: 10.1038/nbt1082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.