Abstract
Context: The purpose of this report was to describe the improvement in walking ability using the Hybrid Assistive Limb® (HAL®) intervention in the case of a patient with paraplegia after spinal cord injury whose condition deteriorated because of a spinal dural arteriovenous fistula (SDAVF).
Findings: A 48-year-old man started the HAL® intervention twice per week (total 10 sessions), after his neurologic improvement had plateaued from 3 to 6 months postoperatively for an SDAVF. During the HAL® intervention, the 10-m walk test (10MWT) without HAL® was performed before and after each session. An electromyography system was used to evaluate muscle activity of both the gluteus maximus (Gmax) and quadriceps femoris (Quad) muscles in synchronization with the Vicon motion capture system. The International Standards for Neurological and Functional Classification of Spinal Cord Injury (ISNCSCI) motor scores of the lower extremities and the Walking Index for Spinal Cord Injury II (WISCI II) score were also assessed to evaluate motor function. The HAL® intervention improved gait speed and cadence during the 10MWT. Before the intervention, both the Gmax and left Quad muscles were not activated. After the intervention, the right Gmax and both Quad muscles were activated in stance phase rhythmically according to the gait cycle. The ISNCSCI motor score also improved from 14 to 16, and the WISCI II scored improved from 7 to 12.
Conclusion/clinical relevance: Our experience with this patient suggests that the HAL® can be an effective tool for improving functional ambulation in patients with chronic spinal cord injury.
Keywords: Hybrid Assistive Limb (HAL®), Spinal dural arteriovenous fistula, Chronic spinal cord injury, Gait analysis, Rehabilitation
Introduction
Spinal dural arteriovenous fistulas (SDAVFs) are spinal vascular lesions with nonspecific symptoms. SDAVFs are difficult to diagnose, and they slowly progress to severe myelopathy with paraplegia and bowel-bladder disturbances. Spontaneous recovery is rare, and a delayed diagnosis causes irreversible spinal cord injury.1–6
The Hybrid Assistive Limb® (HAL®) is a wearable robot suit that assists one in voluntary control of knee joint and hip joint motion by detecting signals from force/pressure sensors in the shoes and very weak bioelectric signals on the surface of the skin. Power units on the hip and knee joints on both sides consist of angular sensors and actuators, and the control system consists of a cybernic voluntary control (CVC) mode and cybernic autonomous control (CAC) subsystem.7 Gait training with the HAL® has been reported to improve gait ability for chronic stroke,8–10 chronic spinal cord injury,10–13 and postoperative patients with thoracic ossification of the posterior longitudinal ligament.14–16
We describe the effects of the HAL® intervention using gait analysis in a patient with chronic spinal cord injury who underwent operation for an SDAVF, and whose postoperative improvement plateaued.
Case report
Patient
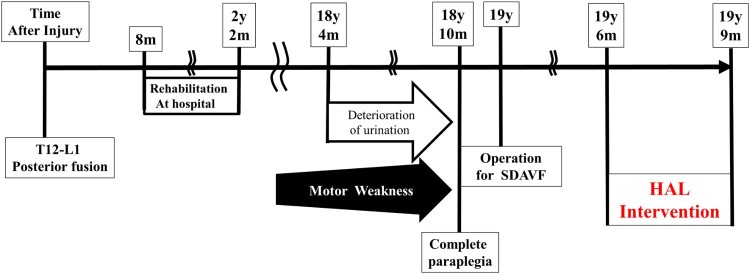
A 48-year-old man with incomplete paraplegia due to chronic spinal cord injury complained of being completely paraplegic. He was referred and diagnosed as having an SDAVF. His clinical course is summarized in Figure 1.
Figure 1.
Summary of the patient's clinical course. y, years; m, months; SDAVF, spinal dural arteriovenous fistula; HAL, Hybrid Assistive Limb®.
He had sustained a burst fracture of the first lumbar vertebra 19 years previously. He had undergone posterior fusion with an instrumented rod at the T12–L1 level at an emergency hospital on the same day, and he was transferred to a rehabilitation hospital 8 months after the injury. Upon admission, he was an incomplete paraplegic: the American Spinal Cord Injury Association (ASIA) impairment scale (AIS17) was grade C; International Standards for Neurological and Functional Classification of Spinal Cord Injury (ISNCSCI)17,18 motor score (both lower extremities) was 20 points; sensory score for a pinprick was 91 points (right: 46 points, left: 45 points); motor neurological level was L3; and sensory neurological levels were L2 and L3 on the right and left sides, respectively. After in-hospital rehabilitation for 1 year and 4 months, he gained the ability to perform activities of daily living independently with a wheelchair and to walk with leg braces (right side, short leg brace; left side, long leg brace [LLB]) at home. He sensed the need for micturition and performed intermittent self-catheterization.
After hospital discharge, he attended follow-up visits every 3 months. He had returned to work as a researcher, and his motor function had not changed for several years; however, he had few opportunities for walking with leg braces.
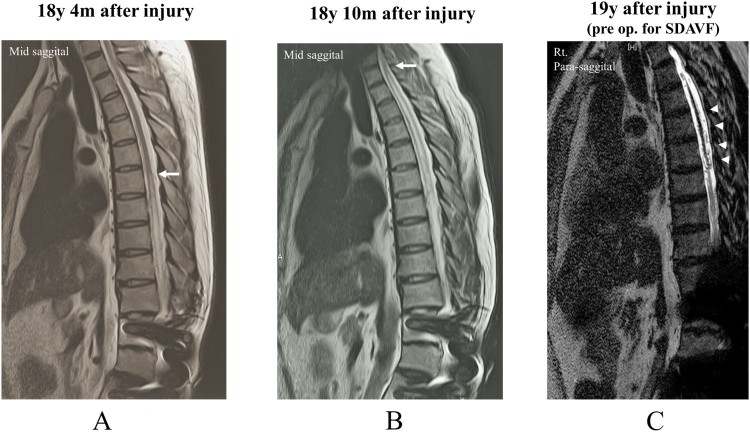
About 10 years post-injury, he had become aware of progressive muscle weakness. He also had gradually lost the ability to perceive the need for micturition 6 months before the first visit to our hospital. His assessment was as follows: ISNCSCI motor score of the lower extremities, 13 points; AIS,17 grade A; and motor neurological levels, L3 and L2 for the right and left sides, respectively. Post-traumatic syringomyelia was suggested based on the T2-weighted magnetic resonance imaging (MRI) scan of the thoracic spine (Fig. 2A). His disorder of urination and motor weakness gradually progressed; therefore, he was observed during regular hospital visits. When his condition worsened, he was admitted.
Figure 2.
(A) Sagittal slice of the T2-weighted (T2WI) magnetic resonance imaging (MRI) scan showing a high-intensity change up to the T7 level in the cord (arrow) at the time of deterioration in urination. (B) T2WI MRI scan obtained when the patient developed complete paraplegia, which shows a high-intensity change in the cord up to the T2 level (arrow). (C) T2 fast-recovery fast spin-echo MRI scan (pre op) showing that the flow void is enlarged around the spinal cord (arrowheads). y, years; m, months.
During his visit for complete paraplegia, syringomyelia was again suggested based on the T2-weighted MRI scan (Fig. 2B), so he was referred to us. Another MRI examination showed edematous change of the spinal cord and the flow void enlarged around the spinal cord in fast-recovery fast spin-echo (T2 DRIVE). He was considered to have an SDAVF (Fig. 2C).
After he was diagnosed as having an SDAVF based on the spinal angiogram, he underwent an operation. He underwent direct surgical intervention and ligation of the SDAVF. The arteriovenous fistula was located near the twelfth thoracic vertebra with a venous varix, which had a single feeder and single drainer. Postoperatively, he could perform voluntary hip flexion and knee extension to a slight degree.
Sixteen days postoperatively, he was discharged; he returned for rehabilitation every week. One month postoperatively, his paralysis improved predominantly on the right side. The ISCNCSCI motor score of the lower extremities was 10 points, AIS was grade A, and motor neurological levels were T10 on both sides. Subsequently, his paralysis gradually improved. Three months postoperatively, he was able to walk with both LLBs and the assistance of a physical therapist. The ISCNCSCI motor score of the lower extremities became 14 points; AIS was grade A; and motor neurological levels were T10 on both sides. The manual muscle testing (MMT) score of both hip flexors was 4, and the MMT scores of the knee extensor were 4 for the right side and 2 for the left side. For the gluteus maximus (Gmax), the MMT scores were 1 for the right side and 0 for the left side. The Walking Index for Spinal Cord Injury II (WISCI II) score19–22 was 3 by 3 months and 7 by 4 months postoperatively. In addition, he had no perception of the need to urinate.
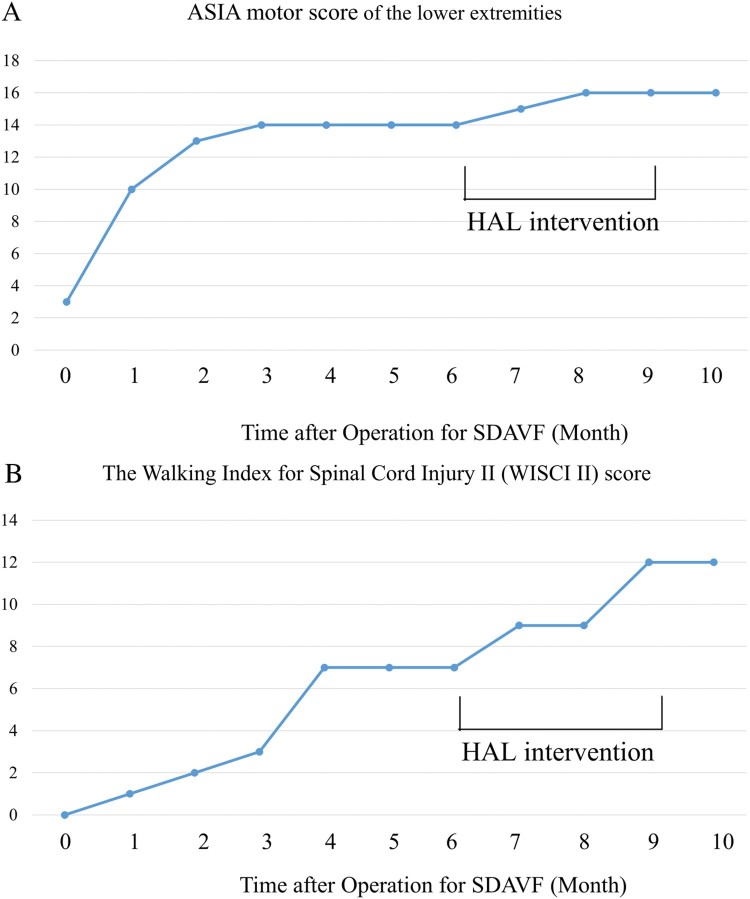
Six months postoperatively, his ISCNCSCI motor and WISCII scores remained unchanged (Fig. 3A-B). Therefore, we concluded that his improvement had plateaued, so we performed the HAL® intervention.
Figure 3.
Progression of the International Standards for Neurological and Functional Classification of Spinal Cord Injury motor score of the lower extremities (A) and the Walking Index for Spinal Cord Injury II score (B). HAL®, Hybrid Assistive Limb®.
HAL® intervention
The patient underwent 10 HAL® sessions (20 minutes each) during 3 months. We defined each locomotion opportunity with the HAL® as a HAL® session and the 10 HAL® sessions as the HAL® intervention. The first 5 sessions were implemented with typical physical therapy once per week, and for the latter 5 sessions, a typical HAL® session proceeded as follows: perform the 10-meter walking test (10MWT) without the HAL®, prepare the electrodes, and put on the HAL® suit (20 minutes); walk with the HAL® (20 minutes, including periods of rest); and take off the HAL® suit and perform the 10MWT (20 minutes). A physiatrist was present in case of an emergency, a therapist and two assistants took the HAL® suit on and off, and an engineer implemented gait analysis. For safety reasons, a walking device (All-in-One Walking Trainer, Ropox A/S, Naestved, Denmark) with a harness was used to prevent falls.
The HAL® suit has a hybrid control system comprising the CVC and CAC modes. The CVC mode of the HAL® suit can support the wearer's voluntary motion by providing assistive torque to each joint according to the voluntary muscle activity. The CAC mode can move the wearer's leg by signals from the force-pressure sensors.7 In the CVC mode, it needs to detect the wearer's muscle activation. However, for patients with severe paralysis of their leg, it is difficult to detect their neuromuscular activities. In these cases, we use the CAC mode, because it applies a pre-designed joint trajectory in accordance with foot landing instead of relying on the detected neuromuscular activities. When this study started, the patient had more severe paralysis in the left leg with weaker neuromuscular activation than in the right leg; therefore, we used the CVC mode for the right side and CAC mode for the left side. As the CVC mode enables the operator to adjust the degree of physical support according to the patient's comfort, we gradually reduced the support as the sessions progressed.
Assessments
Assessments were performed before and after the HAL® intervention. A Trigno™ Lab Wireless EMG System (Delsys, Inc., Boston, MA, USA) was used to evaluate muscle activity of both Quad and Gmax muscles. Each muscle's activity was evaluated using electromyography, which was collected at 2000Hz and filtered with a 30–400-Hz bandwidth passing filter using scripts on MATLAB 8.2 (Mathworks, Natick, MA, USA). Motion capture (Vicon MX with 16 T20S cameras, Vicon, Oxford, UK) was used to evaluate foot motion in synchronization with electromyography. Auto-reflective markers were placed on the feet following VICON plug-in gait marker set, head of the second metatarsal bone for the toe, lateral malleolus for the ankle, and posterior peak of the calcaneus for the heel. The swing phase and stance phase within a gait cycle were extracted according to the movement trajectory of the markers. Heel strikes were detected as the lower peaks of the height of the heel markers, and toe lifts were detected at the lower peaks of the toe markers. The swing phase was detected as the duration starting with a toe lift and ending with the succeeding heel strike on the same side. The stance phase was detected as the duration starting with a heel strike and ending with the succeeding toe lift.
Ambulatory function was assessed using the 10MWT results, measured muscle activity, and WISCI II score. ISCNCSCI motor subscores of the lower extremities were assessed to evaluate motor function.
Results
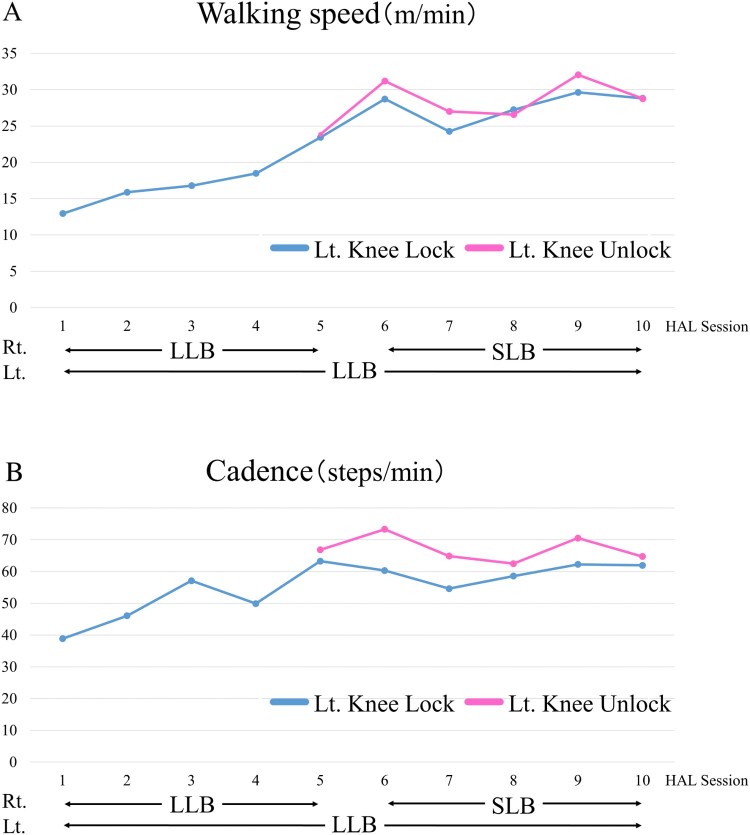
Improvements in gait speed and cadence were observed in the 10MWT. Figures 4A and B show the data before each session. In the 10MWT, from the first to the fifth session, he walked with both LLBs, and from the sixth to tenth session, he used the right short leg brace (SLB) and left LLB with the All-in-One Walking Trainer. Before the HAL® intervention, he could walk with the right LLB in the knee-unlocked position; however, he could only walk in the left knee-locked position (knee extended position). After the fifth session, he walked using an LLB with and without the knee extension locked.
Figure 4.
Walking speed (A) and cadence (B) before each HAL® session are improved after the Hybrid Assistive Limb® (HAL®) intervention. In the first 5 HAL® sessions, the patient used a right long leg brace (LLB) and left LLB, and in the last HAL® sessions, he used a right short leg brace (SLB) and left LLB. From the fifth session, he used a left LLB in both the knee-locked position and knee-unlocked position. Rt., right; Lt., left.
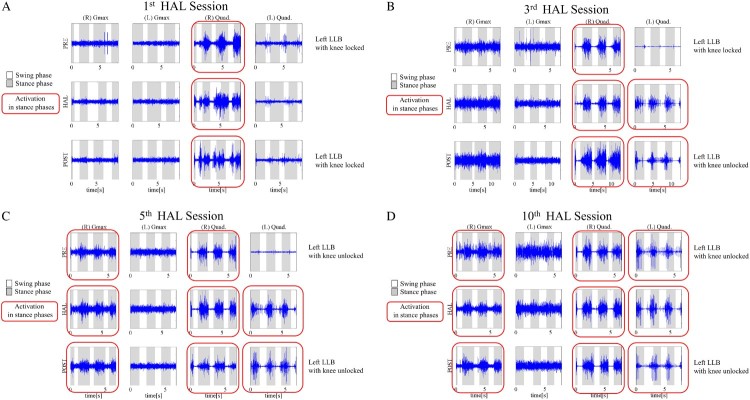
Figures 5A-D demonstrate muscle activities of both Gmax and Quad muscles. Muscle activities in 10MWT before the first HAL® session are shown in Figure 5A. There was no activation of both Gmax muscles. Activities of the right Quad were shown dominantly in stance phase, and those of the left Quad were shown in swing phase. Before the third session, the left Quad was not activated with the LLB in knee-locked position. The HAL® session enabled rhythmical activation of the left Quad in stance phases; therefore, after the session, he could safely walk with the LLB in knee-unlocked positioned for the first time with rhythmical activation of the left Quad in stance phases. Regarding the right Quad, there was more rhythmical activation before, during, and after the third session than during the first session (Fig. 5B). Before the fifth session, the left Quad was not activated with the LLB in knee-unlocked position. However, during and after the HAL® session, there was rhythmical activation of the left Quad in stance phases. On the right side, the Quad and Gmax were activated in stance phases before, during, and after the HAL® session (Fig. 5C). During the first 5 sessions, the right Quad became activated rhythmically in stance phase compared to before the intervention; therefore, we changed the orthosis from an LLB to an SLB on the right side.
Figure 5.
Muscle activities of both gluteus maximus (Gmax) and quadriceps femoris (Quad) muscles before and after the first (A), third (B), fifth (C), and tenth (D) Hybrid Assistive Limb® (HAL®) session. Before the HAL® intervention, both Gmax muscles and the left Quad are not activated. After the HAL® intervention, the right Gmax and both Quad muscles are activated in stance phase rhythmically according to the gait cycle. PRE, preoperatively; POST, postoperatively; LLB, long leg brace.
Before, during, and after the tenth session, the left Quad and right Gmax were activated in stance phase, although before the HAL® intervention, there was no activation of either muscle (Fig. 5D).
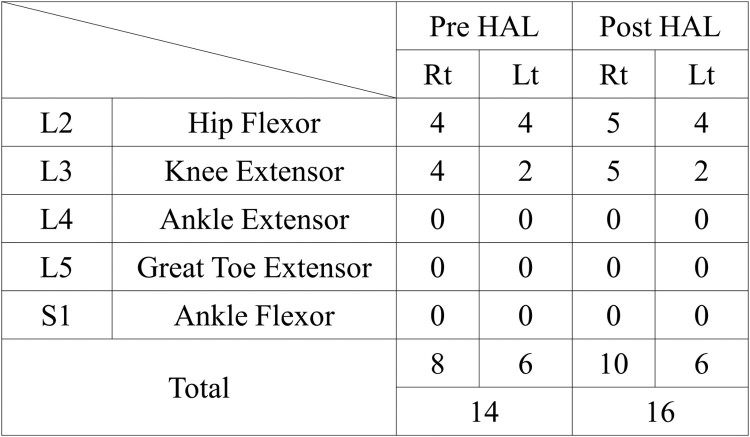
Regarding functional outcome, the ISNCSCI motor score of the lower extremities also improved from 14 to 16 (Figs. 3A, 6). The MMT score of the hip flexor and knee extensor had become 5 from 4 on the right side, and the MMT scores of the hip extensor and knee extensor for the right side had remained as 4 and 2, respectively. The MMT score of the Gmax improved from 1 to 2 on the right side, and from 0 to 1 on the left side. Motor neurological levels changed from L3 to T10 on the right side and remained as T10 on the left side. The WISCI II score also improved from 7 to 12 (Fig. 3B). No adverse events, such as local pain, skin irritation, and a sense of heaviness of the lower back caused by the suit, associated with the HAL® intervention occurred. As the patient had not walked for several years before being a complete paraplegic, he was able to attain better gait ability after the HAL® intervention than before.
Figure 6.
Summary of the International Standards for Neurological and Functional Classification of Spinal Cord Injury motor scores before and after the Hybrid Assistive Limb® (HAL®) intervention. Rt, right; Lt, left; Pre, before; Post, after.
Discussion
In this case, a patient with an SDAVF and chronic spinal cord injury, whose postoperative improvement had plateaued, had improved gait after the HAL® intervention. Improvements in his gait speed and cadence were observed, and he became able to extend his knees in stance phase; therefore, he was able to change his orthosis from the LLB to the SLB on the right side, and from the LLB in the knee-locked positioned to the knee-unlocked position on the left side.
An SDAVF is rare and difficult to diagnose. The recovery of functional ambulation in patients with an SDAVF is related to the time of the treatment, and the early diagnosis and early treatment of an SDAVF have been previously reported as important.1–6 An SDAVF is a circulation disorder in the spinal cord; therefore, once congestion of the spinal cord improves after early treatment, the clinical outcome of rehabilitation should be better. However, complete recovery is reportedly difficult.5 In this patient, his paralysis improved postoperatively; however, 3 month postoperatively, his recovery had plateaued. To gain more improvement in his motor function, we chose to use the HAL® intervention during the period of stagnation of motor recovery.
The HAL® has been reported to be a feasible tool for some types of neuromuscular disorders,8–16 and improvement of ambulation in patients with chronic spinal cord injury has been reported.10–13 No reports have described further gait analysis of the HAL® intervention.
This study used surface electromyography in synchronization with a motion capture system before, during, and after the HAL® intervention. In patients who have difficulty extending the knee, an LLB in knee-locked position is usually needed during walking exercises; therefore, it is difficult for them to train knee extensor movement during gait exercise. Using the HAL®’s function to provide joint motion assist with an appropriate strength, gait with the HAL® enabled our patient to perform knee extensor movement in stance phase according to the gait cycle without knee locking. By analyzing muscle activities of the Quad during the gait cycle, we determined the optimal orthosis for this patient.
According to Daly and Ruff,23 the critical principles of motor learning for central nervous system plasticity are five characteristics: near-normal movements, muscle activation driving movement practice, focused attention, repetition of desired movements, and training specificity. In this case, there were differences in the severity of paralysis between both lower extremities; therefore, HAL® was configured to provide adequate assistance for each of the legs in accordance with muscle activation of each leg. The continuous motion of repeated gait cycles with closer to normal knee motion under adequate assistance from the HAL®, which is derived from volitional contraction of the muscle, may have caused appropriate muscle activation during gait. We previously discussed the effect of the HAL® Single Joint type for motor learning for recovery of upper limb function.24
After the HAL® intervention, muscle activities of the left Quad improved in the stance phase and those of the right Gmax were observed, although MMT showed a level one for these muscles before the HAL® intervention.
Conclusions
Functional improvement after the HAL® intervention was demonstrated in a patient with paraplegia after spinal cord injury and deterioration due to an SDAVF. The HAL® is effective for patients with an SDAVF whose postoperative recovery has plateaued, and it contributes to rhythmical activation of both Quad and right Gmax muscles in the stance phase by providing adequate assistance according to the gait cycle.
Funding Statement
This study was supported by the Industrial Disease Clinical Research Grants of the Ministry of Health, Labor, and Welfare in Japan (14060101-01).
This study was supported by the Industrial Disease Clinical Research Grants of the Ministry of Health, Labor, and Welfare in Japan (14060101-01).
Acknowledgements
We thank Mayuko Sakamaki and Yumiko Ito, Center for Innovative Medicine and Engineering (CIME), University of Tsukuba Hospital, for their excellent technical assistance. This study was supported by the Industrial Disease Clinical Research Grants of the Ministry of Health Labour and Welfare, Japan (14060101-01).
Contributor Statement
A commercial party having a direct financial interest in the results of the research supporting this article has conferred or will confer a financial benefit to one or more of the authors. Yoshiyuki Sankai is CEO of Cyberdyne Inc., Ibaraki, Japan. Hiroaki Kawamoto is a stockholder of the company. Cyberdyne is the manufacturer of the robot suit HAL. This study was proposed by the authors. Cyberdyne was not directly involved in the study design, collection, analysis, interpretation of data, writing of the report, or the decision to submit the paper for publication.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the following authors or on any organization with which these authors are associated: Yukiyo Shimizu, Kei Nakai, Hideki Kadone, Shunsuke Yamauchi, Shigeki Kubota, Tomoyuki Ueno, Aiki Marushima, Kayo Hiruta, Ayumu Endo, Akira Matsumura, Yasushi Hada, and Masashi Yamazaki.
Declarations of interest
None.
Ethics approval
This study was conducted with approval from the Ethics Committee of the Tsukuba. University Faculty of Medicine (approval no.: H26-22).
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
ORCID
Yukiyo Shimizu http://orcid.org/0000-0001-7491-4516
References
- 1. Marcus J, Schwarz J, Singh IP, Sigounas D, Knopman J, Gobin YP, et al. Spinal dural arteriovenous fistulas: a review. Curr Atheroscler Rep 2013;15(7):335. doi: 10.1007/s11883-013-0335-7 [DOI] [PubMed] [Google Scholar]
- 2. Brinjikji W, Nasr DM, Morris JM, Rabinstein AA, Lanzino G.. Clinical outcomes of patients with delayed diagnosis of spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol 2016;37(2):380–6. doi: 10.3174/ajnr.A4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iovtchev I, Hiller N, Ofran Y, Schwartz I, Cohen J, Rubin SA, et al. Late diagnosis of spinal dural arteriovenous fistulas resulting in severe lower-extremity weakness: a case series. Spine J 2015;15(6):e39–44. doi: 10.1016/j.spinee.2013.08.029 [DOI] [PubMed] [Google Scholar]
- 4. Ofran Y, Yovchev I, Hiller N, Cohen J, Rubin SA, Schwartz I, et al. Correlation between time to diagnosis and rehabilitation outcomes in patients with spinal dural arteriovenous fistula. J Spinal Cord Med 2013;36(3):200–6. doi: 10.1179/2045772312Y.0000000029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prieto R, Pascual JM, Gutierrez R, Santos E, Recovery from paraplegia after the treatment of spinal dural arteriovenous fistula: case report and review of the literature. Acta Neurochir (Wien) 2009;151(11):1385–97. doi: 10.1007/s00701-009-0439-6 [DOI] [PubMed] [Google Scholar]
- 6. Sherif C, Gruber A, Bavinzski G, Standhardt H, Widhalm G, Gibson D, et al. Long-term outcome of a multidisciplinary concept of spinal dural arteriovenous fistulae treatment. Neuroradiology 2008;50(1):67–74. doi: 10.1007/s00234-007-0303-4 [DOI] [PubMed] [Google Scholar]
- 7. Kawamoto H, Sankai Y.. Power assist method based on Phase Sequence and muscle force condition for HAL. Advanced Robotics 2005;19(7):717–34. doi: 10.1163/1568553054455103 [DOI] [Google Scholar]
- 8. Kawamoto H, Kamibayashi K, Nakata Y, Yamawaki K, Ariyasu R, Sankai Y, et al. Pilot study of locomotion improvement using hybrid assistive limb in chronic stroke patients. BMC Neurol 2013;13:141. doi: 10.1186/1471-2377-13-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nilsson A, Vreede KS, Haglund V, Kawamoto H, Sankai Y, Borg J.. Gait training early after stroke with a new exoskeleton—the hybrid assistive limb: a study of safety and feasibility. J Neuroeng Rehabil 2014;11:92. doi: 10.1186/1743-0003-11-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wall A, Borg J, Palmcrantz S.. Clinical application of the Hybrid Assistive Limb (HAL) for gait training-a systematic review. Front Syst Neurosci 2015;9:48. doi: 10.3389/fnsys.2015.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aach M, Cruciger O, Sczesny-Kaiser M, Hoffken O, Meindl R, Tegenthoff M, et al. Voluntary driven exoskeleton as a new tool for rehabilitation in chronic spinal cord injury: a pilot study. Spine J 2014;14(12):2847–53. doi: 10.1016/j.spinee.2014.03.042 [DOI] [PubMed] [Google Scholar]
- 12. Sczesny-Kaiser M, Hoffken O, Aach M, Cruciger O, Grasmucke D, Meindl R, et al. HAL(R) exoskeleton training improves walking parameters and normalizes cortical excitability in primary somatosensory cortex in spinal cord injury patients. J Neuroeng Rehabil 2015;12:68. doi: 10.1186/s12984-015-0058-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ikumi A, Kubota S, Shimizu Y, Kadone H, Marushima A, Ueno T, et al. Decrease of spasticity after hybrid assistive limb(R) training for a patient with C4 quadriplegia due to chronic SCI. J Spinal Cord Med 2016:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sakakima H, Ijiri K, Matsuda F, Tominaga H, Biwa T, Yone K, et al. A newly developed robot suit hybrid assistive limb facilitated walking rehabilitation after spinal surgery for thoracic ossification of the posterior longitudinal ligament: a case report. Case Rep Orthop 2013;2013:621405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujii K, Abe T, Kubota S, Marushima A, Kawamoto H, Ueno T, et al. The voluntary driven exoskeleton Hybrid Assistive Limb (HAL) for postoperative training of thoracic ossification of the posterior longitudinal ligament: a case report. J Spinal Cord Med 2016:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kubota S, Tetsuya A, Fujii K, Marushima A, Ueno T, Haginoya A, et al. Improvement of walking ability using Hybrid Assistive Limb training in a patient with severe thoracic myelopathy caused by ossification of the posterior longitudinal ligament - a case report. J Spine 2016;S7:003. [Google Scholar]
- 17. Kirshblum SC, Burns SP, Biering-Sørensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34(6):535–46. doi: 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirshblum SC, Biering-Sørensen F, Betz R, Burns S, Donovan WK, Graves DE, et al. International Standards for Neurological Classification of Spinal Cord Injury: cases with classification challenges. J Spinal Cord Med 2014;37(2):120–7. doi: 10.1179/2045772314Y.0000000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dittuno PL, Ditunno JF Jr. Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord 2001;39(12):654–6. doi: 10.1038/sj.sc.3101223 [DOI] [PubMed] [Google Scholar]
- 20. Kim MO, Burns AS, Ditunno JF Jr, Marino RJ.. The assessment of walking capacity using the walking index for spinal cord injury: self-selected versus maximal levels. Arch Phys Med Rehabil 2007;88(6):762–7. doi: 10.1016/j.apmr.2007.03.021 [DOI] [PubMed] [Google Scholar]
- 21. Ditunno JF Jr, Ditunno PL, Scivoletto G, Patrick M, Dijkers M, Barbeau H, et al. The Walking Index for Spinal Cord Injury (WISCI/WISCI II): nature, metric properties, use and misuse. Spinal Cord 2013;51(5):346–55. doi: 10.1038/sc.2013.9 [DOI] [PubMed] [Google Scholar]
- 22. Scivoletto G, Tamburella F, Laurenza L, Torre M, Molinari MDitunno JF.. Walking Index for Spinal Cord Injury version II in acute spinal cord injury: reliability and reproducibility. Spinal Cord 2014;52(1):65–9. doi: 10.1038/sc.2013.127 [DOI] [PubMed] [Google Scholar]
- 23. Daly JJ, Ruff RL.. Construction of efficacious gait and upper limb functional interventions based on brain plasticity evidence and model-based measures for stroke patients. ScientificWorldJournal 2007;7:2031–45. doi: 10.1100/tsw.2007.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shimizu Y, Kadone H, Kubota S, Ikumi A, Abe T, Marushima A, et al. Active elbow flexion is possible in C4 quadriplegia using hybrid assistive limb (HAL®) technology: a case study. J Spinal Cord Med 2017:1–7. doi: 10.1080/10790268.2017.1329916 [DOI] [PMC free article] [PubMed] [Google Scholar]