Abstract
Objective/Context
To highlight questions with regards to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) and provide historical perspectives to help SCI professionals gain fuller insights into the classification system.
Methods
Frequently asked questions to the ISNCSCI were collected and a review of literature and personal communications with International Standards committee members and Chairs were undertaken.
Results
Background and explanations for nine questions, detailing decision processes and challenging classification rules are presented.
Conclusion
While the ISNCSCI can be challenging, this background and historical explanation may provide a greater understanding and the ability to critically analyze this classification system.
Keywords: Spinal cord injury, Classification, Quadriplegia, Tetraplegia
Introduction
The International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) are the most widely used classification in the field of Spinal Cord Injury (SCI) Medicine. The ISNCSCI defines the terminology used, details the examination, and classifies the severity of the injury utilizing the American Spinal Injury Association (ASIA) Impairment Scale (AIS). This allows for consistency in communication between patients, clinicians, and researchers.
The first edition of the ASIA publication “Standards for Neurologic Classification of spinal cord injury”1 was published in 1982 and drew strongly from works by Frankel,2 Austin,3 and Bracken4 to create a uniform system to classify SCI. The initial five-grade system of classifying traumatic SCI was based on Frankel et al.,2 with divisions into neurologically “complete” (A), three “incomplete” (B-D) injury grades, and complete recovery (E). Neurologically “complete” and “incomplete” injuries were initially based on sparing more than three levels below the neurological level of injury (NLI), termed the “zone of injury”. The Standards further introduced motor testing of key muscle groups, and sensory testing initially in 29 dermatomes (later changed to 28 dermatomes in 1989),5 as well as describing select anatomical incomplete syndromes (e.g. central cord syndrome).
Since this first edition, the International Standards Committee of ASIA has published revised versions of these Standards to clarify scoring methods, patient positioning, and create universal definitions.5–11 Despite these revisions and clarifications, consistent performance and understanding of the International Standards has been challenging, and questions persist. Here we present some common questions regarding the International Standards with explanations that will help professionals in SCI understand the basis for some of the changes that are part of the current ISNCSCI. This document is intended to provide context and clarity to SCI professionals whom are familiar with the ISNCSCI exam. As such, early learners and those seeking classification criteria are directed to the most updated exam revisions and training modules.11,12 Additional historical reviews on the International Standards can be found elsewhere.13–15
Questions:
1. How was the “zone of injury”, which is used to determine motor incomplete injuries, decided to be three levels when classifying between AIS B and C?
The “zone of injury” is currently described as “three levels below the ipsilateral motor level on either side of the body”.11 For an injury to be classified as motor incomplete (AIS C-D), there needs to be motor sparing either by presence of voluntary anal contraction (VAC) or in a patient with a sensory incomplete injury, motor sparing below the “zone of injury” (i.e. more than three levels below the motor level on either side).11
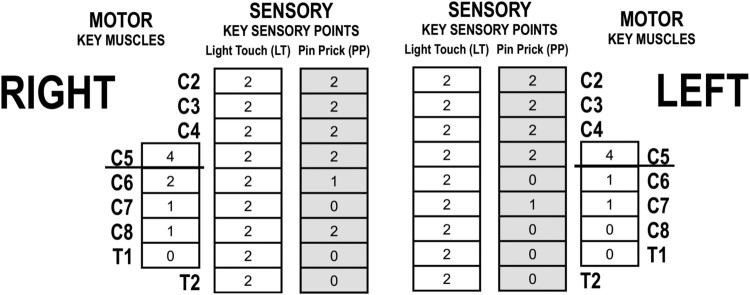
As an example, in Figure 1, the motor level is C5 on each side. Assuming this patient has a sensory incomplete injury based upon sacral sensory sparing, with motor sparing only three levels below the motor level on the right and two on the left, the classification would remain an AIS B. In order for the classification to change to motor incomplete, there would need to be sparing below C8 on at least one side of the body.
Figure 1.
Partial example of ISNCSCI flow sheet of an individual with C5 AIS B.
From a historical standpoint, the term “zone of injury” was initially (1982 classification) defined as up to three neurological segments below the point of damage to the spinal cord.1 This zone of injury was important, as sparing more than three levels below the NLI would define an incomplete injury. For example, at that time any sensory sparing below the zone of injury would lead to a sensory incomplete definition (Frankel B) and motor sparing below the zone of injury defined a motor incomplete injury (Frankel C-D).1 In 1989, the term “zone of injury” was changed to “zone of partial preservation” (ZPP) and was divided into sensory and/or motor function.6 In 1992, when the “sacral sparing” definition was adopted (and the Frankel Scale was changed to being called the AIS) to determine completeness of the injury, a number of terms were changed.7 The ZPP was redefined as sensory and/or motor function that could expand beyond three levels, and was only reserved for complete injuries - causing notable confusion with the previous definition of “zone of injury (ZOI)”. When these changes were adopted, the rule used to differentiate AIS B from AIS C required motor sparing outside of a zone of injury of more than two levels below the motor level.7 (Note that for the initial Frankel Scale motor sparing more than three levels below the neurological level was required, this was changed to two levels below the motor level in the new AIS classification).16 There is no documentation regarding the exact reasoning for this change of the ZOI from three to two levels, though as best as we can determine (personal communication with some of the 1992 Standards Committee members), may have been influenced by a timely article by Waters, et al.,17 noting < 1% chance of functional recovery three levels below the injury level in motor complete injuries. In addition, there were multiple other prevailing articles from that time period that described motor recovery within the first one to two levels below the motor level through clinical exam18,19 and electrodiagnostic evidence of peripheral sprouting.20,21 This two-level definition of the zone of injury for determining motor incomplete injuries remained in place until the 2000 Standards edition,9 when the definition was expanded back to three levels to be consistent with the previous definitions for ‘zone of injury’.5,6 A later report however demonstrated that either the two or three level definition equally predicted the pattern of motor recovery at one year.22
2. Why are there no key muscles tested for levels between T2-L1?
While the spinal levels for T2-L1 innervate functionally significant musculature (intercostals, rectus abdominis, obliques, etc.), it is difficult to test these muscles in isolation or reliably on bedside exam. For this reason, the motor level in these segments defaults to the sensory level as the most discernable estimate of motor capability (in the event that all key muscles above are graded as normal). Recent research23 has demonstrated electrodiagnostic and ultrasound evidence of preservation of abdominal musculature activation in multiple patients with motor complete injuries above T6. However, others24,25 have suggested that these responses may be inaccurate unless controlling for intraabdominal pressures, spasticity and stretch reflexes.
Clinically, testing for trunk control in patients with spinal cord injuries is performed for select Paralympic sports.26,27 Yet even in these controlled environments, sufficient isolation and grading of these muscles remains a barrier. Additionally, Beevor’s sign is a clinical measure of rectus abdominis innervation. In this clinical exam, if partial innervation of the rectus abdominis is present with interruption of the spinal cord signaling from T10 to L2, provocative maneuvers will cause the umbilicus to displace rostrally with abdominal activation. This finding is due to imbalance in the innervation of the portion of the rectus above the umbilicus with the portion below. A negative Beevor’s sign (no umbilical displacement), however, represents a balance of muscle innervation above and below the umbilicus. Without further testing this finding may represent either full innervation or no innervation of abdominal musculature. Currently, assessment of Beevor’s sign is listed as an optional test for the International Standards (with instruction available as part of the International Standards Training e-Learning Program- InSTeP).12
While these tests remain important in the neurologic function of individuals with SCI, the inability to determine an accurate neurologic level from them has prevented their inclusion in the current International Standards.
3. Why was the term changed to “tetraplegia” from “quadriplegia”?
The 1992 revision to the Standards were endorsed by the International Spinal Cord Society (ISCoS, known at the time as the International Medical Society of Paraplegia). Incorporation of these experts’ knowledge led to the 1992 edition clarifying the preferred vernacular as tetraplegia as opposed to quadriplegia 7. This was changed in an effort to have language of origin for the root (tetra- Greek) agree with the suffix (plegia- Greek). The word quadriplegia, by contrast, combines quadra (Latin root) to the Greek suffix plegia. This change in preferred language aligns with the term paraplegia, which also is Greek for both root and suffix.
4. Why sensation for C6-C8 is on the dorsum of the hand as opposed to the palmar aspect?
The development of the sensory points assigned to each dermatome has been accredited to guidance from the spinal surgical text by Austin.3 However, three different dermatomal maps are presented within this text. Through personal communication with Standards Committee previous Chairs, the original source relied upon within this text was felt to be Foerster’s 1933 article.28 While Foerster references several previous dermatomal maps detailing spinal innervation of both the dorsum and palmar surfaces of the hand, he also provides pictorial representations of pathologic dermatomal outlines. While not specifically advocating sensory testing on the dorsum of the hand for C6-C8, this report does demonstrate significant variable case presentations of the C8 dermatome involving the palmar aspect of the hand, perhaps presenting some evidence against using this surface.
Standards committee members however also noted increased variability from keratin deposition on the palmar surface of the hand, as well as the potential for confounding from common conditions like carpal tunnel syndrome and peripheral neuropathy as potential factors contributing to the improved sensory reliability of the dorsum of the hand over the palmar surface. So, while no explicit reference was identified for using the dorsum of the hand for key sensory points, several factors are suggestive that these locations may be more reliable for assessing sensation of C6-C8 dermatomes.
5. Why the “motor level” is used to define AIS B vs C, but the NLI is used for AIS C vs D?
a) Why the motor level is used to define AIS B vs C.
Since 1992, VAC or motor sparing more than three levels below the motor level has been required to meet the criteria to be classified as a motor incomplete injury. The Standards Committee at that time felt that it was more accurate to use motor level to determine this as opposed to the NLI. The benefit of using the motor level in this classification is that often the NLI is determined by the sensory level, with the motor level one to two segments more caudal.29 As such, three levels below the NLI may only be one level below the motor level; resulting in the patient being classified as motor incomplete with very little extended motor preservation. If the NLI (because of a more rostral sensory level) is multiple levels above the motor level, there could be considerable instability in the classification since as the patient potentially improved their sensory scores (which is often seen after an acute injury) they could convert from a motor incomplete (AIS C) to motor complete sensory incomplete (AIS B) status without motor loss.
b) Why the NLI is used for AIS C vs D?
The NLI being used to discriminate AIS C from D has often caused confusion, given that the motor level is used to differentiate AIS B from C.30 Once a patient has met the criteria for motor incomplete status by either having VAC or sacral sensory sparing with motor sparing more than three levels below the motor level, the NLI represents the lowest level where both motor and sensory functions are considered intact. This was felt by the Standards Committee, in 1989, to be the best starting point to determine if the criteria for AIS D are met. A key factor in this decision was that there will always be an even number of muscles being counted by using the NLI. If using the motor level instead, there could be different levels on each side of the body making counting the levels more difficult. The downside of using NLI for this decision is that this may, at times, upgrade patients to a more incomplete grade by counting an antigravity muscle just below the NLI that is above the motor level.31
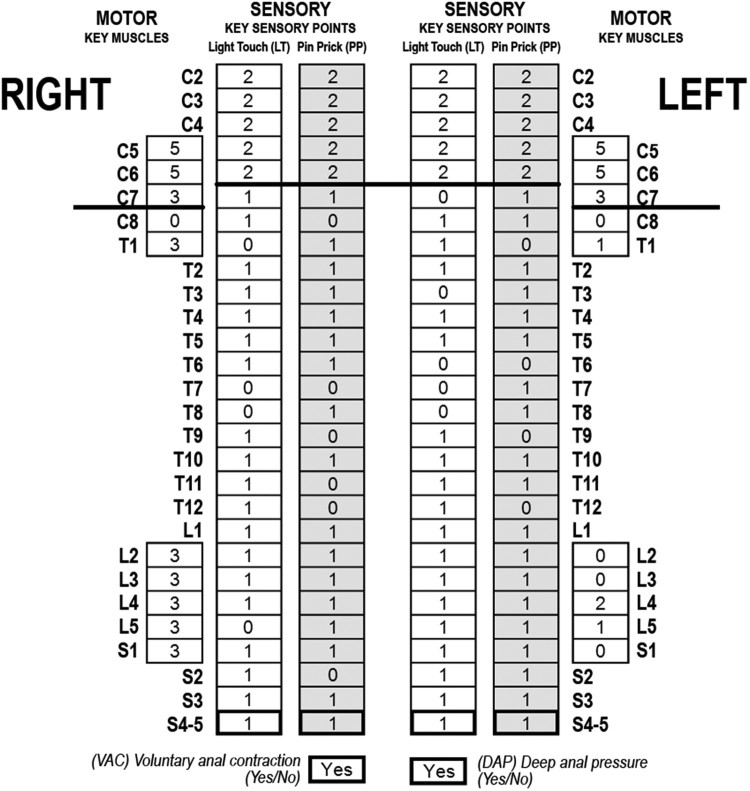
In the example in Figure 2, if the motor level were used then the classification for this case would be AIS C, but since the NLI is used, it is classified as AIS D.
Figure 2.
Example of C6 AIS D with motor level at C7 bilaterally and sensory level/neurological level at C6 bilaterally.
6. Why is the sacral sparing definition used to determine completeness of injury?
As discussed above in question 1, versions of the Standards prior to 1992 defined the severity of a spinal cord injury (complete vs incomplete) based upon the preservation of motor or sensory function more than three levels below the neurological level.1 Since 1992 however, the “sacral sparing” definition to describe the severity of the neurologic injury has been used.7–11 Sacral sparing relates to the presence of sensory function in the lowest sacral (most caudal) segments of the anal mucocutaneous junction (S4-5 dermatome) on either side of the body, and by testing motor function through VAC, and deep anal pressure (DAP) as part of the rectal examination. If any of these components are present, intact or impaired, even if on only one side of the body, the individual has an incomplete injury. If none of these above components are present on examination, then the patient has a neurological complete injury. According to this definition, a patient with a cervical SCI can have sensory and motor function in the trunk or even the legs many dermatomes or myotomes below the NLI, but without sacral sparing, the injury is classified as complete (AIS A) with a large ZPP.
The basis for changing the definition to sacral sparing was that the sacral sparing was found to be a more ‘stable’ definition than the previous classification schema, as fewer patients converted from incomplete back to complete status over time after injury (0% with sacral sparing definition vs. 1.3% with three level definition).32 Interestingly, the key finding of this study, the rate of conversion from incomplete to complete injury using this sacral sparing definition, has not been specifically tested since.
7. Why is there 1 myotome listed for each key muscle when almost all muscles are innervated by more than 1 myotome?
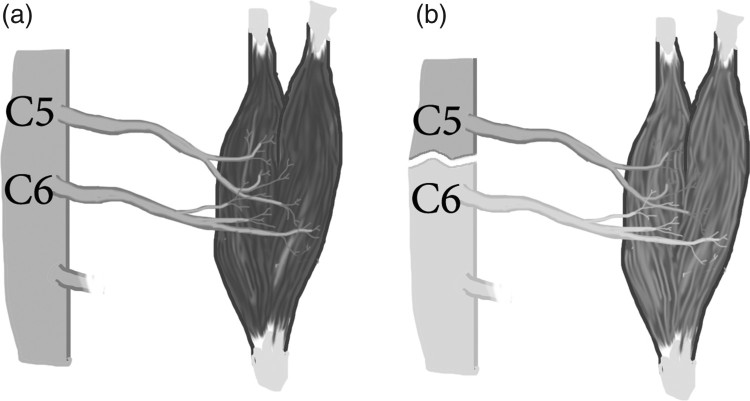
Multiple spinal roots most commonly innervate peripheral skeletal muscles, with the exception of the rhomboids. However, as part of the ISNCSCI exam, motor levels are identified by a single myotome for each of the key muscles tested. While this is a simplification, the key muscles were chosen such that the most rostral spinal nerve is considered the primary contribution for antigravity strength. For example, the biceps brachii, while C5 and C6 both provide partial innervation, C5 provides the most rostral and primary nervous supply (Figure 3A). Using this example, if an individual had a complete spinal cord transection between C5 and C6, one would expect the C5 muscle- biceps brachii (which has now lost its innervation from C6) to be at less than full strength but to be able to grade as 3/5 (See Figure 3B). With this consideration, the ISNCSCI allows this one level of ≥ 3/5 strength to contribute toward the motor level, so long as all rostral muscles are full (5/5) strength.
Figure 3.
(A) Innervation of biceps brachii with contributions coming from C5 and C6 (B) Innervation of biceps brachii following spinal cord injury above C6.
8. Why in the thoracic region does the motor ZPP not defer to the sensory ZPP when the motor level defers to sensory level in that region?
In the regions of the body where motor function is unable to be tested by manual muscle testing, including from C1-4, T2-L1, and caudal to S1, the motor level is presumed to be the same as the sensory level if testable, rostral motor function is fully intact (5/5). While the motor level defers to the sensory level in these regions, motor ZPP does not defer to the sensory ZPP. Rather, the caudal extent of the motor ZPP is based only on the presence of voluntary, testable muscle contraction below the motor level. This represents a key difference between determining the NLI and ZPP. For example, in a person with a neurologically complete injury where the upper extremity key muscles are fully intact with a T9 sensory level and sparing of some sensation at T10 bilaterally with all other motor and sensory functions absent, the motor level and NLI is T9 with a sensory ZPP documented at T10 bilaterally. In this case T9 should be documented on the worksheet for the motor ZPP bilaterally.
The reason motor ZPP does not defer to sensory ZPP is to be consistent with the overall rule of only deferring the motor level to the sensory level when the sensation is fully intact (with all testable upper limb key muscles intact). As the ZPP represents only spared sensation below the sensory level, the motor level does not defer. In a case of a neurologically complete injury where the sensory level is T9, with impaired sensation at T10 and intact sensation at T11, the motor ZPP (assuming bilateral upper extremity key muscle functions were fully intact) would still remain at T9. While the sensation may be fully intact at T11, the rule of “all testable function above is intact as well” is not met, since impaired sensation at T10 prevents deferment of motor level to sensory level caudal to this. Conversely, if this rule was not in place and motor ZPP deferred to sensory ZPP, potential negative outcomes could occur. Namely, if an individual with T6 AIS A paraplegia with no motor or sensory ZPP gained some sensation at T10, this individual would also expand their motor ZPP to T10. This would represent recovery of 4 motor levels without an objective way of measuring motor recovery (see question 2).
It should be noted that the rule of the motor level following the sensory level in areas where there is no muscle to test is not without controversy. Particularly in the upper cervical spine, you may have a C3 sensory level with strong biceps and even wrist extensors in an individual with an otherwise motor complete injury. Such an individual would be classified as a C3 motor level and C3 neurological level based on sensation alone based on the rule of “motor level defers to the sensory level”, despite functionally behaving more like a C5 or C6 level SCI. This has been shown to be ‘counterintuitive’ and an adjustment of this definition may be needed.33
9. Why pinprick/ light touch are used in the standard sensory assessment and NOT temperature or proprioception?
While the International Standards exam is extremely detailed, it is not a comprehensive neurologic test for patients with spinal cord injuries. As such, temperature and proprioception are deferred as part of the standard exam. Since 1992, the Standards has included optional tests including vibration, proprioception, deep pressure sensation, and diaphragm fluoroscopy. These optional tests are available as part of InSTeP online training for a more exhaustive neurologic assessment. Complimentary guidelines on assessing autonomic function are provided by the International Standards for documenting remaining Autonomic Function after SCI.34
The use of pin prick and light touch allows estimation of intact sensory tracts (spinothalamic and dorsal column respectively) and establishment of a sensory level, while still limiting the duration of time required for a thorough and accurate bedside exam. Previous research has consistently demonstrated substantial inter-rater reliability in performing both the light touch and pinprick portions of the exam.32,35,36 In their recent paper, Hales et al.37 note that the sensitivity and validity of sensory testing in the ISNCSCI exam is less reliable for individuals with incomplete as opposed to complete injuries and call for a restructuring of the sensory exam to include additional modalities. While additional sensory (and autonomic) testing has been quantified for patients with SCI,34,38,39 and definitely have a role in comprehensive assessments of spinal tracts, the need for the International Standards exam to be performed at bedside with minimal equipment and in a reasonable amount of time on recently injured individuals limits the addition of other sensory assessment modalities.
Conclusion
The ISNCSCI is the most widely used guide for examination and classification in traumatic SCI, and has undergone many revisions since its initial introduction. The details here will hopefully allow researchers and practitioners to have a greater understanding of the previous revisions that have taken place as well as allow for critical analysis for continued improvement.
Disclaimer statements
Contributors None.
Funding This research was unfunded
Declaration of interest The authors report no declarations of interest
Conflicts of interest None.
Ethics approval None.
ORCID
Ryan Solinsky http://orcid.org/0000-0002-7121-8678
References
- 1. American Spinal Injury Association Standards for Neurological Classification of Spinal Injury Patients. Chicago, IL: ASIA, 1982. [Google Scholar]
- 2. Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7(3):179–92. [DOI] [PubMed] [Google Scholar]
- 3. Austin GM. The spinal cord: Basic aspects and surgical consideration. 2nd ed Springfield, Il: Thomas; 1972:762. [Google Scholar]
- 4. Bracken MB, Webb SB, Wagner FC.. Classification of the severity of acute spinal cord injury: implications for management. Paraplegia; 1977; 15:319–26. [DOI] [PubMed] [Google Scholar]
- 5. American Spinal Injury Association Standard for Neurological Classification of Spinal Injured Patients (3rd edition), Chicago, IL: ASIA, 1989. [Google Scholar]
- 6. American Spinal Injury Association Standard for Neurological Classification of Spinal Injured Patients (2nd edition), Chicago, IL: ASIA, 1987. [Google Scholar]
- 7. American Spinal Injury Association Standard for Neurological Classification of Spinal Injured Patients (4th edition), Chicago, IL: ASIA, 1992. [Google Scholar]
- 8. Maynard FM, Bracken MB, Creasy G, Dittuno JF, Young W, et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury Patients. Spinal Cord. 1997;35: 266–74 doi: 10.1038/sj.sc.3100432 [DOI] [PubMed] [Google Scholar]
- 9. American Spinal Injury Association/ International Medical Society of Paraplegia (2000). International Standards for Neurological and Functional Classification of Spinal Cord Injury Patients (6th edition), Chicago, Illinois. [Google Scholar]
- 10. Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (Revised 2011) J Spinal Cord Med. 2011;34(6):535–46. doi: 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Spinal Injury Association/ International Medical Society of Paraplegia International Standards for Neurological and Functional Classification of Spinal Cord Injury Patients (7th edition, 2015 update). ASIA; Chicago, Illinois. [Google Scholar]
- 12. American Spinal Injury Association ASIA Learning Center [document on the Internet].2017. [cited 2017 March 6]. Available from http://asia-spinalinjury.org/learning/
- 13. Ditunno John F., Jr American spinal injury standards for neurological and functional classification of spinal cord injury: past, present and future: 1992 Heiner Sell Lecture of the American Spinal Injury Association. J Am Para Soc. 1994;17(1):7–11. doi: 10.1080/01952307.1994.11735909 [DOI] [PubMed] [Google Scholar]
- 14. Kirshblum SC, Donovan W.. Neurological Assessment and Classification of Traumatic Spinal Cord Injury. In Kirshblum SC, Campagnolo D, DeLisa JE.. Spinal Cord Medicine. Lippincott/Williams and Wilkins; Philadelphia: 2002;82–95. [Google Scholar]
- 15. Kirshblum S, Waring W.. Updates for the International Standards for Neurological Classification of Spinal Cord Injury. Phys Med Rehabil Clin N. 2014;25(3):505–17. doi: 10.1016/j.pmr.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 16. American Spinal Injury Association Reference Manual for the International Standards for Neurological and Functional Classification of Spinal Cord Injury. ASIA; Chicago, Illinois. 1994. p 52. [Google Scholar]
- 17. Waters RL, Adkins RH, Yakura JS, Sie I.. Motor and sensory recovery following complete tetraplegia. Arch Phys Med Rehabil. 1993;74(3):242–7. [PubMed] [Google Scholar]
- 18. Mange KC, Ditunno JF, Herbison GJ, Jaweed MM.. Recovery of strength at the zone of injury in motor complete and motor incomplete cervical spinal cord injured patients. Arch Phys Med Rehabil. 1990;71(8):562–5. [PubMed] [Google Scholar]
- 19. Wu L, Marino RL, Herbison GJ, Ditunno JF.. Recovery of zero-grade muscles in the zone of partial preservation in motor complete quadiplegia. Arch Phys Med Rehabil. 1992;73:40–4. [PubMed] [Google Scholar]
- 20. Little JW, Moore D, Brooke M, Powers R.. Electromyographic evidence for motor axon sprouting in recovering upper extremities of acute quadriplegics. J Am Para Soc. 1990;13(1):16 [Google Scholar]
- 21. Marino RJ, Herbison GJ, Ditunno JF.. Peripheral sprouting as a mechanism for recovery in the zone of injury in acute quadriplegia: A single-fiber EMG study. Muscle Nerve. 1994;17(12):1466–8. doi: 10.1002/mus.880171218 [DOI] [PubMed] [Google Scholar]
- 22. Kirshblum SC, Memmo P, Kim N, Campagnolo D, Millis S.. Comparison of the revised 2000 American Spinal Injury Association Classification Standards with the 1996 guidelines. Am J Phys Med Rehabil. 2002;81:502–5. doi: 10.1097/00002060-200207000-00006 [DOI] [PubMed] [Google Scholar]
- 23. Bjerkefors A. Diagnostic accuracy of common clinical tests for assessing abdominal muscle function after motor-complete spinal cord injury above T6. Spinal Cord. 2015; 53:114–9. doi: 10.1038/sc.2014.202 [DOI] [PubMed] [Google Scholar]
- 24. Silver JR. Response to: Diagnostic accuracy of common clinical tests for assessing abdominal muscle function after motor-complete spinal cord injury above T6. Spinal Cord. 2015;53(12):891. doi: 10.1038/sc.2015.113 [DOI] [PubMed] [Google Scholar]
- 25. Guttmann L, Silver JR.. Electromyographic studies on reflex activity of the intercostals and abdominal muscles in cervical cord lesions. Paraplegia. 1965;3:1–22 [DOI] [PubMed] [Google Scholar]
- 26. Altmann VC, Groen BE, van Limbeek J, Vanlandewijck YC, Keijsers NL.. Reliability of the revised wheelchair rugby trunk impairment classification system. Spinal Cord. 2013;51:913–8. doi: 10.1038/sc.2013.109 [DOI] [PubMed] [Google Scholar]
- 27. Pernot HF, Lannem AM, Geers RP, Ruijters EF, Bloemendal M, Seelen HA.. Validity of the test-table-test for Nordic skiing for classification of paralympic sit-ski sports participants. Spinal Cord. 2011;49:935–41. doi: 10.1038/sc.2011.30 [DOI] [PubMed] [Google Scholar]
- 28. Forester O. The dermatomes in man. Brain 1933;56(1):1–39. doi: 10.1093/brain/56.1.1 [DOI] [Google Scholar]
- 29. Marino RJ, Ditunno JF, Donovan WH, Maynard F.. Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch Phys Med Rehabil. 1994;80(11):1391–6. doi: 10.1016/S0003-9993(99)90249-6 [DOI] [PubMed] [Google Scholar]
- 30. Schuld C, Wiese J, Franz S, Putz C, Stierle I, Smoor I, et al Effect of formal training in scaling, scoring and classification of the International Standards for Neurological Classification of Spinal Cord Injury. Spinal Cord 2013;51(4):282–8. doi: 10.1038/sc.2012.149 [DOI] [PubMed] [Google Scholar]
- 31. Gündoğdu İ, Akyüz M, Öztürk EA, Cakcı FA.. Can spinal cord injury patients show a worsening in ASIA impairment scale classification despite actually having neurological improvement? The limitation of ASIA Impairment Scale Classification. Spinal Cord. 2014;52(9):667–70. [DOI] [PubMed] [Google Scholar]
- 32. Waters RL, Adkins RH, Yakura JS.. Definition of complete spinal cord injury. Paraplegia. 1991;29:573–81. [DOI] [PubMed] [Google Scholar]
- 33. Franz S, Kirshblum SC, Weidner N, Rupp R, Schuld C; EMSCI study group. Motor levels in high cervical spinal cord injuries: Implications for the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med. 2016;39(5):513–516 doi: 10.1080/10790268.2016.1138602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krassioukov A, Biering-Sorensen F, Donovan W, Kennelly M, Kirshblum S, Krogh K, et al International standards to document remaining autonomic function after spinal cord injury. J Spinal Cord Med. 2012;35:201–10. doi: 10.1179/1079026812Z.00000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Savic G, Bergstrom EMK, Frankel HL, Jamous MA, Jones PW.. Inter-rater reliability of motor and sensory examinations performed according to the American Spinal Injury Association standards. Spinal Cord. 2007;45:444–51 doi: 10.1038/sj.sc.3102044 [DOI] [PubMed] [Google Scholar]
- 36. Marino RJ, Jones L, Kirshblum S, Tal J, Dasgupta A.. Reliability and repeatability of the motor and sensory examination of the international standards. J Spinal Cord Med. 2008;31(2):166–70. doi: 10.1080/10790268.2008.11760707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hales M, Biros E, Reznik JE.. Reliability and Validity of the Sensory Component of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI): A Systematic Review. Top Spinal Cord Inj Rehabil. 2015;21(3):241–9. doi: 10.1310/sci2103-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Savic G, Bergström EMK, Frankel HL, Jamous MA, Ellaway PH, Davey NJ.. Perceptual threshold to cutaneous electrical stimulation in patients with spinal cord injury. Spinal Cord. 2006;44(9):560–6. doi: 10.1038/sj.sc.3101921 [DOI] [PubMed] [Google Scholar]
- 39. Nicofra A, Ellaway P.. Thermal perception thresholds: Assessing the level of human spinal cord injury. Spinal Cord. 2006;44:617–24. doi: 10.1038/sj.sc.3101877 [DOI] [PubMed] [Google Scholar]