Abstract
Context: Sublesional osteoporosis is an important sequel after spinal cord injury (SCI) resulting in a high incidence of fractures and impaired osseous healing due to altered bone metabolism. The following study aims to identify demographic characteristics and outcome of patients with SCI with lower extremity fractures.
Design: Retrospective observational study.
Setting: Level-I cross-regional trauma center.
Participants: All patients with SCI suffering from osteoporotic/pathologic fractures during an 11-year-period (01/2003–12/2013) at the Center for Spinal Cord Injuries (Trauma Center Murnau) were analyzed via a chart review.
Outcome measures: Demographics, surgical and radiologic outcome as well as complication rate were assessed with a special emphasis on union rates and independent risk factors for non-unions.
Results: We identified 132 patients (105 males) who fulfilled the inclusion criteria. Most of them were paraplegic (n=101) and showed motor complete syndromes (n=119). Supracondylar femur fractures were the most prevalent in this study (n=47). We observed a non-union rate of 15.9% (n=21). The development of pseudarthrosis was associated with the time interval since the initial SCI (P < 0.010), delayed in-patient submission (P < 0.038), fracture classification (P < 0.002) and the localization of the fracture (P < 0.0001). The overall complication rate was 16.7%. All dislocated subtrochanteric femur fractures (Garden III and IV) (n=10) developed a non-union, regardless of their management (conservative or surgical). The following independent predictors for non-unions were identified: fracture localization (P < 0.0002), fracture classification (P < 0.056), and fracture management (P < 0.036).
Conclusions: Even though modern techniques allow surgical interventions in bones with reduced mineral density, non-unions remain a common complication in patients with SCI. Risk factors for non-unions of lower extremity fractures are identified.
Keywords: Spinal cord injury, Complication, Osteoporosis, Non-union, Outcome
Introduction
Spinal cord dysfunction may affect almost every organ system within the human body. Bone metabolism and its complex remodeling are, among others, influenced by direct mechanical loading, hormonal regulation and autonomic modulations.1 After spinal cord injury (SCI), these regulatory mechanisms are impaired below the level of lesion.2,3 Sublesional osteoporosis commonly occurs in patients with SCI.4 Bone mineral density is reduced to up to 50% after the first 3–4 years after SCI.5,6 Hence, the SCI population is very susceptible to fractures. The annual incidence of fractures within this cohort has been reported to range between 2–6%, with even higher numbers having been published recently.7 With longer duration of spinal cord malfunction, the probability for fractures is increasing even further.8 The majority of these fractures occur after low-impact traumas that would not cause fractures in patients without SCI.9 Especially the trabecular region is vulnerable for fractures, since in patients with SCI, there is an initial rapid progression of trabecular bone loss with a predominant region around the knee.10
The management of fractures in patients with SCI requires special consideration due to the mentioned sublesional osteoporosis and dysfunction of the spinal cord with all related consequences. The development of intramedullary-nailing systems, however, improved surgical treatment options. This study provides demographic characteristics, distribution patterns, outcome analysis and complication rates of lower extremity fractures in patients with SCI managed in our institution, with a special emphasis on bone healing. In general non-unions are thought to be multifactorial and several risk factors have been identified for this complication in patients without SCI.11 However, in the SCI population, pseudarthrosis probably has been underestimated, since the clinical significance of this complication has been questioned. However, we believe that also in patients with SCI the compromised mechanical stability and increased risk for infections and other complications caused by pseudarthrosis must not be neglected especially ambulatory patients but also in non-walkers. Therefore, non-unions in patients with SCI deserve special attention among physicians.
Methods
Study design and setting
The following study has been performed at the Center for Spinal Cord Injuries (Trauma Center Murnau), a specialized center solely dedicated to the treatment of patients with SCI. The vast majority of patients are admitted on a non-elective basis.
We performed a retrospective chart review. Clinical and radiological follow-up was collected prospectively according to institutional guidelines. All patients admitted to our institution over an 11-year-period (01/2003–12/2013) were retrospectively analyzed. To be included, patients had to suffer from the consequences of a prior SCI and had to sustain a traumatic fracture of the lower extremities. Fractures were classified according to McMaster and Stauffer.12 Only class-II fractures (“pathologic/osteoporotic”)12 were included in this study. Additionally, localization and severity of all fractures were classified according to the AO/OTA classification system (AO Trauma, Davos, Switzerland). Regarding surgical versus conservative treatment options, the physician made recommendations, but the patient made the final treatment decision. Only patients with adequate follow-up data were included in the study.
Follow-up clinical and radiologic (in most cases X-ray studies in 2 planes) assessments were performed regularly over 1 year after the insult. Pseudarthrosis was defined as non-osseous union after 1-year follow-up. After analyzing the study protocol, the responsible ethics committee of the Bavarian Medical Board waived the requirement for an ethical review (2016–090) for this study.
Statistical analysis
All statistical analyses were done using the software SPSS Statistics 19 (IBM Corp, Armonk, NY, USA). Patients with SCI suffering from lower extremity fractures were divided into 2 groups (pseudarthrosis and union). Each variable was tested for normal distribution (by Kolmogorov-Smirnov and Shapiro-Wilk tests) before differences between the two groups were assessed. For continuous variables that were normally distributed, t-tests were used and for other continuous variables Mann-Whitney U tests. Categorical variables (e.g. sex, AIS grade, and SCI level) were compared using the χ2 and Fisher exact test. Univariate correlation analyses were done using Spearman's ranked correlation. To avoid redundancy, complications were excluded from the correlation analyses, since pseudarthrosis is an adverse event per se, which partially overlaps with the complication category implant failure/dislocation. Variables with a significant effect in uni- or bivariate analyses, together with potentially confounding patient characteristics and treatment parameters, were tested as potential predictors of pseudarthrosis. For this, bivariate logistic regression was used, and significant predictor variables (P > 0.05) were added in a forward fashion to the model based on the likelihood quotient. To facilitate the interpretation of the results, the categories for fracture localization were reduced (to tibia vs. distal femur, femur shaft and proximal femur).
Results
We identified a total of 186 patients (146 male). Complete follow-up data was available for 132 patients (105 male). These patients were further analyzed. Patients were stratified between a “no pseudarthrosis” (=union) and a “pseudarthrosis” (=non-union) group. Twenty-one patients (16.0%) showed non-union in the follow-up examination.
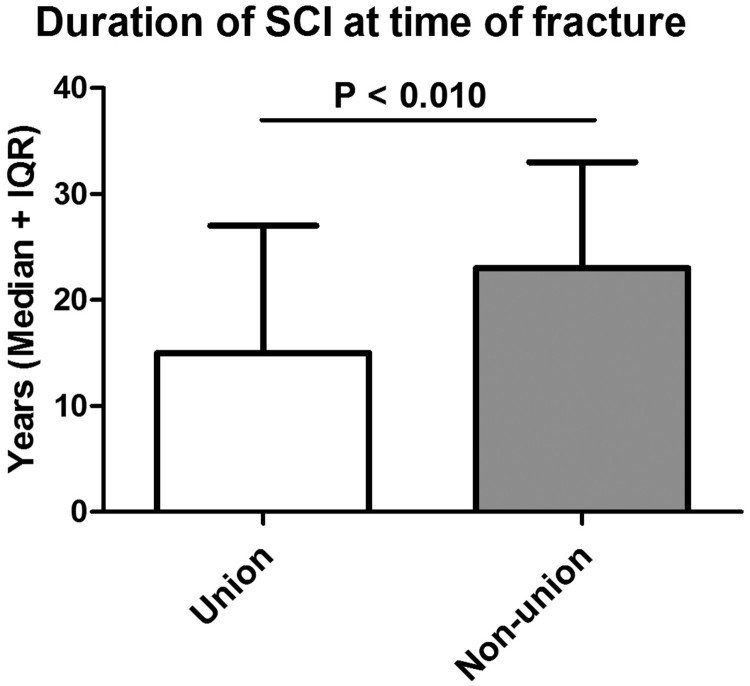
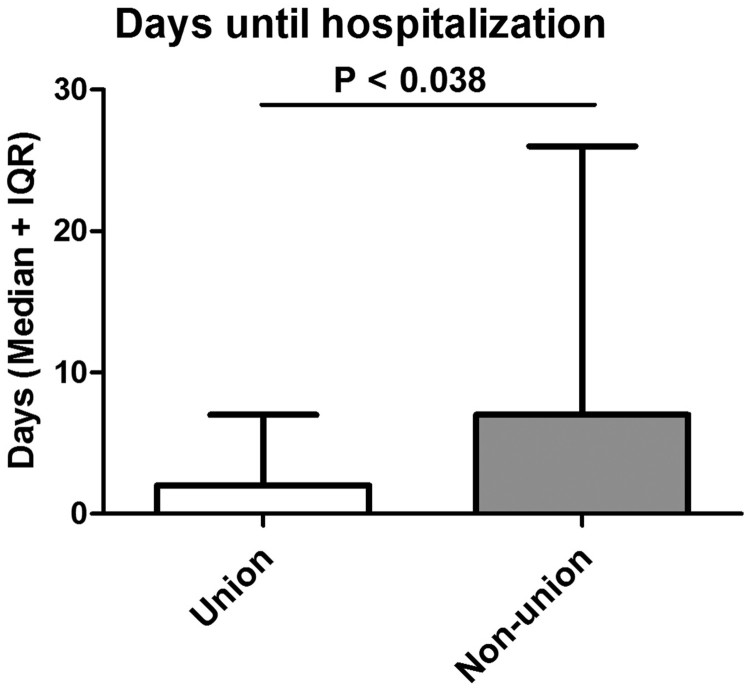
In general, most patients were paraplegic and showed motor complete syndromes as assessed by the AIS (American Spinal Cord Injury Association Impairment Scale) examination according to the ISNCSCI (International Standards for Neurological Classification in Spinal Cord Injury) protocol. Only 7 patients were ambulatory with wheeled walkers. SCI levels (P < 0.781) and AIS grades (P < 0.901) did not differ significantly between both groups. Overall a male predominance was observed. However, there was no statistically significant gender difference between the union and non-union group (P < 0.367). The mean age at the time of the fracture was comparable between both groups. The mean time lag between the SCI and the fracture was 19 years (SD: 12), with significant longer time intervals in the pseudarthrosis group (25 ± 10 versus 18 ± 12 years; P < 0.010). Further, hospitalization occurred delayed in the pseudarthrosis group (15 ± 20 days versus 11 ± 27; P < 0.038) (Table 1; Figs. 1 and 2).
Table 1. Demographic characteristics: Demographic data of all patients with adequate follow-up data. Results are further compared between the union and the non-union group.
| Overall (n = 132) | Union (n = 111) | Non-union (n = 21) | P-value | |
|---|---|---|---|---|
| Sex [n (%)] | P < 0.376 | |||
| Male | 105 (79.5%) | 90 (81.1%) | 15 (71.4%) | |
| Female | 27 (20.5%) | 21 (18.9%) | 6 (28.6%) | |
| SCI level [n (%)] | ||||
| Cervical | 31 (23.5%) | 27 (24.3%) | 4 (19.0%) | P < 0.781 |
| Thoracic/thoracolumbar | 101 (76.5%) | 84 (75.7%) | 17 (81.0%) | |
| AIS Grades [n (%)] | P < 0.901 | |||
| A | 107 (81.1%) | 89 (80.2%) | 18 (85.7%) | |
| B | 12 (9.1%) | 10 (9.0%) | 2 (9.5%) | |
| C | 6 (4.5%) | 6 (5.4%) | 0 (0.0%) | |
| D | 7 (5.3%) | 6 (5.4%) | 1 (4.8%) | |
| Age at time of fracture [Mean years (SD)] | 54 (13) | 53 (13) | 55 (12) | P < 0.560 |
| Duration of SCI at time of fracture [Median years (IQR)] | 18 (22) | 15 (20) | 23 (14) | P < 0.010 |
| Time until hospitalization [Median days (IQR)] | 2 (8) | 2 (7) | 7 (25) | P < 0.038 |
SCI, spinal cord injury; SD, standard deviation; AIS, American Spinal Injury Association Impairment Scale; IQR, interquartile range.
Figure 1.
Time lag between SCI and occurrence of lower extremity fracture. The time period until the fracture occurred is compared between the union and non-union group.
Figure 2.
Days until hospitalization: Time until in-patient submission into the specialized SCI center occurred is shown between both cohorts.
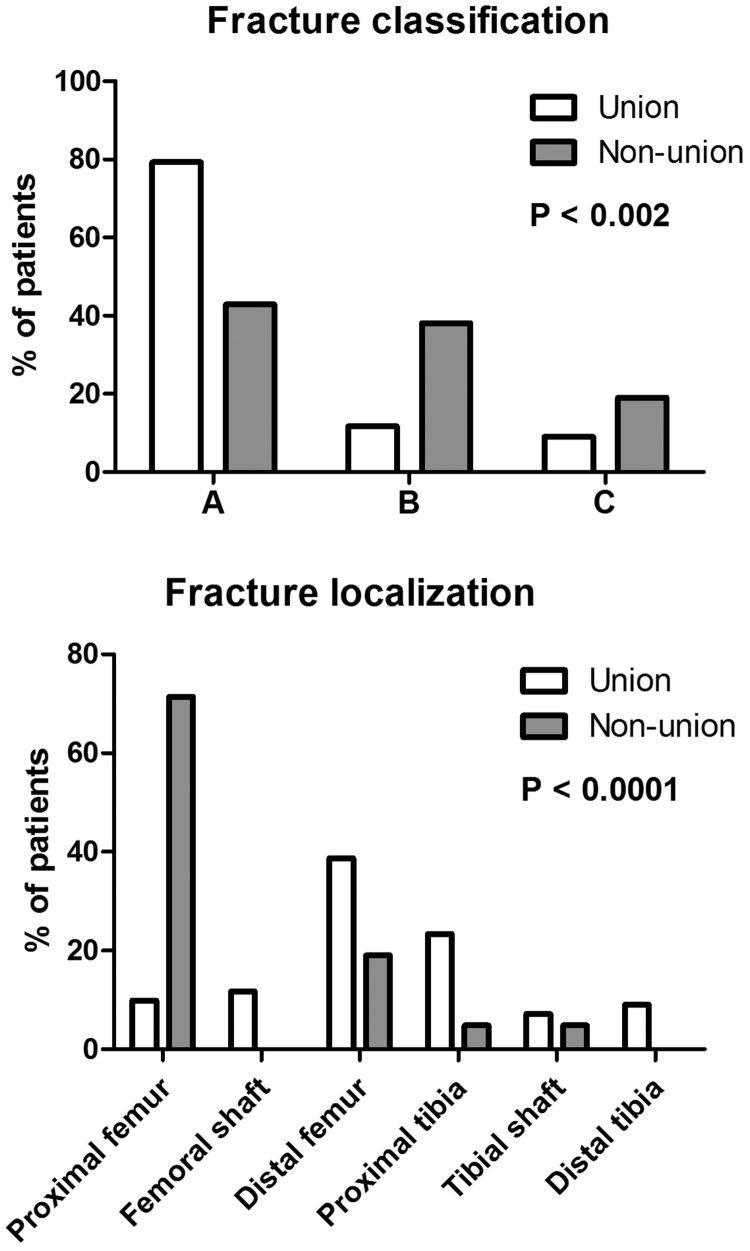
In almost half of all cases, falls out of the wheelchair were the cause for the lower extremity fractures. Together with accidents during transfers and unnoticed traumas, these incidents account for over three quarters of all fractures. The most prevalent fracture region was identified around the knee with a total of 47 fractures of the supracondylar femur (35.6%) and 27 of the proximal tibia (20.5%). These fractures were treated conservatively or surgically. Fractures of the proximal femur (n=26; 19.7%) and shaft (n=13; 9.8%) occurred relatively frequently as well. All tibial shaft fractures (n=9; 6.8%) were treated operatively, whereas this was not the case for distal tibial fractures (n=10; 7.6%). Fracture management is outlined in Table 2. Almost 63% of all fractures were managed surgically. A further subcategorization of treatment strategies did not show significant differences of chosen treatment options between patients whose fractures healed and those suffering from non-unions (Table 3). However, fracture classification and their localization differed significantly between the union and non-union groups (P < 0.002 and P < 0.0001) (Table 2; Fig. 3, Fig. 4).
Table 2. Fracture classification, localization and treatment strategies.
| Overall (n = 132) | Union (n = 111) | Non-union (n = 21) | P value | |
|---|---|---|---|---|
| Fracture classification [n (%)] | P < 0.002 | |||
| A | 97 (73.5%) | 88 (79.3%) | 9 (42.9%) | |
| B | 21 (15.9%) | 13 (11.7%) | 8 (38.1%) | |
| C | 14 (10.6%) | 10 (9.0%) | 4 (19.0%) | |
| Fracture localization [n (%)] | P < 0.0001 | |||
| Proximal femur | 26 (19.7%) | 11 (9.9%) | 15 (71.4%) | |
| Femoral shaft | 13 (9.8%) | 13 (11.7%) | 0 (0.0%) | |
| Distal femur | 47 (35.6%) | 43 (38.7%) | 4 (19.0%) | |
| Proximal tibia | 27 (20.5%) | 26 (23.4%) | 1 (4.8%) | |
| Tibial shaft | 9 (6.8%) | 8 (7.2%) | 1 (4.8%) | |
| Distal tibia | 10 (7.6%) | 10 (9.0%) | 0 (0.0%) | |
| Fracture treatment [n (%)] | P < 0.141 | |||
| Conservative | 49 (37.1%) | 38 (34.2%) | 11 (52.4%) | |
| Operative | 83 (62.9%) | 73 (65.8%) | 10 (47.6%) |
Table 3. Overall complication rate and subcategories of complications.
| Overall (n = 132) | Union (n = 111) | Non-union (n = 21) | P value | |
|---|---|---|---|---|
| Overall complication rate [n (%)] | P < 0.120 | |||
| No | 110 (83.3%) | 95 (85.6%) | 15 (71.4%) | |
| Yes | 22 (16.7%) | 16 (14.4%) | 6 (28.6%) | |
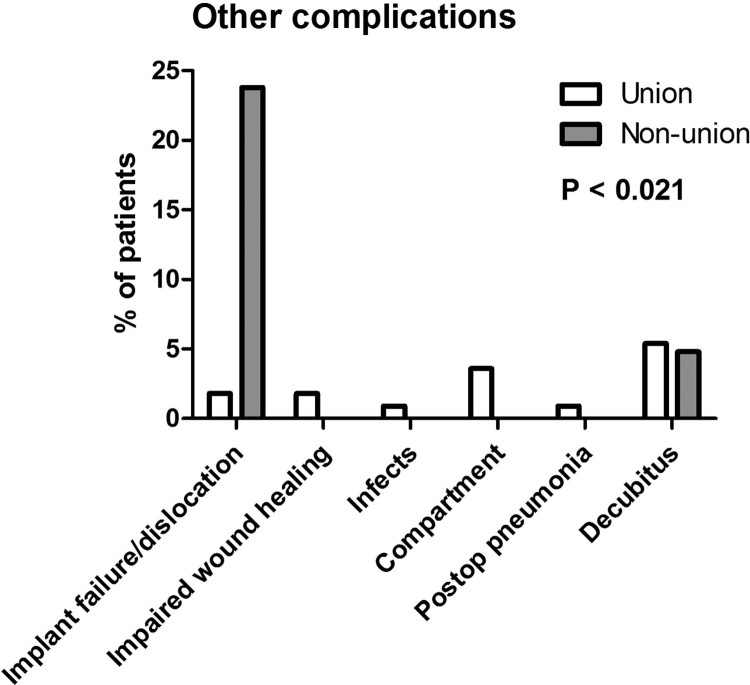
| Complications categorized | P < 0.021 | |||
| No | 110 (83.3%) | 95 (85.6%) | 15 (71.4%) | |
| Implant failure/dislocation | 7 (5.3%) | 2 (1.8%) | 5 (23.8%) | |
| Impaired wound healing | 2 (1.5%) | 2 (1.8%) | 0 (0.0%) | |
| Infection | 1 (0.8%) | 1 (0.9%) | 0 (0.0%) | |
| Compartment | 4 (3.0%) | 4 (3.6%) | 0 (0.0%) | |
| Postoperative pneumonia | 1 (0.8%) | 1 (0.9%) | 0 (0.0%) | |
| Pressure ulcer | 7 (5.3%) | 6 (5.4%) | 1 (4.8%) |
Figure 3.
Fracture classification and localization: Differences between fracture classification and localization between the union and non-union group are presented.
Figure 4.
Complications: Subcategorization of all observed complications are shown between the union and non-union group.
Clinical and radiologic follow-up showed that 84.1% of all fractures in our cohort healed well and resulted in satisfactory osseous unions, despite the changes in bone metabolism reported in patients with SCI. However, we observed a total of 21 non-unions (15.9%) in our cohort. Non-unions were not associated with gender, age at time of the lower extremity fracture, SCI level (cervical versus thoracic/thoracolumbar) and the AIS grade. However, non-union was associated with the time lag between the initial SCI and the fracture. Interestingly, non-unions were also associated with delay of hospitalization in an adequate center.
As a next step, we analyzed our patients according to surgical and non-surgical complication rates. We observed an overall complication rate of 16.7% (n=22). Overall complication rates did not differ significantly between the union and non-union group. However, further subcategorization of all observed complications revealed significant differences between both groups (P < 0.021) (Table 3, Fig. 4).
Based on patient characteristics and the univariate analyses, variables (age at time of fracture, sex, SCI level, AIS grade, duration of SCI, time until hospitalization, fracture classification, fracture localization and management) were tested as potential predictors of pseudarthrosis. Significant predictor variables (P > 0.05) were added stepwise to the logistic regression model based on the likelihood quotient. The best model for predicting pseudarthrosis (Nagelkerke R2: 0.537 P < 5.46*10−9) contained the fracture localization (P < 0.0002), fracture classification (P < 0.056), and fracture management (P < 0.036) as independent predictors (Table 4). The model showed significantly increased odds (OR=64.87, P < 0.00006) for pseudarthrosis in patients who sustained a fracture of the proximal femur compared to patients whose fracture was located at the tibia. Regarding fracture classification, the odds ratio for pseudarthrosis was significantly increased for patients with a fracture class B in comparison to class A according to the AO/OTA classification system (OR=5.23, P < 0.028). Additionally, fracture management was a significant predictor of pseudarthrosis with a higher risk for conservatively treated patients (P < 0.036, OR=5.68).
Table 4. Odds ratios for pseudarthrosis.
| Predictor variables | Comparisons | P-value | Odds ratio (CI 95%) |
|---|---|---|---|
| Localization | |||
| Contrast: Tibia | P < 0.0002 | ||
| Proximal femur | P < 0.00006 | 64.87 (8.54, 492.93) | |
| Femur shaft | n.s. | — | |
| Distal femur | n.s. | — | |
| Classification | |||
| Contrast: A | P < 0.056 | — | |
| B | P < 0.028 | 5.23 (1.19, 22.98) | |
| C | n.s. | — | |
| Fracture management | |||
| Contrast: Operative | |||
| Conservative | P < 0.036 | 5.68 (1.12, 28.78) |
Since there was no significant association of fracture management with pseudarthrosis in univariate (P < 0.141) or bivariate analyses (Spearman rank correlation coefficient for operative-conservative: 1.37, P < 0.116), we did further analyses and found that a correction for fracture localization was sufficient for fracture management to become a significant predictor for pseudarthrosis (P < 0.019, OR=6.67, CI 1.37, 32.64).
Discussion
Musculoskeletal complications are common among patients with SCI.13 Almost three quarters of these patients sustain a long bone fracture over time.14 Because of altered bone metabolism and high complication rates in patients with SCI, the management of fractures in this patient group requires clinical expertise.7 A lot of fractures are managed conservatively.15 But modern surgical techniques allow interventions in this patient cohort as well despite their reduced bone mineral density. However, this study identifies non-unions as a common complication in patients with SCI. Risk factors for non-unions of lower extremity fractures are identified.
As previously described.9,10 fractures occur in a high frequency in active paraplegic patients after low impact injuries during transfer or activities that involve minimal trauma. This was also the case in our study. The level of SCI seems to affect the fracture risk, as patients with thoracic or lumbar medullary injuries seem to be more susceptible to fractures of long bones after minimal trauma than cervical patients with SCI.16 This might be related to the extent of activity, but also reflects the fact that these fractures occur often after transfer maneuvers.17 Further, the seated position leads to high shear forces when a range of movements is induced in the quadriceps muscles.18 Additionally, we want to point out, that the highly used term of “low impact trauma” might be inappropriate in a significant amount of patients with SCI. For instance, in our opinion, falling out of the wheelchair on a hard ground with the knee joint in a flexed position is an “adequate” trauma for causing a lower extremity fracture.
Time intervals between the SCI and the fracture varied greatly (2–68 years after injury) within our cohort. This probably reflects the relatively rapid initial bone loss, which leads to a plateau of reduced bone mineral density in patients with SCI.5,6,19 Hence, patients with SCI remain susceptible for fractures. Interestingly, a lower bone mineral density in patients with SCI with fractures in comparison to those without a bone lesion was identified in previous studies.10,20 A general threshold for fracture risk has not been identified so far, but according to previously published literature a reduction of 50% of the initial bone mineral density seems to be a risk factor for fractures around the knee.10,20
In accordance with preexisting literature most fractures were located around the knee joint.9,21,22 Distal femur and proximal tibial fractures were treated via conservative or surgical management depending on dislocation, soft tissue damage, surgeons’ recommendations and, finally, the decision of the patients. There was a clear trend towards operative management (mostly intramedullary procedures) in femoral and tibial shaft fractures with good outcomes (Table 2).
In general, reports about union rates after long bone fractures in individuals with SCI are scarce and most often limited to incidence rates. To the best of our knowledge, risk factors have not been identified in this population so far. In patients without SCI, several predisposing factors for pseudarthrosis have been found. Among these, open reduction, an open fracture and smoking were most consistently reported.11 Additionally, the type of fracture and osteoporosis were also risk factors.11,23,24
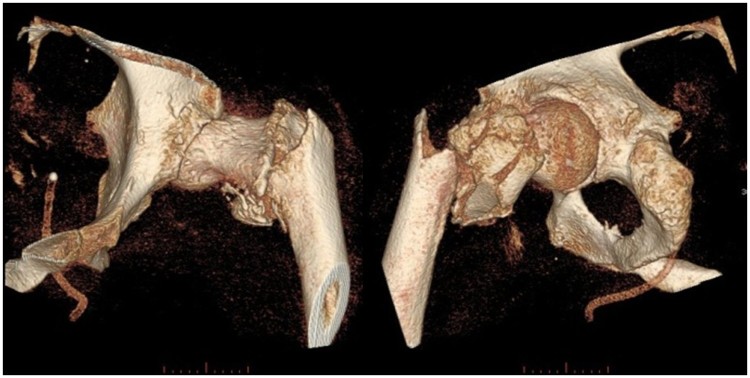
The severity of fractures was related to union rates in our study. More severe fractures (according to the AO/OTA classification) were significantly associated with a higher pseudarthrosis rate (Fig. 3). Additionally, we found a strong relationship of fracture localization with osseous non-unions (Fig. 3). Among these, proximal femur fractures deserve special consideration. We identified 26 patients with proximal femur fractures. The Garden classification is most often used for these fractures. All patients with a Garden III or IV fractures (= with dislocation of the femoral head) (n=10) showed no union independently of their management. 2 patients with Garden 3 fractures were treated surgically and among 8 patients with Garden 4 fractures, 5 were treated conservatively and 3 were managed surgically. This dilemma has already been described over two decades ago.25 An illustrative example of a Garden 4 fracture is presented in Fig. 5. Even modern surgical techniques (i.e. intramedullary devices) failed to show favorable results in our study. Thus, symptomatic treatment should be the goal in this population.
Figure 5.
Case presentation: Presentation of a typical case of a right-sided trochanteric and subtrochanteric femur fracture of a 48-year-old paraplegic male (AIS A) after falling out of his wheelchair.
Femoral shaft and especially supracondylar femur fractures have been associated with a non-favorable outcome in the SCI population.26,27 Our study, however, shows a relatively good outcome in these fractures with only few complications and high union rates (Table 2). In the case of supracondylar femur fractures (n=47)—the most prevalent localization in this study—59.5% (n=25) were treated surgically. Four patients (8.5%) showed non-union in this subpopulation. Among those patients, 3 were treated conservatively, leading to a pseudarthrosis rate of 13.6%.
The complication rate after long bone lower extremity fractures is considerable in individuals with SCI.12,28 In our study, surgically treated fractures tended to be more complex and dislocated. We observed an overall complication rate of 16.7% (n=22) in our cohort. Noteworthy, some complications such as pressure ulcers or compartment syndrome could have been prevented. In the non-union group, implant failure and dislocation of screws were significantly elevated as a direct result of the pseudarthrosis per se (Fig. 4).
In patients with SCI, reported pseudarthrosis rates range from 2% to 10%.1 Here, we found even higher non-union rates (15.9%). However, as discussed, fracture localization and fracture type were associated with non-unions. As a trauma center, we probably receive more severe and dislocated fractures. This might explain the higher rate of non-unions in our study population compared to other studies.29 Within this study, we identified several independent risk factors for non-unions in patients with SCI, especially some susceptible fracture types and locations (Table 4). Interestingly, an adjustment for fracture localization was sufficient for fracture management to become a significant predictor for pseudarthrosis. This may suggest that surgical management might prevent some non-unions, which might otherwise occur after conservative management. However, one should be cautious when interpreting these results, as the overall pseudarthrosis number is relatively small. For definite treatment recommendations, further studies with larger patient numbers are needed.
For the interpretation of the results of this study, some relevant limitations need to be acknowledged. Since study data were collected only at one center, it may be difficult to extrapolate our results to other hospitals and settings, especially if they are not experienced with special needs of the SCI population. However, in our own experience a specialized SCI center is crucial for the treatment of these patients. Additionally, we used a retrospective study design that per se carries the disadvantage of missing values and incomplete datasets in some study patients. This is reflected by the circumstance that data of 54 patients was missing at the end of the follow-up period of one year. A further limitation of this study is the relatively small sample size of patients suffering from pseudarthrosis—for this reason the results from the correlation analyses should interpreted with caution and be taken as a starting point for further studies with a greater cohort.
Conclusion
In summary, this study highlights the clinical significance of lower extremity fractures in patients with SCI—with a special emphasis on union-rates. The management of fractures in patients with SCI is not straightforward as indicated by relative high pseudarthrosis rates in certain fracture types and localizations. At the moment, there is no definitive algorithm available for the pharmacologic and exercise-induced prevention as well as management of these fractures.17,30 Clinical expertise remains fundamental in the clinical decision-making. The functional status of the affected patient needs to be taken into consideration. Several independent risk factors for the development of pseudarthrosis were identified in this study, which should help to identify recognize patients at risk.
Acknowledgments
We would like to thank Mr. Orpheus Mach (Center for Spinal Cord Injuries, Trauma Center Murnau, Murnau, Germany) for his assistance with data management.
Disclaimer statements
Conflict of interest The authors declare no conflict of interest.
Funding None.
Ethics approval None.
Contributors None.
ORCID
Lukas Grassner http://orcid.org/0000-0002-3390-0895
References
- 1. Denise I. Campagnolo and Steven Kirshblum. Spinal Cord Medicine. 2nd ed Wolter Klower / Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 2. Jiang SD, Jiang LS, Dai LY.. Mechanisms of osteoporosis in spinal cord injury. Clin Endocrinol (Oxf). 2006;65(5):555–65. doi: 10.1111/j.1365-2265.2006.02683.x [DOI] [PubMed] [Google Scholar]
- 3. Maimoun L, Fattal C, Micallef JP, Peruchon E, Rabischong P.. Bone loss in spinal cord-injured patients: from physiopathology to therapy. Spinal Cord. 2006;44(4):203–10. doi: 10.1038/sj.sc.3101832 [DOI] [PubMed] [Google Scholar]
- 4. Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C, Liotta FJ, et al Osteoporosis after spinal cord injury. J Orthop Res. 1992;10(3):371–8. doi: 10.1002/jor.1100100309 [DOI] [PubMed] [Google Scholar]
- 5. Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, et al Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 2004;34(5):869–80. doi: 10.1016/j.bone.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 6. Dudley-Javoroski S, Shields RK.. Regional cortical and trabecular bone loss after spinal cord injury. J Rehabil Res Dev 2012;49(9):1365–76. doi: 10.1682/JRRD.2011.12.0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gifre L, Vidal J, Carrasco J, Portell E, Puig J, Monegal A, et al Incidence of skeletal fractures after traumatic spinal cord injury: a 10-year follow-up study. Clin Rehabil 2014;28(4):361–9. doi: 10.1177/0269215513501905 [DOI] [PubMed] [Google Scholar]
- 8. Zehnder Y, Luthi M, Michel D, Knecht H, Perrelet R, Neto I, et al Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int 2004;15(3):180–9. doi: 10.1007/s00198-003-1529-6 [DOI] [PubMed] [Google Scholar]
- 9. Comarr AE, Hutchinson RH, Bors E.. Extremity fractures of patients with spinal cord injuries. Am J Surg 1962;103:732–9. doi: 10.1016/0002-9610(62)90256-8 [DOI] [PubMed] [Google Scholar]
- 10. Garland DE, Adkins RH, Kushwaha V, Stewart C.. Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med 2004;27(3):202–6. doi: 10.1080/10790268.2004.11753748 [DOI] [PubMed] [Google Scholar]
- 11. Santolini E, West R, Giannoudis PV.. Risk factors for long bone fracture non-union: a stratification approach based on the level of the existing scientific evidence. Injury 2015;46 Suppl 8:S8–S19. doi: 10.1016/S0020-1383(15)30049-8 [DOI] [PubMed] [Google Scholar]
- 12. McMaster WC, Stauffer ES.. The management of long bone fracture in the spinal cord injured patient. Clin Orthop Rel Res 1975(112):44–52. [PubMed] [Google Scholar]
- 13. Vogel LC, Krajci KA, Anderson CJ.. Adults with pediatric-onset spinal cord injury: part 2: musculoskeletal and neurological complications. J Spinal Cord Med 2002;25(2):117–23. doi: 10.1080/10790268.2002.11753611 [DOI] [PubMed] [Google Scholar]
- 14. Eser P, Frotzler A, Zehnder Y, Schiessl H, Denoth J.. Assessment of anthropometric, systemic, and lifestyle factors influencing bone status in the legs of spinal cord injured individuals. Osteoporos Int 2005;16(1):26–34. doi: 10.1007/s00198-004-1638-x [DOI] [PubMed] [Google Scholar]
- 15. Bethel M, Bailey L, Weaver F, Le B, Burns SP, Svircev JN, et al Surgical compared with nonsurgical management of fractures in male veterans with chronic spinal cord injury. Spinal Cord 2015;53(5):402–7. doi: 10.1038/sc.2015.5 [DOI] [PubMed] [Google Scholar]
- 16. Vestergaard P, Krogh K, Rejnmark L, Mosekilde L.. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36(11):790–6. doi: 10.1038/sj.sc.3100648 [DOI] [PubMed] [Google Scholar]
- 17. Akhigbe T, Chin AS, Svircev JN, Hoenig H, Burns SP, Weaver FM, et al A retrospective review of lower extremity fracture care in patients with spinal cord injury. J Spinal Cord Med 2015;38(1):2–9. doi: 10.1179/2045772313Y.0000000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McHenry CL, Shields RK.. A biomechanical analysis of exercise in standing, supine, and seated positions: Implications for individuals with spinal cord injury. J Spinal Cord Med 2012;35(3):140–7. doi: 10.1179/2045772312Y.0000000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garland DE, Adkins RH, Stewart CA.. Five-year longitudinal bone evaluations in individuals with chronic complete spinal cord injury. J Spinal Cord Med 2008;31(5):543–50. doi: 10.1080/10790268.2008.11753650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M.. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord 2001;39(4):208–14. doi: 10.1038/sj.sc.3101139 [DOI] [PubMed] [Google Scholar]
- 21. Barlehner C, Bohm V, Flieger R, Meiners T.. [Surgery for fractures of the lower extremities in cases of chronic spinal cord injury]. Der Orthopade 2005;34(2):137–8, 40–3. doi: 10.1007/s00132-004-0757-6 [DOI] [PubMed] [Google Scholar]
- 22. Ragnarsson KT, Sell GH.. Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil 1981;62(9):418–23. [PubMed] [Google Scholar]
- 23. Nikolaou VS, Efstathopoulos N, Kontakis G, Kanakaris NK, Giannoudis PV.. The influence of osteoporosis in femoral fracture healing time. Injury 2009;40(6):663–8. doi: 10.1016/j.injury.2008.10.035 [DOI] [PubMed] [Google Scholar]
- 24. Nicoll EA. Fractures of the Tibial Shaft. A Survey of 705 Cases. J Bone Joint Surg Br 1964;46:373–87. doi: 10.1302/0301-620X.46B3.373 [DOI] [PubMed] [Google Scholar]
- 25. Freehafer AA. Limb fractures in patients with spinal cord injury. Arch Phys Med Rehabil 1995;76(9):823–7. doi: 10.1016/S0003-9993(95)80546-X [DOI] [PubMed] [Google Scholar]
- 26. Freehafer AA, Hazel CM, Becker CL.. Lower extremity fractures in patients with spinal cord injury. Paraplegia 1981;19(6):367–72. [DOI] [PubMed] [Google Scholar]
- 27. Baird RA, Kreitenberg A, Eltorai I.. External fixation of femoral shaft fractures in spinal cord injury patients. Paraplegia 1986;24(3):183–90. [DOI] [PubMed] [Google Scholar]
- 28. Cochran TP, Bayley JC, Smith M.. Lower extremity fractures in paraplegics: pattern, treatment, and functional results. J Spinal Disord 1988;1(3):219–23. doi: 10.1097/00002517-198803000-00007 [DOI] [PubMed] [Google Scholar]
- 29. Frotzler A, Cheikh-Sarraf B, Pourtehrani M, Krebs J, Lippuner K.. Long-bone fractures in persons with spinal cord injury. Spinal Cord 2015;53(9):701–4. doi: 10.1038/sc.2015.74 [DOI] [PubMed] [Google Scholar]
- 30. Bryson JE, Gourlay ML.. Bisphosphonate use in acute and chronic spinal cord injury: a systematic review. J Spinal Cord Med 2009;32(3):215–25. doi: 10.1080/10790268.2009.11760776 [DOI] [PMC free article] [PubMed] [Google Scholar]