Abstract
Systemically delivered pharmacologic thrombolysis for acute deep vein thrombosis long ago gave way to catheter delivery of plasminogen activators within the clot. This simple concept resulted in markedly improved efficacy and safety. In an effort to accelerate thrombus dissolution or extraction, mechanical methods were developed, but the initial techniques left substantial residual thrombus that required subsequent catheter-directed thrombolysis (CDT). It was soon observed that combined pharmacomechanical thrombolysis was more effective than either one alone.
Randomized trials of catheter-based strategies for thrombus removal have documented objective benefit, including improved patency, preserved valve function, and reduced post-thrombotic syndrome. The largest randomized study is the ATTRACT trial published at the end of 2017. Although mild post-thrombotic syndrome (PTS) was no different between the pharmacomechanical catheter-directed thrombolysis (PCDT) and control groups, acute pain and swelling and moderate-to-severe PTS were reduced with PCDT. Additional analyses from this robust data set are forthcoming.
Keywords: acute DVT, deep vein thrombosis, pharmacomechanical thrombolysis, catheter-directed thrombolysis, ATTRACT trial
BACKGROUND
Acute deep venous thrombosis (DVT) is a highly prevalent and growing problem in the United States. Anticoagulation is the standard of care for most, if not all, patients in many communities. However, DVT represents a spectrum of diseases with varying thrombus location and burden among patients. It has become increasingly recognized that post-thrombotic syndrome (PTS) occurs in a large percentage of patients treated with anticoagulation alone, and that PTS has an adverse effect on subsequent quality of life (QOL). Furthermore, the severity of the patients' DVT appeared to be predictive of PTS severity. Physicians have observed that patients treated with a successful strategy of thrombus removal, especially those with extensive iliofemoral DVT, had markedly reduced post-thrombotic morbidity (Figure 1).
Figure 1.
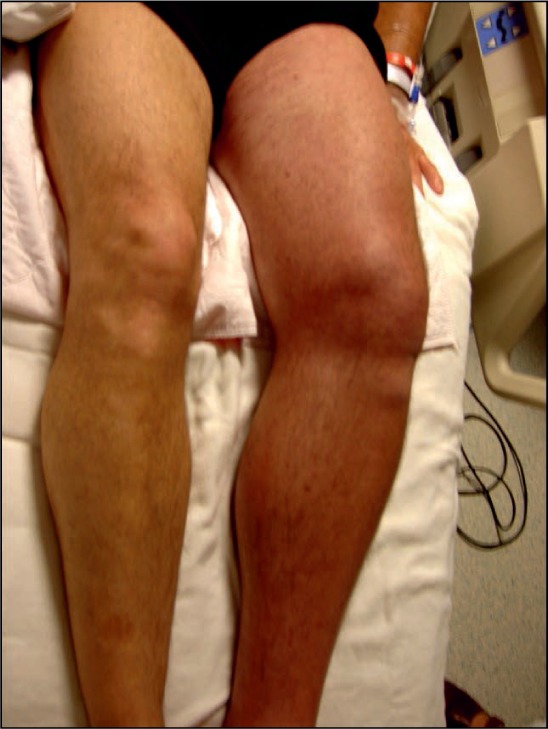
Photograph of swollen, painful, discolored left leg (phlegmasia cerulea dolens) in a man with extensive iliofemoral DVT. The patient had received 5 days of anticoagulation therapy with low-molecular-weight heparin.
Until 2008, national and international guidelines addressing the management of acute DVT only recommended anticoagulation. However, the 8th American College of Chest Physicians Consensus Conference on Antithrombotic and Thrombolytic Therapy,1 The American Heart Association Scientific Statement on the Management of Iliofemoral DVT,2 and the Society of Vascular Surgery Guidelines3 indicate that interventional approaches to thrombus removal reduce the incidence of PTS, particularly in patients with iliofemoral DVT (IFDVT).
The initial randomized trial demonstrating a treatment strategy of thrombus removal was conducted and reported by Plate et al., who performed the ultimate mechanical thrombectomy when they randomized patients with IFDVT to either venous thrombectomy and arteriovenous fistula plus anticoagulation or to anticoagulation alone. They observed increased patency of the iliofemoral venous system, reduced venous pressures, and a reduction in post-thrombotic morbidity in patients treated with venous thrombectomy.4,5 Despite the results of this trial, operative venous thrombectomy has not been well integrated into the treatment of IFDVT by vascular surgeons, and most did not perform a venous thrombectomy during their vascular surgical education. It is apparent that if patients are to benefit from a treatment strategy that eliminates an extensive thrombus occluding venous outflow, catheter-based techniques will be required.
CATHETER-DIRECTED THROMBOLYSIS (DRIP TECHNIQUE)
The initial management of patients using catheter-directed thrombolysis (CDT) involved inserting a catheter into the thrombus with the intent of activating fibrin-bound plasminogen within the clot. Many physicians observed improved efficacy and safety compared to systemic thrombolysis. In fact, this technique has endured the test of time and continues to be used successfully more than two decades since its inception.
Advances in CDT have demonstrated that the multiple-side-hole catheter is better than the end-hole catheter. Progressively lower doses of plasminogen activator have been observed to be effective, especially when infused in larger volumes (≥ 100 mL/h). More precise infusion methods (i.e., infusing the entire clot—but only the clot—to maximize efficacy and safety) may further improve this technique.
The National Venous Registry was the largest data base analyzing CDT for acute DVT using the drip technique. Seventy-one percent of participants had IFDVT.6 Following CDT, thrombosis-free survival correlated with the success of thrombolysis. At 1 year, 78% of patients with complete clot resolution had patent veins compared with 37% who had less than 50% lysis (P < .001). In patients with first-time IFDVT who had successful lysis, 96% of the veins were patent at 1 year. Successful lysis correlated with normal valve function (P < .02).
A cohort-controlled QOL study was subsequently performed to determine whether lytic therapy affected QOL in patients with IFDVT in the National Venous Registry. Results showed that QOL was related to success of thrombolysis, and CDT resulted in better QOL than anticoagulation alone. In those who failed thrombolysis, their QOL was similar to patients treated with anticoagulation alone.7 Unquestionably, advances in technology and our understanding of care have progressed since the Registry's report in 1999. However, many of these early observations remain valid.
ENDOVENOUS MECHANICAL THROMBECTOMY
Mechanical techniques are being developed to more rapidly clear the acutely thrombosed venous system. Vedanthan et al. evaluated the effectiveness of early mechanical thrombectomy devices alone or in combination with pharmacologic thrombolysis. With venographic scoring, 26% of thrombus removal was achieved with mechanical devices alone. Success improved to 86% when pharmacologic (lytic) techniques were added.8
Endovascular aspiration thrombectomy is an increasingly popular technique to debulk and, in some patients, definitively treat extensive large vein thrombosis. Jia et al. performed a retrospective review of 68 patients with IFDVT treated with aspiration thrombectomy and reported a technical success of 100%. Aspiration alone was used in 47 cases (69%), and additional CDT was used in 21 (31%). Thirty-six patients (46%) required venous stenting. Recurrent thrombosis occurred in 11% at 22-month follow-up.9
Catheter-based technology is rapidly evolving, offering more sophisticated aspiration techniques, basket thrombectomy, and thrombus retrieval catheters. These techniques are in clinical evaluation, with many being adopted for management of massive and submassive pulmonary embolism in addition to extensive DVT.
PHARMACOMECHANICAL THROMBOLYSIS
Integrating mechanical thrombectomy with catheter-delivered thrombolysis is a sound and reasonable approach to managing extensive thrombosis, taking advantage of what can be accomplished mechanically and enzymatically. This appears to be the most commonly used approach for interventionalists managing large burdens of thrombus.
Rheolytic thrombectomy uses power-pulse fluid injected into the thrombus, with or without aspiration of the pulsed fluid. Some physicians use the power pulse to penetrate the thrombus with a plasminogen activator, whereas others use it to fragment the thrombus. Rheolytic thrombectomy has been used as the initial approach to debulk the thrombus or following CDT to remove residual thrombus.
Lin et al. reported their 8-year experience of 98 patients managed with pharmacomechanical thrombectomy integrating the rheolytic technique. Forty-six patients received CDT alone and 52 underwent pharmacomechanical management. Rheolytic thrombectomy was associated with significantly shorter hospital stays, fewer blood transfusions, and a reduction in required venograms. Bleeding complications were similar between the CDT and the rheolytic thrombectomy group.10
A smaller cohort of patients reported by Kasirajan et al. showed that mechanical thrombectomy alone was less effective than combined pharmacomechanical thrombolysis.11
Isolated segmental pharmacomechanical thrombolysis (ISPMT) is an interesting technique that isolates the thrombus (or portion of thrombus) between two balloons. The catheter between the two balloons permits the infusion of a plasminogen activator, then assumes a spiral configuration and spins at 3,500 rpm. The principle is to fragment the thrombus and increase its surface area, thereby permitting maximal binding of the plasminogen activator to the fibrin-bound plasminogen and accelerating clot resolution. The liquefied and fragmented thrombus can then be aspirated and, based upon venographic results, the same portion of the vein can be re-treated if necessary, or another portion treated in similar fashion.
Martinez et al. studied the effect of ISPMT versus standard drip CDT on the outcome of 52 consecutive patients treated for IFDVT. Twenty-seven were treated with CDT and 25 treated with ISPMT. Of the 27 limbs treated with CDT, 16 required adjunctive catheter-based therapy (rheolytic thrombectomy, balloon maceration, etc.) compared with 7 of 25 ISPMT patients. Complete lysis was achieved with 11% of CDT patients and 28% of ISPMT-treated patients (P = .077). Treatment time was shorter (23.4 h vs 55.4 h, P < .001) and recombinant tissue plasminogen activator (rtPA) dose was lower (33.4 mg vs 59.3 mg, P = .009) with ISPMT. Only 18% of limbs experienced complete thrombolysis using ISPMT alone. Bleeding complications occurred in 5% of both groups.12 These results are similar to those reported by O'Sullivan et al.13
Ultrasound-accelerated thrombolysis is based on the concept that the addition of ultrasound during lytic infusion increases the surface area of the fibrin, thereby permitting more efficient binding of the plasminogen activator to the fibrin-bound plasminogen. Parikh et al. evaluated a multicenter registry of patients with acute lower- and upper-extremity DVT treated with ultrasound-accelerated thrombolysis. A variety of plasminogen activators were used, and the results were compared to historical observations. Complete lysis (> 90%) was observed in 70% and overall patient lysis in 91%. The median infusion time was 22 hours and 4% of patients had major complications, mainly puncture-site bleeding. Compared to historical observations, treatment time and doses of lytic agent were reduced with ultrasound thrombolysis.14
A trial by Engelberger et al. randomized patients with IFDVT to ultrasound-accelerated thrombolysis or CDT without ultrasound. The primary end point was percent lysis at 15 hours of therapy. Patients received 20 mg of rtPA, and a repeat venogram was performed and read by blinded interpreters. Patients receiving CDT alone had 54% lysis and those receiving ultrasound-accelerated thrombolysis had 55% lysis at 15 hours of treatment (P = .91).15
CATHETER-BASED RANDOMIZED TRIALS
A small randomized trial comparing CDT with anticoagulation alone was performed by Elsharawy et al. The trial was powered to evaluate the outcomes of venous patency and deep vein valve function. They reported that patients treated with CDT had significantly improved venous patency and a reduction in valvular incompetence compared to those treated with anticoagulation alone.16 These observations are frequently overlooked when there is no assessment of QOL and post-thrombotic morbidity using the Villalta score and venous clinical severity score. However, it is fair to say that very few patients will have PTS if they have patent veins with normal valve function.
The CaVent trial randomized 209 patients with IFDVT to anticoagulation alone versus CDT and anticoagulation.17 The primary end points were iliofemoral venous patency at 6 months and PTS at 2 years. CDT was performed with a multi-side-hole catheter. The rtPA was prepared by mixing 20 mg in 500 cc of 0.9 % NaCl, then infused at a dose of 0.01 mg/kg for a maximum of 96 hours. Therefore, a 70-kg patient would be infused with 0.7 mg/h of rtPA in 17.5 mL of infusate. This appears to be an unusually small volume of infusate, which would potentially reduce the degree of thrombolysis.
Results indicated that 43% of patients had complete thrombolysis, 37% had partial lysis, and 10% were unsuccessful. Patients receiving CDT had significantly improved iliofemoral venous patency at 6 months (P = .012) and less PTS at 2 years (P = .047). The lower thrombus scores at the completion of CDT were associated with improved venous patency (P = .04). Patency of the iliofemoral venous system directly correlated with a reduction of PTS (P < .001). There was an absolute risk reduction in PTS of 14.4% with CDT. Major bleeding complications occurred in 3.3% of patients undergoing CDT. Only one inferior vena caval filter was used, and no patient had a symptomatic pulmonary embolus.17
At 2-year follow-up, the number needed to treat to avoid one PTS was 7. Patients were re-evaluated at 5 years. Interestingly, PTS continued to progress in the patients treated with anticoagulation, whereas those treated with CDT were stable. At 5-year follow-up, the number needed to treat with CDT to avoid one PTS dropped to 4.18
THE ATTRACT TRIAL
Sponsored by the National Institutes of Health/National Heart, Lung, and Blood Institute, the ATTRACT trial—with 692 patients—is the largest study to date evaluating PDCT plus anticoagulation versus anticoagulation alone for proximal DVT. Patients in 56 U.S. centers were stratified according to their extent of thrombosis (iliofemoral DVT or femoral-popliteal DVT) then randomized 1:1 to PCDT plus anticoagulation versus anticoagulation alone (Figure 2). Patients aged 16 to 75 with < 14-day history of acute DVT were eligible if they had no contraindication to anticoagulation or thrombolysis. Each group received initial and long-term anticoagulation consistent with guideline-driven care. All patients received below-knee 30- to 40-mm Hg ankle gradient stockings to be worn during their waking hours.19
Figure 2.
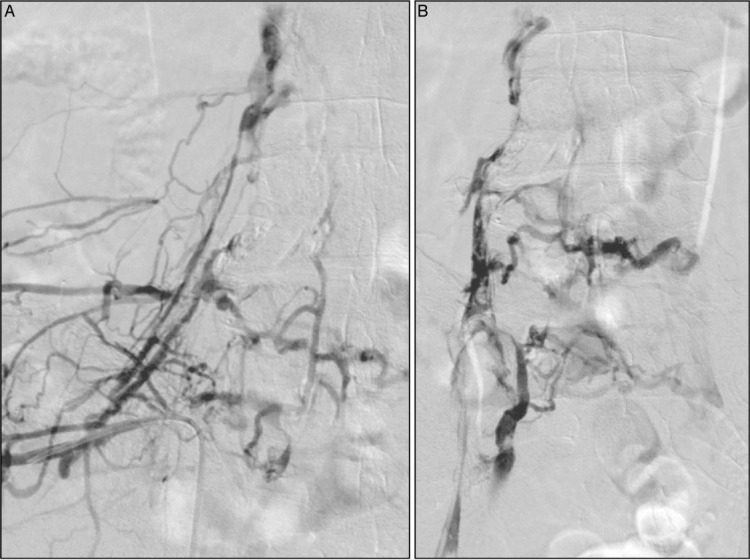
An ascending diagnostic venogram performed via the ipsilateral popliteal vein in the prone position demonstrates extensive iliofemoral venous occlusion, shown in both (A) anteroposterior (AP) and (B) oblique views. Note the superimposed thrombus in the AP view. The patient was treated with pharmacomechanical catheter-directed thrombolysis.
The plasminogen activator used in the PCDT group was rtPA with a maximum dose of 35 mg. Infusions of lytic agent could continue up to 30 hours. Each center chose one of three initial catheter-based treatment options: (1) infusion first by a drip technique through a multi-side-hole catheter, (2) attempt single-session thrombus removal with the AngioJet Rheolytic Thrombectomy System (Boston Scientific), or (3) attempt single-session thrombus removal with the Trellis Peripheral Infusion System (Covidien) and then infuse rtPA for no longer than 24 hours if residual thrombus was present. Balloon maceration and other adjunctive methods were permitted in order to manage residual thrombus. Stenting was encouraged for persistent stenoses covering ≥ 50% of the iliofemoral segment.19
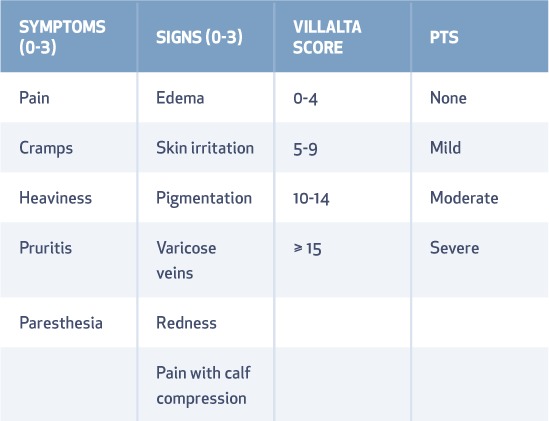
The primary end point of PTS was defined as a Villalta Score > 4 between 6 and 24 months following treatment (Table 1). Secondary end points included changes in acute pain and edema up to 10 and 30 days, general and disease-specific QOL, separate analysis of iliofemoral and femoral popliteal groups, and a cost-benefit analysis.19
Table 1.
Signs and symptoms of post-thrombotic syndrome (PTS) comprising the Villalta Score. Rank the severity of each symptom and sign on a scale of 0 (none) to 3 (severe), then add them up to determine a patient's Villalta Score for PTS.
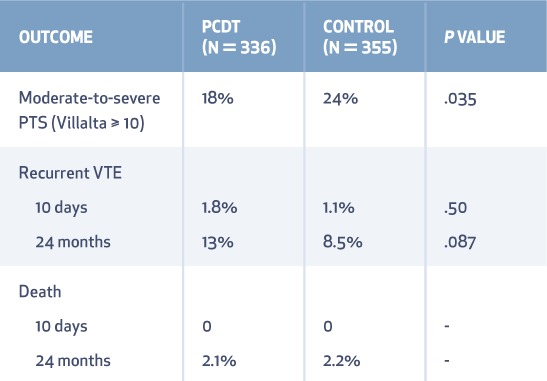
PTS developed in 157 (47%) patients assigned to the PCDT group and 171 (48%) assigned to the control group (RR 0.96; 95% CI 0.82–1.11; P = .56) (Table 2). Moderate-to-severe PTS (Villalta Score > 9) occurred in 18% of the PCDT group and 24% of the control group (RR 0.73; 95% CI 0.54–0.98; P = .04) (Table 3). The severity of PTS was significantly lower in the PCDT group at all visits between 6 and 24 months (P < .01 for the between-group comparison at each time point).19
Table 2.
Primary outcomes of the ATTRACT trial. PDCT: pharmacomechanical catheter-directed thrombolysis; PTS: post-thrombotic syndrome
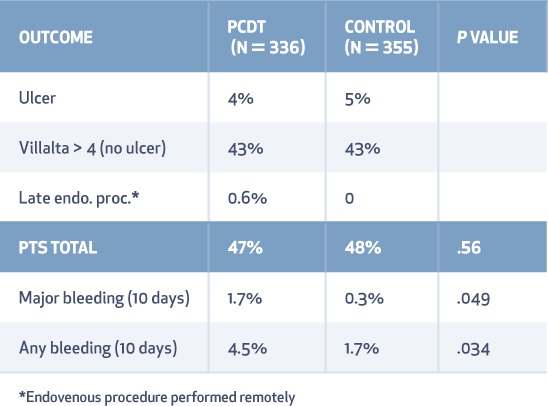
Table 3.
Secondary outcomes of the ATTRACT trial. PDCT: pharmacomechanical catheter-directed thrombolysis; PTS: post-thrombotic syndrome; VTE: venous thromboembolism
Leg pain decreased in the PCDT group at 10 and 30 days compared with the control group (P = .02). Edema likewise decreased in the PCDT group compared with the control group over 10 and 30 days (P < .02 and P < .05, respectively).19
General QOL was similar for each group. Disease-specific QOL measured by the VEINES-QOL questionnaire showed a trend toward improved QOL in the PCDT group (P = .08) (Figures 3, 4). Major bleeding occurred in 1.7% of the PCDT group and 0.3% of the control group (P = .049). Recurrent venous thromboembolism during 24-month follow-up occurred in 12% of the PCDT patients and 8% of the controls.19
Figure 3.
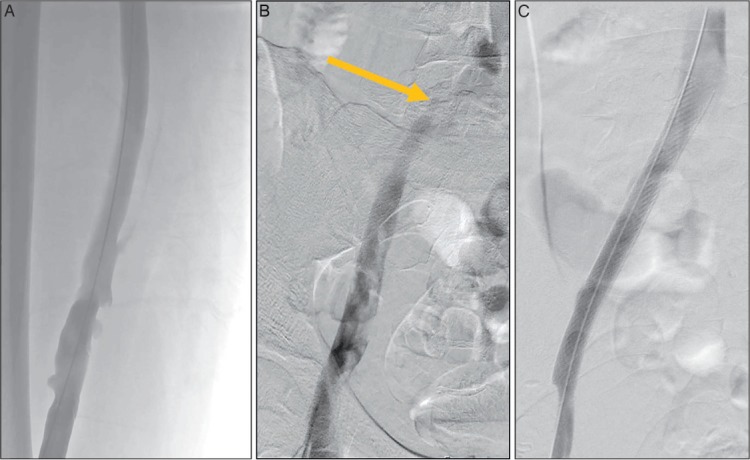
Venogram in the prone position after pharmacomechanical catheter-directed thrombolysis shows (A) patency of the iliofemoral venous system. (B) There is a persistent stenosis of the left common iliac vein due to compression by the right common iliac artery (arrow). (C) After stenting of the residual common iliac vein stenosis, there is unobstructed venous drainage into the vena cava.
Figure 4.
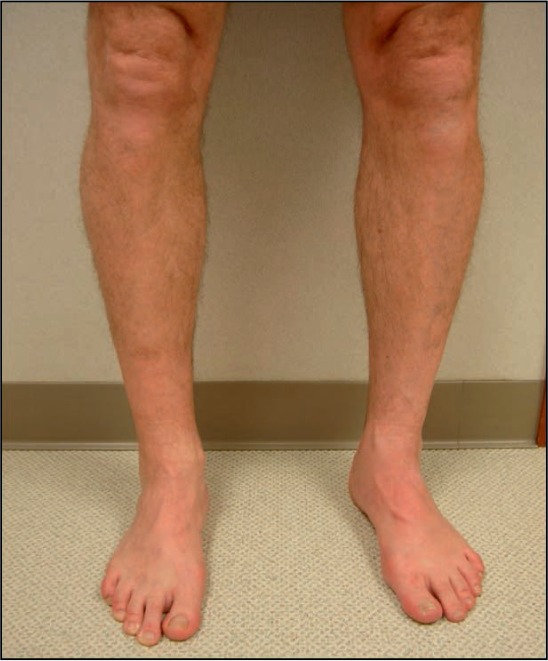
At 12-month follow-up, the patient is asymptomatic with patent veins, no edema, and normal venous valve function.
COMMENT
The ATTRACT trial is a landmark study. At first glance, ATTRACT appears to be a negative study since there was no difference in PTS during the 6- to 24-month follow-up between the PCDT and control groups. However, it is important to recognize that the Villalta scale is a highly sensitive tool for PTS (and perhaps any form of venous disease), and the primary end point was chosen more than 12 years ago when the protocol was written. At that time, the Villalta scale was in its infancy, and the important nuances available today did not exist. The important question asked by all clinicians is, “Do patients with acute DVT—and especially extensive DVT—benefit from PCDT?” If written today, it is likely that protocol developers would choose a different primary end point (moderate-to-severe PTS) to answer the question. Most clinicians would not offer, and most patients would not accept, an invasive procedure with recognized major morbidity to prevent mild PTS. However, when the protocol was written, the tool measuring PTS was not developed enough to adequately differentiate between the differing severities of post-thrombotic morbidity.
The ATTRACT trial had the opportunity to evaluate the benefit of thrombus-removal strategies in patients with femoral-popliteal DVT and patients with iliofemoral DVT. The outcome reported to date includes all randomized patients, while the analyses of the separate groups are underway (Figure 5). Since these two groups (IFDVT and femoral-popliteal DVT) were stratified prior to randomization, the results of these analyses will carry extra weight.
Figure 5.
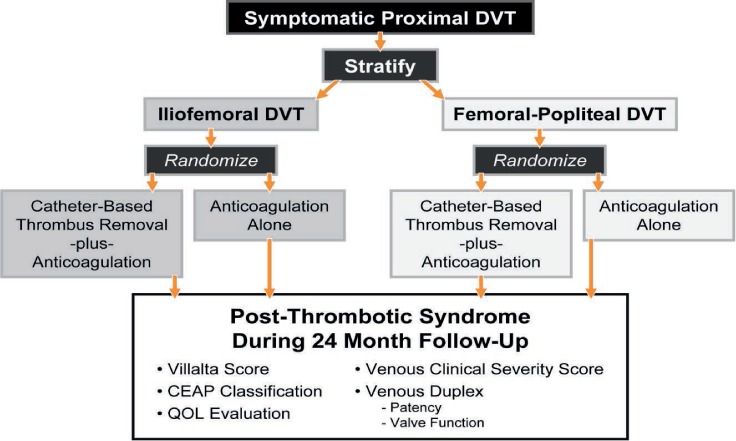
Stratification and randomization sequence and outcome measures for the ATTRACT Trial. DVT: deep vein thrombosis; CEAP: a classification system to standardize the reporting and treatment of chronic venous disorders based on clinical, etiological, anatomical, and pathophysiological manifestations; QOL: quality of life
The contemporary risk of major bleeding with PCDT is lower in ATTRACT than in any prior study. While a 1.7% risk remains higher than that of anticoagulation alone, it demonstrates that proper patient selection and careful attention to technical detail can add considerable safety to the technique.
The ATTRACT trial has advanced patient care and added high-quality evidence to assist in the management of patients with acute DVT. The subsequent analyses of this robust data set are anxiously awaited.
CONCLUSION
Patients with iliofemoral DVT are at higher risk of severe PTS compared to patients with acute DVT limited to the infrainguinal venous location. Prior randomized trials have demonstrated benefits of thrombus removal. The ATTRACT trial taught us that PCDT should not be routine therapy for acute proximal DVT. Although the frequency of PTS was no different between the two treatment groups, its severity was lower in patients treated with PCDT. Analyses addressing QOL, iliofemoral DVT versus infrainguinal DVT, and cost-benefit are underway.
KEY POINTS
Prior randomized trials of thrombus removal strategies for patients with iliofemoral DVT have demonstrated increased patency, preserved venous valve function, and reduced post-thrombotic morbidity.
Integrating pharmacologic with mechanical techniques of thrombus management has resulted in more efficient thrombus removal during shorter treatment times and requires lower doses of plasminogen activators.
The ATTRACT trial randomized patients with acute proximal DVT to standard anticoagulation or PCDT plus anticoagulation. The primary end point of any PTS at 2 years was the same in the two treatment groups.
In the ATTRACT trial, PCDT patients enjoyed more rapid resolution of lower extremity pain and edema at 10 and 30 days and significantly less moderate-to-severe PTS.
Footnotes
Conflict of Interest Disclosure: Dr. Comerota is on the scientific advisory board for Tactile, Inc., on the speaker's bureau for BMS/Pfizer, conducts research on National Institutes of Health ATTRACT trial, and is co-principal investigator of the COOK VIVO Study.
REFERENCES
- 1.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008 Jun;133(6 Suppl):454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 2.Jaff MR, McMurty MS, Archer SL et alManagement of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011 Apr 26;123(16):1788–830. doi: 10.1161/CIR.0b013e318214914f. .; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. [DOI] [PubMed] [Google Scholar]
- 3.Meissner MH, Gloviczki P, Comerota AJ et alEarly thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2012 May;55(5):1449–62. doi: 10.1016/j.jvs.2011.12.081. .; Society for Vascular Surgery; American Venous Forum. [DOI] [PubMed] [Google Scholar]
- 4.Plate G, Einarsson E, Ohlin P, Jensen R, Qvarfordt P, Eklöf B. Thrombectomy with temporary arteriovenous fistula: the treatment of choice in acute iliofemoral venous thrombosis. J Vasc Surg. 1984 Nov;1(6):867–76. doi: 10.1067/mva.1984.avs0010867. [DOI] [PubMed] [Google Scholar]
- 5.Plate G, Akesson H, Einarsson E, Ohlin P, Eklöf B. Long-term results of venous thrombectomy combined with a temporary arterio-venous fistula. Eur J Vasc Surg. 1990 Oct;4(5):483–9. doi: 10.1016/s0950-821x(05)80788-1. [DOI] [PubMed] [Google Scholar]
- 6.Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999 Apr;211(1):39–49. doi: 10.1148/radiology.211.1.r99ap4739. [DOI] [PubMed] [Google Scholar]
- 7.Comerota AJ, Throm RC, Mathias SD, Haughton S, Mewissen M. Catheter-directed thrombolysis for iliofemoral deep venous thrombosis improves health-related quality of life. J Vasc Surg. 2000 Jul;32(1):130–7. doi: 10.1067/mva.2000.105664. [DOI] [PubMed] [Google Scholar]
- 8.Vedantham S, Vesely TM, Parti N, Darcy M, Hovsepian DM, Picus D. Lower extremity venous thrombolysis with adjunctive mechanical thrombectomy. J Vasc Interv Radiol. 2002 Oct;13(10):1001–8. doi: 10.1016/s1051-0443(07)61864-8. [DOI] [PubMed] [Google Scholar]
- 9.Jia Z, Tu J, Zhao J et al. Aspiration thrombectomy using a large-size catheter for acute lower extremity deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2016 Apr;4(2):167–71. doi: 10.1016/j.jvsv.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Lin PH, Zhou W, Dardik A et al. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg. 2006 Dec;192(6):782–8. doi: 10.1016/j.amjsurg.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 11.Kasirajan K, Gray B, Ouriel K. Percutaneous AngioJet thrombectomy in the management of extensive deep venous thrombosis. J Vasc Interv Radiol. 2001 Feb;12(2):179–85. doi: 10.1016/s1051-0443(07)61823-5. [DOI] [PubMed] [Google Scholar]
- 12.Martinez Trabal JL, Comerota AJ, LaPorte FB, Kazanjian S, DiSalle R, Sepanski DM. The quantitative benefit of isolated, segmental, pharmacomechanical thrombolysis (ISPMT) for iliofemoral venous thrombosis. J Vasc Surg. 2008 Dec;48(6):1532–7. doi: 10.1016/j.jvs.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 13.O'Sullivan GJ, Lohan DG, Gough N, Cronin CG, Kee ST. Pharmacomechanical thrombectomy of acute deep vein thrombosis with the Trellis-8 isolated thrombolysis catheter. J Vasc Interv Radiol. 2007 Jun;18(6):715–24. doi: 10.1016/j.jvir.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Parikh S, Motarjeme A, McNamara T et al. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: initial clinical experience. J Vasc Interv Radiol. 2008 Apr;19(4):521–8. doi: 10.1016/j.jvir.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Engelberger RP, Spirk D, Willenberg T et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ Cardiovasc Interv. 2015 Jan;8(1) doi: 10.1161/CIRCINTERVENTIONS.114.002027. [DOI] [PubMed] [Google Scholar]
- 16.Elsharawy M, Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg. 2002 Sep;24(3):209–14. doi: 10.1053/ejvs.2002.1665. [DOI] [PubMed] [Google Scholar]
- 17.Enden T, Haig Y, Kløw NE et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012 Jan 7;379(9810):31–8. doi: 10.1016/S0140-6736(11)61753-4. [DOI] [PubMed] [Google Scholar]
- 18.Haig Y, Enden T, Grøtta O et al. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol. 2016 Feb;3(2):e64–71. doi: 10.1016/S2352-3026(15)00248-3. [DOI] [PubMed] [Google Scholar]
- 19.Vedantham S, Goldhaber SZ, Julian JA et alPharmacomechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis. N Engl J Med. 2017 Dec 7;377(23):2240–52. doi: 10.1056/NEJMoa1615066. .; ATTRACT Trial Investigators. [DOI] [PMC free article] [PubMed] [Google Scholar]


