A 61-year-old female was admitted with acute dyspnea on exertion and diffuse muscle tenderness 1 month after initiation of sorafenib for renal cell carcinoma. On examination, she was tachycardic with elevated jugular venous pressure, ascites, and lower extremity edema. Initial laboratory testing revealed a troponin I of 13.1 ng/mL, elevated creatine kinase of 1,817 ng/mL, elevated aminotransferases, and B-natriuretic peptide of 534 pg/dL. Electrocardiogram showed acute ST segment elevation in the anteroseptal leads, which may suggest right ventricle (RV) myocardial damage more than left ventricle. Echocardiogram revealed a dilated and severely depressed right ventricle with global left ventricular systolic dysfunction (Figure 1 A). A lung ventilation/perfusion scan showed low probability for pulmonary embolism. Coronary angiogram showed normal coronary arteries (Figure 1 B). Right heart catheterization revealed a mean right atrial pressure of 20 mm Hg, a pulmonary capillary wedge pressure of 15 mm Hg, and a Fick cardiac output of 3.5 L/min, indexed to 1.7 L/min/m2. Endocardial biopsies (Figure 1 C, D) showed lymphohistiocytic active myocarditis with prominent endocardial plasma cells and eosinophilic infiltrate consistent with drug-mediated myocarditis. Reverse transcription polymerase chain reaction and staining were negative for viral or fungal etiologies.
Figure 1.
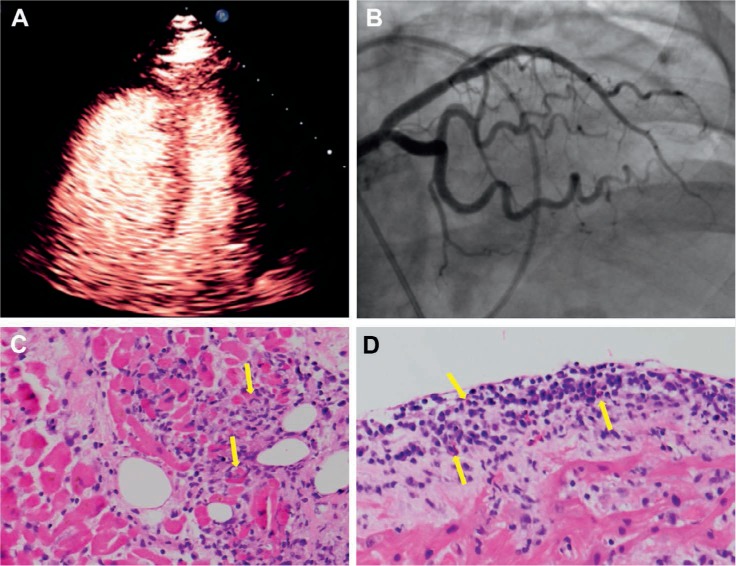
(A) Contrast echocardiogram shows right ventricular dilation and reduced biventricular function. (B) Cardiac angiogram shows no obstructive lesion in the left coronary system. (C, D) Endomyocardial biopsy with hematoxylin & eosin stain shows infiltrating lymphocytes with associated myocyte injury. Endocardial infiltrate includes lymphocytes and eosinophils (yellow arrows).
The patient was started on inotropic support and methylprednisone for presumed drug-mediated myocarditis. The patient did not have risk factors to predispose her to RV dysfunction prior to her presentation. Patient prognosis was poor given her cancer. Her condition deteriorated, and a percutaneous RV assist device was offered; however, the patient refused and chose comfort care. She passed away soon afterwards.
To the best of our knowledge, this is the first case of acute myocarditis and myositis secondary to a short course of sorafenib treatment. Sorafenib is a tyrosine kinase inhibitor that targets more than 30 kinases, including vascular endothelial growth factor receptors.1,2 Sorafenib can induce cardiotoxicity effects, including hypertension, thromboembolism, and left ventricular dysfunction.3 The mechanisms underlying cardiomyopathy associated with sorafenib are complex and multifactorial and include reversible mitochondrial injury, impairment of stress-induced paracrine angiogenic capacity, reduced myocardial capillary density, hypoxic signaling induction in the myocardium, and myocardial hibernation and necrosis. An animal model of myocyte necrosis demonstrated that inhibition of c-kit+ stem cell proliferation by inducing apoptosis exacerbates damage by decreasing endogenous cardiac repair.3
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1.Schmidinger M, Zielinski CC, Vogl UM et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008 Nov 10;26(32):5204–12. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 2.Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013 Aug;123(8):3190–200. doi: 10.1172/JCI70212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duran JM, Makarewich CA, Trappanese D et al. Sorafenib cardiotoxicity increases mortality after myocardial infarction. Circ Res. 2014 May 23;114(11):1700–12. doi: 10.1161/CIRCRESAHA.114.303200. [DOI] [PMC free article] [PubMed] [Google Scholar]


