Abstract
We have explored orally effective thyrotropin-releasing hormone (TRH) mimetics, showing oral bioavailability and brain penetration by structure–activity relationship (SAR) study on the basis of in vivo antagonistic activity on reserpine-induced hypothermia in mice. By primary screening of the synthesized TRH mimetics, we found a novel TRH mimetic: l-pyroglutamyl-[3-(thiazol-4-yl)-l-alanyl]-l-prolinamide with a high central nervous system effect compared with TRH as a lead compound. Further SAR optimization studies of this lead compound led to discovery of a novel orally effective TRH mimetic: 1-{N-[(4S,5S)-(5-methyl-2-oxooxazolidine-4-yl)carbonyl]-3-(thiazol-4-yl)-l-alanyl}-(2R)-2-methylpyrrolidine trihydrate (rovatirelin hydrate), which was selected as a candidate for clinical trials.
Introduction
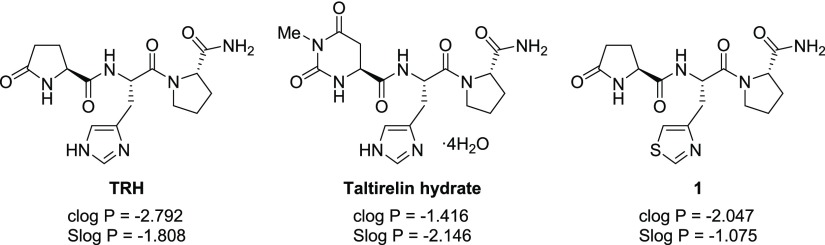
Thyrotropin-releasing hormone (TRH), first isolated from pig or sheep, is a hypothalamic hormone that is synthesized in various areas of the brain. TRH, composed of l-pyroglutamate, l-histidine and l-prolinamide (l-pGlu–l-His–l-Pro-NH2) (Figure 1),1,2 is the smallest molecule among the known peptide hormones. TRH is distributed in high concentrations in the brain and accelerates the synthesis and secretion of thyrotropin-stimulating hormone (TSH)3 and prolactin from the anterior pituitary. TRH also displays physiological activities on the central nervous system (CNS), acting as a neurotransmitter or a neuromodulator,4−6 and has been used for the treatment of CNS disorders. Intact TRH (protirelin tartrate)7 has been clinically used for symptomatic therapy of spinocerebellar degeneration (SCD) in Japan.
Figure 1.
Chemical structure of TRH (left), taltirelin hydrate (middle) and lead compound 1 (right) and their lipophilicities.
It has been reported that two subtypes of TRH receptor (TRH receptor type 1: TRH-R18 and TRH receptor type 2: TRH-R29,10) exist and TRH-R1 is related to endocrine effects and TRH-R2 to CNS effects.11 On the other hand, it has been reported that a single type of TRH receptor is expressed in humans and that the human TRH receptor is similar to TRH-R1.12,13 To separate the CNS and the endocrine effects, a large number of TRH analogues such as taltirelin (TA-0910),14 orotirelin (CG-3509),15 montirelin (CG-3707),16 DN-1417,17 azetirelin (YM-14673),18 JTP-2942,19 posatirelin (RGH-2202),20 MK-771,21 and RX7736822 were synthesized and developed before 2000. Because of the short half-life times in serum, low lipophilicity, and oral absorption, most TRH mimetics have been developed as intravenous injections and not for oral administration. Against such a background, we have been carrying out structure–activity relationship (SAR) studies with TRH to find orally effective TRH mimetics since the 1990s. Among them, only taltirelin hydrate (CEREDIST), which is an agonist at the human TRH-R has been launched in Japan as an orally effective agent for treatment of SCD (Figure 1).14,23,24 After 2000, new types of TRH analogues such as NP-647, which binds to TRH-R subtype selectively and has only CNS effects25 or prodrugs for brain targeting,26 were found and evaluated.
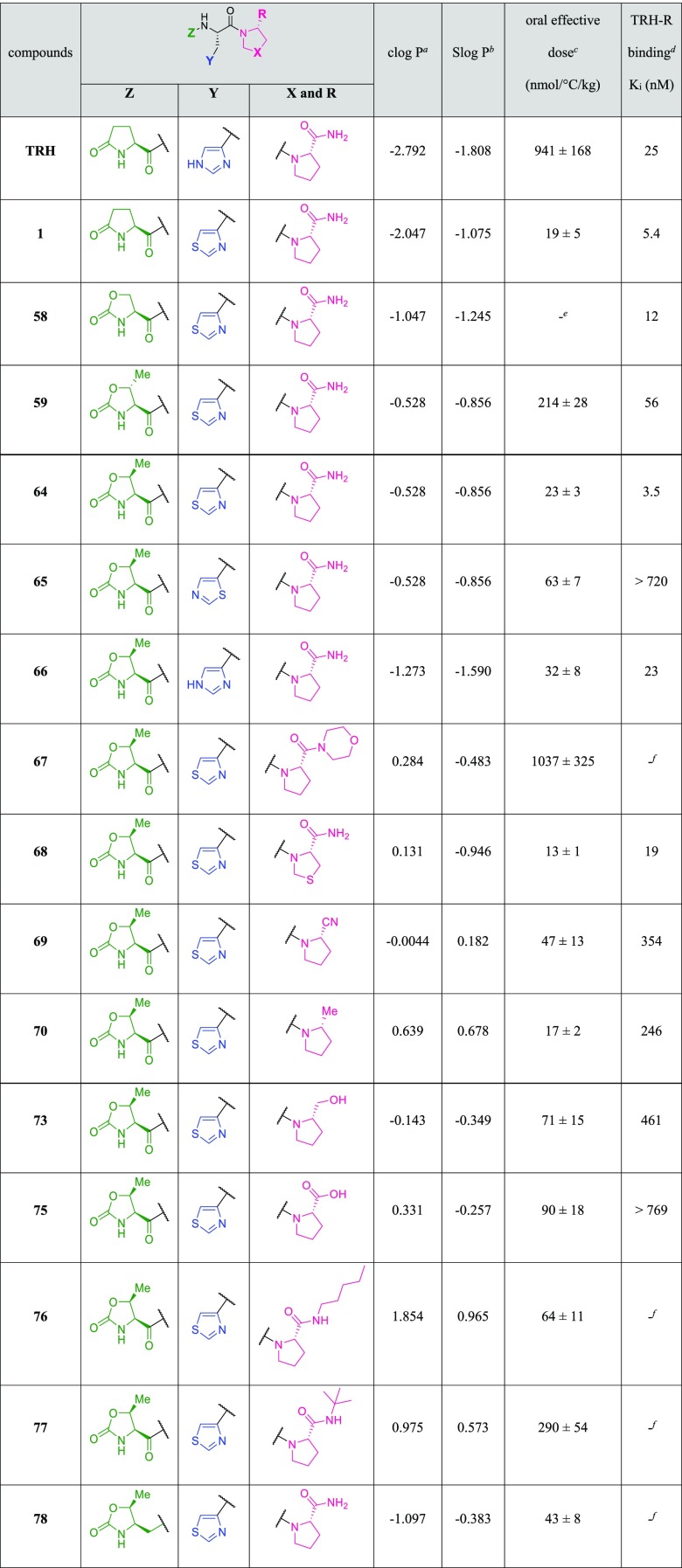
In a primary screening study, we found a novel TRH mimetic: l-pyroglutamyl-[3-(thiazol-4-yl)-l-alanyl]-l-prolinamide 1(27) (Figure 1), which was used as a lead compound. It has an unnatural amino acid 3-(thiazol-4-yl)-l-alanine moiety in the middle part of TRH instead of l-histidine. The most important feature of TRH mimetic 1 is its better lipophilicity than TRH, and 1 was found to have high in vivo CNS effect (antagonistic effect on reserpine-induced hypothermia) compared with TRH (see Table 1).
Table 1. SAR Study of TRH Mimetics on Physicochemical Properties, CNS Effects, and Rat TRH Receptor Binding Affinities.
clog P was calculated using ChemBioDraw Ultra.
Slog P was calculated using MOE.
Effective dose for increasing rectal body temperature by 1 °C per h (nmol/°C/kg body weight) in reserpine-induced hypothermia mice. Each value represents the mean ± SD of at least three animals. The formula for computation is as follows: AUC0–7h: area under the rectal temperature–time curve for 7 h, Administration dose (5 or 50 μmol/kg)/((AUC0–7h of test compounds – temperature at time 0 × 7 h of test compounds) – (AUC0–7h of saline temperature at time 0 × 7 h of saline)/7 h).
Ki values were obtained from the inhibition to [3H]-(3-Me-His2)-TRH using a membrane preparation of the rat whole brain.
No data.
Not assayed.
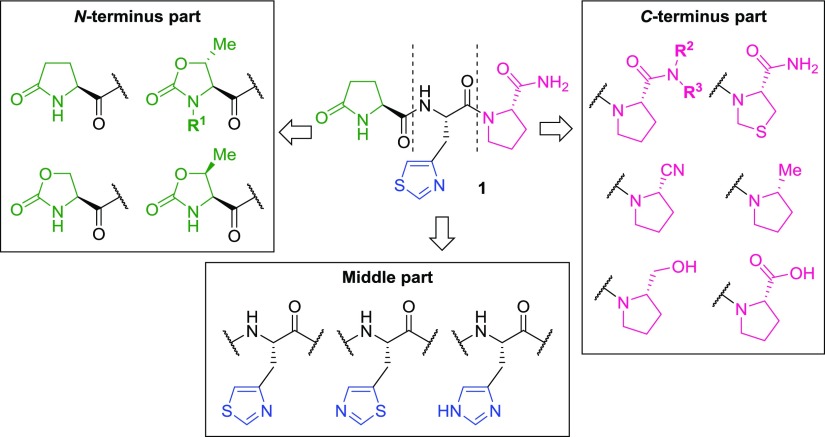
In this paper, we report a SAR study of lead compound 1 on the basis of its physicochemical properties, in vivo antagonistic activity on reserpine-induced hypothermia in mice, TRH-R binding affinity, and pharmacokinetic properties to find orally effective TRH mimetics (Figure 2). We finally selected a TRH mimetic 1-{N-[(4S,5S)-(5-methyl-2-oxooxazolidin-4-yl)carbonyl]-3-(thiazol-4-yl)-l-alanyl}-(2R)-2-methylpyrrolidine trihydrate (rovatirelin hydrate) as a candidate for clinical development.
Figure 2.
Outline of the SAR study from lead compound 1.
Results and Discussion
Chemistry
N-Terminus Fragments
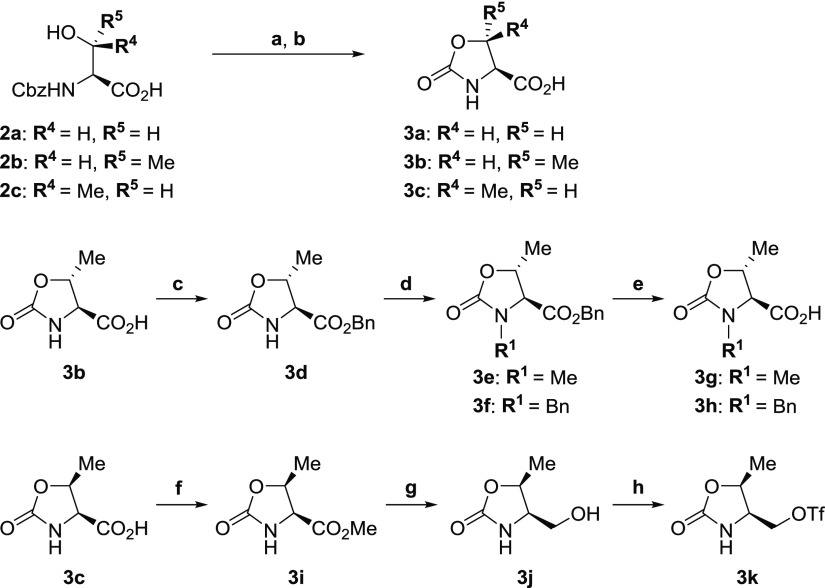
All N-terminus fragments 3a–k were synthesized from readily available N-Cbz protected l-serine 2a, l-threonine 2b, and allo-l-threonine 2c (Scheme 1).
Scheme 1. Synthesis of N-Terminus Fragments 3a–k.
Reagents and conditions: (a) aq. NaOH, MeOH, rt; (b) aq. HCl, 49–73%; (c) BnOH, DMAP, DCC, DMF, rt, 91%; (d) MeI or BnBr, NaH, DMF, 0 °C, 86–88%; (e) H2, Pd–C, MeOH, rt or aq. LiOH, THF-DME, rt, 80–98%; (f) SOCl2, MeOH, 0 °C to rt, quant; (g) NaBH4, EtOH, 0 °C, 77%; and (h) Tf2O, pyridine, CH2Cl2, −35 °C, 45%.
N-Terminus amino acids, 2-oxooxazolidine-4-carboxylic acid 3a, (4S,5R)- or (4S,5S)-5-methyl-2-oxooxazolidine-4-carboxylic acids 3b and 3c, were synthesized on the basis of previous reports28−32 in one step by treatment of 2a–c with sodium hydroxide, with oxazolidinone ring formation occurring rapidly. N-Methyl or N-benzyl (4S,5R)-5-methyl-2-oxooxazolidine-4-carboxylic acids 3g and 3h were both synthesized from carboxylic acid 3b, via esterification with benzyl alcohol to afford benzyl ester 3d. The benzyl ester 3d was alkylated with iodomethane or benzyl bromide to afford N-methyl derivative 3e or N-benzyl derivative 3f, respectively. The benzyl ester 3e was deprotected by catalytic hydrogenation to give N-methyl-4-carboxylic acid 3g. On the other hand, the benzyl ester 3f was deprotected with aqueous lithium hydroxide to give N-benzyl-4-carboxylic acid 3h.
Trifluoromethanesulfonate 3k was synthesized from carboxylic acid 3c via esterification with thionyl chloride in methanol to afford methyl ester 3i, which was reduced with sodium borohydride to give alcohol 3j. The alcohol derivative 3j was sulfonylated with trifluoromethanesulfonic anhydride to give trifluoromethanesulfonate 3k.
Middle-Part Fragments
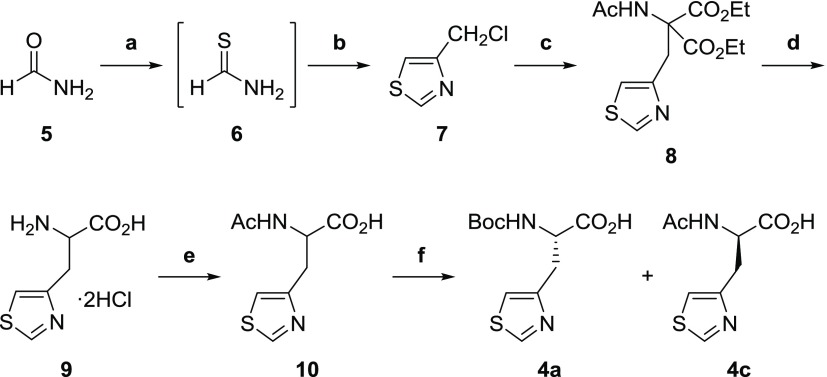
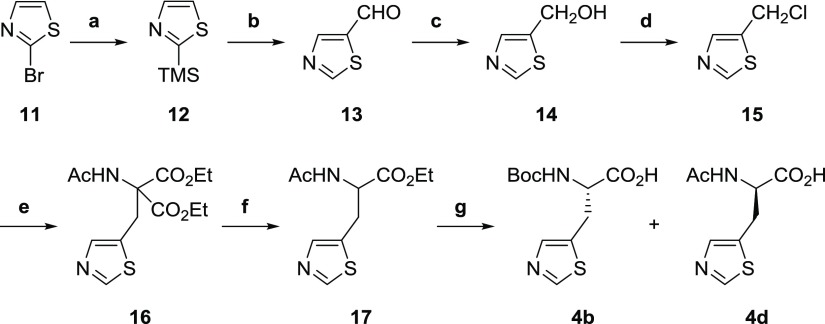
N-Boc-3-(thiazol-4-yl)-l-alanine 4a and N-Boc-3-(thiazol-5-yl)-l-alanine 4b were synthesized by alkylation of diethyl acetamidomalonate (Schemes 2 and 3).
Scheme 2. Synthesis of N-Boc-3-(Thiazol-4-yl)-l-alanine 3a.
Reagents and conditions: (a) Lawesson’s reagent, THF, 0 °C; (b) 1,3-dichloroacetone, THF, 0 °C, 63%; (c) diethyl acetamidomalonate, NaOEt, KI, EtOH, reflux, 89%; (d) conc. aq. HCl, reflux, 74%; (e) aq. NaOH, Ac2O; and (f) 1) aminoacylase, 37 °C, pH 7.2; 2) Boc2O, THF, rt, 29%.
Scheme 3. Synthesis of N-Boc-3-(Thiazol-5-yl)-l-alanine 4b.
Reagents and conditions: (a) TMS-Cl, n-BuLi, Et2O, −78 °C, 89%; (b) N-formylmorpholine, Et2O, −78 °C, 50%; (c) NaBH4, MeOH, rt, 61%; (d) SOCl2 neat, rt, quant; (e) diethyl acetamidomalonate, NaOEt, KI, EtOH, reflux, 71%; (f) (1) aq. NaOH; (2) aq. HCl, reflux, 52%; and (g) (1) aq. NaOH; (2) aminoacylase, 37 °C, pH 7.3; (3) Boc2O, THF, rt, 44%.
N-Boc-3-(thiazol-4-yl)-l-alanine 4a was synthesized on the basis of previous reports.33−37 Here, we describe the synthesis of 4a from readily available formamide 5 (Scheme 2). Formamide 5 was converted to thioformamide 6 with Lawesson’s reagent. Thiazole ring formation was accomplished by treatment of thioformamide 6 with 1,3-dichloroacetone to afford 4-chloromethylthiazole 7. The chloro derivative 7 was converted to the desired (thiazol-4-yl)-l-alanine 4a via alkylation of diethyl acetamidomalonate to give 8, with hydrolysis to provide unprotected (thiazol-4-yl)-dl-alanine 9 and acetylation to give 10, which was not isolated. The acetyl derivative 10 was optically resolved by enzymatic deacetylation, with the amino group of the deacetylated l-enantiomer protected with Boc2O to give l-isomer 4a, with 99% ee.
Similarly, the synthesis of N-Boc-3-(thiazol-5-yl)-l-alanine 4b was accomplished from readily available 2-bromothiazole 11 (Scheme 3). The intermediates 2-trimethylsilylthiazole 12 and 5-formylthiazole 13 were synthesized on the basis of Dondoni’s method.38 The formyl group of 13 was reduced with sodium borohydride to afford hydroxymethylthiazole 14.39−41 The hydroxy group of 14 was chlorinated with thionyl chloride to afford 5-chloromethylthiazole 15. The chloro derivative 15 was converted to (thiazol-5-yl)-l-alanine 4b via diethyl ester 16 and ethyl ester 17 in a manner similar to Scheme 2.
C-Terminus Fragments
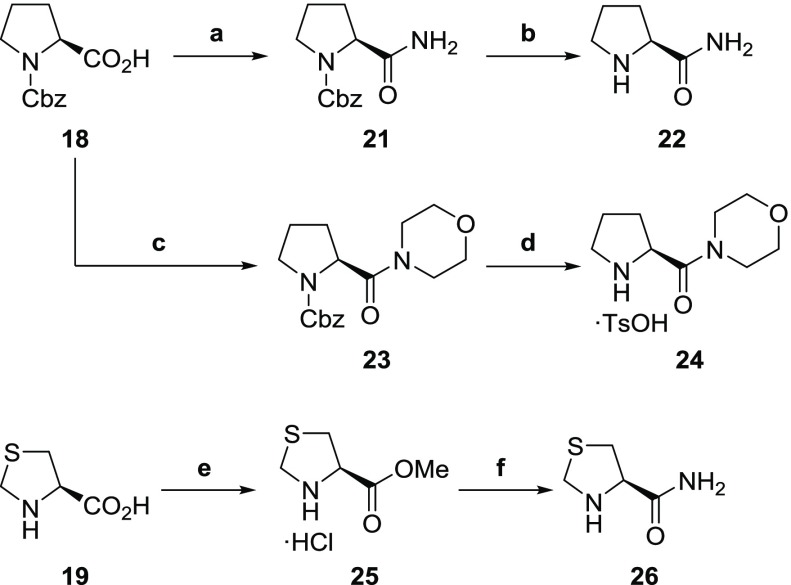
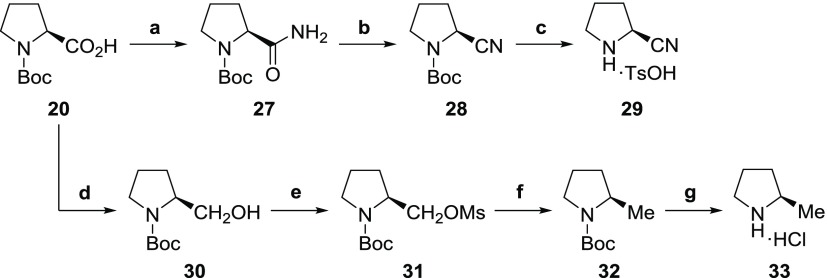
All C-terminus fragments were synthesized from readily available N-Cbz-l-proline 18, (4R)-4-thiazolidinecarboxylic acid 19 and N-Boc-l-proline 20 as starting materials on the basis of previous reports (Schemes 4 and 5).21,42−44
Scheme 4. Synthesis of l-Prolinamide 22, l-Prolylmorpholine p-Toluenesulfonate 24 and (4R)-4-Thiazolidinecarboxamide 26.
Reagents and conditions: (a) (1) ClCO2Et, Et3N, THF, −25 °C; (2) aq. NH3, THF, −25 °C, 87%; (b) H2, Pd–C, MeOH, quant; (c) morpholine, HOBt, Et3N, DCC, DMF, 0 °C to rt, 70%; (d) H2, Pd–C, p-TsOH·H2O, MeOH, rt, quant; and (e) SOCl2, MeOH, rt, 99%; (f) liq. NH3, rt, 78%.
Scheme 5. Synthesis of (2S)-2-Cyanopyrrolidine p-Toluenesulfonate 29 and (2R)-2-Methylpyrrolidine Hydrochloride 33.
Reagents and conditions: (a) (1) ClCO2Et, Et3N, THF, −25 °C; (2) aq. NH3, THF, −25 °C, 86%; (b) POCl3, imidazole, pyridine, 0 °C to rt, 90%; (c) (1) TFA, anisole, 0 °C; (2) p-TsOH·H2O, quant; (d) BH3-THF complex, THF, 0 °C, 98%; (e) MsCl, Et3N, THF, 0 °C, 95%; (f) NaBH4, DMSO, 80 °C, 46%; and (g) HCl in 1,4-dioxane, rt, quant.
l-Prolinamide 22,42l-prolylmorpholine p-toluenesulfonate 24, and (4R)-4-thiazolidinecarboxamide 26(21) were synthesized in two steps from 18 and 19 as starting materials, respectively (Scheme 4).
Both (2S)-2-cyanopyrrolidine p-toluenesulfonate 29 and (2R)-2-methylpyrrolidine hydrochloride 33 were synthesized from 20 on the basis of previous reports43,44 (Scheme 5).
TRH Mimetics
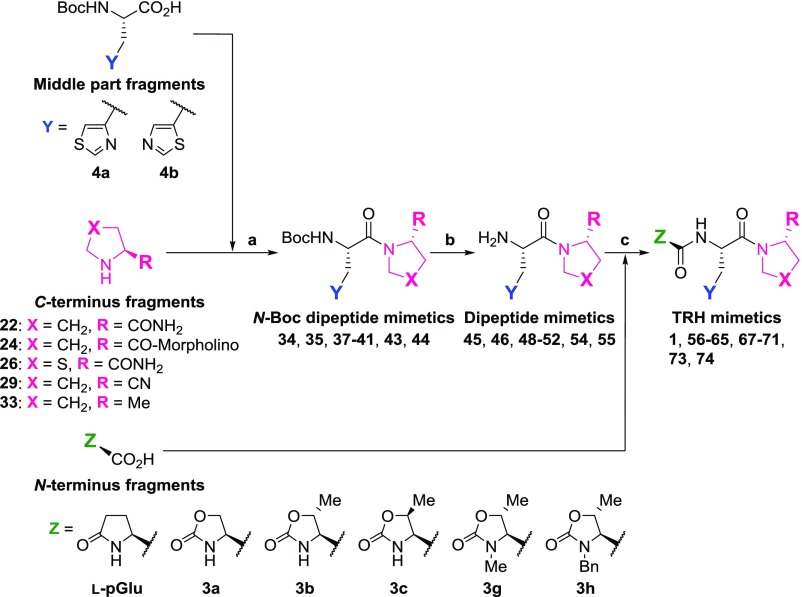
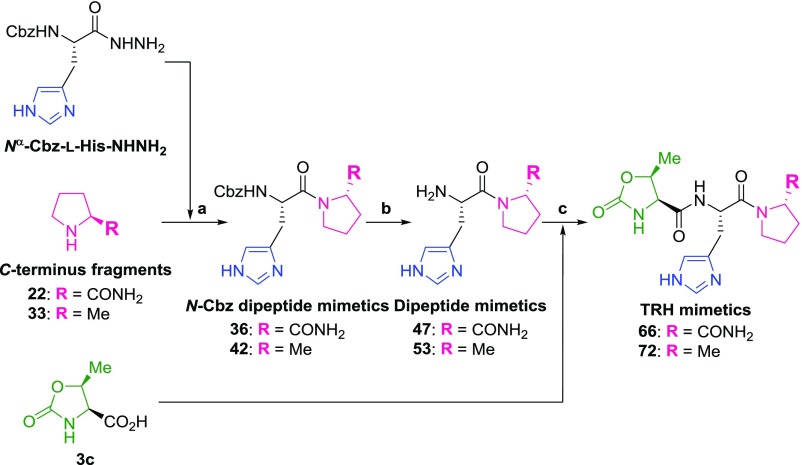
All fragments were coupled from the C-terminus to the N-terminus by a general peptide synthetic route to afford the TRH mimetics shown in Schemes 6 and 7.45,46
Scheme 6. General Synthetic Route of TRH Mimetics Including 4a and 4b as Middle-Part Fragments.
Reagents and conditions: (a) DCC, HOBt or HOSu, DMF, 0 °C to rt; (b) HCl or TFA or p-TsOH·H2O, 0–50 °C; and (c) DCC, HOSu, Et3N, DMF, 0 °C to rt.
Scheme 7. General Synthetic Route of TRH Mimetics Including l-Histidine as the Middle-Part Fragment.
Reagents and conditions: (a) (1) aq. HCl, NaNO2, H2O, 0 °C; (2) K2CO3, H2O, 0 °C to rt or (1) HCl in 1,4-dioxane, isoamyl nitrite, DMF, −78 to 0 °C; (2) Et3N, 0 °C to rt; (b) 25% HBr in acetic acid, rt or H2, Pd–C, MeOH, rt; and (c) DCC, HOSu, Et3N, DMF, 0 °C to rt.
When 4a and 4b were used as the middle part, the synthetic route was as shown in Scheme 6. The C-terminus fragments 22, 24, 26, 29, and 33 were coupled with the middle-part fragments 4a and 4b using DCC and HOBt or HOSu to obtain N-Boc dipeptide mimetics 34, 35, 37–41, 43, and 44, respectively. The Boc groups of intermediates 34, 35, 37–41, 43, and 44 were deprotected under acidic conditions (HCl in solvents, TFA, or p-toluenesulfonic acid monohydrate) to afford dipeptide mimetics 45, 46, 48–52, 54, and 55, respectively. Intermediates 45, 46, 48–52, 54, and 55 were coupled with N-terminus fragments l-pyroglutamic acid, 3a–c, to afford TRH mimetics 1, 56–65, 67–71, and 73–77, respectively.
When using commercially available Nα-Cbz-l-histidine hydrazide47 as the middle-part fragment, the synthetic route was as shown in Scheme 7. The C-terminus fragments 22 and 33 were coupled with Nα-Cbz-l-histidine hydrazide by the azide method to afford N-Cbz dipeptide mimetics 36 and 42, respectively. The Cbz group of intermediate 36 was deprotected with 25% hydrogen bromide in acetic acid to afford dipeptide 47. On the other hand, the Cbz group of intermediate 42 was deprotected by catalytic hydrogenation to afford dipeptide mimetic 53. Finally, dipeptide mimetics 47 and 53 were coupled with 3c using DCC and HOSu to obtain TRH mimetics 66 and 72, respectively.
The chemical structure of the TRH mimetics 1, 56–74 are shown in Table 1. The yields of the N-Boc or N-Cbz dipeptide mimetics 34–44, dipeptide mimetics 45–55, and TRH mimetics 1, 56–74 are shown in the Supporting Information.
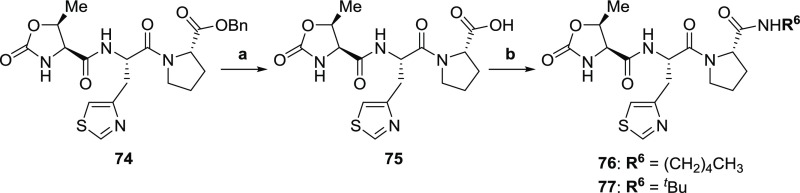
The synthetic method to obtain TRH mimetics with carboxyl group 75 or secondary amide group 76 and 77 at the C-terminus is shown in Scheme 8.
Scheme 8. Synthesis of TRH Mimetics 75–77.
Reagents and conditions: (a) LiOH·H2O, MeOH–H2O, rt, 80%; (b) amylamine or tert-butylamine, HOBt, DCC, DMF, 0 °C to rt, 62–65%.
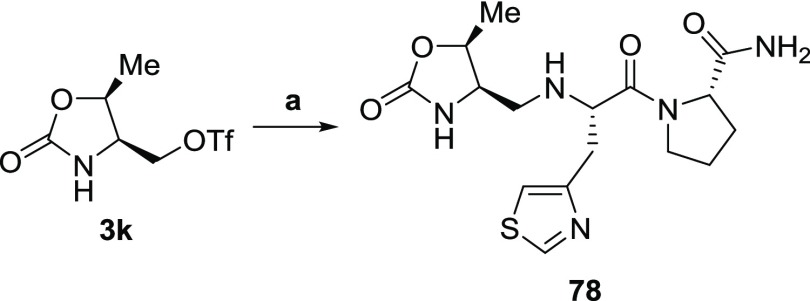
The synthetic method to obtain TRH mimetic with N-terminus 2-oxooxazolidine-4-yl-methyl moiety 78 is shown in Scheme 9.
Scheme 9. Synthesis of TRH Mimetic 78.
Reagents and conditions: (a) 45, Et3N, DMF, 0 °C to rt, 27%.
The synthesized TRH mimetics 1 and 56–78 were purified by gel-filtration column chromatography packed with styrene-divinylbenzene copolymer resin (MCI GEL CHP-20P) using aqueous methanol as an eluent and/or silica gel column chromatography using chloroform–methanol(−water) as an eluent.
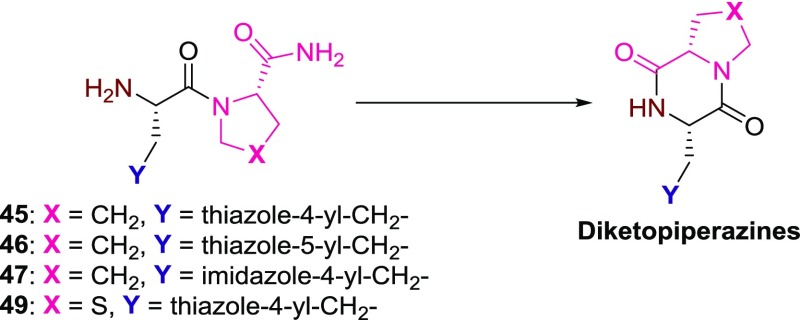
In the process of conversion from 45–47 and 49 having the l-prolinamide moiety or (4R)-4-thiazolidinecarboxamide moiety to TRH mimetics 1, 56–66, and 68, diketopiperazines were produced as byproducts7,21 (Scheme 10). The diketopiperazines were produced immediately after addition of triethylamine in the coupling reaction. On the other hand, in the case of 69–72 having the cyano or the methyl group, no diketopiperazines were produced.
Scheme 10. Diketopiperazines Produced from Dipeptide Mimetics 45–47 and 49.
Physicochemical Properties of TRH Mimetics
The physicochemical properties were estimated by clog P and Slog P. clog P, which is the log of the octanol/water partition coefficient, was calculated using ChemBioDraw Ultra. Slog P, which is the log of the octanol/water partition coefficient (including implicit hydrogens), was calculated using Molecular Operating Environment (MOE). This property is an atomic contribution model48 obtained by calculating log P from the given structure; that is, the correct protonation state (washed structures). Results may vary with the log P (o/w) descriptor. The training set for Slog P was ∼7000 structures. All results are shown in Table 1.
In Vivo CNS Effect of TRH Mimetics
We selected the reserpine-induced hypothermia model as the classical assay method for SAR including CNS effects in the first tier assay for compound selection. The CNS effects of TRH mimetics (1, 56–78) were evaluated by their antagonistic effect on reserpine-induced hypothermia in mice.14f,14g The mice used had rectal temperatures of 30 °C or lower about 18 h after subcutaneous administration of reserpine (3 mg/kg). Rectal temperature was measured by thermistor before and up to 7 h after, oral administration of TRH and TRH mimetics at a dose of 50 and 5 μmol/kg, respectively. The CNS effect of the test drugs on reserpine-induced hypothermia was evaluated on the basis of the area under the temperature–time curve after dosing (AUC0–7h) (see the Experimental Section). All results are shown as the oral effective dose in Table 1. All animal studies were conducted under the approval of the Institutional Animal Care and Use Committees of Shionogi Research Laboratories. The results of the SAR study of the TRH mimetics are shown in Table 1.
We first conducted optimization from the N-terminus, examining TRH mimetics 1, 58–61, 64, and 78. The l-pyroglutamate of 1 was converted to one having the 2-oxooxazolidine carbonyl moiety 58. The biological activity was maintained by introducing an oxygen atom (data not shown). Introducing a methyl group on the 5-position of oxazolidinone ring, the lipophilicity was increased (clog P = −0.528, Slog P = −0.856). The CNS effects vary depending on stereochemistry. The cis configuration 64 had a higher effect than the trans configuration 59. Introducing a methyl group 60 or a benzyl group 61 on the 3-position of the oxazolidinone ring decreased the CNS effects (data not shown). Therefore, the NH of the 3-position of the oxazolidinone ring was essential for the high activity. Converting the carbonyl group 64 to methylene 78 increased the lipophilicity (clog P = −1.097, Slog P = −0.383), but the CNS effect decreased slightly. As a result, (4S,5S)-5-methyl-2-oxooxazolidine-4-carbonyl moiety 64 was considered to be the best for the N-terminus.
Next, we optimized the middle part of the mimetics. For the middle part, when the 3-(thiazol-4-yl)-l-alanine moiety 64 was introduced in place of l-histidine moiety 66, 64 had a higher CNS effect than 66. Increasing the lipophilicity seemed to lead to higher oral absorption and brain penetration. The substitution position on the thiazole ring was significantly important. The lipophilicity of the 3-(thiazol-4-yl)-l-alanine moiety 64 and the 3-(thiazol-5-yl)-l-alanine moiety 65 were equal (clog P = −0.528, Slog P = −0.856); however, the biological activity of 65 was much lower than that of 64. This indicated that the positioning of the nitrogen and sulfur atoms in the thiazole ring was critical for the activity.49−51 As a result, the 3-(thiazol-4-yl)-l-alanine moiety 64 seemed to be the best for the middle part.
Finally, we optimized the C-terminus of the mimetics by replacing the l-prolinamide moiety with other moieties to increase the lipophilicity to improve the CNS effect, with TRH mimetics 67–70 and 73–77. As for the C-terminus part of TRH mimetics 67–70, 73–77 were examined. It is known that the l-prolinamide moiety is important for the formation of the hydrogen bond with Arg283 of TRH receptor52,53 to have expression of the biological activities. In the case of the TRH mimetic having the (4R)-4-thiazolidinecarboxamide moiety 68, both lipophilicity (clog P = 0.131, Slog P = −0.946) and CNS effect increased. When the l-prolinamide moiety was converted to l-proline secondary amide moiety 76 and 77 or tertiary amide moiety 67, the lipophilicities increased; however, the CNS effects decreased remarkably. On the other hand, when l-prolinamide moiety 64 was converted to the (2S)-cyanopyrrolidine moiety 69, lipophilicity (clog P = −0.0044, Slog P = 0.182) increased but the CNS effect decreased slightly. However, replacement of the l-prolinamide moiety of 64 with the (2R)-methylpyrrolidine moiety 70 led to much higher lipophilicity (clog P = 0.639, Slog P = 0.678) and CNS effect than 64. When l-prolinamide moiety 64 was converted to l-prolinol moiety 73 and l-proline moiety 75, the lipophilicities (73: clog P = −0.143, Slog P = −0.349, 75: clog P = 0.331, Slog P = −0.257) increased but the CNS effects decreased markedly. TRH mimetics 56, 57, 62, 63, 71, 72, and 74 were synthesized and evaluated; however, the CNS effects of these compounds were less than that of 64. As a result, l-prolinamide moiety 64, (4R)-4-thiazolidinecarboxamide moiety 68, (2S)-cyanopyrrolidine moiety 69, and (2R)-methylpyrrolidine moiety 70 were considered to be suitable for the C-terminus part.
TRH Receptor Binding in Rat
TRH receptor binding of TRH and TRH mimetics were evaluated using a rat brain preparation. As the TRH receptor included both TRH-R1 and TRH-R2 in the rat brain preparation, it was not possible to establish whether TRH mimetics were binding preferentially to TRH-R1 or TRH-R2. The results of the TRH receptor binding assay are shown in Table 1.
TRH mimetics 1, 58, 59, 64–66, and 68, which have l-prolinamide moiety or (4R)-4-thiazolidinecarboxamide moiety at the C-terminus indicated much higher affinity than that of TRH mimetics, which have (2S)-cyanopyrrolidine moiety 69, (2R)-methylpyrrolidine moiety 70, (2S)-hydroxymethylpyrrolidine moiety 73, and l-proline moiety 75. When the C-terminus (2R)-methylpyrrolidine moiety 70 was oxidized to (2S)-hydroxymethylpyrrolidine moiety 73 and l-proline moiety 75, the affinities to TRH receptor decreased. As for the middle part, the TRH mimetic, which has 3-(thiazol-4-yl)-l-alanine moiety 64, had a higher affinity than l-histidine moiety 66. The TRH mimetic with 3-(thiazol-5-yl)-l-alanine moiety 65 did not show any affinity at all. Therefore, the positioning of the nitrogen and sulfur atoms in the thiazole ring was critical not only for the CNS effect but also for binding to TRH receptor. On the other hand, l-histidine moiety 66 showed the same affinity as TRH. As for the N-terminus part, 2-oxooxazolidine carbonyl moiety 58 had almost equal affinity to l-pyroglutamate moiety 1. The cis configuration 64 between the methyl group and the carbonyl group had much higher affinity than the trans configuration 59. It is considered that the CNS effects among TRH mimetics are not equal to the binding affinity to TRH receptor.
As a result of their CNS effect in mice, physicochemical properties, and TRH receptor binding affinity, four TRH mimetics 64, 68–70 were selected for further investigation of PK parameters in rats.
PK Properties of TRH Mimetics 64, 68–70 in Rat
The clearance (CL), half-life times (t1/2), volume of distribution at steady state (Vdss), and mean residence times (MRT) of the four TRH mimetics 64, 68–70 in rat after intravenous (i.v.) administration at a dose of 1 μmol/kg were evaluated. Also, the bioavailability (BA) of these four TRH mimetics in rat after per oral administration was evaluated. Moreover, free fraction ratio (fu) in serum was evaluated in vitro examinations. These PK properties are shown in Table 2. The CL of four TRH mimetics was lower than that of TRH. However, the t1/2 and MRT of four TRH mimetics which have l-prolinamide moiety 64, (4R)-4-thiazolidinecarboxamide moiety 68, (2S)-cyanopyrrolidine moiety 69, and (2R)-methylpyrrolidine moiety 70 at the C-terminus were much longer than that of TRH; TRH mimetics 64 and 68 were shorter than those for the TRH mimetics 69 or 70. Although the Vdss of TRH mimetics 64, 68, and 69 were lower than that of TRH, TRH mimetic 70 showed the highest value. Therefore, the (2R)-methylpyrrolidine moiety is significant to increase the tissue distribution. The BA of TRH mimetics 64 and 70 were 1.1 and 4.7%, respectively. Oral BA for TRH mimetics 68 and 69 could not be calculated due to low absorption. As a consequence, TRH mimetic 70 had the best total PK properties among the four TRH mimetics and a favorable effect would be predicted in rats.
Table 2. PK Properties of TRH and TRH Mimetics 64, 68–70 in Rat Plasmaa.
| compounds | CL (mL/min/kg) | t1/2 (min)b | Vdss (L/kg) | MRT (min)c | BA (%)d | fu (%)e |
|---|---|---|---|---|---|---|
| TRH | 89.2 | 3.0 | 0.33 | 3.7 | NCf | NCf |
| 64 | 13.0 | 13.5 | 0.19 | 14.3 | 1.1 | >99 |
| 68 | 14.2 | 9.8 | 0.16 | 11.0 | NCf | >99 |
| 69 | 17.4 | 26.2 | 0.25 | 14.6 | NCf | >99 |
| 70 | 30.6 | 21.6 | 0.58 | 19.1 | 4.7 | >99 |
Each value represents the mean of two animals.
t1/2 of the plasma concentration (Cp,iv) of TRH mimetic after intravenous administration to rats were calculated by interpolation for plasma concentration of terminal phase.
MRT for the plasma concentration (Cp,iv) of TRH mimetic after intravenous administration to rats was calculated as defined in eq 1 by numerical integration using a linear trapezoidal formula and extrapolation to infinite time based on a monoexponential equation.54
fu: free fraction ratio in serum (%).
NC: not calculated.
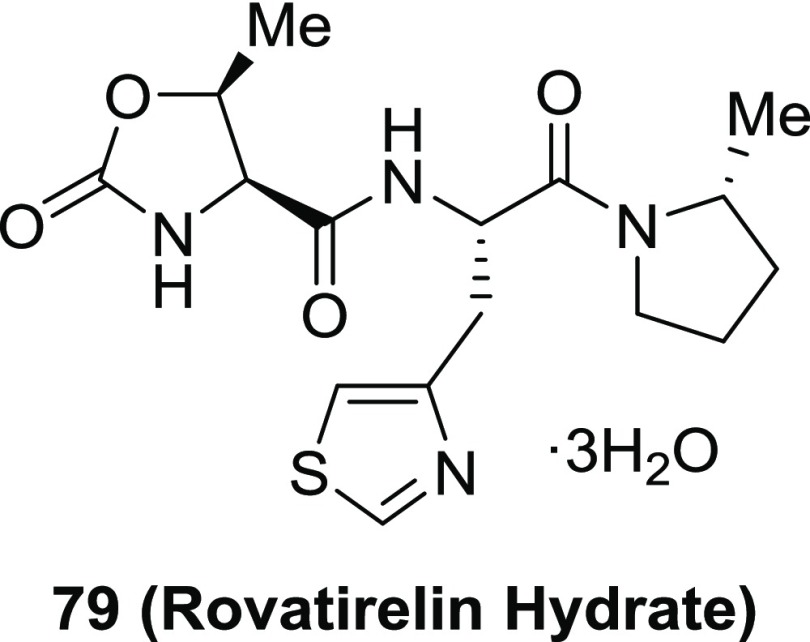
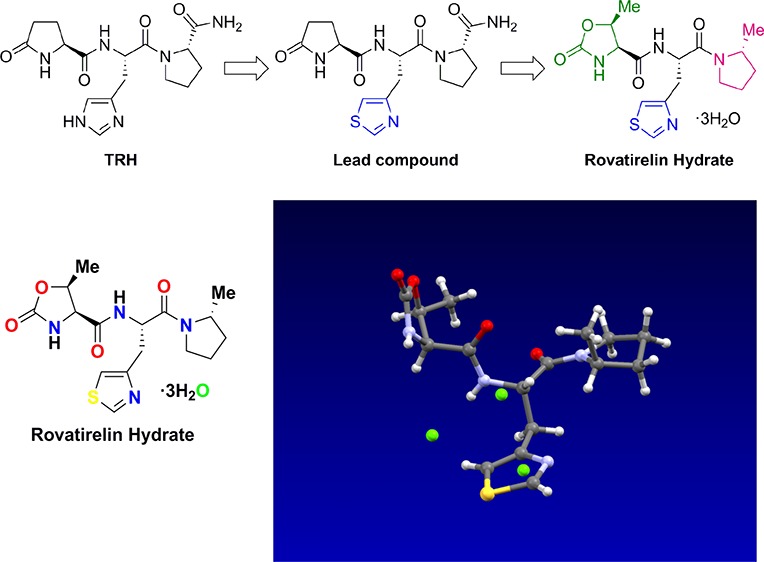
From the data of the CNS effect, PK properties, TRH receptor affinity, and the synthetic viewpoint that the diketopiperazine was not produced in the process of the coupling reaction, we selected TRH mimetic 70 as a candidate for further development. Moreover, in the process of scale-up synthesis of 70, the trihydrate of 70 was obtained as a stable crystalline solid. We selected this trihydrate 79 (rovatirelin hydrate)27,55,56 as the preferred candidate (Figure 3).
Figure 3.
Chemical structure of 79 (rovatirelin hydrate).
X-ray Crystallography of 79
Single crystals of 79 were recrystallized by gradual cooling of a saturated aqueous solution and obtained as the trihydrate. 79 has four asymmetric carbons; their absolute stereochemistry was determined by X-ray structural analysis. The X-ray crystal structure is shown in Figure 4. Three water molecules of 79 were omitted to compare the X-ray crystal structure of TRH,57 in which tartrate molecule and water molecules were omitted. 79 has three heterocyclic rings (oxazolidinone ring, thiazole ring, and pyrrolidine ring) and these groups are oriented to form a “propeller-shaped” conformation. This conformation is similar to the proposed TRH bioactive conformation.58,59 Moreover, the “propeller-shaped” conformation seemed to be extremely important for high receptor binding. Three water molecules of 79 were coordinated to the amide bond, thiazole ring, and oxazolidinone ring. Each of them was used to form intramolecular hydrogen bonding (see the Supporting Information).
Figure 4.
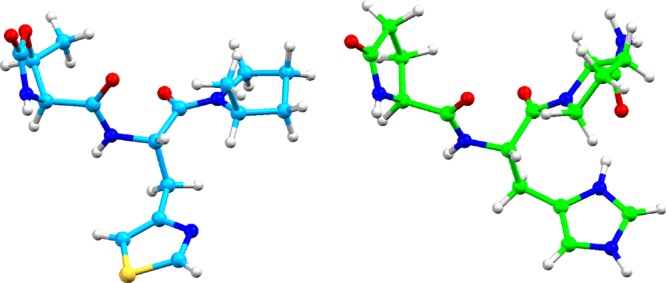
X-ray crystal structures of 79 (CCDC-1442496) (left) and TRH (CCDC-1275682) (right).57 Crystallographic data are summarized in Tables S4–S9.
In vitro binding activities of TRH and 79 for human TRH-R have been reported previously.56 The Ki values of TRH and 79 for human TRH-R are 128 and 702 nM, respectively. As the human TRH-R is more similar to TRH-R1 than TRH-R2,12,13 the effect of TRH analogues may be related to TRH-R1 in human. The homology of the TRH-R1 receptor is also well conserved in many species. The human TRH-R is 90.3 and 89.2% homologous to that of mouse and rat at the DNA level, respectively.11 Thus, the pharmacological effects of 79 in mouse and rat may be explained by the human TRH-R binding activity.
In Vivo CNS Effects of 79
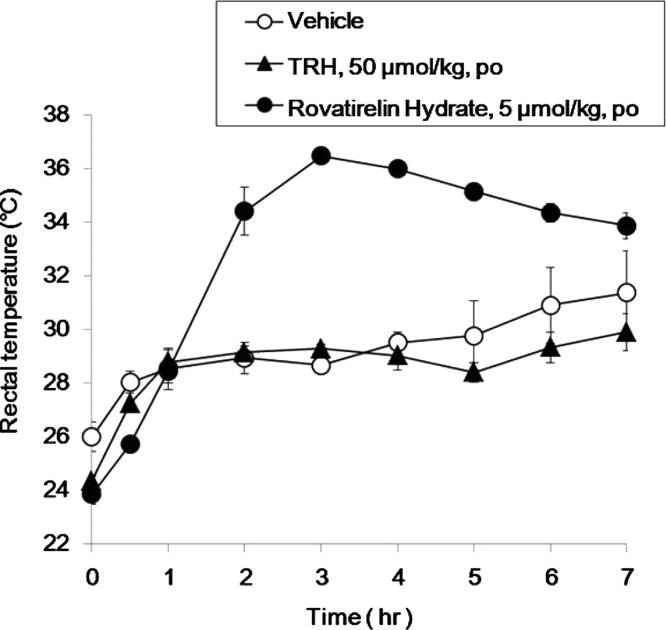
The CNS effects of TRH and 79 were tested by examining their in vivo antagonistic activity on reserpine-induced hypothermia in mice.14 A representative figure for the rectal temperature–time curve is shown in Figure 5. After oral administration of 79 at a dose of 5 μmol/kg, the rectal temperature was maintained sustainably over a 2.5 °C temperature range from 2 to 7 h and compared with saline levels. TRH, at a dose of 50 μmol/kg, led to almost the same low rectal temperature profiles as the saline levels from 0 to 7 h after oral administration. The area under the rectal temperature–time curve up to 7 h (AUC) of 79 was higher than that of saline and TRH (Table 3). The saline-stripped AUC (ΔAUC) of 79 was higher than that of TRH in spite of a 10-fold dose difference (5 vs 50 μmol/kg).
Figure 5.
Rectal temperature–time profiles in reserpine-induced hypothermia mice after oral administration of TRH and 79 at a dose of 50 and 5 μmol/kg, respectively. Each point represents the mean ± SD of at least four animals.
Table 3. Area under the Rectal Temperature–Time Curve after Oral Administration to Reserpine-Induced Hypothermia Mice of TRH and 79 at a Dose of 50 and 5 μmol/kg, Respectivelya.
| rectal
temperature |
||
|---|---|---|
| compounds (dose) | AUC0–7h (°C·h)b | ΔAUC0–7h (°C·h)c |
| vehicle | 205.4 ± 11.1 | |
| TRH (50 μmol/kg) | 201.4 ± 3.3 | –4.0 ± 3.3 |
| 79 (5 μmol/kg) | 233.4 ± 4.3 | 28.0 ± 4.3 |
Each value represents the mean ± SD of at least three animals.
AUC0–7h was calculated by using the trapezoidal method.
ΔAUC = AUC0–7h(compounds) – AUC0–7h(saline).
Improvement Effect of 79 on Cerebellar Ataxia in Rats
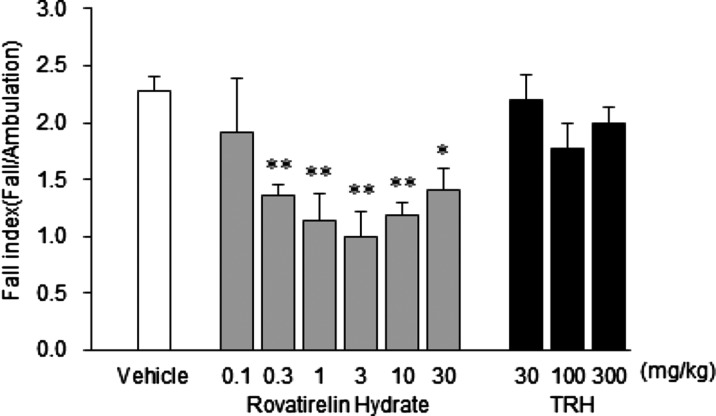
We investigated improvement effects of 79 on ataxia in animals with cerebellar ataxia induced by administration of cytosine arabinoside (Ara-C) in the neonatal period. Neonate Sprague-Dawley rats were subcutaneously injected 60 mg/kg at age of 2 and 3 days (the day of confirming delivery: age of 0 day). Ara-C administered rats were subjected to the experiment at age of 4 weeks. 79 and TRH were orally administered once daily for 7 days to assess ataxia at 24 h after final administration. Severity of ataxia was assessed by the open field test which measured the number of ambulation events and falls. In brief, each animal was placed on the center of the circular open field (75 cm in diameter, 25 parts). The number of crossing parts (number of ambulation) and the number of falls for 3 min were measured to calculate the fall index ([number of falls]/[number of ambulation]). On the basis of the fall index, the severity of ataxia was assessed. The fall index in the control group was 2.27 (mean). In the 79 0.1 mg/kg group, no significant decrease was observed in the fall index. In the 79 0.3 mg/kg group, a significant decrease to 1.36 was observed in the fall index. The decrease in the fall index was dose-dependent up to 3 mg/kg. Although significant decrease was observed in the fall index in the 79 10 and 30 mg/kg groups compared with that in the control group, the fall index increased more than the 3 mg/kg group. On the other hand, after administration of TRH, no significant decrease was observed in the fall index at any doses (30, 100, and 300 mg/kg). When TRH was intravenously administered once to assess ataxia at 30 min post-dose, a significant decrease was observed in the fall index at the doses of 10 and 30 mg/kg [fall index: mean ± SE (n = 10–17), vehicle: 2.06 ± 0.09, 10 mg/kg: 1.05 ± 0.22, and 30 mg/kg: 0.87 ± 0.17] (Figure 6).
Figure 6.
Improvement effects of 7 day repeated oral administration of 79 and TRH on ataxia in rats with Ara-C-induced cerebellar damage (age of 4 weeks). Data are expressed as mean ± SE (n = 10–18). *p < 0.05, **p < 0.01 vs vehicle group (Dunnett’s test).
TSH Releasing Activity of 79
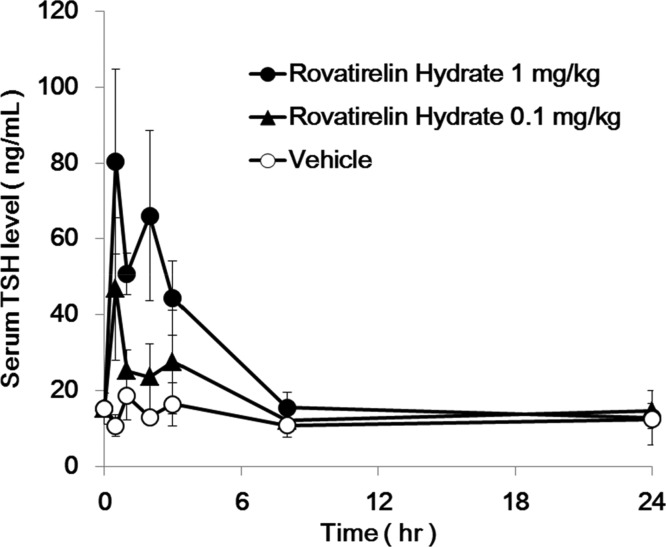
We examined the TSH releasing activity of 79 after oral administration in rats. After oral administration of 79 at a dose of 0.1 and 1 mg/kg, the plasma TSH levels at 0.5 h elevated up to 46.8 and 80.4 ng/mL, respectively. Then, plasma TSH level recovered to vehicle levels at 24 h (Figure 7).
Figure 7.
Plasma TSH level–time profiles after oral administration of 79 to male rats at a dose of 0.1 and 1.0 mg/kg, respectively. Each point represents the mean ± SD of at least four animals.
PK Properties of 79
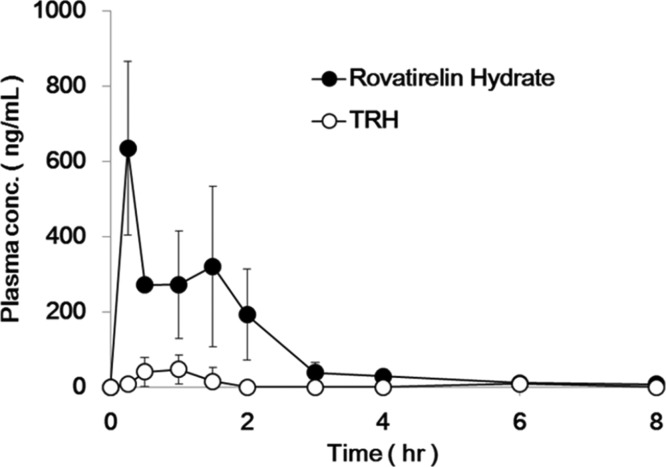
Plasma concentration–time profiles of TRH and 79 after oral administration to fasted conscious rats at a dose of 40 mg/kg are presented in Figure 8. The PK parameters are summarized in Table 4. After oral administration, the profiles of plasma concentration of 79 were higher than those of TRH up to the experiment period (8 h) and, Cmax and AUC0–8h values of 79 were over 12 times higher than that of TRH. It seems that the stronger and more sustained efficacies of 79 for reserpine-induced hypothermia in mice and cerebellar ataxia in rats could also be attributed to the longer sustained plasma exposure and high brain Kp to 79 compared with TRH.
Figure 8.
Plasma concentration–time profiles of TRH and 79 after oral administration to conscious rats at a dose of 40 mg/kg under fasted condition. Each point represents the mean ± SD of at least four animals.
Table 4. PK Parameters of TRH and 79 after Oral Administration to Fasted Conscious Rats at a Dose of 40 mg/kga.
| compounds | Cmax (ng/mL) | Tmax (h) | AUC0–8h (ng·h/mL)b | brain Kpc |
|---|---|---|---|---|
| TRH | 50 ± 39 | 0.75 ± 0.29 | 68 ± 72 | NDd |
| 79 | 636 ± 232 | 0.25 ± 0.00 | 817 ± 349 | 0.06 ± 0.03 |
Each value represents the mean ± SD of at least four animals.
AUC0–8h was calculated by using the trapezoidal method.
No data.
Stability Study of 79
Stability of 79 in rat plasma and brain homogenate was evaluated. The amount of 79 remaining after incubation at 37 °C is shown in Table 5. Almost no degradation of 79 was seen in rat plasma, cerebrum, and cerebellum homogenate samples even after 180 min. It was reported that taltirelin and TRH were degraded by the brain homogenate with t1/2 of 64.4 and 7.9 min, respectively,60 suggesting that 79 is more stable in the brain than taltirelin and TRH.
Table 5. Stability of 79 in Rat Plasma, Cerebrum, and Cerebellum Homogenate at 37 °Ca.
| compound
remaining |
|||
|---|---|---|---|
| time (min) | plasma (%) | cerebrum (%) | cerebellum (%) |
| 15 | 100 ± 0.1 | 100.2 ± 0.2 | 100 ± 0.0 |
| 30 | 99.8 ± 0.1 | 100.2 ± 0.1 | 99.9 ± 0.0 |
| 60 | 99.6 ± 0.3 | 100.3 ± 0.1 | 99.7 ± 0.0 |
| 120 | 99.8 ± 0.2 | 99.9 ± 0.1 | 99.6 ± 0.1 |
| 180 | 99.8 ± 0.1 | 99.7 ± 0.1 | 99.5 ± 0.1 |
Values are mean ± SD of five experiments.
CYP Inhibition and Safety Information of 79
79 did not directly inhibit the 10 CYP enzymes (CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4/5, and CYP4A11) examined by more than 50% at the highest concentration tested (300 μM). 79 caused no more than 10% inhibition of CYP activity except for CYP2C8, which displayed approximately 20% inhibition. The acute lethal dose of 79 in rats was over 2000 mg/kg in a single oral administration.
Summary and Conclusions
We conducted SAR studies of TRH to find orally effective TRH mimetics. The oxygen atom was introduced to the five-membered ring in the N-terminus part 58, leading to better lipophilicity than l-pyroglutamate. Introduction of a methyl group to the 5-position of oxazolidinone ring 64 led to increased CNS effect and affinity to TRH receptor. The relative configuration between the methyl group and the carbonyl group was significant. Cis configuration 64 showed higher CNS effect and affinity to TRH receptor than trans configuration 59. Moreover, the carbonyl group of 64 was important because when a methylene was introduced instead of carbonyl group 78, the CNS effect decreased. It seemed that the carbonyl group was used to form a hydrogen bond with the target receptor or maintain the active conformation of the molecule. A thiazole ring in the middle-part, as in 1 and 64, was more suitable than the imidazole ring, but the substitution position of the thiazole ring was significantly important. The 4-position substituent 64 showed high CNS effect, but the 5-position substituent 65 had no activity. The position of the nitrogen atom of the thiazole ring seemed to be important for forming a hydrogen bond with the target receptor and also for higher biological activity. It was speculated that converting the imidazole to thiazole lead to increasing the lipophilicity of molecule, with accompanying improvements in passing the blood–brain barrier. TRH mimetics that had the (4R)-4-thiazolidinecarboxamide moiety 68, (2S)-cyanopyrrolidine moiety 69, or the (2R)-methylpyrrolidine moiety 70 as the C-terminus part instead of the l-prolinamide moiety 64 maintained high biological activities. The l-prolinamide moiety was not essential for a high CNS effect. As a result of the SAR study, and taking into consideration the CNS effect, PK properties, and physicochemical characteristics, along with synthetic tractability, we selected 70 as a candidate, despite 70 having a weaker binding affinity for the TRH receptor than TRH and some of the TRH mimetics. Subsequently, TRH mimetic 70 was developed in clinical trials as the trihydrate 79 named rovatirelin hydrate.55,56 Although rovatirelin hydrate showed approximately six times less in vitro binding activity for human TRH receptor than TRH,56 in vivo efficacy of rovatirelin hydrate for reserpine-induced hypothermia in mice and on cerebellar ataxia in rats was stronger than that of TRH. The strong CNS effect of rovatirelin hydrate may be due to increased oral BA, higher distribution to the target brain region, and greater stability in plasma, cerebrum, and cerebellum than TRH. Rovatirelin hydrate was designed for higher lipophilicity than TRH for better intestinal and brain permeability. Therefore, rovatirelin hydrate, which was first found by our group,55 is currently in a phase III clinical trial (NCT02889302) in SCD patients.
Experimental Section
General
All solvents and reagents were obtained from commercial sources and were used as received. Melting points (mp) were measured with a Yanagimoto melting point apparatus and were uncorrected. Infrared spectra were recorded on a Nicolet 20SXB FT-IR spectrometer. 1H and 13C NMR spectra were taken with a Varian VXR-200 or Gemini-200300 FT-NMR spectrometer using tetramethylsilane as an internal standard. Optical rotations were determined with a PerkinElmer 430 polarimeter. Elemental analyses were performed by Shionogi Research Laboratories (Shionogi & Co., Ltd. 3-1-1, Futaba-cho, Toyonaka-shi, Osaka 561-0825, Japan). For silica gel column chromatography, Kiesel gel 60 (0.063–0.20 mm, Merck) was employed for the purification. For gel-filtration chromatography, MCI GEL CHP-20P (75–150 g, Mitsubishi Chemical Industries) was utilized with aqueous MeOH as an eluent. Thin-layer chromatography (TLC) was carried out with Merck silica gel 603–254 plates mainly using the following three solvent systems: (a) CHCl3/MeOH (9/1), (b) CHCl3/MeOH/H2O (32/6/0.5), and (c) CHCl3/MeOH/H2O (6/4/1). The spots were detected under ultraviolet irradiation at 254 nm and by the use of phosphomolybdic acid in ethanol solution and ninhydrin sprays.
General Procedure for the Synthesis of TRH Mimetics
l-Pyroglutamyl-[3-(thiazol-4-yl)-l-alanyl]-l-prolinamide (1)
To an ice-cooled solution of l-pyroglutamic acid (1.76 g, 13.6 mmol) and HOSu (1.73 g, 15.0 mmol) in DMF (50.0 mL), DCC (3.09 g, 15.0 mmol) was added, and the mixture was stirred for 2 h at the same temperature. [3-(Thiazol-4-yl)-l-alanyl]-l-prolinamide dihydrochloride (45) (6.67 g, 15.0 mmol) and triethylamine (4.60 mL, 33.0 mmol) were added to the solution, and the mixture was stirred for 1 h. The ice bath was removed and the mixture was stirred overnight. The precipitate was filtered off and the filtrate was concentrated under reduced pressure. A small amount of water was added to the residue, and the precipitate was filtered off. The filtrate was purified by gel-filtration chromatography (MCI GEL CHP-20P, 200 mL, eluent: H2O/MeOH). The purified fractions were concentrated in vacuo and the residue was lyophilized to afford the title compound 1 (2.54 g, 49%) as a white amorphous powder.
IR (KBr): 3294, 1683, 1639, 1541, 1518, 1444, 1263 cm–1.
1H NMR (200 MHz, CD3OD): δ 8.95 (d, J = 2.0 Hz, 1H), 7.43 and 7.34 (d each, J = 2.0 Hz, total 1H), 4.95 (t, J = 7.0 Hz, 1H), 4.42 and 4.34 (m each, total 1H), 4.17 (m, 1H), 3.80 (m, 1H), 3.60–3.10 (m, 3H), 2.50–1.80 (m, 8H).
13C NMR (75 MHz, CD3OD): δ 182.20, 182.10, 177.54, 176.74, 175.28, 175.19, 174.69, 172.64, 155.99, 154.06, 118.59, 118.31, 62.32, 62.14, 58.52, 58.47, 58.40, 53.36, 53.27, 53.20, 50.24, 48.54, 35.25, 33.94, 33.41, 31.32, 30.99, 30.94, 27.16, 27.09, 26.38, 23.66.
[α]D24 −42.9° (c 1.0, MeOH).
Anal. Calcd for C16H21N5O4S·H2O: C, 48.35; H, 5.83; N, 17.62; S, 8.07. Found: C, 48.35; H, 5.85; N, 17.36; S, 8.31.
TLC Rf (b) 0.24, (c) 0.65.
In a similar manner, other TRH mimetics (56–74) were prepared.
l-Pyroglutamyl-[3-(thiazol-5-yl)-l-alanyl]-l-prolinamide (56)
The condensation of l-pyroglutaminic acid (0.380 g, 2.93 mmol) and [3-(thiazol-5-yl)-l-alanyl]-l-prolinamide dihydrochloride (46) (1.00 g, 2.93 mmol) yielded the title compound 56 (1.00 g, 2.93 mmol) as a white amorphous powder.
IR (KBr): 3393, 3081, 1684, 1639, 1540, 1443, 1247 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.89 (s, 1H), 7.76 and 7.71 (s each, total 1H), 4.90 (dd, J = 9.3, 4.5 Hz, 1H), 4.42 and 4.26 (dd each, J = 8.4, 4.5 Hz, total 1H), 4.18 (dd, J = 8.7, 4.8 Hz, 1H), 3.74 (m, 2H), 3.50 (dd, J = 15.3, 4.5 Hz, 1H), 3.24 (dd, J = 15.3, 9.3 Hz, 1H), 2.50–1.80 (m, 8H).
13C NMR (75 MHz, CD3OD): δ 182.22, 182.06, 177.45, 176.67, 175.52, 174.84, 171.61, 171.50, 155.92, 155.76, 143.81, 143.64, 136.16, 135.51, 62.25, 62.07, 58.56, 58.47, 54.23, 54.14, 33.68, 31.44, 30.90, 30.80, 29.53, 27.30, 27.13, 26.46, 23.68.
[α]D23 −53.6° (c 1.0, MeOH).
Anal. Calcd for C16H21N5O4S·1.6H2O: C, 47.07; H, 5.97; N, 17.15; S, 7.85. Found: C, 47.05; H, 5.92; N, 17.34; S, 7.72.
TLC Rf (b) 0.20, Rf (c) 0.61.
3-{N-l-Pyroglutamyl-[3-(thiazol-4-yl)-l-alanyl]}-(4R)-4-thiazolidinecarboxamide (57)
The condensation of l-pyroglutaminic acid (0.646 g, 5.00 mmol) and [3-(thiazol-4-yl)-l-alanyl]-(4R)-4-thiazolidinecarboxamide dihydrochloride (49) (1.65 g, 4.00 mmol) yielded the title compound 57 (0.783 g, 39%) as a white amorphous powder.
IR (KBr): 3301, 2936, 1685, 1518, 1419, 1330, 1262 cm–1.
1H NMR (200 MHz, CD3OD): δ 8.95 (d, J = 1.8 Hz, 1H), 7.43 and 7.37 (d each, J = 1.8 Hz, total 1H), 5.05 (t, J = 6.8 Hz, 1H), 4.99 (d, J = 8.6 Hz, 1H), 4.86 (m, 1H), 4.45 (d, J = 8.6 Hz, 1H), 4.18 (dd, J = 8.6, 5.0 Hz, 1H), 3.50–3.10 (m, 4H), 2.50–1.90 (m, 4H).
[α]D23 −87.6° (c 1.0, H2O).
Anal. Calcd for C15H19N5O4S2·1.2H2O: C, 42.99; H, 5.15; N, 16.71; S, 15.30. Found: C, 42.92; H, 5.07; N, 16.73; S, 15.18.
TLC Rf (b) 0.35.
{[(4S)-(2-Oxooxazolidine-4-yl)carbonyl]-3-(thiazol-4-yl)-l-alanyl}-l-prolinamide (58)
The condensation of (4S)-2-oxooxazolidine-4-carboxylic acid (3a) (0.239 g, 1.82 mmol) and [3-(thiazol-4-yl)-l-alanyl]-l-prolinamide dihydrochloride (45) (0.932 g, 2.00 mmol) yielded the title compound 58 (0.368 g, 53%) as a white amorphous powder.
IR (KBr): 3294, 1752, 1676, 1637, 1542, 1519, 1446, 1407, 1237 cm–1.
1H NMR (200 MHz, CD3OD): δ 8.95 (d, J = 2.0 Hz, 1H), 7.43 and 7.33 (d each, J = 2.0 Hz, total 1H), 4.96 (t, J = 7.2 Hz, 1H), 4.60–4.20 (m, 4H), 3.80 (m, 1H), 3.60–3.10 (m, 3H), 2.30–1.90 (m, 4H).
[α]D25 −53.0° (c 1.0, H2O).
Anal. Calcd for C15H19N5O5S·0.9H2O: C, 45.31; H, 5.27; N, 17.61; S, 8.06. Found: C, 45.27; H, 5.31; N, 17.65; S, 7.90.
TLC Rf (b) 0.22.
{[(4S,5R)-(5-Methyl-2-oxooxazolidine-4-yl)carbonyl]-3-(thiazol-4-yl)-l-alanyl}-l-prolinamide (59)
The condensation of (4S,5R)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3b) (0.264 g, 1.82 mmol) and [3-(thiazol-4-yl)-l-alanyl]-l-prolinamide dihydrochloride (45) (0.932 g, 2.00 mmol) yielded the title compound 59 (0.410 g, 57%) as a white amorphous powder.
IR (KBr): 3398, 1752, 1677, 1641, 1517, 1445, 1229 cm–1.
1H NMR (200 MHz, CD3OD): δ 8.95 (d, J = 2.0 Hz, 1H), 7.43 and 7.33 (d each, J = 2.0 Hz, total 1H), 4.97 (t, J = 7.0 Hz, 1H), 4.60–4.30 (m, 2H), 3.93 (d, J = 5.4 Hz, 1H), 3.78 (m, 1H), 3.60–3.10 (m, 3H), 2.30–1.70 (m, 4H), 1.45 (d, J = 6.6 Hz, 3H).
[α]D25 −30.3° (c 1.0, H2O).
Anal. Calcd for C16H21N5O5S·0.9H2O: C, 46.68; H, 5.58; N, 17.01; S, 7.79. Found: C, 46.69; H, 5.62; N, 17.19; S, 7.93.
TLC Rf (b) 0.30.
{[(4S,5R)-3,5-Dimethyl-2-oxooxazolidine-4-carbonyl]-3-(thiazol-4-yl)-l-alanyl}-l-prolinamide (60)
The condensation of (4S,5R)-3,5-dimethyl-2-oxooxazolidine-4-carboxylic acid (3g) (0.159 g, 1.00 mmol) and [3-(thiazol-4-yl)-l-alanyl]-l-prolinamide dihydrochloride (45) (0.535 g, 1.10 mmol) yielded the title compound 60 (0.201 g, 49%) as a white amorphous powder.
IR (KBr): 3412, 1751, 1678, 1519, 1544, 1519, 1437, 1401, 1237 cm–1.
1H NMR (200 MHz, CD3OD): δ 8.96 (d, J = 2.0 Hz, 1H), 7.44 and 7.35 (d each, J = 2.0 Hz, total 1H), 5.04 (dd, J = 7.8, 6.2 Hz, 1H), 4.40 (m, 2H), 3.88 (d, J = 5.4 Hz, 1H), 3.80 (m, 1H), 3.60–3.10 (m, 3H), 2.67 (s, 3 H), 2.30–1.80 (m, 4H), 1.43 (d, J = 6.4 Hz, 3H).
[α]D23 −31.9° (c 1.0, H2O).
Anal. Calcd for C17H23N5O5S·1.4H2O: C, 46.97; H, 5.98; N, 16.11; S, 7.38. Found: C, 46.98; H, 5.91; N, 16.27; S, 7.37.
TLC Rf (b) 0.40.
{[(4S,5R)-3-Benzyl-5-Methyl-2-oxooxazolidine-4-carbonyl]-3-(thiazol-4-yl)-l-alanyl}-l-prolinamide (61)
The condensation of (4S,5R)-3-benzyl-5-methyl-2-oxooxazolidine-4-carboxylic acid (3h) (0.235 g, 1.00 mmol) and [3-(thiazol-4-yl)-l-alanyl]-l-prolinamide dihydrochloride (45) (0.535 g, 1.10 mmol) yielded the title compound 61 (0.309 g, 64%) as a white amorphous powder.
IR (KBr): 3412, 1752, 1679, 1644, 1543, 1516, 1442, 1415, 1227, 1206, 1092, 1066 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.99 (d, J = 2.1 Hz, 1H), 7.41 (d, J = 2.1 Hz, 1H), 7.50–7.10 (m, 5H), 4.95 (m, 1H), 4.73 (d, J = 15.3 Hz, 1H), 4.42 (m, 2H), 3.87 (d, J = 15.3 Hz, 1H), 3.78 (m, 1H), 3.68 (d, J = 5.1 Hz, 1H), 3.60–3.00 (m, 3H), 2.30–1.80 (m, 4H), 1.35 (d, J = 6.3 Hz, 3H).
[α]D24.5 −43.7° (c 1.0, H2O).
Anal. Calcd for C23H27N5O5S·1.2H2O: C, 54.47; H, 5.84; N, 13.81; S, 6.32. Found: C, 54.33; H, 5.86; N, 13.97; S, 6.30.
TLC Rf (b) 0.39.
{[(4S,5R)-(5-Methyl-2-oxooxazolidine-4-yl)carbonyl]-3-(thiazol-5-yl)-l-alanyl]}-l-prolinamide (62)
The condensation of (4S,5R)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3b) (0.190 g, 1.29 mmol) and [3-(thiazol-5-yl)-l-alanyl]-l-prolinamide dihydrochloride (46) (0.440 g, 1.29 mmol) yielded the title compound 62 (0.220 g, 45%) as a white amorphous powder.
IR (KBr): 3397, 2979, 1753, 1677, 1639, 1527, 1446, 1403, 1230 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.89 (s, 1H), 7.76 and 7.70 (s each, total 1H), 4.92 (dd, J = 9.3, 3.9 Hz, 1H), 4.50–4.20 (m, 2H), 3.95 and 3.94 (d each, J = 5.1 Hz, total 1H), 3.90–3.60 (m, 2H), 3.50 (dd, J = 15.3, 3.9 Hz, 1H), 3.26 (dd, J = 9.3, 3.9 Hz, 1H), 2.30–1.80 (m, 4H), 1.46 and 1.45 (d each, J = 6.6 Hz, total 3H).
[α]D23 −25.8° (c 1.0, MeOH).
Anal. Calcd for C16H21N5O5S·1.4H2O: C, 45.68; H, 5.70; N, 16.65; S, 7.62. Found: C, 45.63; H, 5.63; N, 16.82; S, 7.64.
TLC Rf (b) 0.22.
3-{N-[(4S,5R)-(5-Methyl-2-oxooxazolidine-4-yl)carbonyl]-3-(thiazol-4-yl)-l-alanyl}-(4R)-4-thiazolidinecarboxamide (63)
The condensation of (4S,5R)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3b) (0.528 g, 3.64 mmol) and [3-(thiazol-4-yl)-l-alanyl]-(4R)-4-thiazolidinecarboxamide dihydrochloride (49) (1.65 g, 4.00 mmol) yielded the title compound 63 (0.632 g, 42%) as a white amorphous powder.
IR (KBr): 3397, 2980, 2932, 1752, 1677, 1649, 1519, 1413, 1227 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.95 (d, J = 1.8 Hz, 1H), 7.43 and 7.36 (each d, J = 1.8 Hz, total 1H), 5.07 (t, J = 6.6 Hz, 1H), 4.98 (d, J = 8.7 Hz, 1H), 4.86 (m, 1H), 4.60–4.40 (m, 1H), 4.45 (d, J = 8.7 Hz, 1H), 3.95 (d, J = 5.1 Hz, 1H), 3.50–3.10 (m, 4H), 1.46 (d, J = 6.3 Hz, 3H).
[α]D23 −58.8° (c 1.0, H2O).
Anal. Calcd for C15H19N5O5S2·1.1H2O: C, 41.58; H, 4.93; N, 16.16; S, 14.80. Found: C, 41.55; H, 4.92; N, 16.27; S, 14.82.
TLC Rf (b) 0.35.
{[(4S,5S)-(5-Methyl-2-oxooxazolidine-4-yl)carbonyl]-3-(thiazol-4-yl)-l-alanyl}-l-prolinamide (64)
The condensation of (4S,5S)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3c) (0.264 g, 1.82 mmol) and [3-(thiazol-4-yl)-l-alanyl]-l-prolinamide dihydrochloride (45) (0.973 g, 2.00 mmol) yielded the title compound 64 (0.356 g, 50%) as a white amorphous powder.
IR (KBr): 3392, 2982, 1752, 1676, 1637, 1542, 1519, 1447, 1234 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.95 (d, J = 1.8 Hz, 1H), 7.43 and 7.35 (d each, J = 1.8 Hz, total 1H), 5.02 (t, J = 7.2 Hz, 1H), 4.90 (m, 1H), 4.38 (m, 1H), 4.33 (d, J = 8.7 Hz, 1H), 3.88 (m, 1H), 3.60–3.10 (m, 3H), 2.30–1.90 (m, 4H), 1.26 and 1.20 (d each, J = 6.6 Hz, total 3H).
13C NMR (75 MHz, CD3OD): δ 176.45, 171.44, 170.21, 161.30, 154.99, 152.95, 117.48, 76.10, 61.23, 59.42, 52.08, 32.94, 30.30, 25.31, 22.70, 15.93, 15.79.
[α]D26 −52.1° (c 1.0, H2O).
Anal. Calcd for C16H21N5O5S·1.5H2O: C, 45.49; H, 5.73; N, 16.58; S, 7.59. Found: C, 45.53; H, 5.71; N, 16.85; S, 7.60.
TLC Rf (b) 0.35.
{[(4S,5S)-(5-Methyl-2-oxooxazolidine-4-yl)carbonyl]-3-(thiazol-5-yl)-l-alanyl}-l-prolinamide (65)
The condensation of (4S,5S)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3c) (0.210 g, 1.47 mmol) and [3-(thiazol-5-yl)-l-alanyl]-l-prolinamide dihydrochloride (46) (0.500 g, 1.47 mmol) yielded the title compound 65 (0.270 g, 45%) as a white amorphous powder.
IR (KBr): 3406, 2982, 1752, 1677, 1638, 1542, 1447, 1404, 1343, 1300, 1237 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.90 and 8.89 (s each, total 1H), 7.77 and 7.73 (s each, total 1H), 5.00–4.90 (m, 2H), 4.41 (dd, J = 8.7, 4.8 Hz, 1H), 4.39 and 4.35 (d each, J = 8.7 Hz, total 1H), 3.91 (m, 1H), 3.71 (m, 1H), 3.50 (dd, J = 15.6, 3.6 Hz, 1H), 3.25 (dd, J = 15.6, 9.6 Hz, 1H), 2.30–1.80 (m, 4H), 1.26 and 1.18 (d each, J = 6.3 Hz, total 3H).
13C NMR (75 MHz, CD3OD): δ 176.26, 170.47, 161.27, 154.66, 142.59, 135.04, 76.17, 76.01, 60.95, 59.45, 59.35, 53.17, 48.06, 32.58, 30.40, 28.54, 25.43, 22.75, 15.93, 15.78.
[α]D25 −59.3° (c 1.0, H2O).
Anal. Calcd for C16H21N5O5S·1.5H2O: C, 45.49; H, 5.73; N, 16.58; S, 7.59. Found: C, 45.39; H, 5.67; N, 16.75; S, 7.50.
TLC Rf (b) 0.29.
[(4S,5S)-(5-Methyl-2-oxooxazolidine-4-yl)carbonyl]-l-histidyl-l-prolinamide (66)
The condensation of (4S,5S)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3c) (2.37 g, 16.3 mmol) and l-histidyl-l-prolinamide dihydrobromide (47) (7.53 g, 16.3 mmol) yielded the title compound 66 (0.340 g, 5.5%) as a white amorphous powder.
IR (KBr): 3282, 2984, 2882, 1749, 1675, 1636, 1543, 1448, 1343, 1235, 1097 cm–1.
1H NMR (300 MHz, CD3OD): δ 7.64 and 7.60 (s each, total 1H), 6.96 and 6.90 (s each, total 1H), 5.00–4.80 (m, 1H), 4.41 (dd, J = 8.4, 4.2 Hz, 1H), 4.36 and 4.35 (d each, J = 8.4 Hz, total 1H), 3.85 (m, 1H), 3.43 (m, 1H), 3.12 and 2.98 (dd each, J = 14.4, 7.2 Hz, total 2H), 2.30–1.70 (m, 4H), 1.28 and 1.21 (d each, J = 6.6 Hz, total 3H).
[α]D25 −48.4° (c 1.0, MeOH).
Anal. Calcd for C16H22N6O5·1.8H2O: C, 46.78; H, 6.28; N, 20.46. Found: C, 46.83; H, 6.27; N, 20.72.
TLC Rf (b) 0.12.
1-{N-[(4S,5S)-5-Methyl-2-oxooxazolidine-4-carbonyl]-3-(thiazol-4-yl)-l-alanyl}-l-prolylmorpholine (67)
The condensation of (4S,5S)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3c) (0.300 g, 2.07 mmol) and [3-(thiazol-4-yl)-l-alanyl]-l-prolylmorpholine dihydrochloride (48) (0.850 g, 2.07 mmol) yielded the title compound 67 (0.560 g, 58%) as a white amorphous powder.
IR (KBr): 3411, 3284, 1760, 1638, 1518, 1445, 1233, 1112 cm–1.
1H NMR (200 MHz, CD3OD): δ 8.98 and 8.94 (d each, J = 2.0 Hz, total 1H), 7.42 and 7.32 (d each, J = 2.0 Hz, total 1H), 5.09 (dd, J = 9.4, 4.6 Hz, 1H), 5.00–4.70 (m, 2H), 4.33 and 4.30 (d each, J = 8.6 Hz, total 1H), 4.10–3.50 (m, 10H), 3.38 (dd, J = 15.0, 4.6 Hz, 1H), 3.15 (dd, J = 15.0, 9.4 Hz, 1H), 2.40–1.60 (m, 4H), 1.21 and 1.17 (d each, J = 6.6 Hz, total 3H).
[α]D25 −45.8° (c 1.0, MeOH).
Anal. Calcd for C20H27N5O6S·1.4H2O: C, 48.95; H, 6.12; N, 14.27; S, 6.53. Found: C, 48.84; H, 6.13; N, 14.44; S, 6.43.
TLC Rf (b) 0.64.
3-{N-[(4S,5S)-(5-Methyl-2-oxooxazolidine-4-yl)carbonyl]-3-(thiazol-4-yl)-l-alanyl}-(4R)-4-thiazolidinecarboxamide (68)
The condensation of (4S,5S)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3c) (0.528 g, 3.64 mmol) and [3-(thiazol-4-yl)-l-alanyl]-(4R)-4-thiazolidinecarboxamide dihydrochloride (49) (1.65 g, 4.00 mmol) yielded the title compound 68 (0.704 g, 47%) as a white amorphous powder.
IR (KBr): 3309, 2986, 2937, 1752, 1678, 1650, 1519, 1415, 1333, 1231, 1097 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.99 and 8.95 (d each, J = 2.1 Hz, total 1H), 7.43 and 7.39 (d each, J = 2.1 Hz, total 1H), 5.11 (t, J = 6.6 Hz, 1H), 5.10 (d, J = 8.7 Hz, 1H), 5.00–4.70 (m, 2H), 4.48 (d, J = 8.7 Hz, 1H), 4.34 (d, J = 8.7 Hz, 1H), 3.50–3.10 (m, 4H), 1.22 (d, J = 6.6 Hz, 3H).
13C NMR (75 MHz, CD3OD): δ 174.23, 171.06, 170.26, 161.32, 155.21, 152.74, 117.62, 76.14, 64.06, 59.28, 52.08, 49.97, 33.55, 33.15, 15.86.
[α]D25.5 −80.4° (c 1.0, MeOH).
Anal. Calcd for C15H19N5O5S2·H2O: C, 41.75; H, 4.91; N, 16.23; S, 14.86. Found: C, 41.73; H, 4.94; N, 16.23; S, 14.82.
TLC Rf (b) 0.37.
1-{N-[(4S,5S)-(5-Methyl-2-oxooxazolidine-4-yl)carbonyl]-3-(thiazol-4-yl)-l-alanyl}-(2S)-2-cyanopyrrolidine (69)
The condensation of (4S,5S)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3c) (0.210 g, 1.43 mmol) and 1-N-[3-(thiazol-4-yl)-l-alanyl]-(2S)-2-cyanopyrrolidine di-trifluoroacetate (50) (0.970 g, 1.43 mmol) yielded the title compound 69 (0.330 g, 61%) as a white amorphous powder.
IR (KBr): 3299, 2984, 2242, 1754, 1653, 1541, 1517, 1443, 1341, 1233, 1097 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.98 (d, J = 2.1 Hz, 1H), 7.35 (d, J = 2.1 Hz, 1H), 5.00–4.85 (m, 2H), 4.70 (dd, J = 7.8, 3.6 Hz, 1H), 4.39 and 4.34 (d each, J = 8.4 Hz, total 1H), 3.77 (m, 1H), 3.43 (m, 1H), 3.40–3.20 (m, 2H), 2.30–1.80 (m, 4H), 1.23 (d, J = 6.3 Hz, 3H).
13C NMR (75 MHz, CD3OD): δ 171.34, 170.45, 161.35, 155.16, 152.38, 118.93, 117.75, 76.21, 59.23, 52.08, 47.42, 47.28, 33.15, 30.44, 25.69, 15.83.
[α]D25 −35.0° (c 1.0, MeOH).
Anal. Calcd for C16H19N5O4S·1.1H2O: C, 48.38; H, 5.38; N, 17.63; S, 8.07. Found: C, 48.38; H, 5.39; N, 17.82; S, 7.94.
TLC Rf (b) 0.46.
1-{N-[(4S,5S)-(5-Methyl-2-oxooxazolidine-4-yl)carbonyl]-3-(thiazol-4-yl)-l-alanyl}-(2R)-2-methylpyrrolidine (70)
The condensation of (4S,5S)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3c) (3.51 g, 24.2 mmol) and 1-N-[3-(thiazol-4-yl)-l-alanyl]-(2R)-2-methylpyrrolidine dihydrochloride (51) (7.56 g, 24.2 mmol) yielded the title compound 70 (5.25 g, 59%) as a white amorphous powder.
IR (KBr): 3279, 2972, 2877, 1763, 1655, 1626, 1548, 1456, 1384, 1231 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.97 and 8.96 (d each, J = 2.1 Hz, total 1H), 7.34 and 7.33 (d each, J = 2.1 Hz, total 1H), 5.19 and 5.05 (t each, J = 7.5 Hz, total 1H), 4.92 (dq, J = 8.7, 6.6 Hz, 1H), 4.37 and 4.35 (d each, J = 8.7 Hz, total 1H), 4.07 and 3.92 (m each, total 1H), 3.75 (m, 1H), 3.41 (m, 1H), 3.22 (m, 2H), 2.00–1.50 (m, 4H), 1.28 and 1.22 (d each, J = 6.6 Hz, total 3H), 1.21 and 1.02 (d each, J = 6.6 Hz, total 3H).
13C NMR (75 MHz, CD3OD): δ 170.47, 170.33, 170.16, 169.83, 161.30, 155.04, 154.94, 152.97, 152.92, 117.33, 76.12, 76.05, 59.40, 59.33, 54.43, 54.17, 51.94, 51.82, 47.63, 46.11, 34.63, 33.54, 33.26, 32.20, 24.37, 21.86, 20.61, 18.87, 15.83.
[α]D23 −4.9° (c 1.0, MeOH).
Anal. Calcd for C16H22N4O4S·H2O: C, 49.99; H, 6.29; N, 14.57; S, 8.34. Found: C, 49.83; H, 6.22; N, 14.83; S, 8.37.
TLC Rf (b) 0.56.
1-{N-[(4S,5S)-(5-Methyl-2-oxooxazolidine-4-yl)carbonyl]-3-(thiazol-5-yl)-l-alanyl}-(2R)-2-methylpyrrolidine (71)
The condensation of (4S,5S)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3c) (0.140 g, 0.960 mmol) and 1-N-[3-(thiazol-5-yl)-l-alanyl]-(2R)-2-methylpyrrolidine dihydrochloride (52) (0.300 g, 0.960 mmol) yielded the title compound 71 (0.130 g, 37%) as a white amorphous powder.
IR (KBr): 3274, 3079, 2973, 2875, 1761, 1680, 1626, 1539, 1445, 1401, 1383, 1344, 1232 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.90 (s, 1H), 7.73 and 7.72 (s each, total 1H), 5.00–4.80 (m, 1H), 5.04 and 4.89 (t each, J = 7.5 Hz, total 1H), 4.40 and 4.37 (d each, J = 8.7 Hz, total 1H), 4.09 and 3.85 (m each, total 1H), 3.90–3.20 (m, 4H), 2.10–1.50 (m, 4H), 1.23 (d, J = 6.3 Hz, 3H), 1.25 and 1.09 (d each, J = 6.3 Hz, total 3H).
13C NMR (75 MHz, CD3OD): δ 170.74, 170.53, 170.20, 170.01, 161.80, 155.35, 155.22, 143.22, 134.82, 76.54, 76.47, 59.75, 55.21, 54.88, 53.56, 53.35, 48.22, 33.67, 32.72, 30.54, 29.53, 24.85, 22.33, 21.11, 19.41, 16.24.
[α]D22 −12.3° (c 1.0, MeOH).
Anal. Calcd for C16H22N4O4S·1.2H2O: C, 49.52; H, 6.34; N, 14.44; S, 8.26. Found: C, 49.39; H, 6.18; N, 14.69; S, 8.25.
TLC Rf (b) 0.50.
1-{N-[(4S,5S)-(5-Methyl-2-oxooxazolidine-4-yl)carbonyl]-l-histidyl}-(2R)-2-methylpyrrolidine (72)
The condensation of (4S,5S)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3c) (0.600 g, 4.12 mmol) and 1-N-(l-histidyl)-(2R)-2-methylpyrrolidine (53) (1.00 g, 4.50 mmol) yielded the title compound 72 (0.420 g, 29%) as a white amorphous powder.
IR (KBr): 3398, 3271, 2975, 2877, 1749, 1626, 1541, 1449, 1385, 1345, 1232 cm–1.
1H NMR (300 MHz, CD3OD): δ 7.63 and 7.62 (d each, J = 1.2 Hz, total 1H), 6.88 (s, 1H), 5.00–4.85 (m, 1H), 5.04 and 4.89 (t each, J = 7.5 Hz, total 1H), 4.37 and 4.35 (d each, J = 8.7 Hz, total 1H), 4.08 and 3.86 (m each, total 1H), 3.80–3.20 (m, 2H), 3.02 and 2.93 (dd each, J = 14.7, 7.5 Hz, total 1H), 2.10–1.50 (m, 4H), 1.22 (d, J = 6.6 Hz, 3H), 1.27 and 1.04 (d each, J = 6.6 Hz, total 3H).
13C NMR (75 MHz, CD3OD): δ 171.28, 171.12, 170.58, 170.24, 161.80, 136.63, 136.49, 134.59, 117.86, 76.61, 76.54, 59.79, 59.72, 54.71, 54.58, 52.74, 52.69, 48.01, 46.46, 33.59, 32.63, 31.66, 30.53, 24.77, 22.31, 20.91, 19.25, 16.23.
[α]D21 −7.4° (c 1.0, MeOH).
Anal. Calcd for C16H23N5O4·1.3H2O: C, 51.55; H, 6.92; N, 18.79. Found: C, 51.57; H, 6.72; N, 18.86.
TLC Rf (b) 0.21.
1-{N-[(4S,5S)-5-Methyl-2-oxooxazolidine-4-carbonyl]-3-(thiazol-4-yl)-l-alanyl}-l-Prolinol (73)
The condensation of (4S,5S)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3c) (0.299 g, 1.00 mmol) and [3-(thiazol-4-yl)-l-alanyl]-l-prolinol dihydrochloride (54) (0.101 g, 1.00 mmol) yielded the title compound 73 (0.168 g, 44%) as a white amorphous powder.
IR (KBr): 3276, 1750, 1627, 1542, 1518, 1450, 1407, 1385, 1342, 1231, 1097 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.98 and 8.95 (d each, J = 2.1 Hz, total 1H), 7.36 and 7.35 (d each, J = 2.1 Hz, total 1H), 5.21 and 5.06 (t each, J = 7.5 Hz, total 1H), 4.91 (m, 1H), 4.37 and 4.35 (d each, J = 8.7 Hz, total 1H), 4.06 (m, 1H), 3.90–3.70 (m, 1H), 3.51 (dd, J = 10.8, 3.9 Hz, 1H), 3.43 (dd, J = 10.8, 6.3 Hz, 1H), 3.40 (m, 1H), 3.25 (m, 2H), 2.00–1.60 (m, 4H), 1.25 and 1.22 (d each, J = 6.3 Hz, total 3H).
[α]D25 −10.7° (c 0.50, H2O).
Anal. Calcd for C16H22N4O5S·1.5H2O: C, 46.93; H, 6.15; N, 13.68, S, 7.83. Found: C, 46.97; H, 6.20; N, 13.66; S, 7.65.
TLC Rf (b) 0.37.
{[(4S,5S)-5-Methyl-2-oxooxazolidine-4-carbonyl]-3-(thiazol-4-yl)-l-alanyl}-l-proline Benzyl Ester (74)
The condensation of (4S,5S)-5-methyl-2-oxooxazolidine-4-carboxylic acid (3c) (0.316 g, 2.17 mmol) and [3-(thiazol-4-yl)-l-alanyl]-l-proline benzyl ester dihydrochloride (55) (0.865 g, 2.17 mmol) yielded the title compound 74 (0.496 g, 47%) as a white amorphous powder.
IR (KBr): 3363, 2632, 2550, 1746, 1728, 1685, 1653, 1412, 1224, 1156, 1060 cm–1.
1H NMR (200 MHz, CD3OD): δ 8.93 (d, J = 2.0 Hz, 1H), 7.35 (m, 5H), 7.30 (d, J = 2.0 Hz, 1H), 5.15 (s, 2H), 5.11 (m, 1H), 4.90 (m, 1H), 4.49 (m, 1H), 4.31 (d, J = 8.6 Hz, 1H), 3.90 (m, 1H), 3.60 (m, 1H), 3.17 (m, 2H), 2.25 (m, 1H), 1.97 (m, 3H), 1.17 (d, J = 6.6 Hz, 3H).
[α]D26 −55.9° (c 0.51, MeOH).
Anal. Calcd for C23H26N4O6S·0.4H2O: C, 55.95; H, 5.47; N, 11.35; S, 6.49. Found: C, 55.83; H, 5.53; N, 11.52; S, 6.48.
TLC Rf (b) 0.62.
{[(4S,5S)-5-Methyl-2-oxooxazolidine-4-carbonyl]-3-(thiazol-4-yl)-l-alanyl}-l-proline (75)
To a solution of {[(4S,5S)-5-methyl-2-oxooxazolidine-4-carbonyl]-3-(thiazol-4-yl)-l-alanyl}-l-proline benzyl ester (74) (1.99 g, 4.09 mmol) in MeOH (10.0 mL)–H2O (10.0 mL), lithium hydroxide hydrate (0.858 g, 20.5 mmol) was added and stirred for 1 h at room temperature. To the mixture, 1 M aqueous hydrochloric acid solution (20.5 mL, 20.5 mmol) was added and concentrated to about half volume under reduced pressure. To the mixture, ethyl acetate (50.0 mL) was added. After separation, the aqueous layer was washed with ethyl acetate (50.0 mL × 2) and then concentrated in vacuo. The residue was purified by gel-filtration chromatography (MCI GEL CHP-20P, 200 mL, eluent: H2O/MeOH). The purified fractions were concentrated in vacuo, and the residue was lyophilized to afford the title compound 75 (1.29 g, 80%) as a white amorphous powder.
IR (KBr): 3398, 3299, 1749, 1636, 1523, 1450, 1230 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.98 and 8.95 (d each, J = 2.1 Hz, total 1H), 7.40 and 7.33 (d each, J = 2.1 Hz, total 1H), 5.09 (dd, J = 8.4, 5.4 Hz, 1H), 4.90 (m, 1H), 4.42 (dd, J = 8.4, 3.6 Hz, 1H), 4.37 and 4.32 (d each, J = 8.7 Hz, total 1H), 3.91 (m, 1H), 3.61 (m, 1H), 3.30 (m, 1H), 3.17 (dd, J = 14.7, 8.4 Hz, 1H), 2.25 (m, 1H), 2.01 and 1.83 (m each, total 3H), 1.25 and 1.18 (d each, J = 6.9 Hz, total 3H).
[α]D23 −52.0° (c 1.0, H2O).
Anal. Calcd for C16H20N4O6S·1.5H2O: C, 45.38; H, 5.48; N, 13.23; S, 7.57. Found: C, 45.61; H, 5.34; N, 13.28; S, 7.69.
TLC Rf (c) 0.35.
1-{N-[(4S,5S)-5-Methyl-2-oxooxazolidine-4-carbonyl]-3-(thiazol-4-yl)-l-alanyl}-(2S)-N-pentylpyrrolidine-2-carboxamide (76)
To an ice cooled solution of {[(4S,5S)-5-methyl-2-oxooxazolidine-4-carbonyl]-3-(thiazol-4-yl)-l-alanyl}-l-proline (75) (0.300 g, 0.757 mmol) and HOSu (0.087 g, 0.757 mmol) in DMF (30.0 mL), DCC (0.170 g, 0.840 mmol) was added and stirred for 1 h. After the ice bath was removed, the reaction mixture was stirred continuously for 3 h. Next, n-pentylamine (0.130 g, 1.49 mmol) was added, and the stirring was continued for 16 h. The precipitate was filtered off and the filtrate was concentrated under reduced pressure. The residue was purified by gel-filtration chromatography (MCI GEL CHP-20P, 200 mL, eluent: H2O/MeOH). The purified fractions were concentrated in vacuo, and the residue was lyophilized to afford the title compound 76 (0.230 g, 65%) as a white amorphous powder.
IR (KBr): 3292, 3084, 1753, 1646, 1542, 1445, 1234, 1098 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.97 and 8.95 (d each, J = 2.1 Hz, total 1H), 7.44 and 7.35 (d each, J = 2.1 Hz, total 1H), 5.00 (t, J = 6.9 Hz, 1H), 4.91 (m, 1H), 4.37 (dd, J = 10.5, 4.2 Hz, 1H), 4.35 and 4.33 (d each, J = 9.0 Hz, total 1H), 3.87 (m, 1H), 3.60–3.30 (m, 5H), 2.30–1.70 (m, 4H), 1.51 (m, 2H), 1.31 (m, 4H), 1.25 and 1.20 (d each, J = 6.6 Hz, total 3H), 0.90 (t, J = 6.9 Hz, 3H).
[α]D25 −53.9° (c 1.0, MeOH).
Anal. Calcd for C21H31N5O5S·H2O: C, 52.16; H, 6.88; N, 14.48; S, 6.63. Found: C, 51.92; H, 6.88; N, 14.73; S, 6.48.
TLC Rf (b) 0.53.
1-{N-[(4S,5S)-5-Methyl-2-oxooxazolidine-4-carbonyl]-3-(thiazol-4-yl)-l-alanyl}-(2S)-N-(tert-butyl)pyrrolidine-2-carboxamide (77)
To an ice cooled solution of {[(4S,5S)-5-methyl-2-oxooxazolidine-4-carbonyl]-3-(thiazol-4-yl)-l-alanyl}-l-proline (75) (0.300 g, 0.757 mmol) and HOSu (0.087 g, 0.757 mmol) in DMF (30.0 mL), DCC (0.170 g, 0.840 mmol) was added and stirred for 1 h. After the ice bath was removed and the reaction mixture was stirred continuously for 4 h, tert-butylamine (0.110 g, 1.52 mmol) was added and stirred for 16 h. The precipitate was filtered off and the filtrate was concentrated under reduced pressure. The residue was purified by gel-filtration chromatography (MCI GEL CHP-20P, 200 mL, eluent: H2O/MeOH) and by silica gel column chromatography (eluent: CHCl3/MeOH). The purified fractions were concentrated in vacuo, and the residue was lyophilized to afford the title compound 77 (0.210 g, 62%) as a white amorphous powder.
IR (KBr): 3326, 1753, 1647, 1542, 1449, 1227, 1096 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.97 and 8.95 (d each, J = 1.8 Hz, total 1H), 7.42 and 7.34 (d each, J = 1.8 Hz, total 1H), 5.06 (dd, J = 8.1, 5.4 Hz, 1H), 4.90 (m, 1H), 4.34 (t, J = 8.7 Hz, 1H), 4.31 (d, J = 8.7 Hz, 1H), 3.90–3.60 (m, 2H), 3.37 (dd, J = 15.3, 5.4 Hz, 1H), 3.19 (dd, J = 15.3, 8.1 Hz, 1H), 2.30–1.70 (m, 4H), 1.33 (s, 9H), 1.25 and 1.18 (d each, J = 6.3 Hz, total 3H).
[α]D25 −55.3° (c 1.0, MeOH).
Anal. Calcd for C20H29N5O5S·1.5H2O: C, 50.20; H, 6.74; N, 14.63; S, 6.70. Found: C, 50.21; H, 6.68; N, 14.78; S, 6.85.
TLC Rf (b) 0.53.
{[(4R,5S)-5-Methyl-2-oxooxazolidine-4-ylmethyl]-3-(thiazol-4-yl)-l-alanyl}-l-prolinamide (78)
To an ice cooled solution of [3-(thiazol-4-yl)-l-alanyl]-l-prolinamide dihydrochloride (45) (0.556 g, 1.09 mmol) in DMF (5.00 mL), triethylamine (0.560 mL, 4.02 mmol) and (4R,5S)-5-methyl-2-oxooxazolidin-4-ylmethyl trifluoromethanesulfonate (3k) (0.262 g, 0.995 mmol) were added under stirring for 10 min. After the ice bath was removed and the reaction mixture was stirred continuously for 10 h, the precipitate was filtered off, and the filtrate was concentrated under reduced pressure. The residue was purified by gel-filtration chromatography (MCI GEL CHP-20P, 200 mL, eluent: H2O/MeOH). The purified fractions were concentrated in vacuo, and the residue was lyophilized to afford the title compound 78 (0.086 g, 27%) as a white amorphous powder.
IR (KBr): 3397, 2979, 2949, 1742, 1678, 1631, 1515, 1437, 1325, 1245, 1079 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.98 and 8.93 (d each, J = 2.1 Hz, total 1H), 7.39 and 7.29 (d each, J = 2.1 Hz, total 1H), 4.86–4.68 (m, 1H), 4.46 (dd, J = 8.7, 3.6 Hz, 1H), 4.13 and 3.86 (t each, J = 5.4 Hz, total 1H), 3.82–3.70 (m, 1H), 3.62 and 3.38 (m each, total 2H), 3.15 and 3.02 (m each, total 2H), 2.80–2.70 (m, 1H), 2.49 and 2.40 (dd each, J = 12.3, 7.5 Hz, total 1H), 2.25–1.70 (m, 4H), 1.28 and 1.26 (d each, J = 6.3 Hz, total 3H).
[α]D24 −10.1° (c 0.50, H2O).
Anal. Calcd for C16H23N5O4S·0.6H2O: C, 48.99; H, 6.22; N, 17.85; S, 8.17. Found: C, 48.98; H, 6.12; N, 17.81; S, 6.26.
TLC Rf (b) 0.36.
1-{N-[(4S,5S)-(5-Methyl-2-oxooxazolidine-4-yl)carbonyl]-3-(thiazol-4-yl)-l-alanyl}-(2R)-2-methylpyrrolidine trihydrate (79)
1-{N-[(4S,5S)-(5-Methyl-2-oxooxazolidine-4-yl)carbonyl]-3-(thiazol-4-yl)-l-alanyl}-(2R)-2-methylpyrrolidine (70) (5.00 g, 13.6 mmol) was dissolved in hot water (120 mL). After the solution was cooled to room temperature, seed crystals were added and concentrated under reduced pressure until the total volume was about one-sixth volume. The resultant slurry was filtered and washed with cold water to give the title compound 79 (5.34 g, 93%) as colorless crystals.
mp 194–196 °C.
IR (Nujol): 3498, 3276, 1754, 1682, 1609, 1550, 1464, 1378, 1235, 1089 cm–1.
1H NMR (300 MHz, CD3OD): δ 8.97 and 8.96 (d each, J = 2.1 Hz, total 1H), 7.34 and 7.33 (d each, J = 2.1 Hz, total 1H), 5.19 and 5.04 (t each, J = 7.5 Hz, total 1H), 4.92 (dq, J = 8.7, 6.6 Hz, 1H), 4.36 and 4.35 (d each, J = 8.7 Hz, total 1H), 4.07 and 3.92 (m each, total 1H), 3.78 (m, 1H), 3.42 (m, 1H), 3.22 (m, 2H), 2.00–1.50 (m, 4H), 1.28 and 1.22 (d each, J = 6.6 Hz, total 3H), 1.21 and 1.02 (d each, J = 6.6 Hz, total 3H).
13C NMR (75 MHz, CD3OD): δ 170.47, 1760.33, 170.16, 169.83, 161.30, 155.04, 154.94, 152.97, 152.92, 117.33, 76.12, 76.05, 59.40, 59.33, 54.43, 54.17, 51.94, 51.82, 47.63, 46.11, 34.63, 33.54, 33.26, 32.20, 24.37, 21.86, 20.61, 18.87, 15.83.
[α]D25 −1.9° (c 1.0, H2O).
Anal. Calcd for C16H22N4O4S·3H2O: C, 45.70; H, 6.71; N, 13.33; S, 7.63; H2O, 12.9. Found: C, 45.52; H, 6.49; N, 13.46; S, 7.59; H2O, 12.8 (K.F.).
TLC Rf (b) 0.56.
Chiral HPLC analysis was performed by using an isocratic solvent system of 0.05 M aqueous phosphate buffer (pH 6.0)/CH3CN (80/20) using a CAPCELLPACK (Shiseido) C-18 column with a flow rate of 1 mL/min.
tR = 8.07 min.
Experiments Using Laboratory Animals
All experiments using laboratory animals were conducted in accordance with the guideline by the Animal Care and Use Committee in Shionogi.
Anti Reserpine-Induced Hypothermia Effect in Mice
Male ddY mice were purchased from SLC Japan Inc. at the age of 6 weeks. After quarantine for 1 week, the mice were placed in animal compartments with a controlled room temperature of approximately 25 °C and relative humidity of approximately 60%, and a light cycle time of 12 h [light (8:00–20:00)/dark (20:00–8:00)]. Reserpine-induced hypothermia was conducted by the following method.14 The mice used had rectal temperatures of 30 °C or lower about 18 h after subcutaneous administration of reserpine (3 mg/kg, 1 mg/mL reserpine injection; Daiichi, Tokyo, Japan). TRH and TRH mimetics were dissolved in saline. Rectal temperature was measured with a thermistor (MGA-III, Nihon Kohden) before and after oral administration of TRH and TRH mimetics up to 7 h after a dose of 50 and 5 μmol/kg/mL, respectively. The antagonistic effect of the test drugs on reserpine-induced hypothermia was evaluated on the basis of the area under the temperature–time curve after dosing (AUC0–7h). All of the mice used in the experiments were killed immediately after the last measurement.
TRH Receptor Binding
Male Sprague-Dawley rats (300–400 g body weight) were sacrificed and dissected on ice to obtain the whole brain excluding the olfactory bulb. The brain was homogenized with glass/Teflon homogenizer (20 strokes) in 20-fold volume of 20 mM phosphate buffer (pH 7.4) and centrifuged at 40 000g for 30 min. The pellet was washed and re-centrifuged under the same conditions. The obtained pellet was suspended in 50-fold volume of 20 mM phosphate buffer (pH 7.4). The TRH receptor binding was conducted with [3H]-(3-Me-His2)-TRH (Daiichi Chemical, specific activity 82.5 Ci/mmol) and the crude membrane preparation (ca. 0.4 mg protein). The tracer, test compounds, and the membrane preparation were incubated in 0.2 mL of phosphate buffer (20 mM, pH 7.4) in ice cold water for 2 h. After the incubation, the sample was filtered through a Whatman GF/C filter, and the filter was washed three times with the buffer, and then the retained radioactivity was counted with a liquid scintillation counter. The nonspecific binding was measured in the presence of 10 μM TRH (BACHEM). The saturation and inhibition binding studies were conducted with 0.125–8 and 2 nM of [3H]-(3-Me-His2)-TRH. The Ki value was calculated with the following formula: Ki = IC50/1 + (ligand/Kd).
PK Experiments of TRH Mimetics 64, 68–70 in Rat
Male Sprague-Dawley rats were purchased from Charles River Laboratories Japan, Inc. at the age of 7 weeks. After quarantine for 1 week in the Animal Care Laboratory (Shionogi & Co., Ltd.), the rats were placed in animal compartments with a controlled room temperature of approximately 25 °C and relative humidity of approximately 60%, and a light cycle time of 12 h [light (8:00–20:00)/dark (20:00–8:00)]. All animal studies were conducted with the approval of the Institutional Animal Care and Use Committees of Shionogi Research Laboratories (n = 2, 302–364 g body weight). For oral administration, 2 μmol portions of TRH and TRH mimetics mixture as cassette dosing61,62 were accurately weighed and dissolved in the dimethyl sulfoxide/0.5% methyl cellulose aq. sol. = 1/4 to obtain the dosing solution with the target concentration and dose of 2 μmol/5 mL/kg. For intravenous administration, 1 μmol portions of TRH and TRH mimetics mixture as cassette dosing were accurately weighed and dissolved in the dimethyl sulfoxide/propylene glycol = 1/1 to obtain the dosing solution with the target concentration and dose of 1 μmol/1 mL/kg. Blood (approximately 0.3 mL) was collected from the jugular vein directory using a heparinized syringe at the scheduled time after administration. The samples were immediately centrifuged in approximately 10 000g for 5 min at 4 °C to obtain plasma, which was transferred into a tube and stored in a freezer at approximately −20 °C until the determination of the concentrations of TRH and TRH mimetics. Methanol/acetonitrile = 1/1 mixture was added to the plasma with mixing in a tube with a mixer and centrifugation in approximately 10 000g for 5 min at 4 °C to obtain the supernatant. The supernatant was injected into LC/MS/MS systems (ESI positive mode), which was a Qtrap 5500 system (AB SCIEX Pte. Ltd.) to determine the plasma concentration of TRH and TRH mimetics.
Ara-C-Induced Cerebellar Ataxia in Rat
Male and female neonates from Sprague-Dawley pregnant rats (purchased from Clea Japan at 13–17 days of pregnancy) were used. Cytosine arabinoside (Ara-C) was prepared using physiological saline before use and was subcutaneously injected at 60 mg/kg into the dorsal region at age of 2 days and age of 3 days (the day of confirming delivery: age of 0 day). Ara-C-administered animals were housed in room temperature: 22–27 °C, relative humidity: 25–74%, lighting period: 12 h from 7:00. Animals were allowed free access to diets (Clea Japan, CA-1) and water (tap water) by the experiment at age of 3–5 weeks. On the measurement of ataxia, each animal was placed on the center of the circular open field (75 cm in diameter, 25 parts). The number of crossing parts (number of ambulation) and the number of falls for 3 min were measured to calculate fall index ([number of falls]/[number of ambulation]). On the basis of the fall index, the severity of ataxia was assessed. To equalize the severity of ataxia among animals, the fall index ([number of falls]/[number of ambulation]) was measured at baseline (in the morning on the day of experiment for the day before initiation of the experiment for the 7 day repeated-dose experiment) for each animal except animals that did not fall down. Animals with the baseline fall index ranging 1.71–2.19 were included in the study. The severity of ataxia was assessed at 24 h after final administration in the 7 days of repeated oral dose. Animals in the control group were administered physiological saline.
TSH Releasing Activity in Rat
Animal rearing conditions: male Sprague-Dawley rats were purchased from Nippon Clea Co. Ltd at the age of 7 weeks. After quarantine for 1 week in the Animal Care Laboratory (Shionogi & Co., Ltd.), the rats were placed in animal compartments with a controlled room temperature of approximately 25 °C and relative humidity of approximately 60%, and a light cycle time of 12 h [light (7:00–19:00)/dark (19:00–7:00)]. At least n = 4, 258–320 g body weight. For oral administration, 2 mg portions of TRH mimetics were accurately weighed and dissolved in the water to obtain the dosing solution with the target concentration and dose of 0.1 and 1 mg/2 mL/kg. Blood (approximately 3 mL) was collected by decapitation at the scheduled time after oral administration to fed rats. The blood samples were placed for 30 min at room temperature (25 °C) and centrifuged in approximately 10 000g for 5 min at 4 °C using a centrifuge to obtain serum, which was transferred into a tube and stored in a freezer at approximately −20 °C until determination of the concentrations of TSH levels. Serum TSH levels were determined by the assay kit which was BIOTRAKTM Rat thyroid stimulating hormone [125I] assay system with magnetic separation, Amersham Pharmacia Biotech, code: RPA554.
PK Experiments of 79 in Rat
Male Wistar rats were purchased from SLC Japan Inc. at the age of 10 or 11 weeks. After quarantine for 1 week in the Animal Care Laboratory (Shionogi & Co., Ltd.), the rats were placed in animal compartments with a controlled room temperature of approximately 25 °C and relative humidity of approximately 60%, and a light cycle time of 12 h [light (8:00–20:00)/dark (20:00–8:00)]. In the fasting study, the rats were reared under fasting condition from the evening of the day before administration until the 12 h-sampling after administration. Each rat was subjected to surgery to insert a cannula tube into the jugular vein under isoflurane anesthesia at 2 or 3 days before administration. At least n = 4, 230–274 g body weight. For oral administration, 40 mg portions of TRH and TRH mimetics were accurately weighed and dissolved in the saline to obtain the dosing solution with the target concentration and dose of 40 mg/4 mL/kg. For intravenous administration, 8 mg portions of TRH and TRH mimetics were accurately weighed and dissolved in the saline to obtain the dosing solution with the target concentration and dose of 8 mg/1.6 mL/kg. Blood (approximately 0.3 mL) was collected from the cannula inserted into the jugular vein using a heparinized syringe at the scheduled time after administration. The samples were immediately centrifuged in approximately 10 000g for 5 min at 4 °C using a centrifuge to obtain plasma, which was transferred into a tube and stored in a freezer at approximately −20 °C until determination of the concentrations of TRH and TRH mimetics. Methanol was added to the plasma with mixing in a tube with a mixer and centrifugation at approximately 10 000g for 5 min at 4 °C to obtain the supernatant. The supernatant was injected into HPLC systems, which was Waters 805 system (Waters 717 auto sampler, Waters 486 Tunable Absorbance detector, Waters 600 Controller) to determine the plasma concentration of TRH and TRH mimetics.
Brain Kp Experiments of 79 in Rat
Male Sprague-Dawley rats were purchased from Nippon Clea Co. Ltd at the age of 7 weeks. After quarantine for 1 week in the Animal Care Laboratory (Shionogi & Co., Ltd.), the rats were placed in animal compartments with a controlled room temperature of approximately 23 °C and relative humidity of approximately 55%, and a light cycle time of 12 h [light (8:00–20:00)/dark (20:00–8:00)]. In the fasting study, the rats were reared under fasting condition from the evening of the day before administration until the 12 h-sampling after administration. n = 4, 262–312 g body weight. Each rat was subjected to surgery to insert a cannula tube into the jugular vein and micro dialysis cannula tube into ventral tegmental area of brain under pentobarbital anesthesia at 3 days before administration. For oral administration, 40 mg portions of 79 was accurately weighed and dissolved in the saline to obtain the dosing solution with the target concentration and dose of 40 mg/2 mL/kg. Blood (approximately 0.3 mL) was collected from the cannula inserted into the jugular vein using a heparinized syringe at the scheduled time after administration. The samples were immediately centrifuged in approximately 3000g for 5 min at 4 °C using a centrifuge to obtain plasma, which was transferred into a tube and stored in a freezer at approximately −20 °C until determination of the concentrations of 79. Methanol was added to the plasma with mixing in a tube with a mixer and centrifugation at 10 000g for 5 min at 4 °C to obtain the supernatant. Micro dialysis solution was collected from the cannula inserted into the ventral tegmental area of brain using a micro syringe pump (1 μL/min of Ringer solution) at the scheduled time after administration.
The supernatant and dialysis solution were injected into HPLC systems with a mass spectrometer, which was Waters 606 system (Waters 717 auto sampler, Thermo Quest TSQ7000 mass spectrometer) to determine the plasma and brain dialysis concentration of 79.
Stability Study in Rat Plasma and Brain Homogenate
Animal rearing conditions: male Sprague-Dawley rats were purchased from Nippon Clea Co. Ltd at the age of 7 weeks. After quarantine for 1 week in the Animal Care Laboratory (Shionogi & Co., Ltd.), the rats were placed in animal compartments with a controlled room temperature of approximately 25 °C and relative humidity of approximately 60%, and a light cycle time of 12 h [light (8:00–20:00)/dark (20:00–8:00)]. 79 in vitro stability test in plasma and brain homogenate (n = 5, 292–306 g body weight).
Test concentration: approximately 1 μg/mL. Nonfasted rats were anesthetized under ether and exsanguinated from the abdominal aorta. The blood was centrifuged (3000g, 5 min) and the plasma collected. Brains were then promptly extracted, divided into cerebrum and cerebellum, nine volumes of phosphate buffered saline (PBS) solution (Dulbecco’s PBS (-): Nissui Co.) added, and 10% homogenates prepared in a Polytron homogenizer. Five milliliter quantities of the plasma, cerebrum, and cerebellum homogenates (5 samples of each) were placed in 50 mL capacity tubes, cooled in ice, and mixed with 50 μL of each drug sample solution adjusted to approximately 0.1 mg/kg. Sampling (0 min) was then promptly carried out. Each test solution was left on a water bath at 37 °C, and sampling of 0.1 mL quantities was carried out after 5, 15, 30, 60, 120, and 180 min. Three volumes of methanol was added and mixed with each sample of test solution, the mixture was centrifuged, and the supernatant was collected and stored at -20 °C until determined by TLC. The supernatants were spotted onto TLC plates (20 × 20 cm silica gel 60 F254, Merck Co.) and promptly developed with the following mixed solvents: CHCl3/MeOH/H2O = 32/6/0.5. After TLC development, [14C]-79 was kept in contact with X-ray film for 2–3 weeks, and the positions of the labeled entities was confirmed after development. Unchanged drug and other metabolites were separately scraped off the plates and put into glass vials. Distilled water (1 mL) and 15 mL of Pico-Fluor 40 were added and left overnight, and then the radioactivity in each sample was measured by liquid scintillation counter.
Acknowledgments
We thank R. Nishimura, K. Yoshino, K. Kuwano, and R. Ikenishi for the elemental analysis, M. Sugita for the IR spectroscopy and optical rotation data, Y. Takayama and J. Kikuchi for the NMR spectroscopy data.
Glossary
Abbreviations
- TRH
thyrotropin-releasing hormone;
- CNS
central nervous system
- SCD
spinocerebellar degeneration
- TSH
thyrotropin-stimulating hormone
- Me
methyl
- Bn
benzyl
- tBu
tert-butyl
- MeOH
methanol
- EtOH
ethanol
- BnOH
benzyl alcohol
- Et2O
diethyl ether
- MEK
methyl ethyl ketone
- Et3N
triethylamine
- TsOH·H2O
p-toluenesulfonic acid monohydrate
- Tf2O
trifluoromethanesulfonic anhydride
- Tf
trifluoromethanesulfonyl
- Ac2O
acetic anhydride
- DMAP
4-dimethylaminopyridine
- Boc2O
di-tert-butyl dicarbonate
- MeI
iodomethame
- BnBr
benzyl bromide
- HOBt
N-hydroxybenzotriazole
- HOSu
N-hydroxysuccinimide
- TLC
thin-layer chromatography
- tR
retention time
- K.F.
Karl Fischer
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01481.
Synthetic procedure and analytical data for the N-terminus part fragments 3a–h, the middle-part fragments 4a–b, 7–9, 12–17, the C-terminus part fragments 21–33, the N-Boc and the N-Cbz dipeptide mimetics 34–44, and the dipeptide mimetics 45–55 and X-ray crystallographic data of 79 (PDF)
Accession Codes
Crystal structure coordinates and structure factors for 79 have been deposited to the CCDC with the accession number 1442496.
Author Contributions
¶ These authors contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Bøler J.; Enzmann F.; Folkers K.; Bowers C. Y.; Shally A. V. The Identity of Chemical and Hormonal Properties of the Thyrotropin Releasing Hormone and Pyroglutamyl-Histidyl-Proline amide. Biochem. Biophys. Res. Commun. 1969, 37, 705–710. 10.1016/0006-291x(69)90868-7. [DOI] [PubMed] [Google Scholar]
- Burgus R.; Dunn T. F.; Desiderio D.; Ward D. N.; Vale W.; Uillemin R. Characterization of Ovine Hypothalamic Hypophysiotropic TSH-Releasing Factor. Nature 1970, 226, 321–325. 10.1038/226321a0. [DOI] [PubMed] [Google Scholar]
- Stwertka S. A.; Vincent G. P.; Gamzu E. R.; MacNeil D. A.; Verderese A. G. TRH Protection Against Memory Retrieval Deficits is Independent of Endocrine Effects. Pharmacol. Biochem. Behav. 1991, 41, 145–152. 10.1016/0091-3057(92)90074-p. [DOI] [PubMed] [Google Scholar]
- Yarbrough G. G. Thyrotropin Releasing Hormone and CNS Cholinergic Neurons. Life Sci. 1983, 33, 111–118. 10.1016/0024-3205(83)90403-4. [DOI] [PubMed] [Google Scholar]
- Griffiths E. Thyrotrophin Releasing Hormone: Endocrine and Central Effects. Psychoneuroendocrinology 1985, 10, 225–235. 10.1016/0306-4530(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Horita A.; Carino M. A.; Lai H. Pharmacology of Thyrotropin-Releasing Hormone. Annu. Rev. Pharmacol. Toxicol. 1986, 26, 311–332. 10.1146/annurev.pa.26.040186.001523. [DOI] [PubMed] [Google Scholar]
- a Fujino M.; Kobayashi S.; Obayashi M.; Fukuda T.; Shinagawa S.; Nishimura O. The use of N-hydroxy-5-norbornene-2,3-dicarboximide acitive esters in peptide synthesis. Chem. Pharm. Bull. 1974, 22, 1857–1863. 10.1248/cpb.22.1857. [DOI] [PubMed] [Google Scholar]; b Hatanaka C.; Obayashi M.; Nishimura O.; Toukai N.; Fujino M. An Improved Synthesis of Thyrotropin Releasing Hormone (TRH) and Crystallization of the Tartrate. Biochem. Biophys. Res. Commun. 1974, 60, 1345–1350. 10.1016/0006-291x(74)90345-3. [DOI] [PubMed] [Google Scholar]; c Hatanaka C.; Nakamachi H.; Kaneko M.; Yonezawa H.; Toukai N.; Fujino M. Synthesis and Polymorphs of TRH L-Tartrate. J. Takeda Res. Lab. 1976, 35, 16–22. [Google Scholar]
- Straub R. E.; Frech G. C.; Joho R. H.; Gershengorn M. C. Expression Cloning of a cDNA Encoding the Mouse Pituitary Thyrotropin-Releasing Hormone Receptor. Proc. Natl. Acad. Sci. U.S.A. 1990, 87, 9514–9518. 10.1073/pnas.87.24.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J.; O’Donnell D.; Vu H.; Payza K.; Pou C.; Godbout C.; Jakob A.; Pelletier M.; Lembo P.; Ahmad S.; Walker P. Cloning and Characterization of a cDNA Encoding a Novel Subtype of Rat Thyrotropin-releasing Hormone Receptor. J. Biol. Chem. 1988, 273, 32281–32287. 10.1074/jbc.273.48.32281. [DOI] [PubMed] [Google Scholar]
- Itadani H.; Nakamura T.; Itoh J.; Iwaasa H.; Kanatani A.; Borkowski J.; Ihara M.; Ohta M. Cloning and Characterization of a New Subtype of Thyrotropin-Releasing Hormone Receptors. Biochem. Biophys. Res. Commun. 1998, 250, 68–71. 10.1006/bbrc.1998.9268. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Lu X.; Gershengorn M. C. Thyrotropin-releasing hormone receptors -- similarities and differences. J. Mol. Endocrinol. 2003, 30, 87–97. 10.1677/jme.0.0300087. [DOI] [PubMed] [Google Scholar]
- Duthie S. M.; Taylor P. L.; Eidne K. A. Characterization of the mouse thyrotrophin-releasing hormone receptor gene: an exon corresponds to a deletion in the rat cDNA. J. Mol. Endocrinol. 1993, 11, 141–149. 10.1677/jme.0.0110141. [DOI] [PubMed] [Google Scholar]
- Matre V.; Karlsen H. E.; Wright M. S.; Lundell I.; Fjeldheim A. K.; Gabrielsen O. S.; Larhammar D.; Gautvik K. M. Molecular Cloning of a Functional Human Thyrotropin-Releasing Hormone Receptor. Biochem. Biophys. Res. Commun. 1993, 195, 179–185. 10.1006/bbrc.1993.2027. [DOI] [PubMed] [Google Scholar]
- a Sugano H.; Ishida R.; Yamamura M.. Peptides and Process for Preparing the same. U.S. Patent US4,563,306, 1986.; b Sugano H.; Ishida R.; Yamamura M.. Novel Peptides and Processes for Preparing the Same.U.S. Patent US4,711,878, 1987.; c Suzuki M.; Sugano H.; Matsumoto K.; Yamamura M.; Ishida R. Synthesis and Central Nervous System Actions of Thyrotropin-Releasing Hormone Analog Containing a Dihydroorotic Acid Moiety. J. Med. Chem. 1990, 33, 2130–2137. 10.1021/jm00170a014. [DOI] [PubMed] [Google Scholar]; d Yamamura M.; Kinoshita K.; Nakagawa H.; Tanaka T.; Maeda K.; Ishida R. Pharmacological study of TA-0910, a new thyrotropin-releasing hormone(TRH) analog. (I). Effects on the central nervous system by oral administration. Jpn. J. Pharmacol. 1990, 53, 451–461. 10.1254/jjp.53.451. [DOI] [PubMed] [Google Scholar]; e Maeda H.; Suzuki M.; Sugano H.; Yamamura M.; Ishida R. Synthesis and Central Nervous System Actions of Thyrotropin-Releasing Hormone Analogs Containing a 1-Substituted 2-oxoimidazolidine Moiety. Int. J. Pept. Protein Res. 1989, 33, 403–411. 10.1111/j.1399-3011.1989.tb00216.x. [DOI] [PubMed] [Google Scholar]; f Maeda H.; Suzuki M.; Sugano H.; Yamamura M.; Ishida R. Synthesis and central nervous system actions of thyrotropin-releasing hormone analogs containing a 1-oxo-1,2,3,4-tetrahydroisoquinoline moiety. Chem. Pharm. Bull. 1988, 36, 190–201. 10.1248/cpb.36.190. [DOI] [PubMed] [Google Scholar]; g Suzuki M.; Maeda H.; Kondo K.; Sugano H.; Matsumoto K. Practical syntheses of optically pure 1- and 3-substituted dihydroorotic acids. Chem. Pharm. Bull. 1989, 37, 1764–1769. 10.1248/cpb.37.1764. [DOI] [Google Scholar]
- Sharif N. A.; To Z.; Michel A. D.; Whiting R. L. Differential Affinities of TRH Analogs at the Mammalian Spinal Cord TRH Receptor: Implications for Therapy in Spinal Injuries. Neurosci. Lett. 1989, 104, 183–188. 10.1016/0304-3940(89)90352-2. [DOI] [PubMed] [Google Scholar]
- a Kimura K.; Ogasawara T.; Mushiroi T.. Antiepileptic Pharmaceuticals Containing Histidylproline Derivatives. U.S. Patent US4,877,784, 1989.; b Faden A. I.; Yum S. W.; Lemke M.; Vink R. Effects of TRH-Analog Treatment on Tissue Cations, Phospholipids and Energy Metabolism after Spinal Cord Injury. J. Pharmacol. Exp. Ther. 1990, 255, 608–614. [PubMed] [Google Scholar]
- a Inanaga K.; Nagawa Y.. Antiepileptic Composition. U.S. Patent US4,421,745, 1983.; b Miyamoto M.; Fukuda N.; Narumi S.; Nagai Y.; Saji Y.; Nagawa Y. γ-butyrolactone-γ-carbonyl-histidyl-prolinamide citrate (DN-1417) : A novel TRH analog with potent effects on the central nervous system. Life Sci. 1981, 28, 861–869. 10.1016/0024-3205(81)90047-3. [DOI] [PubMed] [Google Scholar]; c Fukuda N.; Nishimura O.; Shikata M.; Hatanaka C.; Miyamoto M.; Saji Y.; Nakayama R.; Fujino M.; Nagawa Y. Synthesis and Pharmacology of TRH Analogs to Separate Central Nervous Action from Endocrine Activity. Chem. Pharm. Bull. 1980, 28, 1667–1672. 10.1248/cpb.28.1667. [DOI] [PubMed] [Google Scholar]
- a Iwamoto H.; Yoshida M.; Yamamoto M.; Tamura T.. 4-Substituted-2-azetidinone Compound. U.S. Patent US4,610,821, 1986.; b Faden A. I. TRH Analog YM-14673 Improves Outcome Following Traumatic Brain and Spinal Cord Injury in Rats: Dose-Response Studies. Brain Res. 1989, 486, 228–235. 10.1016/0006-8993(89)90509-x. [DOI] [PubMed] [Google Scholar]; c Ono H.; Nagano N.; Yamada M.; Fukuda H. Effects of a new analog of thyrotropin-releasing hormone, Nα-[{(s)-4-oxo-2-azetidinyl} carbonyl]-l-histidyl-l-prolinamide dehydrate (YM-14673) on spinal reflex potentials and flexor reflexes in spinalized rats. Neuropharmacology 1990, 29, 69–74. 10.1016/0028-3908(90)90085-6. [DOI] [PubMed] [Google Scholar]; d Mori M.; Iriuchijima T.; Yamada M.; Murakami M.; Kobayashi S. A Novel TRH Analog, YM14673, Stimulates Intracellular Signaling Systems in the Brain more Potently than Predicted by its Pituitary Actions. Res. Commun. Chem. Pathol. Pharmacol. 1991, 71, 17–26. [PubMed] [Google Scholar]
- a Uchida I.; Saito A.; Yasuda A.; Iwata K.; Hari H.; Hara K.; Matsushita M.; Anami K.; Haruta J.; Furukawa N.. Preparation of Peptide Thyrotropin Releasing Hormone (TRH) Analogs as Central Nervous System Agents. U.S. Patent US5,151,497, 1992.; b Toide K.; Shinoda M.; Takase M.; Iwata K.; Yoshida H. Effects of a Novel Thyrotropin-Releasing Hormone Analogue, JTP-2942, on Extracellular Acetylcholine and Choline Levels in the Rat Frontal Cortex and Hippocampus. Eur. J. Pharmacol. 1993, 233, 21–28. 10.1016/0014-2999(93)90344-h. [DOI] [PubMed] [Google Scholar]; c Matsushita M.; Yonemori F.; Furukawa N.; Ohta A.; Toide K.; Uchida I.; Iwata K. Effects of the Novel Thyrotropin-Releasing Hormone Analogue Nα-((1S,2R)-2-Methyl-4-oxocyclopentylcarbonyl)-L-histidyl-L-prolinamide Monohydrate on the Central Nervous System in Mice and Rats. Arzneimittelforschung 1993, 43, 813–817. [PubMed] [Google Scholar]; d Matsushita M.; Yonemori F.; Hamada A.; Toide K.; Iwata K. Effect of JTP-2942, a Novel Thyrotropin-Releasing Hormone Analogue, on Pentobarbital-Induced Anesthesia in Rats. Eur. J. Pharmacol. 1995, 276, 177–182. 10.1016/0014-2999(95)00034-i. [DOI] [PubMed] [Google Scholar]
- a Kisfaludy L.; Szirtes T.; Balaspiri L.; Palosi E.; Szporny L.; Sarkadi A.. Tripeptide Amides and Pharmaceutical Compositions Containing Them. U. S. Patent US4,299,821, 1983.; b Szirtes T.; Kisfaludy L.; Pálosi É.; Szporny L. Synthesis of Thyrotropin-Releasing Hormone Analogs. 1. Complete Dissociation of Central Nervous System Effects from Thyrotropin-Releasing Activity. J. Med. Chem. 1984, 27, 741–745. 10.1021/jm00372a006. [DOI] [PubMed] [Google Scholar]; c Szirtes T.; Kisfaludy L.; Pálosi É.; Szporny L. Synthesis of Thyrotropin-Releasing Hormone Analogs. 2. Tripeptides Structurally Greatly Different from TRH with High Central Nervous System Activity. J. Med. Chem. 1986, 29, 1654–1658. 10.1021/jm00159a015. [DOI] [PubMed] [Google Scholar]
- a Veber D. F.; Holly F. W.; Nutt R.; Varga S. L.. Analogs of Thyrotropin-Releasing Hormone. U.S. Patent US3,959,248, 1976.; b Nutt R. F.; Holly F. W.; Homnick C.; Hirschmann R.; Vaber D. F.; Arison B. H. Synthesis of Thyrotropin-Releasing Hormone Analogs with Selective Central Nervous System Effects. J. Med. Chem. 1981, 24, 692–698. 10.1021/jm00138a010. [DOI] [PubMed] [Google Scholar]
- a Brewster D.; Dettmar P. W.; Metcalf G. Biologically Stable Analogues of TRH with Increased Neuropharmcological Potency. Neuropharmacology 1981, 20, 497–503. 10.1016/0028-3908(81)90184-2. [DOI] [PubMed] [Google Scholar]; b Yanagisawa K.; Yang H.; Walsh J. H.; Taché Y. Role of Acetylcholine, Histamine and Gastrin in the Acid Response to Intracisternal Injection of TRH Analog, RX 77368, in the Rat. Regul. Pept. 1990, 27, 161–170. 10.1016/0167-0115(90)90036-v. [DOI] [PubMed] [Google Scholar]
- Thirunarayanan N.; Nir E. A.; Raaka B. M.; Gershengorn M. C. Thyrotropin-Releasing Hormone Receptor Type 1 (TRH-R1), not TRH-R2, Primarily Mediates Taltirelin Actions in the CNS of Mice. Neuropsychopharmacology 2013, 38, 950–956. 10.1038/npp.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirunarayanan N.; Raaka B. M.; Gershengorn M. C. Taltirelin is a Superagonist at the Human Thyrotropin-Releasing Hormone Receptor. Front. Endocrinol. 2012, 3, 120–124. 10.3389/fendo.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Jain R.; Singh J.; Perlman J. H.; Gershengorn M. C. Synthesis and Biology of New Thyrotropin-Releasing Hormone (TRH) Analogues. Bioorg. Med. Chem. 2002, 10, 189–194. 10.1016/s0968-0896(01)00265-6. [DOI] [PubMed] [Google Scholar]; b Kaur N.; Lu X.; Gershengorn M. C.; Jain R. Thyrotropin-Releasing Hormone (TRH) Analogues That Exhibit Selectivity to TRH Receptor Subtype 2. J. Med. Chem. 2005, 48, 6162–6165. 10.1021/jm0505462. [DOI] [PubMed] [Google Scholar]; c Kaur N.; Monga V.; Josan J. S.; Lu X.; Gershengorn M. C.; Jain R. Synthesis, Receptor Binding, and Activation Studies of N(1)-Alkyl-L-histidine Containing Thyrotropin-Releasing Hormone (TRH) Analogues. Bioorg. Med. Chem. 2006, 14, 5981–5988. 10.1016/j.bmc.2006.05.031. [DOI] [PubMed] [Google Scholar]; d Kaur N.; Monga V.; Lu X.; Gershengorn M. C.; Jain R. Modifications of the Pyroglutamic Acid and Histidine Residues in Thyrotropin-Releasing Hormone (TRH) Yield Analogs with Selectivity for TRH Receptor Type 2 over Type 1. Bioorg. Med. Chem. 2007, 15, 433–443. 10.1016/j.bmc.2006.09.045. [DOI] [PubMed] [Google Scholar]; e Khomane K. S.; Meena C. L.; Jain R.; Bansal A. K. Novel Thyrotropin-Releasing Hormone Analogs: A Patent Review. Expert Opin. Ther. Patents 2011, 21, 1673–1691. 10.1517/13543776.2011.623127. [DOI] [PubMed] [Google Scholar]; f Meena C. L.; Thakur A.; Nandekar P. P.; Sangamwar A. T.; Sharma S. S.; Jain R. Synthesis of CNS Active Thyrotropin-Releasing Hormone (TRH)-Like Peptides: Biological Evaluation and Effect on Cognitive Impairment Induced by Cerebral Ischemia in Mice. Bioorg. Med. Chem. 2015, 23, 5641–5653. 10.1016/j.bmc.2015.07.022. [DOI] [PubMed] [Google Scholar]
- a Prokai-Tatrai K.; Perjési P.; Zharikova A. D.; Li X.; Prokai L. Design, Synthesis, and Biological Evaluation of Novel, Centrally-Acting Thyrotropin-Releasing Hormone Analogues. Bioorg. Med. Chem. Lett. 2002, 12, 2171–2174. 10.1016/s0960-894x(02)00368-2. [DOI] [PubMed] [Google Scholar]; b Prokai L. Central Nervous System Effects of Thyrotropin-Releasing Hormone and and its Analogues: Opportunities and Perspectives for Drug Discovery and Development. Prog. Drug Res. 2002, 59, 133–169. 10.1007/978-3-0348-8171-5_5. [DOI] [PubMed] [Google Scholar]; c Prokai-Tatrai K.; Nguyen V.; Zharikova A. D.; Braddy A. C.; Stevens S. M. Jr.; Prokai L. Prodrugs to enhance central nervous system effects of the TRH-like peptide pGlu-Glu-Pro-NH2. Bioorg. Med. Chem. Lett. 2003, 13, 1011–1014. 10.1016/s0960-894x(03)00081-7. [DOI] [PubMed] [Google Scholar]; d Prokai L.; Prokai-Tatrai K.; Zharikova A. D.; Nguyen V.; Perjesi P.; Stevens S. M. Jr. Centrally Acting and Metabolically Stable Thyrotropin-Releasing Hormone Analogues by Replacement of Histidine with Substituted Pyridinium. J. Med. Chem. 2004, 47, 6025–6033. 10.1021/jm020531t. [DOI] [PubMed] [Google Scholar]
- Sugawara T.; Yoshikawa T.; Tada Y.. Novel Peptide Derivatives Having Thiazolyl-Alanine Residue. WO/1998/008867, 1998.
- Inui T.; Tanaka S.; Takino M. Oxazolidone Derivatives of Hydroxyamino Acid. VI. Reactions of Phosgene onN-Acylserine Esters. Bull. Chem. Soc. Jpn. 1970, 43, 1582–1584. 10.1246/bcsj.43.1582. [DOI] [Google Scholar]
- Saeed A.; Young D. W. Synthesis of L-β-hydroxyaminoacids using serine hydroxymethyltransferase. Tetrahedron 1992, 48, 2507–2514. 10.1016/s0040-4020(01)88770-6. [DOI] [Google Scholar]
- Yonezawa Y.; Shin C.-g.; Ohtsu A.; Yoshimura J. Synthesis of New 2-Oxazolinones from N-Carbonyl α-dehydroamino Acid Anhydrides. Chem. Lett. 1982, 11, 1171–1172. 10.1246/cl.1982.1171. [DOI] [Google Scholar]
- Herborn C.; Zietlow A.; Steckhan E. (4RS,5R)- und (4RS,5S)-4-Methoxy-5-methyloxazolidin-2-on-Derivate aus Threonin - interessante chirale Amidoalkylierungsreagentien. Angew. Chem. 1989, 101, 1392–1394. 10.1002/ange.19891011016. [DOI] [Google Scholar]
- Kaneko T.; Inui T. Oxazolidone Derivatives of Hydroxyamino Acids. III. Inversion of the erythro- to the threo-Form of Threonine via Oxazolidone Ester. Bull. Chem. Soc. Jpn. 1962, 35, 1145–1149. 10.1246/bcsj.35.1145. [DOI] [Google Scholar]
- Caldwell W. T.; Fox S. M. The Synthesis of 4-Chloromethylthiazole Hydrochloride and β-(4-Thiazolyl)-alanine Hydrochloride1. J. Am. Chem. Soc. 1951, 73, 2935–2936. 10.1021/ja01150a502. [DOI] [Google Scholar]
- Watanabe H.; Kuwata S.; Sakata T.; Matsumura K. Studies of Unnatural Amino Acids and Their Peptides. II. The Syntheses of DL-β-(4-Thiazolyl)-α-alanine and Its Peptides. Bull. Chem. Soc. Jpn. 1966, 39, 2473–2476. 10.1246/bcsj.39.2473. [DOI] [PubMed] [Google Scholar]
- Nishi T.; Saito F.; Nagahori H.; Kataoka M.; Morisawa Y.; Yabe Y.; Sakurai M.; Higashida S.; Shoji M.; Matsushita Y.; Iijima Y.; Ohizumi K.; Koike H. Syntheses and Biological Activities of Renin Inhibitors Containing Statine Analogues. Chem. Pharm. Bull. 1990, 38, 103–109. 10.1248/cpb.38.103. [DOI] [PubMed] [Google Scholar]
- Hsiao C.-N.; Leanna M. R.; Bhagavatula L.; Lara E. D.; Zydowsky T. M.; Horrom B. W.; Morton H. E. Synthesis of N-(Tert-butoxycarbonyl)-3-(4-thiazoIyl)-L-alanine. Synth. Commun. 1990, 20, 3507–3517. 10.1080/00397919008051594. [DOI] [Google Scholar]
- Leanna M. R.; Morton H. E. N-(Boc)-L-(2-Bromoallyl)-glycine: A versatile intermediate for the synthesis of optically active unnatural amino acids. Tetrahedron Lett. 1993, 34, 4485–4488. 10.1016/0040-4039(93)88065-q. [DOI] [Google Scholar]
- a Medici A.; Pedrini P.; Dondoni A. Reactions of trimethylsilylthiazoles with ketens: a new route to regioselective functionalisation of the thiazole ring. J. Chem. Soc. Chem. Commun. 1981, 13, 655–656. 10.1039/c39810000655. [DOI] [Google Scholar]; b Dondoni A.; Mastellari A. R.; Medici A.; Negrini E.; Pedrini P. Synthesis of Stannylthiazoles and Mixed Stannylsilylthiazoles and their Use for a Convenient Preparation of Mono- and Bis-halothiazoles. Synthesis 1986, 1986, 757–760. 10.1055/s-1986-31766. [DOI] [Google Scholar]; c Dondoni A.; Fantin G.; Fogagnolo M.; Medici A.; Pedrini P. A New Convenient Preparation of 2-, 4-, and 5- Thiazolecarboxaldehydes and Their Conversion into the Corresponding CarbonitrileN-Oxides: Synthesis of 3-Thiazolylisoxazoles and 3-Thiazolylisoxazolines. Synthesis 1987, 1987, 998–1001. 10.1055/s-1987-28146. [DOI] [Google Scholar]
- Kerdesky F. A. J.; Seif L. S. A Novel and Efficient Synthesis of 5-(Hydroxymethyl) Thiazole: An Important Synthon for Preparing Biologically Active Compounds. Synth. Commun. 1995, 25, 2639–2645. 10.1080/00397919508011810. [DOI] [Google Scholar]
- Berlin A. Y.; Bronovitskaya V. P. p-Di-(2-Chloroethyl)-Aminophenylalanine (Sarcolysin) and Its Derivatives VI. Amides of N-Acetylsarcolysin and Certain Amines of the Thiazol Series. Zhur. Obshcei Kim. 1961, 31, 1255–1259. [Google Scholar]
- Scheeren J. W.; Ooms P. H. J.; Nivard R. J. F. A General Procedure for the Conversion of a Carbonyl Group into a Thione Group with Tetraphosphorus Decasulfide. Synthesis 1973, 1973, 149–151. 10.1055/s-1973-22150. [DOI] [Google Scholar]
- Inouye K.; Namba K.; Otsuka H. A Synthesis of Thyrotropin-Releasing Factor. Bull. Chem. Soc. Jpn. 1971, 44, 1689–1691. 10.1246/bcsj.44.1689. [DOI] [Google Scholar]
- Ashworth D. M.; Atrash B.; Baker G. R.; Baxter A. J.; Jenkins P. D.; Jones D. M.; Szelke M. 2-cyanopyrrolidides as potent, stable inhibitors of dipeptidyl peptidase IV. Bioorg. Med. Chem. Lett. 1996, 6, 1163–1166. 10.1016/0960-894x(96)00190-4. [DOI] [Google Scholar]
- Rosen T.; Chu D. T. W.; Lico I. M.; Fernandes P. B.; Marsh K.; Shen L.; Cepa V. G.; Pernet A. G. Design, Synthesis, and Properties of (4S)-7-(4-Amino-2-Substituted-Pyrrolidin-1-yl)quinolone-3-Carboxylic Acids. J. Med. Chem. 1988, 31, 1598–1611. 10.1021/jm00403a020. [DOI] [PubMed] [Google Scholar]
- Flouret G. Synthesis of Pyroglutamylhistidylprolineamide by Classical and Solid Phase Methods. J. Med. Chem. 1970, 13, 843–845. 10.1021/jm00299a011. [DOI] [PubMed] [Google Scholar]
- Flouret G.; Morgan R.; Gendrich R.; Wilber J.; Seibel M. Synthesis of the Thyrotropin-Releasing Hormone Enantiomer and Some Diastereoisomers and in vitro Studies of their Biological Activity. J. Med. Chem. 1973, 16, 1137–1140. 10.1021/jm00268a015. [DOI] [PubMed] [Google Scholar]
- Holley R. W.; Sondheimer E. The Synthesis of L-Histidyl Peptides1. J. Am. Chem. Soc. 1954, 76, 1326–1328. 10.1021/ja01634a040. [DOI] [Google Scholar]
- Wildman S. A.; Crippen G. M. Prediction of Physicochemical Parameters by Atomic Contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. 10.1021/ci990307l. [DOI] [Google Scholar]
- Burgus R.; Dunn T. F.; Desiderio D. M.; Ward D. N.; Vale W.; Guillemin R.; Felix A. M.; Gillessen D.; Studer R. O. Biological Activity of Synthetic Polypeptide Derivatives Related to the Structure of Hypothalamic TRF. Endocrinology 1970, 86, 573–582. 10.1210/endo-86-3-573. [DOI] [PubMed] [Google Scholar]
- Vale W.; Rivier J.; Burgus R. Synthetic TRF (Thyrotropin Releasing Factor) Analogues1: II. pGlu-N3imMe-His-Pro-NH2: A Synthetic Analogue with Specific Activity Greater than that of TRF2. Endocrinology 1971, 89, 1485–1488. 10.1210/endo-89-6-1485. [DOI] [PubMed] [Google Scholar]
- Rivier J.; Vale W.; Monahan M.; Ling N.; Burgus R. Synthetic Thyrotropin-Releasing Factor Analogs. 3. Effect of Replacement or Modification of Histidine Residue on Biological Activity. J. Med. Chem. 1972, 15, 479–482. 10.1021/jm00275a010. [DOI] [PubMed] [Google Scholar]
- Perlman J. H.; Laakkonen L.; Guarnieri F.; Osman R.; Gershengorn M. C. A Model of the Thyrotropin-Releasing Hormone (TRH) Receptor Binding Pocket. Evidence for a Second Direct Interaction between Transmembrane Helix 3 and TRH. J. Biol. Chem. 1994, 269, 23383–23386. 10.1021/bi952202r. [DOI] [PubMed] [Google Scholar]
- Perlman J. H.; Thaw C. N.; Laakkonen L.; Bowers C. Y.; Osman R.; Gershengorn M. C. Hydrogen Bonding Interaction of Thyrotropin-Releasing Hormone (TRH) with Transmembrane Tyrosine 106 of the TRH Receptor. J. Biol. Chem. 1994, 269, 1610–1613. [PubMed] [Google Scholar]
- Yamaoka K.; Nakagawa T.; Uno T. Statistical Moments in Pharmacokinetics. J. Pharmacokinet. Biopharm. 1978, 6, 547–558. 10.1007/bf01062109. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T.; Katsuura G.. A Pharmaceutical Composition for Treating Ataxia, Multiple System Atrophy or Balance Disorders. WO/2006/028277, 2006.
- Ijiro T.; Nakamura K.; Ogata M.; Inada H.; Kiguchi S.; Maruyama K.; Nabekura J.; Kobayashi M.; Ishibashi H. Effect of rovatirelin, a novel thyrotropin-releasing hormone analog, on the central noradrenergic system. Eur. J. Pharmacol. 2015, 761, 413–422. 10.1016/j.ejphar.2015.05.047. [DOI] [PubMed] [Google Scholar]
- Kamiya K.; Takamoto M.; Wada Y.; Fujino M.; Nishikawa M. Molecular Conformation of Thyrotropin-Releasing Hormone from the X-Ray Structural Analysis of its Tartrate. J. Chem. Soc. Chem. Commun. 1980, 438–439. 10.1039/c39800000438. [DOI] [Google Scholar]
- Eckle E.; Stezowski J. J. Conformational Properties of Central Nervous System Active Thyrotropin Releasing Hormone Analogs: Probing Structure-Activity Relationships at the Molecular Level. J. Med. Chem. 1985, 28, 125–137. 10.1021/jm00379a022. [DOI] [PubMed] [Google Scholar]
- Olson G. L.; Cheung H.-C.; Chiang E.; Madison V. S.; Sepinwall J.; Vincent G. P.; Winokur A.; Gary K. A. Peptide Mimetics of Thyrotropin-Releasing Hormone Based on a Cyclohexane Framework: Design, Synthesis, and Cognition-Enhancing Properties. J. Med. Chem. 1995, 38, 2866–2879. 10.1021/jm00015a009. [DOI] [PubMed] [Google Scholar]
- Kodama H.; Fukushima T.; Chishima S.; Sugihara J.; Yoshikawa M. Disposition of Taltirelin. (3): The Transfer into the Brain and Brain Distribution in Rats. Drug Metab. Pharmacokinet. 1997, 12, 483–490. 10.2133/dmpk.12.483. [DOI] [Google Scholar]
- Urayama A.; Yamada S.; Hirano K.; Deguchi Y.; Kimura R. Brain Receptor Binding Characteristics and Pharmacokinetic-Pharmacodynamic Analysis of Thyrotropin-Releasing Hormone Analogues. Life Sci. 2001, 70, 647–657. 10.1016/s0024-3205(01)01445-x. [DOI] [PubMed] [Google Scholar]
- Ohkawa T.; Ishida Y.; Kanaoka E.; Takahashi K.; Okabe H.; Matsumoto T.; Nakamoto S.; Tamada J.; Koike M.; Yoshikawa T. A New Generic Column Switching System for Quantitation in Cassette Dosing Using LC/MS/MS. J. Pharm. Biomed. Anal. 2003, 31, 1089–1099. 10.1016/s0731-7085(02)00649-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.