Interferon- and cell cycle–associated gene transcript abundance levels in the peripheral blood of dengue vaccine recipients on days 8 and 9 postvaccination were associated with dengue neutralizing antibody titers on day 42, and mirrored responses in primary dengue infection, suggesting the possibility of predicting protective immunity.
Keywords: dengue, vaccine, gene expression, correlates of protection, neutralizing antibody
Abstract
Background
Several promising live attenuated dengue vaccines are in development, but information about innate immune responses and early correlates of protection is lacking.
Methods
We characterized human genome-wide transcripts in whole blood from 10 volunteers at 11 time points after immunization with the dengue virus type 3 (DENV-3) component of the National Institutes of Health dengue vaccine candidate TV003 and from 30 hospitalized children with acute primary DENV-3 infection. We compared day-specific gene expression patterns with subsequent neutralizing antibody (NAb) titers.
Results
The transcriptional response to vaccination was largely confined to days 5–20 and was dominated by an interferon-associated signature and a cell cycle signature that peaked on days 8 and 14, respectively. Changes in transcript abundance were much greater in magnitude and scope in symptomatic natural infection than following vaccination (maximum fold-change >200 vs 21 postvaccination; 3210 vs 286 transcripts with significant fold-change), but shared gene modules were induced in the same sequence. The abundances of 131 transcripts on days 8 and 9 postvaccination were strongly correlated with NAb titers measured 6 weeks postvaccination.
Conclusions
Live attenuated dengue vaccination elicits early transcriptional responses that mirror those found in symptomatic natural infection and provide candidate early markers of protection against DENV infection.
Clinical Trials Registration
Each year, the 4 dengue virus serotypes (DENV-1–4) infect an estimated 390 million individuals globally [1]. Although most of these infections are asymptomatic, approximately 100 million individuals develop clinically apparent disease, from uncomplicated fever to life-threatening illness. Despite the high disease burden, there are no licensed therapeutics for DENV infection. Several promising candidate dengue vaccines are in phase 3 clinical trials, and the live attenuated chimeric dengue vaccine Dengvaxia was recently licensed for use in children 9 years of age and older in DENV-endemic areas. However, the efficacy and duration of protection were limited or uncertain, and DENV-naive vaccine recipients were hospitalized for dengue and severe dengue at a higher rate than placebo recipients, possibly due to antibody-dependent enhancement [2].
Studies of natural DENV infection and live attenuated flavivirus vaccines have identified immune responses needed for protection against dengue disease. Preexisting neutralizing antibody (NAb) titers correlate with a lack of symptomatic disease in subsequent infections [3–6] and are used as the primary measure of candidate vaccine immunogenicity. However, the risk of severe disease is elevated after a second infection with a heterotypic dengue virus [7]. The recognition of effective homotypic immunity after natural infection has led to a common vaccine development strategy of inducing homotypic NAbs to all 4 serotypes simultaneously.
Little is known about the role of early innate immune responses in enhancing NAb production and promoting protective immune memory against dengue. Studies of innate immunity have been hampered by the difficulty in identifying individuals with early infection, when innate immune responses are most active, particularly those with mild or subclinical infections. Trials of live attenuated vaccines provide a unique opportunity to examine early immune responses in a setting where the time, dose, and viral serotype are known. Genome-wide transcript responses to vaccines have provided important clues about early steps in the generation of humoral and cellular immunity [8–13]. Transcript profiling of peripheral blood also incorporates information from cell populations that are difficult to examine in clinical settings, and has led to signatures associated with dengue disease severity, identified links between innate responses and humoral immunity in secondary DENV infection, and illustrated the dynamic nature of these responses [14–20].
In this study, we characterized the transcript response to rDEN3Δ30/31, the DENV-3 component of TV003, a tetravalent live attenuated vaccine candidate developed by the National Institutes of Health (NIH). TV003 is a single-dose vaccine that has proven to be both safe and immunogenic and is being evaluated in a phase 3 efficacy trial [21, 22]. We examined temporal changes in transcript abundance and identified early signatures correlated with NAb titers measured 6 weeks postvaccination. We also compared these results with transcript patterns we observed in patients with symptomatic wild-type primary DENV-3 infection. Despite the anticipated differences in the magnitude of expression, we observed the induction of common gene expression programs in the same temporal sequence, with a similar relationship to the induction of NAb. These results reveal candidate biomarkers of early protective DENV immune responses against dengue and suggest a path toward validation and deployment.
METHODS
Vaccine Study Population
Samples for this study were collected from a phase 1 clinical trial of the live attenuated dengue vaccine rDEN3Δ30/31–7164 (DENV-3), described previously [23]. In brief, healthy, flavivirus-naive adult volunteers were randomized to receive a single 0.5-mL subcutaneous dose of 1000 plaque-forming units (PFU) of DENV-3 vaccine or a placebo (0.5 mL of vaccine diluent). Blood samples, including whole blood for RNA profiling, were collected immediately prior to vaccination and on days 2, 5, 6, 8, 9, 12, 14, 20, 29, 42, and 180. Samples from each of these time points were available from 9 of 10 vaccinees and from all placebo recipients. Subject 9 had samples available for all days except days 8 and 12; 166 samples in total were used for analysis. Serum virus titers (viremia) were measured using a standard plaque assay as described previously [24]. Serum NAb was determined by 60% plaque reduction neutralization titer (PRNT60) [25]. Seroconversion was defined by a ≥4-fold increase in PRNT60 on study day 28 or 42 relative to day 0 and corresponds to a postvaccination titer >10 [23].
Dengue Patient Population
Patients (6 months to 14 years old) presenting with fever and suspected dengue during the 2010 dengue season were enrolled at the Hospital Infantil Manuel de Jesús Rivera in Managua, Nicaragua. Inclusion criteria, recruitment, and laboratory testing have been described previously [26] (Supplementary Data). Blood samples from healthy subjects were collected as part of a separate prospective cohort study in which healthy children in the same general population were enrolled without regard to dengue status [27].
Ethics Statement
The trial of rDEN3Δ30/31 was approved by the Committee for Human Research at the University of Vermont, and written informed consent was obtained from all subjects following a review of risks and benefits and a comprehension assessment. The study in Nicaragua was approved by the institutional review boards of the University of California, Berkeley, and the Nicaraguan Ministry of Health, and by the Stanford University Administrative Panel on Human Subjects in Medical Research. For further details, see the Supplementary Data.
RNA Sample Processing and Transcriptome Analysis
PAXgene RNA was amplified and hybridized to human exonic evidence-based oligonucleotide microarrays [14]. Microarray data were submitted to the Princeton University MicroArray database for normalization and gene filtering and are deposited at Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/; accession numbers GSE96656 and GSE98053). A full description of both sample processing and analysis steps is available in the Supplementary Data.
RESULTS
Temporal Patterns of the Transcriptional Responses to Live Dengue Vaccination
To identify the temporal pattern of the early human transcriptional response to dengue vaccination, we examined changes in genome-wide transcript abundance in serial whole blood samples from 10 volunteers infected with 1000 PFU of rDEN3∆30/31, the dose included in TV003, and 4 volunteers inoculated with placebo (L-15 medium). Nine of 10 vaccinees seroconverted 28 days postvaccination, defined as PRNT60 >10 (Table 1). Four of the vaccinees had low-level viremia on 1 or more days within the first 10 days postvaccination, 5 developed a mild maculopapular rash, and none were febrile. The 4 placebo recipients remained seronegative for DENV serotypes.
Table 1.
Characteristics of Subjects in Vaccine Trial
| Subject | Age, y | Sex | Viremiaa | Rashb | Day 28 PRNT60 | Day 42 PRNT60 | Day 180 PRNT60 |
|---|---|---|---|---|---|---|---|
| 1 (Vaccine) | 19 | F | … | Days 12–20 | 54 | 70 | 30 |
| 2 (Vaccine) | 26 | F | … | … | 22 | 15 | <5 |
| 3 (Vaccine) | 25 | M | Days 8–9 | … | 52 | 106 | 22 |
| 4 (Vaccine) | 20 | M | Days 8–9 | Days 12–16 | 26 | 32 | <5 |
| 5 (Vaccine) | 20 | M | Day 6 | Days 12–20 | 33 | 19 | <5 |
| 6 (Vaccine) | 22 | M | … | … | <5 | <5 | <5 |
| 7 (Vaccine) | 19 | M | … | … | 18 | 8 | 8 |
| 8 (Vaccine) | 22 | F | Days 5–8 | Days 12–20 | 34 | 29 | 8 |
| 9 (Vaccine) | 19 | F | … | … | 25 | 33 | <5 |
| 10 (Vaccine) | 46 | F | … | Days 12–16 | 70 | 152 | 64 |
| 11 (Placebo) | 18 | F | … | … | <5 | <5 | <5 |
| 12 (Placebo) | 19 | M | … | … | <5 | <5 | <5 |
| 13 (Placebo) | 45 | M | … | … | <5 | <5 | <5 |
| 14 (Placebo) | 21 | F | … | … | <5 | <5 | <5 |
Abbreviations: F, female; M, male; PRNT60, reciprocal serum dilution providing 60% reduction in plaque formation.
aVirus detected in serum from tissue culture plaque formation assay.
bFirst and last day on which maculopapular rash was observed..
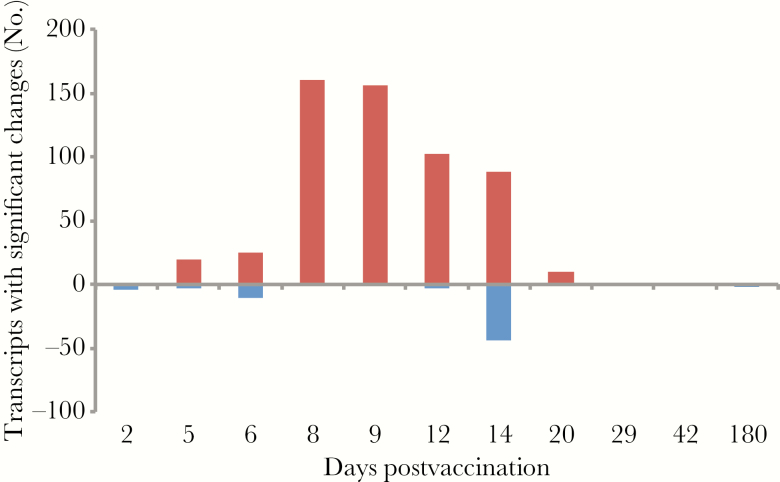
We collected whole blood for isolation of RNA immediately before vaccination (day 0), and on days 2, 5, 6, 8, 9, 12, 14, 20, 29, 42, and 180 postvaccination from all volunteers and measured genome-wide transcript abundance levels. Data were available for the full time course from 8 of the 9 participants who seroconverted. For each of these 8 subjects, we compared transcript abundances for each postvaccination day with those for the matched prevaccination sample (Supplementary Data). Almost all significant changes in transcript abundance occurred 5–20 days after vaccination, with a peak of 161 and 156 transcripts changing in abundance (days 8 and 9, respectively), and 286 transcripts with a significant change in abundance on at least 1 day (Figure 1). Fewer transcripts met criteria for significance when comparing vaccinees to placebo recipients (n = 131), but the direction of change for 271 of the 286 transcripts from vaccinees was the same whether the comparison was with day-matched placebo recipients or each subject’s baseline sample (Supplementary Figure 1).
Figure 1.
Significant differences in transcript abundance postvaccination (false discovery rate <1%; minimum 2-fold change compared to prevaccination sample).
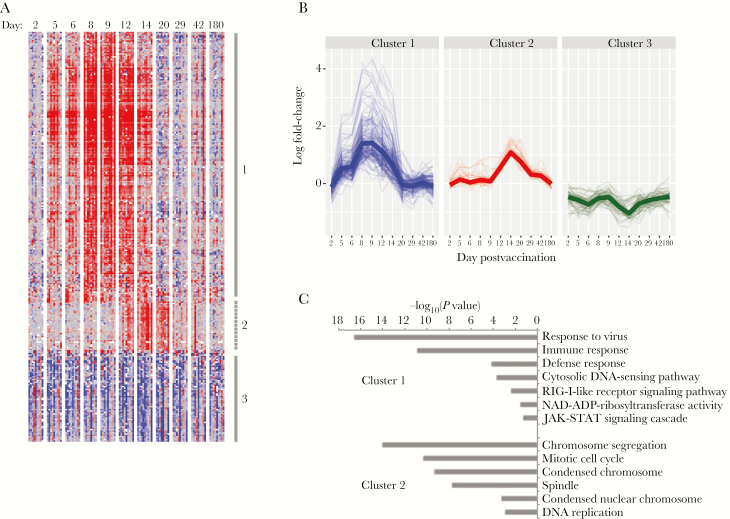
To infer the functional implications of these changes in transcript abundance, we used hierarchical clustering to organize the transcripts and compared gene membership in Gene Ontology and the KEGG pathways using the DAVID bioinformatics resource [28]. Gene transcripts were grouped in 3 clusters (Figure 2 and Supplementary Figure 2). Transcripts in cluster 1 were more abundant after vaccination (Figure 2C), peaked on days 8 and 9 postvaccination, and included canonical interferon-stimulated gene (ISG) transcripts; the IFI44, IFI44L, IFI27, HERC5, IFIT1, USP18, and ISG15 transcripts all increased 10- to 22-fold compared to baseline. Cluster 1 was strongly enriched for genes involved in the innate immune response to viruses and highly enriched for genes that we previously found to be expressed after treatment of peripheral blood mononuclear cells (PBMCs) with type I interferon (IFN) (P < 10-36) [29].
Figure 2.
Changes in transcript abundances over time in vaccinees. A, Hierarchical clustering of the 286 transcripts whose abundance was significantly different from baseline on >1 day. Lines and numbers to the right of the heat map mark sets of coexpressed genes (average cluster, r > 0.5). B, Change over time in abundance for each transcript in each gene cluster. Heavy line indicates median expression of all genes in each cluster. C, Gene ontologies associated with gene clusters described in (A) and (B). There were no significant gene ontologies for cluster 3.
Gene transcripts in clusters 2 and 3 showed maximal changes on day 14, with cluster 2 transcripts increasing and cluster 3 transcripts decreasing in abundance from baseline (Figure 2A and 2B). Cluster 2 included TYMS, CEP55, CCNA2, and NEK2, whose genes products are involved in DNA replication and cell division, and other genes associated with mitosis (P < 2x10-9; Figure 2C). Genes in cluster 3 were enriched in both reticulocytes (P = 10-20) and neutrophils (P = 2x10-7) [30]. We did not measure reticulocyte counts, but we did measure neutrophils and the relative neutrophil abundance in vaccinees did not change significantly with time (P = .55, paired t test), suggesting that decreased expression of these genes was not due to decreased neutrophil abundance.
Changes Observed After Vaccination Are a Subset of Those Observed in Natural Symptomatic DENV-3 Infection
To establish which features of the early response to vaccination are shared with the response to natural symptomatic infection, we examined transcript responses in Nicaraguan children hospitalized with acute dengue. We previously demonstrated that a history of previous DENV exposure is the most prominent source of variation in gene expression in dengue patients [14]. To ensure that DENV immune status, as well as serotype, did not confound our analysis, we identified 30 children diagnosed with acute primary DENV-3 infection during a single year (24 with dengue fever and 6 with dengue hemorrhagic fever; Supplementary Table 1), and compared transcript abundance in whole blood with measurements from 9 healthy individuals. Principal components analysis confirmed previous findings that there are significant day-to-day changes in the transcript response to natural infection [14, 31] (Supplementary Figure 4); thus, we subsequently performed analyses stratified by day of fever. There were no significant differences in transcript abundance between children with dengue fever and children with dengue hemorrhagic fever after matching for sex and day of fever. This result is consistent with previous studies in the same population [14, 18], although the small sample size could affect the results of our analysis.
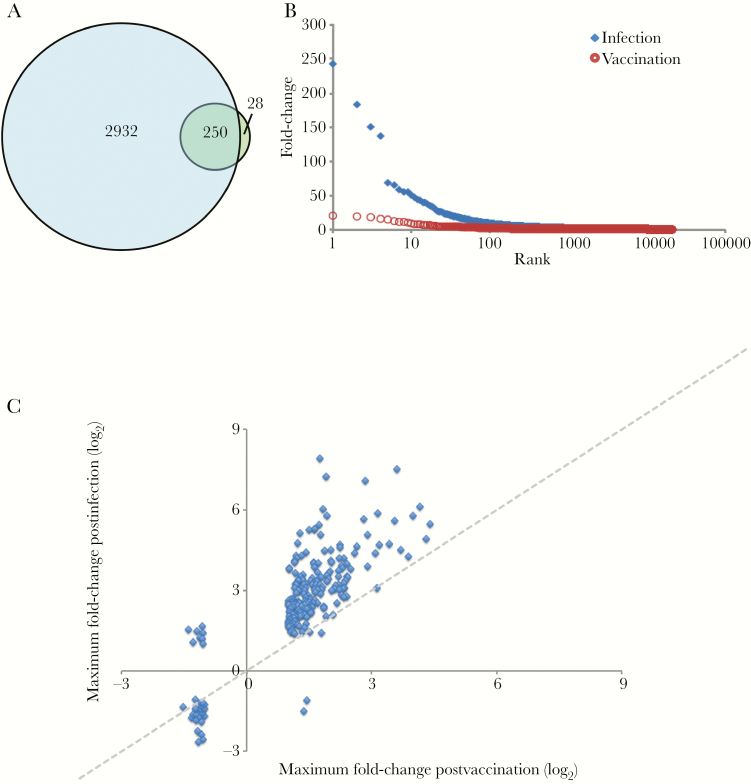
Despite having fewer days available for comparison and lacking baseline samples for each patient, we identified many more transcripts with significant changes in abundance postinfection compared to those found in vaccinees: Among the 20623 transcripts measured in both datasets, we identified 3210 transcripts that differed significantly on at least 1 day of fever, compared with 278 transcripts following vaccination (Figure 3A and Supplementary Figure 5A). The magnitude of the maximum change in abundance postinfection was also nearly 10-fold greater: there was a 200-fold difference postinfection compared to a maximum 21-fold difference postvaccination (Figure 3B). The transcripts with the greatest differences in relative abundance during natural infection were MT2A (242-fold) and USP18 (183-fold), both of which are IFN-induced; HESX1 (150-fold), which is expressed in activated dendritic cells; and SPAT2SL (137-fold), which may be involved in activation and differentiation of multiple cell types.
Figure 3.
Comparison of postvaccination and postinfection transcript abundance changes. A, Transcripts with significant changes on day 2, 3, 4, or 5 of fever in patients with primary dengue virus type 3 infection (blue circle) and on any day postvaccination (green circle). Numbers indicate transcripts unique to vaccination, infection, or shared (overlap, n = 246). B, Maximum fold-change in transcript abundance following vaccination (red circles) or during infection (blue diamonds). C, Maximum fold-change in abundance for transcripts with significant changes postvaccination or during infection. Dotted diagonal line at equal fold-change included for reference.
Despite differences in response magnitude and number, the response following natural symptomatic infection included 90% (250/278) of transcripts that changed after vaccination, and the direction of change was the same for 96% of these transcripts (240/250) (Figure 3C). The transcripts that changed the most postvaccination (IFI44, IFI44L, IFI27, and HERC5) were among the 20 transcripts with the biggest differences in abundance following natural infection, and relative increases in transcript abundance were strongly correlated across the 2 groups (Spearman r2 = 0.75).
Responses to Dengue Vaccination and Symptomatic Natural Infection Share a Common Temporal Sequence
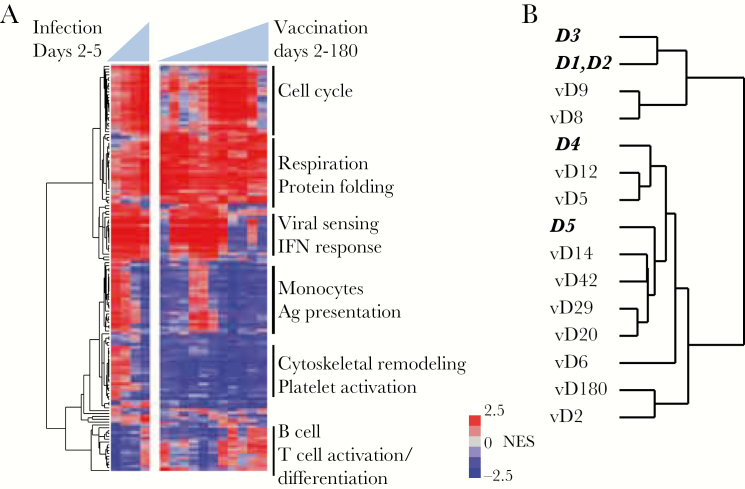
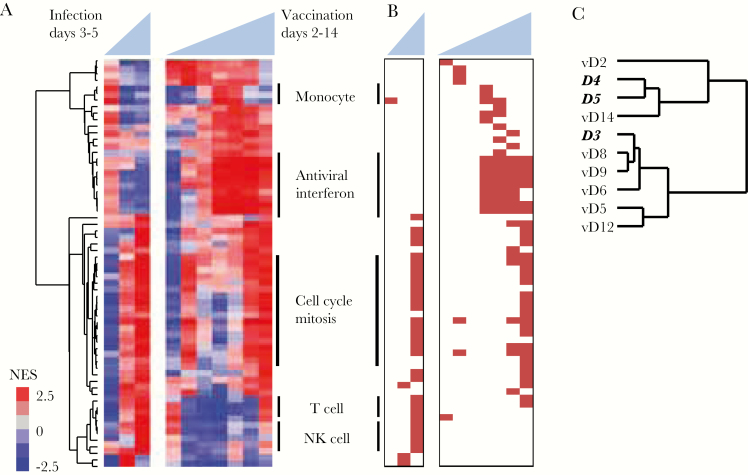
We used gene set enrichment analysis and information from all measured transcripts to identify 141 blood transcript gene modules that changed in abundance following either immunization or infection [8] (false discovery rate <1%). Many of these modules demonstrated similar changes in both vaccinees and patients (Figure 4A). Modules enriched for ISG expression were elevated on days 5–14 postvaccination and were also persistently elevated after natural DENV infection. Modules representing monocyte-associated transcripts were elevated on days 1–3 of natural infection and on days 8–9 postvaccination, whereas modules associated with the mitotic cell cycle were elevated on later days in both groups, with the highest levels on day 5 of natural infection and on day 14 postvaccination. When we compared the overall profiles of the gene modules in the 2 groups, we found that the responses to natural infection on fever days 1–3 were most similar to responses to vaccination on days 8–9 (Pearson r ≥ 0.60; peak on day 9), while fever day 4 was most similar to vaccination day 12 (r > 0.75, peak on day 12), and fever day 5 was most similar to vaccination day 14 and subsequent time points (r ≥ 0.70, peak on day 14) (Figure 4B and Supplementary Data 1). Thus, the enrichment of common modules in the same sequence indicates a similar progression in the early host response to vaccination and to natural infection.
Figure 4.
Gene modules affected by dengue virus vaccination and natural infection. A, Blood transcript modules with transcripts that were significantly up- or down-regulated on at least 1 day (false discovery rate <1%) were hierarchically clustered. Vertical lines on right denote module clusters described in the text. B, Hierarchical clustering of each day postvaccination or postinfection using the normalized enrichment score from (A). Days in bold italics represent days of fever for infected patients; days preceded by “v” represent days postvaccination. Abbreviations: Ag, antigen; IFN, interferon; NES, normalized enrichment score.
We note there was also a cluster of 16 gene modules, 6 associated with platelet activation and cytoskeletal remodeling, that were elevated in natural infection but not vaccinees (Figure 4A and Supplementary Data 1). Previous studies have demonstrated that platelet activation and transforming growth factor beta (TGF-β) expression are elevated in DENV infection and higher in patients with more severe disease [32]. TGF-β, which is expressed at high levels in platelets [33], was elevated on fever days 1–2 in dengue patients but was never elevated postvaccination (Supplementary Figure 6).
Early Transcriptional Responses Linked to NAb Production
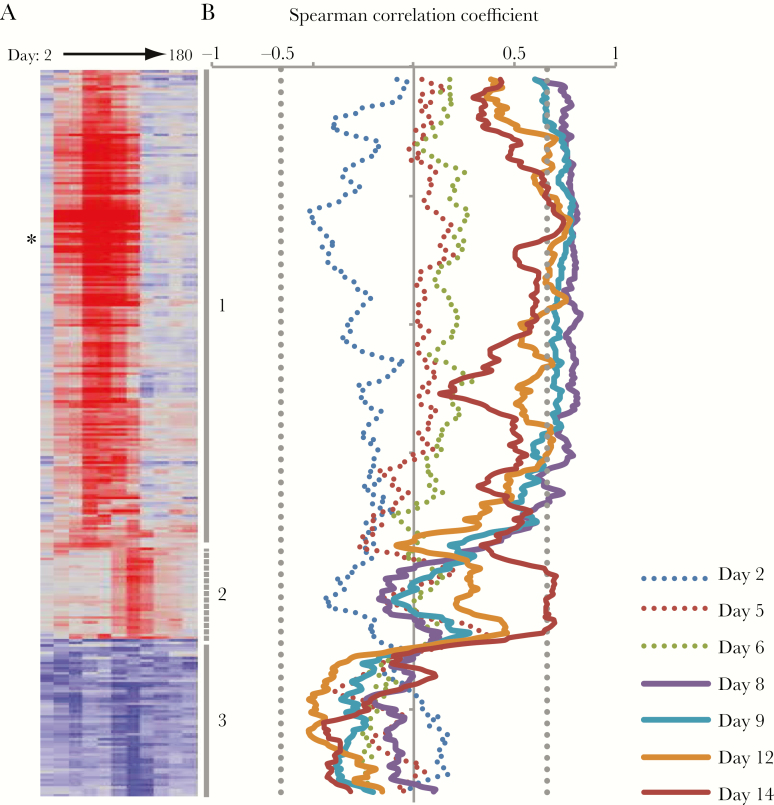
DENV-specific NAbs are the primary endpoint for assessing vaccine responses in clinical trials and are associated with protection from both symptomatic infection and severe disease [3–5]. To determine whether changes in host transcript patterns predicted differences in NAb titer, we calculated the correlation between the change in abundance of each transcript on each day and the NAb titer on postvaccination day 42, when NAbs are generally at peak titer (Table 1 and Supplementary Figure 7A). During the first 6 days postvaccination, we found no significant correlations with NAb titer, but by day 8, expression of the ISGs in cluster 1 positively correlated with the day 42 NAb titer (P < .01; Figure 5). This correlation was equally strong on day 9, and 131 transcripts were significantly correlated with day 42 NAb titer on both days. Among the individual ISG transcripts most strongly correlated with day 42 NAb titer on both days 8 and 9 was IFI44, the transcript whose abundance changed the most postvaccination (Supplementary Figure 7B). IFI44 was also elevated at one time point in each of 2 placebo recipients, but the timing of elevated expression was different and correlated with unrelated respiratory viral infections in each instance (Supplementary Figure 8). Twelve of the 131 transcripts were also associated with subsequent development of a rash, which was the only significant correlate with positive NAb titer in a clinical trial of TV003 [21] (Supplementary Figure 9). Interestingly, the one vaccinee who failed to develop neutralizing antibodies showed little evidence of increased abundance in cluster 1 genes (Supplementary Figure 3). The association of IFN-related transcript abundance and later NAb titer diminished on days 12 and 14, but BUB1 (r = 0.86) and other transcripts associated with the mitotic cell cycle were correlated with subsequent NAb titers on day 14 (Figure 5 and Supplementary Figure 7C).
Figure 5.
Correlation of transcript abundance and the 60% plaque reduction neutralization titer (PRNT60) at day 42 among vaccine recipients. A, Average fold-change in abundance by day for all transcripts with significant differences from baseline postvaccination. Transcripts are ordered and clusters labeled as in Figure 2. Asterisk marks IFI44. B, Spearman correlation of each transcript and day 42 PRNT60 using a moving average of window size 9. Solid lines indicate days postvaccination on which a significant correlation was identified (P < .01, indicated by vertical dotted gray line).
When we performed similar comparisons for naturally infected patients, we found no transcript clusters significantly correlated with either convalescent or 3-month NAb titer (Supplementary Figure 5B and 5C). However, the pattern of blood transcript module enrichment indicated a similar relationship between day-specific gene expression and later production of NAb; gene enrichment for both IFN-stimulated and cell cycle–associated gene modules was associated with higher NAb titer in both vaccinees and patients (Figure 6), albeit more weakly in patients, and cell cycle–associated modules were correlated with NAb titer later in both groups.
Figure 6.
Gene modules correlated with subsequent neutralizing antibody response. A, Blood transcript modules that were significantly enriched for transcripts positively correlated with 60% plaque reduction neutralization titer at day 42 (vaccinees) or 50% neutralization titer at convalescence (patients) on at least 1 day (false discovery rate <1%) were hierarchically clustered. Vertical lines delineate module clusters described in the text. B, Significant modules (false discovery rate <1%) are marked in red. Modules and samples are organized as in (A). C, Hierarchical clustering of gene module expression from each day postvaccination or postinfection using the normalized enrichment score from (A). Day labels in bold italics represent fever day for infected patients; day labels preceded by “v” represent days postvaccination. Abbreviations: NES, normalized enrichment score; NK, natural killer.
There are at least 3 subpopulations of monocytes with distinct transcript profiles [34]; Kwissa et al identified an increase in CD14+CD16+ intermediate-phenotype population after secondary DENV infection, and showed that in vitro these cells stimulated formation of the plasmablasts that secrete antibodies weeks after infection, mediated in part by secretion of the ISG cytokine BAFF [19]. In our study, gene set enrichment analysis indicated enrichment of transcripts for both intermediate and nonclassical monocytes at multiple time points in both vaccinees and patients, while BAFF transcripts were most abundant on fever days 1 and 2 in the patients and days 8 and 9 in the vaccinees (Supplementary Figure 10).
DISCUSSION
In this study, we used intensive longitudinal sampling to characterize the transcriptional response to dengue vaccination, compared results with those from natural infection with the same DENV serotype, and identified early features that may predict a protective immune response. We found that vaccination and natural infection induced common gene expression programs, and the abundance of individual IFN-stimulated transcripts 8 days postvaccination was correlated with NAb titers measured 5 weeks later, representing the earliest identified correlates of a protective adaptive immune response following dengue vaccination.
An IFN response signature has been observed in other studies profiling viral vaccine transcriptional responses. Inactivated influenza and meningococcal vaccines both induce a mild IFN response during the first week postvaccination, but the response is particularly strong after vaccination with live attenuated vaccines [9, 12, 35]. We reported that ISG expression was much stronger in cynomolgus macaques infected with wild-type DENV compared with live attenuated virus [35]. Here, we found that ISG expression was much stronger in symptomatic dengue patients than vaccinees, presumably due to higher viral load after infection with wild-type virus. Expression of ISGs was correlated with viral load in the patients, as seen in other studies [19, 36]. However, this association did not persist when patients were stratified by day of fever, highlighting the importance of temporal variation in the innate immune response and in viral load, and suggesting that factors in addition to viral replication influence ISG expression. Several studies have found stable interindividual differences in the response to IFN, suggesting that genetic and environmental features may affect the relationship between viral infection and the IFN response [37, 38]. We were not able to assess directly the impact of these features on transcriptional responses in this study, but their contributions to the differences between children with symptomatic infection and vaccinated adults are likely to be much less than the contribution of differences in virulence of vaccine and wild-type viruses (Supplementary Data).
The links between type I IFN production and NAb production probably involve multiple cell types. Plasmacytoid dendritic cells (pDCs) contribute to B-cell differentiation and antibody production after viral infection [39]. In this study, increases in monocyte-associated gene expression coincided with ISG expression, and we found features related to multiple monocyte phenotypes in both natural infection and vaccination. Gene module analysis also suggested that T cells were responsible for the increase in cell cycle-associated transcripts 2 weeks after vaccination that was linked to NAb titers. Future targeted studies of pDCs, monocytes, and T-cell populations during the first 2 weeks postvaccination will help clarify their role in establishing long-lasting antibody responses. In addition, the link between an early IFN response and later NAb titer was only apparent in natural infection when we used a module analysis approach. This may indicate a plateau, or saturation effect, in the relationship between ISG expression and antibody titer. Alternatively, it may reflect the variability in pathogen dose, prior health status, and/or days of infection absent in clinical trials but inherent in observational studies.
Comparison with live attenuated virus vaccination also provides a framework for identification of features associated with pathogenic vs nonpathogenic infection. A recent study compared PBMC gene expression in asymptomatic and clinically significant secondary DENV infection and identified differences in antigen presentation and lymphocyte activation [36]. In this study examining whole blood gene expression during primary infection, we found an increased abundance of transcripts associated with platelet activation in natural (pathogenic) infection but not vaccination (nonpathogenic infection), consistent with the hypothesis that platelet activation contributes to dengue pathogenesis [40].
Neutralizing antibody titers were used as an endpoint for these vaccine studies because many studies have shown that these antibodies play an important role in protective immunity. However, recent work has demonstrated that NAbs measured in vitro are an imperfect correlate of in vivo protection [41, 42]. Immunity mediated by NAbs may be neither life-long nor sterilizing [43, 44] and will be affected by the quality as well as the quantity of NAbs [5, 26, 45]. Recent studies also highlight a likely role for cytotoxic T cells in mediating protection against DENV reinfection and severe disease [46–49]. The NIH tetravalent vaccine, of which rDEN3Δ30/31 is a component, elicits CD4+ T-cell responses similar to those seen in natural infection [50]. It will be important to establish whether the early transcript-based features we measured in this study are associated with DENV-specific responses in memory T-cell populations.
Our findings reflect the integration of data across multiple time points and thousands of transcripts, and provide a robust basis for further investigation. We previously found that early IFN-associated transcriptional responses were associated with antibody formation in nonhuman primates exposed to a tetravalent dengue vaccine [35]. We believe it is likely that the same relationship will exist in humans immunized with tetravalent live attenuated dengue vaccines. The initiation of phase 3 clinical trials of TetraVax-DV-TV003 provides an opportunity to validate the relationship between these early responses and NAb titers and to identify specific transcripts as early surrogate markers of both immunogenicity and protection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Cassandra Ventrone for sample collection and logistics, Chunling Wang for viral load data, Ellen Sebastian for data processing, and Elizabeth Costello for helpful suggestions. We thank past and present members of the study team based at the Hospital Infantil Manuel de Jesús Rivera, the Centro de Salud Sócrates Flores Vivas, and the National Virology Laboratory in the Centro Nacional de Diagnóstico y Referencia of the Nicaraguan Ministry of Health, as well as the Sustainable Sciences Institute in Nicaragua, for their dedication and high-quality work, and we are grateful to the study participants and their families.
Financial support. This work was supported by the Division of Intramural Research and Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases (grant numbers U19 AI109761 to D. A. R. and U54 AI065359 to A. B.); the Thomas C. and Joan M. Merigan Endowment at Stanford University (to D. A. R.); the Chan Zuckerberg Biohub (to D. A. R.); and the Pediatric Dengue Vaccine Initiative of the Bill & Melinda Gates Foundation (grant number VE-1 to E. H.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hadinegoro SR, Arredondo-García JL, Capeding MR, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373:1195–6. [DOI] [PubMed] [Google Scholar]
- 3. Endy TP, Nisalak A, Chunsuttitwat S, et al. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis 2004; 189:990–1000. [DOI] [PubMed] [Google Scholar]
- 4. Corbett KS, Katzelnick L, Tissera H, Amerasinghe A, de Silva AD, de Silva AM. Preexisting neutralizing antibody responses distinguish clinically inapparent and apparent dengue virus infections in a Sri Lankan pediatric cohort. J Infect Dis 2015; 211:590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katzelnick LC, Montoya M, Gresh L, Balmaseda A, Harris E. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc Natl Acad Sci U S A 2016; 113:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg 1952; 1:30–50. [DOI] [PubMed] [Google Scholar]
- 7. Sangkawibha N, Rojanasuphot S, Ahandrik S, et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol 1984; 120:653–69. [DOI] [PubMed] [Google Scholar]
- 8. Li S, Rouphael N, Duraisingham S, et al. Molecular signatures of antibody responses derived from a systems biological study of 5 human vaccines. Nat Immunol 2014; 15:195–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakaya HI, Wrammert J, Lee EK, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol 2011; 12:786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bucasas KL, Franco LM, Shaw CA, et al. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. J Infect Dis 2011; 203:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Obermoser G, Presnell S, Domico K, et al. Systems scale interactive exploration reveals quantitative and qualitative differences in response to influenza and pneumococcal vaccines. Immunity 2013; 38:831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Querec TD, Akondy RS, Lee EK, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol 2009; 10:116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kazmin D, Nakaya HI, Lee EK, et al. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc Natl Acad Sci U S A 2017; 114:2425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Popper SJ, Gordon A, Liu M, Balmaseda A, Harris E, Relman DA. Temporal dynamics of the transcriptional response to dengue virus infection in Nicaraguan children. PLoS Negl Trop Dis 2012; 6:e1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long HT, Hibberd ML, Hien TT, et al. Patterns of gene transcript abundance in the blood of children with severe or uncomplicated dengue highlight differences in disease evolution and host response to dengue virus infection. J Infect Dis 2009; 199:537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simmons CP, Popper S, Dolocek C, et al. Patterns of host genome-wide gene transcript abundance in the peripheral blood of patients with acute dengue hemorrhagic fever. J Infect Dis 2007; 195:1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nascimento EJ, Braga-Neto U, Calzavara-Silva CE, et al. Gene expression profiling during early acute febrile stage of dengue infection can predict the disease outcome. PLoS One 2009; 4:e7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loke P, Hammond SN, Leung JM, et al. Gene expression patterns of dengue virus-infected children from Nicaragua reveal a distinct signature of increased metabolism. PLoS Negl Trop Dis 2010; 4:e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kwissa M, Nakaya HI, Onlamoon N, et al. Dengue virus infection induces expansion of a CD14(+)CD16(+) monocyte population that stimulates plasmablast differentiation. Cell Host Microbe 2014; 16:115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van de Weg CAM, van den Ham H-J, Bijl MA, et al. Time since onset of disease and individual clinical markers associate with transcriptional changes in uncomplicated dengue. PLoS Negl Trop Dis 2015; 9:e0003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Durbin AP, Kirkpatrick BD, Pierce KK, et al. A single dose of any of four different live attenuated tetravalent dengue vaccines is safe and immunogenic in flavivirus-naive adults: a randomized, double-blind clinical trial. J Infect Dis 2013; 207:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirkpatrick BD, Whitehead SS, Pierce KK, et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci Transl Med 2016; 8:330ra36. [DOI] [PubMed] [Google Scholar]
- 23. Lindow JC, Durbin AP, Whitehead SS, Pierce KK, Carmolli MP, Kirkpatrick BD. Vaccination of volunteers with low-dose, live-attenuated, dengue viruses leads to serotype-specific immunologic and virologic profiles. Vaccine 2013; 31:3347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Durbin AP, McArthur J, Marron JA, et al. The live attenuated dengue serotype 1 vaccine rDEN1Delta30 is safe and highly immunogenic in healthy adult volunteers. Hum Vaccin 2006; 2:167–73. [DOI] [PubMed] [Google Scholar]
- 25. Durbin AP, Karron RA, Sun W, et al. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3ʹ-untranslated region. Am J Trop Med Hyg 2001; 65:405–13. [DOI] [PubMed] [Google Scholar]
- 26. Puschnik A, Lau L, Cromwell EA, Balmaseda A, Zompi S, Harris E. Correlation between dengue-specific neutralizing antibodies and serum avidity in primary and secondary dengue virus 3 natural infections in humans. PLoS Negl Trop Dis 2013; 7:e2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Balmaseda A, Standish K, Mercado JC, et al. Trends in patterns of dengue transmission over 4 years in a pediatric cohort study in Nicaragua. J Infect Dis 2010; 201:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4:44–57. [DOI] [PubMed] [Google Scholar]
- 29. Waddell SJ, Popper SJ, Rubins KH, et al. Dissecting interferon-induced transcriptional programs in human peripheral blood cells. PLoS One 2010; 5:e9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kupershmidt I, Su QJ, Grewal A, et al. Ontology-based meta-analysis of global collections of high-throughput public data. PLoS One 2010; 5:e13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun P, García J, Comach G, et al. Sequential waves of gene expression in patients with clinically defined dengue illnesses reveal subtle disease phases and predict disease severity. PLoS Negl Trop Dis 2013; 7:e2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pandey N, Jain A, Garg RK, Kumar R, Agrawal OP, Lakshmana Rao PV. Serum levels of IL-8, IFNγ, IL-10, and TGF β and their gene expression levels in severe and non-severe cases of dengue virus infection. Arch Virol 2015; 160:1463–75. [DOI] [PubMed] [Google Scholar]
- 33. Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem 1983; 258:7155–60. [PubMed] [Google Scholar]
- 34. Wong KL, Tai JJ, Wong WC, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011; 118:e16–31. [DOI] [PubMed] [Google Scholar]
- 35. Strouts FR, Popper SJ, Partidos CD, Stinchcomb DT, Osorio JE, Relman DA. Early transcriptional signatures of the immune response to a live attenuated tetravalent dengue vaccine candidate in non-human primates. PLoS Negl Trop Dis 2016; 10:e0004731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simon-Lorière E, Duong V, Tawfik A, et al. Increased adaptive immune responses and proper feedback regulation protect against clinical dengue. Sci Transl Med 2017; 9:eaal5088. [DOI] [PubMed] [Google Scholar]
- 37. Whitney AR, Diehn M, Popper SJ, et al. Individuality and variation in gene expression patterns in human blood. Proc Natl Acad Sci U S A 2003; 100:1896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rani MRS, Xu Y, Lee J, et al. Heterogeneous, longitudinally stable molecular signatures in response to interferon-β. Ann N Y Acad Sci 2009; 1182:58–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deal EM, Lahl K, Narváez CF, Butcher EC, Greenberg HB. Plasmacytoid dendritic cells promote rotavirus-induced human and murine B cell responses. J Clin Invest 2013; 123:2464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hottz ED, Medeiros-de-Moraes IM, Vieira-de-Abreu A, et al. Platelet activation and apoptosis modulate monocyte inflammatory responses in dengue. J Immunol 2014; 193:1864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sabchareon A, Wallace D, Sirivichayakul C, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 2012; 380:1559–67. [DOI] [PubMed] [Google Scholar]
- 42. Moodie Z, Juraska M, Huang Y, et al. Neutralizing antibody correlates analysis of tetravalent dengue vaccine efficacy trials in Asia and Latin America. J Infect Dis 2018; 217:742–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waggoner JJ, Balmaseda A, Gresh L, et al. Homotypic dengue virus reinfections in Nicaraguan children. J Infect Dis 2016; 214:986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forshey BM, Reiner RC, Olkowski S, et al. Incomplete protection against dengue virus type 2 re-infection in Peru. PLoS Negl Trop Dis 2016; 10:e0004398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Henein S, Swanstrom J, Byers AM, et al. Dissecting antibodies induced by a chimeric yellow fever-dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) in naive and dengue-exposed individuals. J Infect Dis 2017; 215:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weiskopf D, Bangs DJ, Sidney J, et al. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc Natl Acad Sci U S A 2015; 112:E4256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weiskopf D, Angelo MA, de Azeredo EL, et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A 2013; 110:E2046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hatch S, Endy TP, Thomas S, et al. Intracellular cytokine production by dengue virus-specific T cells correlates with subclinical secondary infection. J Infect Dis 2011; 203:1282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duangchinda T, Dejnirattisai W, Vasanawathana S, et al. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc Natl Acad Sci U S A 2010; 107:16922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Angelo MA, Grifoni A, O’Rourke PH, et al. Human CD4+ T cell responses to an attenuated tetravalent dengue vaccine parallel those induced by natural infection in magnitude, hla restriction, and antigen specificity. J Virol 2017; 91:e02147–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.