High HPV vaccine uptake in a single-cohort program aimed at largely HPV-naive girls reduced vaccine types by 90% in vaccinated and 54% in unvaccinated girls. Indication of cross-protection in both vaccinated and unvaccinated girls was also observed.
Keywords: human papillomavirus, HPV vaccine, immunization program, effectiveness, vaccine impact
Abstract
Background
In 2009, quadrivalent human papillomavirus (HPV) vaccine was introduced in a school-based single-cohort program targeting 12-year-old girls in Norway. We estimated the impact of the Norwegian HPV immunization program.
Methods
Three birth cohorts of 17-year-old girls, 2 nonvaccine-eligible cohorts (born 1994 or 1996) and 1 vaccine-eligible cohort (born 1997) were invited to deliver urine samples. The samples were analyzed for 37 HPV genotypes. HPV prevalence was compared between birth cohorts and between vaccinated and unvaccinated girls within and across birth cohorts after linkage to the Norwegian Immunisation Registry.
Results
In total, 17749 urine samples were analyzed. A 42% (95% confidence interval [CI], 37%–47%) reduction in any HPV type and 81% (95% CI, 76%–85%) reduction in vaccine types (HPV-6/11/16/18) were observed in the vaccine-eligible cohort compared to the 1994 cohort. Vaccine types were reduced by 54% (95% CI, 39%–66%) and 90% (95% CI, 86%–92%) in unvaccinated and vaccinated girls, respectively, from the 1997 cohort, compared with unvaccinated girls born in 1994. A significant reduction was also observed for several nonvaccine types. Vaccine-type prevalence was reduced by 77% (95% CI, 65%–85%) in vaccinated compared with unvaccinated girls from the 1997 cohort.
Conclusions
In this largely HPV-naive population, we observed a substantial reduction in vaccine and nonvaccine types in vaccinated and unvaccinated girls following introduction of HPV vaccination.
Human papillomavirus (HPV) infection is the most common sexually transmitted infection. Based on the oncogenic potential, HPV genotypes are classified as high-risk (HR), probably/possibly HR, or undetermined or low-risk (LR) types [1]. HPV infection is the underlying cause of cervical and other anogenital cancers, as well as cancers in the oropharynx [1, 2]. HPV-16 and HPV-18 are responsible for 70% of all cases of cervical cancer, whereas HPV-6 and HPV-11 cause 90% of genital warts [2].
In Norway, the HPV vaccine has been offered in a school-based immunization program with single-cohort delivery to 12-year-old girls since 2009. In contrast to most Western countries, no catch-up vaccination of older girls was initially offered.
Since the HPV vaccine was first introduced in national programs in 2007, several studies have described real-life effectiveness including reduction of precancerous cervical lesions among vaccinated women [3–7]. In addition to the direct effect against vaccine types, the population-level effectiveness will depend on cross-protection against nonvaccine types as well as the extent of herd protection achieved in a given setting. To our knowledge, previous effectiveness studies have only been performed in countries with catch-up vaccination. HPV vaccines are prophylactic and do not clear ongoing infections. In clinical trials in women aged 16–26 years, the efficacy of HPV vaccines is higher in HPV-naive populations than in total vaccinated cohorts, reflecting the lack of protection against ongoing HPV infections [8, 9]. Thus, protection is likely to be less effective in older catch-up populations, in comparison to young and mostly HPV-naive populations. Effectiveness data from school-based programs in countries with single-cohort delivery only have not been reported.
Three HPV vaccines including antigens from 2, 4, or 9 HPV types are currently licensed. The quadrivalent vaccine, including antigens against HPV-6/11/16/18, was used in Norway until 2017. The HPV vaccine uptake increased from 70% in the first birth cohort that was eligible for HPV vaccination (born in 1997) [10] to 89% among girls born in 2004 [11].
In Norway, organized cervical screening is not offered until the age of 25. Thus, screening data are not suitable to assess early vaccine impact. Consequently, a national HPV surveillance program comprising a series of nationwide, population-based cross-sectional studies was set up, assessing HPV prevalence in urine samples from girls and young women not yet targeted by the national screening program [12].
The aim of the current study was to assess the impact of the Norwegian school-based HPV immunization program, by comparing the HPV prevalence in urine samples from 17-year-old girls in 3 birth cohorts: the first vaccine-eligible cohort and 2 birth cohorts not eligible for routine HPV vaccination. We studied the direct effect in vaccinated individuals and the potential herd effect in nonvaccinated individuals. Furthermore, the cross-protective effect against nonvaccine types and possible type replacement were assessed. Finally, the total effectiveness of the program (direct plus indirect effects) was estimated.
METHODS
Inclusion and Sampling
Girls from 3 birth cohorts (1994, 1996, and 1997) were eligible for inclusion. Girls born in 1994 (baseline cohort) and 1996 were not eligible for HPV vaccination. Girls born in 1997 (vaccine cohort) were the first birth cohort eligible for HPV vaccination. The Norwegian Central Population Registry was used to obtain information on all girls in the 3 birth cohorts residing in Norway as of 1 February the year they turned 17 (2011, 2013, and 2014 for girls born in 1994, 1996, and 1997, respectively). Girls were invited by mail around their 17th birthday. Due to an administrative error, 5260 girls born in November/December 1994 were not invited. In total, 25811 girls born in 1994, 31749 girls born in 1996, and 31389 girls born in 1997 were invited.
Upon informed consent, girls received a urine sampling kit and were asked to return a first-void urine sample to the Norwegian Institute of Public Health. The sample device contained boric acid to prevent bacterial growth. All samples were registered, aliquoted, and stored at –80°C prior to analysis at the Norwegian HPV Reference Laboratory at Akershus University Hospital. Participants received 2 cinema tickets as compensation. All samples received by 26 November 2014 were analyzed for HPV by 19 August 2015 and have been included in the statistical analyses.
The study was approved by the Regional Committee for Medical and Health Research Ethics, South East Norway and the Norwegian Data Protection Authority.
HPV Vaccination
Individual records on HPV vaccination were retrieved from the Norwegian Immunisation Registry. Notification of all vaccinations provided through the national childhood immunization program is mandatory without the need for consent [13]. HPV vaccinations provided outside the immunization program are notifiable, but the vaccinees or their parents/guardians may oppose notification. Thus, vaccination outside the program may be subject to some underreporting. However, the effect on our results is most likely negligible as only a total of 1000–2000 girls/women per year were prescribed HPV vaccine outside the immunization program from 2007 to 2014 [14].
Vaccine doses administered <15 days prior to urine sampling were unlikely to have a clinical impact and were not taken into account.
When comparing invited and participating girls, vaccine uptake was calculated as of 1 January the year of urine sampling for the respective birth cohorts.
Girls who had not received any doses of HPV vaccine were defined as unvaccinated, whereas girls who had received all 3 doses were defined as vaccinated. Partially vaccinated girls were excluded from analysis.
HPV Testing
DNA extraction and HPV genotyping were performed at the Norwegian HPV Reference Laboratory as previously described [12]. In brief, the presence of HPV DNA was investigated using a modified GP5+/GP6+ polymerase chain reaction (PCR) protocol [15], followed by Luminex-based genotype detection [16]. Sample adequacy was evaluated through a β-globin PCR assay. The assay detects 37 genotypes: 12 HR HPV types (HPV types 16/18/31/33/35/39/45/51/52/56/58/59), 1 probably HR type (HPV-68), 9 possibly HR types (HPV types 26/30/53/66/67/69/70/73/82), and 15 LR types (HPV types 6/11/40/42/43/54/61/74/81/83/86/87/89/90/91) [1]. The samples were analyzed consecutively with overlaps between birth cohorts, according to the same genotyping protocol.
The HPV prevalence data was linked to data from the Central Population Registry and to the immunization records, using the unique personal identifier allocated to all Norwegian citizens.
Statistical Analysis
We calculated the prevalence with corresponding 95% Wilson score confidence intervals (CIs) [17] for each individual HPV type and for the following combinations: “any HPV type,” “any HR type,” “any probably/possibly HR type,” “any LR type,” “vaccine types (HPV-6/11/16/18),” “HPV-16/18,” “HPV-6/11,” “any nonvaccine HR type,” “any HR type other than HPV-16/18/31/33/45,” “HPV-31/33/45 (HR types for which cross-protection has been suggested),” “HPV-31/33/45/52/58 (additional HR types in the nonavalent vaccine),” “HPV-6/11/16/18/31/33/45/52/58” (nonavalent vaccine types), as well as for multiple HPV infection.
To compare HPV prevalence between groups, we calculated relative risks (RRs) with 95% Koopman CIs [18]. Girls born in 1996 and girls born in 1997 were compared to girls born in 1994 (baseline cohort). Girls born in 1997 were also compared to girls born in 1996. To estimate total effectiveness of the HPV immunization program (direct effect plus herd effect), we compared vaccinated girls born in 1997 (vaccine cohort) to unvaccinated girls in the baseline cohort [19]. To estimate herd effect, we compared unvaccinated girls in the vaccine cohort to unvaccinated girls in the baseline cohort. Finally, we compared vaccinated and unvaccinated girls within the vaccine cohort. Adjusting for region of residence using log-binomial regression did not change the estimates and is not included in the results section.
All tests were 2-sided, and P values <.05 were considered statistically significant. The data were analyzed with Stata/SE 15.0 (StataCorp) software.
RESULTS
Approximately 20% of the invited girls participated by delivering a urine sample (Table 1). We observed small differences in participation rates between the northern, middle, western, southern, eastern, and capital regions of Norway, ranging from 19.8% to 23.1% for the 1994 cohort, from 17.0% to 20.0% for the 1996 cohort, and from 21.7% to 24.5% for the 1997 cohort. In total, 17749 urine samples were analyzed.
Table 1.
Uptake of Human Papillomavirus (HPV) Vaccine by Birth Cohort and Participation Status Among 88949 17-Year-Old Norwegian Girls Born in 1994, 1996, or 1997
| Birth Year | Participation Status | Population | Initiated HPV Vaccinationa | Completed HPV Vaccinationb | Completed HPV Vaccinationb, % of Those Who Initiated | Age at First Dose of HPV Vaccine, y, Mean (SD) |
|---|---|---|---|---|---|---|
| 1994 | Invited | 25811 (100) | 500 (1.9) | 271 (1.0) | 54.2 | 15.2 (0.95) |
| Consented to participate | 6778 (26.3) | 212 (3.1) | 112 (1.7) | 52.8 | 15.2 (1.01) | |
| Delivered urine sample | 5528 (21.4) | 186 (3.4) | 96 (1.7) | 51.6 | 15.1 (1.02) | |
| HPV results available | 5468 (21.2) | 185 (3.4) | 95 (1.7) | 51.4 | 15.1 (1.02) | |
| 1996 | Invited | 31749 (100) | 1161 (3.7) | 855 (2.7) | 73.6 | 14.3 (1.12) |
| Consented to participate | 7489 (23.6) | 394 (5.3) | 283 (3.8) | 71.8 | 14.6 (1.12) | |
| Delivered urine sample | 6016 (18.9) | 326 (5.4) | 231 (3.8) | 70.9 | 14.6 (1.11) | |
| HPV results available | 5921 (18.6) | 322 (5.4) | 227 (3.8) | 70.5 | 14.6 (1.11) | |
| 1997 | Invited | 31389 (100) | 22178 (70.7) | 21396 (68.2) | 96.5 | 12.5 (0.52) |
| Consented to participate | 9297 (29.6) | 7366 (79.2) | 7152 (76.9) | 97.1 | 12.5 (0.51) | |
| Delivered urine sample | 7457 (23.8) | 5904 (79.2) | 5740 (77.0) | 97.2 | 12.5 (0.51) | |
| HPV results available | 6360 (20.3) | 5033 (79.1) | 4899 (77.0) | 97.3 | 12.5 (0.49) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: HPV, human papillomavirus; SD, standard deviation.
aReceived at least 1 dose of HPV vaccine before 1 January the year urine was sampled from the birth cohort (1 January 2011 for girls born in 1994, 1 January 2013 for girls born in 1996, and 1 January 2014 for girls born in 1997).
bReceived all 3 doses of HPV vaccine before 1 January the year urine was sampled from the birth cohort (1 January 2011 for girls born in 1994, 1 January 2013 for girls born in 1996, and 1 January 2014 for girls born in 1997).
Vaccine uptake was higher in participating than in all invited girls (Table 1). Among participating girls born in 1994, 1996, and 1997, 1.7%, 3.8%, and 77.0%, respectively, had received 3 doses of HPV vaccine. The mean age at first dose was higher in the 1994 and 1996 cohorts than in the 1997 cohort, but similar among invited and participating girls within each cohort (15.1, 14.6, and 12.5 years, respectively).
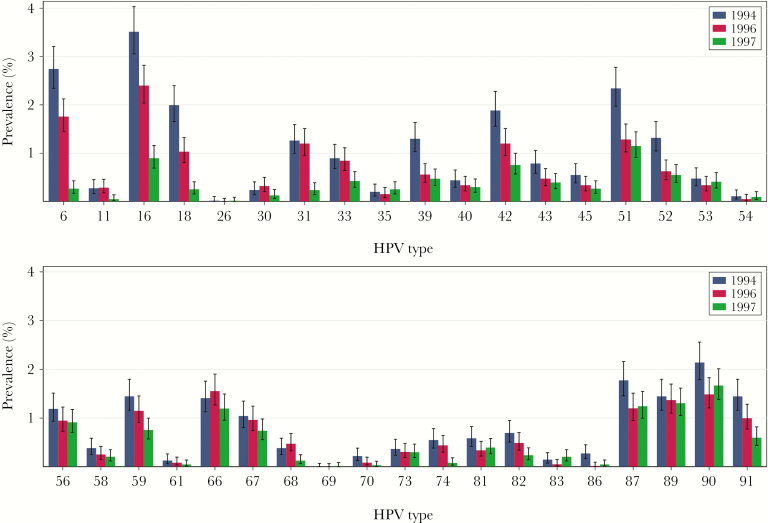
At the cohort level, the prevalence of any HPV type declined from 19.9% (95% CI, 18.8%–21.0%) in the baseline cohort to 11.5% (95% CI, 10.8%–12.3%) in the vaccine cohort (RR, 0.58 [95% CI, .53–.63]), corresponding to an overall reduction, irrespective of HPV type, of 42% (95% CI, 37%–47%) (Table 2). The prevalence of vaccine types declined from 7.4% (95% CI, 6.7%–8.1%) in the baseline cohort to 1.4% (95% CI, 1.1%–1.7%) in the vaccine cohort (RR, 0.19 [95% CI, .15–.24]), corresponding to a reduction of 81% (95% CI, 76%–85%). Furthermore, significant reductions were observed for combinations of nonvaccine types as well as for several single nonvaccine types, including LR types, probably/possibly HR types, and the HR types HPV-31, -33, -39, -45, -51, -52, and -59 (Table 2, Figure 1, and Supplementary Table 1). Estimates for all of the single HPV types according to their classification in species are presented in Supplementary Table 1. The proportion of HPV-positive girls with >1 HPV type declined from 46.4% (95% CI, 43.4%–49.3%) in the baseline cohort to 32.2% (95% CI, 28.9%–35.7%) in the vaccine cohort.
Table 2.
Human Papillomavirus (HPV) Prevalence in Urine Samples From 17-Year-Old Norwegian Girls, by Birth Cohort (N = 17749)
| HPV Type | Birth Cohorts Not Eligible for Routine HPV Vaccination | Birth Cohort Eligible for Routine HPV Vaccination | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Girls Born in 1994 (n = 5468) | Girls Born in 1996 (n = 5921) | Girls Born in 1997 (n = 6360) | |||||||
| No. | % (95% CI) | RR | No. | % (95% CI) | RR (95% CI) | No. | % (95% CI) | RR (95% CI) | |
| Any HPV type | 1087 | 19.9 (18.8–21.0) | 1 (ref) | 916 | 15.5 (14.6–16.4) | 0.78 (.72–.84) | 733 | 11.5 (10.8–12.3) | 0.58 (.53–.63) |
| Any HR typea | 611 | 11.2 (10.4–12.0) | 1 (ref) | 450 | 7.6 (7.0–8.3) | 0.68 (.61–.76) | 314 | 4.9 (4.4–5.5) | 0.44 (.39–.50) |
| Any probably/possibly HR typeb | 243 | 4.4 (3.9–5.0) | 1 (ref) | 239 | 4.0 (3.6–4.6) | 0.91 (.76–1.08) | 186 | 2.9 (2.5–3.4) | 0.66 (.55–.79) |
| Any LR typec | 597 | 10.9 (10.1–11.8) | 1 (ref) | 462 | 7.8 (7.1–8.5) | 0.71 (.64–.80) | 366 | 5.8 (5.2–6.4) | 0.53 (.47–.60) |
| Vaccine types | |||||||||
| Vaccine typesd | 403 | 7.4 (6.7–8.1) | 1 (ref) | 285 | 4.8 (4.3–5.4) | 0.65 (.56–.76) | 88 | 1.4 (1.1–1.7) | 0.19 (.15–.24) |
| HPV-16 or -18 | 280 | 5.1 (4.6–5.7) | 1 (ref) | 189 | 3.2 (2.8–3.7) | 0.62 (.52–.75) | 73 | 1.1 (.9–1.4) | 0.22 (.17–.29) |
| HPV-6 or -11 | 164 | 3.0 (2.6–3.5) | 1 (ref) | 121 | 2.0 (1.7–2.4) | 0.68 (.54–.86) | 20 | 0.3 (.2–.5) | 0.10 (.07–.17) |
| HPV-6 | 150 | 2.7 (2.3–3.2) | 1 (ref) | 104 | 1.8 (1.5–2.1) | 0.64 (.50–.82) | 17 | 0.3 (.2–.4) | 0.10 (.06–.16) |
| HPV-11 | 15 | 0.3 (.2–.5) | 1 (ref) | 17 | 0.3 (.2–.5) | 1.05 (.53–2.07) | 3 | 0.05 (.02–.1) | 0.17 (.05–.55) |
| HPV-16 | 192 | 3.5 (3.1–4.0) | 1 (ref) | 142 | 2.4 (2.0–2.8) | 0.68 (.55–.85) | 57 | 0.9 (.7–1.2) | 0.26 (.19–.34) |
| HPV-18 | 109 | 2.0 (1.7–2.4) | 1 (ref) | 61 | 1.0 (.8–1.3) | 0.52 (.38–.70) | 16 | 0.3 (.2–.4) | 0.13 (.08–.21) |
| Nonvaccine types | |||||||||
| Any nonvaccine HR type | 440 | 8.0 (7.4–8.8) | 1 (ref) | 330 | 5.6 (5.0–6.2) | 0.69 (.60–.79) | 256 | 4.0 (3.6–4.5) | 0.50 (.43–.58) |
| Any HR type other than 16, 18, 31, 33, and 45 | 370 | 6.8 (6.1–7.5) | 1 (ref) | 254 | 4.3 (3.8–4.8) | 0.63 (.54–.74) | 228 | 3.6 (3.2–4.1) | 0.53 (.45–.62) |
| HPV-31, -33, -45, -52, or -58 | 211 | 3.9 (3.4–4.4) | 1 (ref) | 176 | 3.0 (2.6–3.4) | 0.77 (.63–.94) | 98 | 1.5 (1.3–1.9) | 0.40 (.32–.51) |
| HPV-31, -33, or 45 | 141 | 2.6 (2.2–3.0) | 1 (ref) | 132 | 2.2 (1.9–2.6) | 0.86 (.68–1.09) | 57 | 0.9 (.7–1.2) | 0.35 (.26–.47) |
| HPV-31 | 69 | 1.3 (1.0–1.6) | 1 (ref) | 71 | 1.2 (1.0–1.5) | 0.95 (.68–1.32) | 15 | 0.2 (.1–.4) | 0.19 (.11–.32) |
| HPV-33 | 49 | 0.9 (.7–1.2) | 1 (ref) | 50 | 0.8 (.6–1.1) | 0.94 (.64–1.39) | 27 | 0.4 (.3–.6) | 0.47 (.30–.75) |
| HPV-35 | 11 | 0.2 (.1–.4) | 1 (ref) | 9 | 0.2 (.08–.3) | 0.76 (.32–1.77) | 16 | 0.3 (.2–.4) | 1.25 (.59–2.65) |
| HPV-39 | 71 | 1.3 (1.0–1.6) | 1 (ref) | 33 | 0.6 (.4–.8) | 0.43 (.29–.65) | 30 | 0.5 (.3–.7) | 0.36 (.24–.55) |
| HPV-45 | 30 | 0.5 (.4–.8) | 1 (ref) | 20 | 0.3 (.2–.5) | 0.62 (.35–1.07) | 17 | 0.3 (.2–.4) | 0.49 (.27–.87) |
| HPV-51 | 128 | 2.3 (2.0–2.8) | 1 (ref) | 76 | 1.3 (1.0–1.6) | 0.55 (.41–.73) | 73 | 1.1 (.9–1.4) | 0.49 (.37–.65) |
| HPV-52 | 72 | 1.3 (1.0–1.7) | 1 (ref) | 37 | 0.6 (.5–.8) | 0.47 (.32–.70) | 35 | 0.6 (.4–.8) | 0.42 (.28–.62) |
| HPV-56 | 65 | 1.2 (.9–1.5) | 1 (ref) | 56 | 0.9 (.7–1.2) | 0.80 (.56–1.13) | 58 | 0.9 (.7–1.2) | 0.77 (.54–1.09) |
| HPV-58 | 21 | 0.4 (.3–.6) | 1 (ref) | 15 | 0.3 (.2–.4) | 0.66 (.34–1.26) | 13 | 0.2 (.1–.3) | 0.53 (.27–1.05) |
| HPV-59 | 79 | 1.4 (1.2–1.8) | 1 (ref) | 68 | 1.1 (.9–1.5) | 0.79 (.58–1.10) | 48 | 0.8 (.6–1.0) | 0.52 (.37–.74) |
| Other | |||||||||
| Nonavalent HPV typese | 541 | 9.9 (9.1–10.7) | 1 (ref) | 409 | 6.9 (6.3–7.6) | 0.70 (.62–.79) | 179 | 2.8 (2.4–3.3) | 0.28 (.24–.34) |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; HR, high risk; LR, low risk; RR, relative risk.
aHPV type 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, or 59.
bHPV type 26, 30, 53, 66, 67, 68, 69, 70, 73, or 82.
cHPV type 6, 11, 40, 42, 43, 54, 61, 74, 81, 83, 86, 87, 89, 90, or 91.
dHPV type 6, 11, 16, or 18.
eHPV type 6, 11, 16, 18, 31, 33, 45, 52, or 58.
Figure 1.
Type-specific human papillomavirus (HPV) prevalence with 95% confidence intervals in urine samples from 17-year-old Norwegian girls born in 1994, 1996, or 1997 (N = 17749).
A significant reduction in prevalence of any HPV type as compared to the baseline cohort was also observed in the 1996 cohort (RR, 0.78 [95% CI, .72–.84]; Table 2). The prevalence of vaccine types was also significantly reduced (RR, 0.65 [95% CI, .56–.76]). Several nonvaccine-type combinations were also significantly reduced.
When comparing the vaccine cohort to the 1996 cohort, we observed a significant decline in the prevalence of any HPV type, vaccine types, and several nonvaccine type combinations, as well as some single nonvaccine types (Supplementary Table 2).
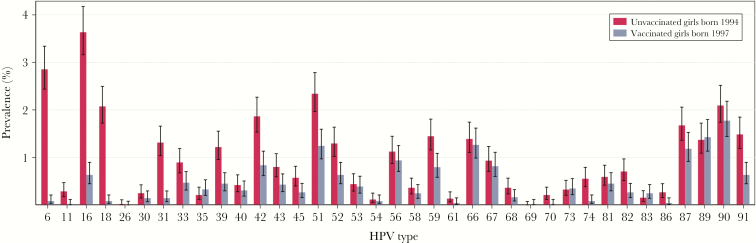
Compared to unvaccinated girls in the baseline cohort, the prevalence of any HPV type was significantly reduced in both unvaccinated and vaccinated girls in the vaccine cohort (RR, 0.54 [95% CI, .46–.63] and 0.59 [95% CI, .54–.65], respectively; Table 3). For vaccine types, the prevalence declined from 7.7% (95% CI, 7.0%–8.4%) in unvaccinated girls in the baseline cohort to 3.5% (95% CI, 2.6%–4.6%) in unvaccinated girls and to 0.8% (95% CI, 0.6%–1.1%) in vaccinated girls born in 1997. This corresponds to a reduction of 54% (95% CI, 39%–66%) in unvaccinated and 90% (95% CI, 86%–92%) in vaccinated girls born in 1997. Among vaccinated girls born in 1997, significant reductions in prevalence were also observed for several single nonvaccine types, including LR types, probably/possibly HR types, and the HR types 31, 33, 39, 45, 51, 52, and 59 (Figure 2). The reduction of HPV-31/33/45 combined was 68% (95% CI, 54%–77%). The reduction of nonavalent vaccine types was 76% (95% CI, 71%–80%). Significant reductions in the prevalence of several single nonvaccine types and combinations were also observed among unvaccinated girls born in 1997.
Table 3.
Human Papillomavirus (HPV) Prevalence in Urine Samples From Norwegian 17-Year-Old Unvaccinated and Vaccinated Girls Born in 1997 Compared to Unvaccinated Girls Born in 1994 (N = 11479)
| HPV Type | Unvaccinateda Girls Born in 1994 (n = 5254) | Unvaccinateda Girls Born in 1997 (n = 1321) | Fully Vaccinatedb Girls Born in 1997 (n = 4904) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % (95% CI) | RR | No. | % (95% CI) | RR (95% CI) | No. | % (95% CI) | RR (95% CI) | |
| Any HPV type | 1041 | 19.8 (18.8–20.9) | 1 (ref) | 141 | 10.7 (9.1–12.5) | 0.54 (.46–.63) | 573 | 11.7 (10.8–12.6) | 0.59 (.54–.65) |
| Any HR typec | 588 | 11.2 (10.4–12.1) | 1 (ref) | 62 | 4.7 (3.7–6.0) | 0.42 (.32–.54) | 244 | 5.0 (4.4–5.6) | 0.44 (.38–.51) |
| Any probably/possibly HR typed | 226 | 4.3 (3.8–4.9) | 1 (ref) | 30 | 2.3 (1.6–3.2) | 0.53 (.36–.77) | 153 | 3.1 (2.7–3.6) | 0.73 (.59–.89) |
| Any LR typee | 569 | 10.8 (10.0–11.7) | 1 (ref) | 69 | 5.2 (4.1–6.6) | 0.48 (.38–.61) | 288 | 5.9 (5.2–6.6) | 0.54 (.47–.62) |
| Vaccine types | |||||||||
| Vaccine typesf | 402 | 7.7 (7.0–8.4) | 1 (ref) | 46 | 3.5 (2.6–4.6) | 0.46 (.34–.61) | 39 | 0.8 (.6–1.1) | 0.10 (.08–.14) |
| HPV-16 or -18 | 279 | 5.3 (4.7–5.9) | 1 (ref) | 35 | 2.6 (1.9–3.7) | 0.50 (.35–.70) | 35 | 0.7 (.5–1.0) | 0.13 (.09–.19) |
| HPV-6 or -11 | 164 | 3.1 (2.7–3.6) | 1 (ref) | 14 | 1.1 (.6–1.8) | 0.34 (.20–.58) | 5 | 0.1 (.04–.2) | 0.03 (.01–.08) |
| HPV-6 | 150 | 2.9 (2.4–3.3) | 1 (ref) | 12 | 0.9 (.5–1.6) | 0.32 (.18–.57) | 4 | 0.08 (.03–.2) | 0.03 (.01–.07) |
| HPV-11 | 15 | 0.3 (.2–.5) | 1 (ref) | 2 | 0.2 (.04–.6) | 0.53 (.14–2.07) | 1 | 0.02 (.004–.1) | 0.07 (.01–.42) |
| HPV-16 | 191 | 3.6 (3.2–4.2) | 1 (ref) | 24 | 1.8 (1.2–2.7) | 0.50 (.33–.76) | 31 | 0.6 (.4–.9) | 0.17 (.12–.25) |
| HPV-18 | 109 | 2.1 (1.7–2.5) | 1 (ref) | 11 | 0.8 (.5–1.5) | 0.40 (.22–.74) | 4 | 0.08 (.03–.2) | 0.04 (.02–.10) |
| Nonvaccine types | |||||||||
| Any nonvaccine HR type | 418 | 8.0 (7.3–8.7) | 1 (ref) | 40 | 3.0 (2.2–4.1) | 0.38 (.28–.52) | 210 | 4.3 (3.8–4.9) | 0.54 (.46–.63) |
| Any HR type other than 16, 18, 31, 33, and 45 | 349 | 6.6 (6.0–7.3) | 1 (ref) | 34 | 2.6 (1.8–3.6) | 0.39 (.27–.55) | 189 | 3.9 (3.4–4.4) | 0.58 (.49–.69) |
| HPV-31, -33, -45, -52, or 58 | 204 | 3.9 (3.4–4.4) | 1 (ref) | 16 | 1.2 (.7–2.0) | 0.31 (.19–.51) | 80 | 1.6 (1.3–2.0) | 0.42 (.33–.54) |
| HPV-31, -33, or -45 | 139 | 2.6 (2.2–3.1) | 1 (ref) | 13 | 1.0 (.6–1.7) | 0.37 (.21–.65) | 42 | 0.9 (.6–1.2) | 0.32 (.23–.46) |
| HPV-31 | 69 | 1.3 (1.0–1.7) | 1 (ref) | 8 | 0.6 (.3–1.2) | 0.46 (.23–.94) | 7 | 0.1 (.07–.3) | 0.11 (.05–.23) |
| HPV-33 | 47 | 0.9 (.7–1.2) | 1 (ref) | 2 | 0.2 (.04–.6) | 0.17 (.05–.63) | 23 | 0.5 (.3–.7) | 0.52 (.32–.86) |
| HPV-35 | 11 | 0.2 (.1–.4) | 1 (ref) | 0 | 0 (0–.3) | 0 (.0001–1.39) | 16 | 0.3 (.2–.5) | 1.56 (.74–3.30) |
| HPV-39 | 64 | 1.2 (1.0–1.6) | 1 (ref) | 6 | 0.5 (.2–1.0) | 0.37 (.17–.84) | 22 | 0.4 (.3–.7) | 0.37 (.23–.59) |
| HPV-45 | 30 | 0.6 (.4–.8) | 1 (ref) | 4 | 0.3 (.1–.8) | 0.53 (.20–1.44) | 13 | 0.3 (.2–.5) | 0.46 (.25–.88) |
| HPV-51 | 123 | 2.3 (2.0–2.8) | 1 (ref) | 10 | 0.8 (.4–1.4) | 0.32 (.17–.61) | 61 | 1.2 (1.0–1.6) | 0.53 (.39–.72) |
| HPV-52 | 68 | 1.3 (1.0–1.6) | 1 (ref) | 3 | 0.2 (.08–.7) | 0.18 (.06–.53) | 31 | 0.6 (.4–.9) | 0.49 (.32–.74) |
| HPV-56 | 59 | 1.1 (.9–1.4) | 1 (ref) | 11 | 0.8 (.5–1.5) | 0.74 (.39–1.39) | 46 | 0.9 (.7–1.2) | 0.84 (.57–1.22) |
| HPV-58 | 19 | 0.4 (.2–.6) | 1 (ref) | 1 | 0.08 (.01–.4) | 0.21 (.04–1.23) | 12 | 0.2 (.1–.4) | 0.68 (.33–1.37) |
| HPV-59 | 76 | 1.4 (1.2–1.8) | 1 (ref) | 9 | 0.7 (.4–1.3) | 0.47 (.24–.92) | 39 | 0.8 (.6–1.1) | 0.55 (.38–.81) |
| Other | |||||||||
| Nonavalent HPV typesg | 533 | 10.1 (9.4–11.0) | 1 (ref) | 56 | 4.2 (3.3–5.5) | 0.42 (.32–.55) | 119 | 2.4 (2.0–2.9) | 0.24 (.20–.29) |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; HR, high risk; LR, low risk; RR, relative risk.
aReceived no doses of HPV vaccine 15 days before sampling.
bReceived 3 doses of HPV vaccine at least 15 days before sampling.
cHPV type 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, or 59.
dHPV type 26, 30, 53, 66, 67, 68, 69, 70, 73, or 82.
eHPV type 6, 11, 40, 42, 43, 54, 61, 74, 81, 83, 86, 87, 89, 90, or 91.
fHPV type 6, 11, 16, or 18.
gHPV type 6, 11, 16, 18, 31, 33, 45, 52, or 58.
Figure 2.
Type-specific human papillomavirus (HPV) prevalence with 95% confidence intervals in urine samples from 17-year-old unvaccinated girls born in 1994 vs 17-year-old vaccinated girls born in 1997 (N = 10158).
We observed no difference in the prevalence of any HPV type between vaccinated and unvaccinated girls within the vaccine cohort (RR, 1.09 [95% CI, .92–1.30]), but found a significantly lower prevalence of vaccine types in vaccinated girls (RR, 0.23 [95% CI, .15–.35]) (Table 4). A significantly higher combined prevalence of any nonvaccine HR types was seen in vaccinated as compared to unvaccinated girls born in 1997 (RR, 1.41 [95% CI, 1.02–1.97]).
Table 4.
Human Papillomavirus (HPV) Prevalence in Urine Samples From 17-Year-Old Norwegian Girls Born in 1997, by HPV Vaccination Status (N = 6225)
| HPV Type | Unvaccinateda (n = 1321) | Fully Vaccinatedb (n = 4904) | ||||
|---|---|---|---|---|---|---|
| No. | % (95% CI) | RR | No. | % (95% CI) | RR (95% CI) | |
| Any HPV type | 141 | 10.7 (9.1–12.5) | 1 (ref) | 573 | 11.7 (10.8–12.6) | 1.09 (.92–1.30) |
| Any HR typec | 62 | 4.7 (3.7–6.0) | 1 (ref) | 244 | 5.0 (4.4–5.6) | 1.06 (.81–1.39) |
| Any probably/possibly HR typed | 30 | 2.3 (1.6–3.2) | 1 (ref) | 153 | 3.1 (2.7–3.6) | 1.37 (.94–2.02) |
| Any LR typee | 69 | 5.2 (4.1–6.6) | 1 (ref) | 288 | 5.9 (5.2–6.6) | 1.12 (.87–1.45) |
| Vaccine types | ||||||
| Vaccine typesf | 46 | 3.5 (2.6–4.6) | 1 (ref) | 39 | 0.8 (.6–1.1) | 0.23 (.15–.35) |
| HPV-16 or -18 | 35 | 2.6 (1.9–3.7) | 1 (ref) | 35 | 0.7 (.5–1.0) | 0.27 (.17–.43) |
| HPV-6 or -11 | 14 | 1.1 (.6–1.8) | 1 (ref) | 5 | 0.1 (.04–.2) | 0.10 (.04–.26) |
| HPV-6 | 12 | 0.9 (.5–1.6) | 1 (ref) | 4 | 0.08 (.03–.2) | 0.09 (.03–.26) |
| HPV-11 | 2 | 0.2 (.04–.6) | 1 (ref) | 1 | 0.02 (.004–.1) | 0.13 (.02–1.03) |
| HPV-16 | 24 | 1.8 (1.2–2.7) | 1 (ref) | 31 | 0.6 (.4–.9) | 0.35 (.21–.59) |
| HPV-18 | 11 | 0.8 (.5–1.5) | 1 (ref) | 4 | 0.08 (.03–.2) | 0.10 (.03–.29) |
| Nonvaccine types | ||||||
| Any nonvaccine HR type | 40 | 3.0 (2.2–4.1) | 1 (ref) | 210 | 4.3 (3.8–4.9) | 1.41 (1.02–1.97) |
| Any HR type other than 16, 18, 31, 33, and 45 | 34 | 2.6 (1.8–3.6) | 1 (ref) | 189 | 3.9 (3.4–4.4) | 1.50 (1.05–2.14) |
| HPV-31, -33, -45, -52, or 58 | 16 | 1.2 (.7–2.0) | 1 (ref) | 80 | 1.6 (1.3–2.0) | 1.35 (.80–2.28) |
| HPV-31, -33, or -45 | 13 | 1.0 (.6–1.7) | 1 (ref) | 42 | 0.9 (.6–1.2) | 0.87 (.47–1.60) |
| HPV-31 | 8 | 0.6 (.3–1.2) | 1 (ref) | 7 | 0.1 (.07–.3) | 0.24 (.09–.62) |
| HPV-33 | 2 | 0.2 (.04–.6) | 1 (ref) | 23 | 0.5 (.3–.7) | 3.1 (.81–11.85) |
| HPV-35 | 0 | 0 (0–.3) | 1 (ref) | 16 | 0.3 (.2–.5) | … |
| HPV-39 | 6 | 0.5 (.2–1.0) | 1 (ref) | 22 | 0.4 (.3–.7) | 0.99 (.41–2.37) |
| HPV-45 | 4 | 0.3 (.1–.8) | 1 (ref) | 13 | 0.3 (.2–.5) | 0.88 (.30–2.55) |
| HPV-51 | 10 | 0.8 (.4–1.4) | 1 (ref) | 61 | 1.2 (1.0–1.6) | 1.64 (.86–3.16) |
| HPV-52 | 3 | 0.2 (.08–.7) | 1 (ref) | 31 | 0.6 (.4–.9) | 2.78 (.91–8.57) |
| HPV-56 | 11 | 0.8 (.5–1.5) | 1 (ref) | 46 | 0.9 (.7–1.2) | 1.13 (.59–2.15) |
| HPV-58 | 1 | 0.08 (.01–.4) | 1 (ref) | 12 | 0.2 (.1–.4) | 3.23 (.54–19.37) |
| HPV-59 | 9 | 0.7 (.4–1.3) | 1 (ref) | 39 | 0.8 (.6–1.1) | 1.17 (.58–2.37) |
| Other | ||||||
| Nonavalent HPV typesg | 56 | 4.2 (3.3–5.5) | 1 (ref) | 119 | 2.4 (2.0–2.9) | 0.57 (.42–.78) |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; HR, high risk; LR, low risk; RR, relative risk.
aReceived no doses of HPV vaccine 15 days before sampling
bReceived 3 doses of HPV vaccine at least 15 days before sampling
cHPV type 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, or 59.
dHPV type 26, 30, 53, 66, 67, 68, 69, 70, 73, or 82.
eHPV type 6, 11, 40, 42, 43, 54, 61, 74, 81, 83, 86, 87, 89, 90, or 91.
fHPV type 6, 11, 16, or 18.
gHPV type 6, 11, 16, 18, 31, 33, 45, 52, or 58.
In a supplementary analysis among unvaccinated girls only, we observed a significant reduction in the prevalence of any HPV type in the 1996 cohort compared with the baseline cohort and a further reduction in the vaccine cohort (RR, 0.79 [95% CI, .73–.86] and 0.54 [95% CI, .46–.63], respectively; Supplementary Table 3). Similar patterns were observed for vaccine types and several nonvaccine-type combinations.
DISCUSSION
In this large, population-based, cross-sectional study, we found a significant reduction in HPV prevalence after introduction of the HPV vaccine in Norway. The overall reduction of any HPV type was 42%, and the reduction in vaccine types was 81% in the vaccine cohort, as compared to the baseline cohort. The total effectiveness of the HPV immunization program against vaccine types was 90%. A reduction in several nonvaccine types was also observed. The reduction of HPV-31/33/45 combined was 68%. Within the vaccine cohort, the prevalence of vaccine types was reduced by 77% in vaccinated as compared to unvaccinated girls. Significant reductions in vaccine types and several nonvaccine types were also observed in unvaccinated girls born in 1997, indicating herd effect.
A major strength of the current study is the large sample size and the population-based design. The study is one of the largest of its kind, with 17749 samples tested for 37 HPV genotypes. The vaccine impact was assessed at age 17 years, 5 years after vaccination. In studies in cervical screening populations, vaccine impact is generally measured at a higher age.
Individual records from the Norwegian Immunisation Registry enabled linkage of HPV prevalence data to individual HPV vaccination status. Notification to the Norwegian Immunisation Registry is mandatory, and information from the electronic patient record systems is transferred electronically to the immunization registry [13]. Thus, registration is considered nearly complete.
Another strength is the high uptake of HPV vaccine in 12- to 13-year-old girls, in general not yet sexually active, and thus probably HPV naive. The mean age at first vaccine dose was 12.5 years. In a recent study from Norway, only 3% of students in upper secondary school reported to have had sexual intercourse before the age of 14 [20]. No catch-up vaccination was offered in Norway until 2016, and vaccine uptake was low in age cohorts not eligible for routine HPV vaccination. Thus, the presented results are likely to reflect the effectiveness of the vaccination program in a largely HPV-naive population.
Urine sampling is noninvasive and reported to be preferred over self-collecting cervicovaginal swabs [21, 22]. In young prescreening populations, as in our study, urine sampling may provide higher response rates than self-collected genital swabs, as demonstrated in nonattenders to cervical screening at age 21 [23]. The HPV prevalence may be lower in urine samples than in cervical samples [24–26]. However, several studies have found a good agreement between HPV detected in urine and cervical samples, and urine sampling has been suggested as an adequate alternative to monitor the impact of HPV immunization programs or to increase uptake in screening [22, 25–28].
The response rate in our study was quite low, although higher than reported from similar studies in the same age group [29–31]. The low response rate may have caused selection bias. We have no information on sexual history or socioeconomic factors, and participating and nonparticipating girls may differ in these respects. We would, however, expect any association between these factors and the willingness to participate to be similar across the invited birth cohorts. The recruitment procedure was identical for all birth cohorts. The median age at first in Norway, intercourse has been stable in the period 2002–2017 [20, 32, 33]. Thus, we believe that the comparisons between birth cohorts are valid.
The HPV vaccine uptake was higher in participants than in nonparticipants. However, in several previous studies, including one with Norwegian data, no association between HPV vaccination and sexual behavior was seen [30, 34–37]. The vast majority of HPV-vaccinated girls in our study were vaccinated before becoming sexually active, further reducing the probability of an association between HPV vaccination and sexual behavior. Nevertheless, due to possible selection bias, we cannot rule out the possibility that unvaccinated and vaccinated participants in our study differ with regard to sexual behavior. If non–sexually active unvaccinated girls were more willing to participate than sexually active unvaccinated girls, the measured HPV prevalence in unvaccinated girls would be lower than in the total unvaccinated population of girls born in 1997. This could partly explain the slightly higher prevalence of nonvaccine types observed in vaccinated as compared to unvaccinated girls born in 1997. Given such selection bias, the vaccine effectiveness would be underestimated, whereas the herd effect would be overestimated.
Samples from the baseline cohort were collected in 2011, 2 years into the HPV immunization program. The girls in the first vaccine-eligible birth cohort were only 14 years old in 2011. We therefore believe the herd effect in the 3-years-older unvaccinated baseline cohort to be minimal.
The HPV prevalence in the 2 nonvaccine-eligible cohorts is comparable to findings in urine samples from 15- to 18-year-old girls in Scotland, prior to HPV vaccine introduction [29]. Other population-based studies in similar age groups have reported somewhat higher prevalence of any HPV type, but lower prevalence of vaccine types in self-collected cervical samples [30, 38].
We observed a substantial reduction in HPV vaccine types, consistent with reports from other countries [3, 4, 6, 39–41]. Moreover, in line with previous studies [3, 4, 6], we found a strong herd effect in unvaccinated girls.
Previous meta-analyses, including data on both quadrivalent and bivalent vaccine, have found evidence of cross-protection against HPV-31 or the combination HPV-31/33/45 [3, 5], whereas a slight increase in HPV-39 and -52 was found in one meta-analysis [5]. Cross-protection against HPV-31, -33, -35, -45, and -52 has been reported for the bivalent vaccine [6, 41]. We observed significant reduction in several single nonvaccine types, including LR types, probably/possibly HR types, and the HR types HPV-31, -33, -39, -45, -51, -52, and -59, both when comparing the vaccine cohort to the baseline cohort and when comparing vaccinated girls in the 1997 cohort to unvaccinated girls in the baseline cohort. The observed reduction of nonvaccine types may be due to changes in sexual behavior, natural variation, cross-protection, or unknown causes. There is no indication of a change in sexual behavior in Norway during recent years [20, 32, 33]. Natural variation may explain some of the decline in HPV types where cross-protection has previously not been suggested. However, natural variation alone seems unlikely to have caused such a large reduction, in particular for vaccine types and known cross-protective types. Alternatively, the reduction of nonvaccine types could be linked to the absence of HPV-16/18, which is known to impair the immune response in the cervix, in particular through depletion of Langerhans cells [42, 43]. The reduction could also be attributable to vaccine-induced cross-reactivity of CD4 T-helper cells, as suggested in an earlier study [44]. They observed a significant reduction in HPV-6, -11, and -74 in HPV-naive girls and women vaccinated with bivalent vaccine, not targeting HPV-6/11. Although a higher level of cell-mediated immune response has been found for the bivalent AS04 adjuvanted vaccine than for the quadrivalent vaccine [45], our findings may suggest a role for cross-protective cellular immunity in naive cohorts vaccinated with non-AS04 adjuvanted vaccines.
Interestingly, we also observed a significant reduction in several single nonvaccine types in unvaccinated girls in the vaccine cohort, suggesting a possible cross-protective herd effect, also reported for the bivalent vaccine [6].
As previously reported, we also observed a significant reduction in HPV prevalence in the 1996 cohort [12]. Given the large herd protection in unvaccinated girls, it seems likely that the reduction in HPV prevalence in the 1996 cohort, at least partially, is caused by herd effect, and not entirely by natural fluctuation in the occurrence of HPV. Girls born in 1996 are only 1 year older than the first vaccine-eligible cohort, and urine samples were collected in 2013, 4 years into the vaccination program.
We observed no indication of type replacement after the introduction of the HPV immunization program in Norway, in line with recent findings from Scotland [6]. This must, however, be followed closely over the coming years as more birth cohorts enter the program.
Although the prevalence of vaccine types was lower among vaccinated than unvaccinated girls within the 1997 cohort, no difference in prevalence of any HPV type was observed, as previously reported [31, 40]. A slightly higher prevalence of some nonvaccine types was observed in vaccinated compared with unvaccinated girls in the 1997 cohort. However, in both groups the prevalence was significantly lower than in unvaccinated girls in the baseline cohort. Similar findings were recently reported in Australia [40]. This may be explained by unmasking of nonvaccine types due to lack of primer competition in the PCR assay in the absence of HPV-16/18 in samples from vaccinated girls [40, 46].
The prevalence of multiple infections was high, consistent with findings from other studies [47, 48], but declined following vaccination. The interplay between multiple HPV infections and changes in HPV prevalence following introduction of HPV vaccination programs warrants further investigation.
CONCLUSIONS
Single-cohort delivery of HPV vaccine to 12- to 13-year-old girls was associated with a reduction in vaccine types of 90% in vaccinated and 54% in unvaccinated girls in the first vaccine-eligible cohort 5 years after vaccination. A substantial reduction in nonvaccine types was observed in both vaccinated and unvaccinated girls, suggesting cross-protection and herd effect. No indication of type replacement was observed. The early impact of the HPV immunization program in this largely HPV-naive Norwegian population seems more beneficial than anticipated upon introduction of the vaccine in 2009, but more effectiveness studies in HPV-naive populations are needed to confirm the findings. With the growing amount of evidence for sustained long-term protection of HPV vaccine [49, 50], we feel optimistic that the observed reduction in HPV prevalence in urine samples may translate into a future decline in the incidence of precancerous lesions and cervical cancer in Norway.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: European Research Organisation on Genital Infection and Neoplasia, Amsterdam, The Netherlands, 8–11 October 2017. Abstract 00476
Acknowledgments. We thank all of the study participants. We are also thankful to Patricia Schreuder, Nina Hovland, Erna Davidsen (deceased), Ranveig Heiberg Andersen, and Grethe Karin Eriksen at the Norwegian Institue of Public Health (NIPH) for their support in the recruitment of study participants; Ole-Martin Kvinge at NIPH for data management; the Department of Biobanks at NIPH, in particular Nina Kristin Stensrud, Kari Harbak, Kaja Klykken Aas, Gholam Davarpanah, Olive Oliva, and Rolf Erik Kolstad for distribution of sampling kits, receipt, and processing of urine samples; and Ellen Myrvang, Alexander Eieland, and Nermin Zecic at the HPV Reference Laboratory for technical support with HPV genotyping.
Financial support. This work was supported by the NIPH and the Norwegian Ministry of Health and Care Services.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. International Agency for Research on Cancer. Monographs on the evaluation of carcinogenic risks to humans. A review of human carcinogens. Part B: biological agents. IARC 2012; 100:1–441. [PMC free article] [PubMed] [Google Scholar]
- 2. Serrano B, Brotons M, Bosch FX, Bruni L. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet Gynaecol 2018; 47:14–26. [DOI] [PubMed] [Google Scholar]
- 3. Drolet M, Bénard É, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garland SM, Kjaer SK, Muñoz N, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis 2016; 63:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mesher D, Soldan K, Lehtinen M, et al. Population-level effects of human papillomavirus vaccination programs on infections with nonvaccine genotypes. Emerg Infect Dis 2016; 22:1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis 2017; 17:1293–302. [DOI] [PubMed] [Google Scholar]
- 7. Cameron RL, Kavanagh K, Cameron Watt D, et al. The impact of bivalent HPV vaccine on cervical intraepithelial neoplasia by deprivation in Scotland: reducing the gap. J Epidemiol Community Health 2017; 71:954–60. [DOI] [PubMed] [Google Scholar]
- 8. Lehtinen M, Dillner J. Clinical trials of human papillomavirus vaccines and beyond. Nat Rev Clin Oncol 2013; 10:400–10. [DOI] [PubMed] [Google Scholar]
- 9. Brotherton JML, Bloem PN. Population-based HPV vaccination programmes are safe and effective: 2017 update and the impetus for achieving better global coverage. Best Pract Res Clin Obstet Gynaecol 2018; 47:42–58. [DOI] [PubMed] [Google Scholar]
- 10. Bergsaker M, Ege MS, Hagerup-Jenssen M, Seterelv SS, Stålcrantz J, Wiklund B. The Childhood Immunisation Programme in Norway. Report for 2013 [in Norwegian]. Oslo: Norwegian Institute of Public Health, 2014. https://www.fhi.no/publ/2014/barnevaksinasjonsprogrammet-i-norge/. Accessed 24 April 2018. [Google Scholar]
- 11. Norwegian Institute of Public Health. Statistics on HPV vaccination in the childhood immunisation programme [in Norwegian]. https://www.fhi.no/hn/helseregistre-og-registre/sysvak/dekkningsstatistikk/. Accessed 24 April 2018. [Google Scholar]
- 12. Molden T, Feiring B, Ambur OH, et al. Human papillomavirus prevalence and type distribution in urine samples from Norwegian women aged 17 and 21 years: a nationwide cross-sectional study of three non-vaccinated birth cohorts. Papillomavirus Res 2016; 2:153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trogstad L, Ung G, Hagerup-Jenssen M, Cappelen I, Haugen IL, Feiring B. The Norwegian immunisation register—SYSVAK. Euro Surveill 2012; 17 pii:20147 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20147. [PubMed] [Google Scholar]
- 14. Norwegian Institute of Public Health. Statistics from the Norwegian Prescription Database http://www.norpd.no/Prevalens.aspx. Accessed 24 April 2018.
- 15. Söderlund-Strand A, Carlson J, Dillner J. Modified general primer PCR system for sensitive detection of multiple types of oncogenic human papillomavirus. J Clin Microbiol 2009; 47:541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmitt M, Bravo IG, Snijders PJ, Gissmann L, Pawlita M, Waterboer T. Bead-based multiplex genotyping of human papillomaviruses. J Clin Microbiol 2006; 44:504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998; 17:857–72. [DOI] [PubMed] [Google Scholar]
- 18. Fagerland MW, Lydersen S, Laake P. Recommended confidence intervals for two independent binomial proportions. Stat Methods Med Res 2015; 24:224–54. [DOI] [PubMed] [Google Scholar]
- 19. Halloran ME. Overview of vaccine field studies: types of effects and designs. J Biopharm Stat 2006; 16:415–27. [DOI] [PubMed] [Google Scholar]
- 20. Bakken A. Ungdata. Nasjonale resultater 2017. Norwegian Social Research report 10/17 [in Norwegian]. Oslo: NOVA, 2017. http://www.hioa.no/Om-HiOA/Senter-for-velferds-og-arbeidslivsforskning/NOVA/Publikasjonar/Rapporter/2017/Ungdata-2017. Accessed 24 April 2018. [Google Scholar]
- 21. Senkomago V, Des Marais AC, Rahangdale L, Vibat CR, Erlander MG, Smith JS. Comparison of urine specimen collection times and testing fractions for the detection of high-risk human papillomavirus and high-grade cervical precancer. J Clin Virol 2016; 74:26–31. [DOI] [PubMed] [Google Scholar]
- 22. Franciscatto LG, Silva CM, Barcellos RB, et al. Comparison of urine and self-collected vaginal samples for detecting human papillomavirus DNA in pregnant women. Int J Gynaecol Obstet 2014; 125:69–72. [DOI] [PubMed] [Google Scholar]
- 23. Sinka K, Lacey M, Robertson C, et al. Acceptability and response to a postal survey using self-taken samples for HPV vaccine impact monitoring. Sex Transm Infect 2011; 87:548–52. [DOI] [PubMed] [Google Scholar]
- 24. Vorsters A, Micalessi I, Bilcke J, Ieven M, Bogers J, Van Damme P. Detection of human papillomavirus DNA in urine. A review of the literature. Eur J Clin Microbiol Infect Dis 2012; 31:627–40. [DOI] [PubMed] [Google Scholar]
- 25. Enerly E, Olofsson C, Nygård M. Monitoring human papillomavirus prevalence in urine samples: a review. Clin Epidemiol 2013; 5:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pathak N, Dodds J, Zamora J, Khan K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis. BMJ 2014; 349:g5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cuschieri K, Nandwani R, McGough P, et al. Urine testing as a surveillance tool to monitor the impact of HPV immunization programs. J Med Virol 2011; 83:1983–7. [DOI] [PubMed] [Google Scholar]
- 28. Van Keer S, Tjalma WAA, Pattyn J, et al. Human papillomavirus genotype and viral load agreement between paired first-void urine and clinician-collected cervical samples. Eur J Clin Microbiol Infect Dis 2018; 37:859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O’Leary MC, Sinka K, Robertson C, et al. HPV type-specific prevalence using a urine assay in unvaccinated male and female 11- to 18-year olds in Scotland. Br J Cancer 2011; 104:1221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jacot-Guillarmod M, Pasquier J, Greub G, Bongiovanni M, Achtari C, Sahli R. Impact of HPV vaccination with Gardasil in Switzerland. BMC Infect Dis 2017; 17:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donken R, King AJ, Bogaards JA, Woestenberg PJ, Meijer CJLM, de Melker HE. High effectiveness of the bivalent human papillomavirus (HPV) vaccine against incident and persistent HPV infections up to 6 years after vaccination in young Dutch women. J Infect Dis 2018; 217:1579–89. [DOI] [PubMed] [Google Scholar]
- 32. Træen B, Stigum H, Magnus P.. Rapport fra seksualvaneundersøkelsene i 1987, 1992, 1997 og 2002 [in Norwegian]. Oslo: Norwegian Institute of Public Health, 2003. https://www.fhi.no/publ/eldre/rapport-fra-seksualvane-undersokels/. Accessed 2 July 2018. [Google Scholar]
- 33. Jensen KE, Munk C, Sparen P, et al. Women’s sexual behavior. Population-based study among 65000 women from four Nordic countries before introduction of human papillomavirus vaccination. Acta Obstet Gynecol Scand 2011; 90:459–67. [DOI] [PubMed] [Google Scholar]
- 34. Bednarczyk RA, Davis R, Ault K, Orenstein W, Omer SB. Sexual activity-related outcomes after human papillomavirus vaccination of 11- to 12-year-olds. Pediatrics 2012; 130:798–805. [DOI] [PubMed] [Google Scholar]
- 35. Forster AS, Marlow LA, Stephenson J, Wardle J, Waller J. Human papillomavirus vaccination and sexual behaviour: cross-sectional and longitudinal surveys conducted in England. Vaccine 2012; 30:4939–44. [DOI] [PubMed] [Google Scholar]
- 36. Hansen BT, Kjær SK, Arnheim-Dahlström L, et al. Human papillomavirus (HPV) vaccination and subsequent sexual behaviour: evidence from a large survey of Nordic women. Vaccine 2014; 32:4945–53. [DOI] [PubMed] [Google Scholar]
- 37. Smith LM, Kaufman JS, Strumpf EC, Lévesque LE. Effect of human papillomavirus (HPV) vaccination on clinical indicators of sexual behaviour among adolescent girls: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ 2015; 187:E74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–9. [DOI] [PubMed] [Google Scholar]
- 39. Kahn JA, Widdice LE, Ding L, et al. Substantial decline in vaccine-type human papillomavirus (HPV) among vaccinated young women during the first 8 years after HPV vaccine introduction in a community. Clin Infect Dis 2016; 63:1281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Machalek DA, Garland SM, Brotherton JML, et al. Very low prevalence of vaccine human papillomavirus types among 18- to 35-year old Australian women 9 years following implementation of vaccination. J Infect Dis 2018; 217:1590–600. [DOI] [PubMed] [Google Scholar]
- 41. Woestenberg PJ, King AJ, van Benthem BHB, et al. . Bivalent vaccine effectiveness against type-specific HPV positivity: evidence for cross-protection against oncogenic types among Dutch STI clinic visitors. J Infect Dis 2018; 217:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matthews K, Leong CM, Baxter L, et al. Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated down regulation of E-cadherin. J Virol 2003; 77:8378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moerman-Herzog A, Nakagawa M. Early defensive mechanisms against human papillomavirus infection. Clin Vaccine Immunol 2015; 22:850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Szarewski A, Skinner SR, Garland SM, et al. Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against low-risk HPV types (PATRICIA randomized trial): an unexpected observation. J Infect Dis 2013; 208:1391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Einstein MH, Baron M, Levin MJ, et al. . Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18–45 years. Hum Vaccin 2011; 7:1359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tota JE, Ramanakumar AV, Jiang M, et al. Epidemiologic approaches to evaluating the potential for human papillomavirus type replacement postvaccination. Am J Epidemiol 2013; 178:625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chaturvedi AK, Katki HA, Hildesheim A, et al. . Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. J Infect Dis 2011; 203:910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kavanagh K, Pollock KG, Potts A, et al. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer 2014; 110:2804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lehtinen M, Lagheden C, Luostarinen T, et al. Ten-year follow-up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end-point-registry-based follow-up of three cohorts from randomized trials. BMJ Open 2017; 7:e015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kjaer SK, Nygård M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis 2018; 66:339–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.