The data demonstrate a novel role of Fli-1 in pericyte loss and vascular dysfunction in cecal ligation and puncture-induced murine sepsis by regulating inflammation and pyroptosis, suggesting that Fli-1 is a potential therapeutic target for sepsis.
Keywords: Fli-1, pericytes, sepsis, vascular leak
Abstract
Background
Pericytes are vascular mural cells and are embedded in the basement membrane of the microvasculature. Recent studies suggest a role for pericytes in lipopolysaccharide (LPS)-induced microvascular dysfunction and mortality, but the mechanisms of pericyte loss in sepsis are largely unknown.
Methods
By using a cecal ligation and puncture (CLP)-induced murine model of sepsis, we observed that CLP led to lung and renal pericyte loss and reduced lung pericyte density and pericyte/endothelial cell (EC) coverage.
Results
Up-regulated Friend leukemia virus integration 1 (Fli-1) messenger ribonucleic acid (RNA) and protein levels were found in lung pericytes from CLP mice in vivo and in LPS-stimulated lung pericytes in vitro. Knockout of Fli-1 in Foxd1-derived pericytes prevented CLP-induced pericyte loss, vascular leak, and improved survival. Disrupted Fli-1 expression by small interfering RNA inhibited LPS-induced inflammatory cytokines and chemokines in cultured lung pericytes. Furthermore, CLP-induced pericyte pyroptosis was mitigated in pericyte Fli-1 knockout mice.
Conclusions
Our findings suggest that Fli-1 is a potential therapeutic target in sepsis.
Sepsis is a systemic inflammatory response caused by microbial infection and remains one of the most frequent causes of mortality in intensive care unit patients [1, 2]. Decreased capillary density and increased endothelial permeability occur in both experimental and human sepsis [3, 4]. This microvascular dysfunction plays a crucial role in the development of sepsis-related multiorgan failure [4, 5]; however, its underlying pathophysiology has not been completely elucidated. Pericytes are key regulators of endothelial function [6, 7], but the contribution of pericytes to the microvascular dysfunction of sepsis is unknown.
Pericytes are vascular smooth muscle lineage cells embedded in the basement membrane of the microvasculature that wrap around the microvascular endothelial cells (ECs) [8]. Pericytes, characterized with common expressed markers platelet-derived growth factor receptor β (PDGFRβ) and neural/glial antigen 2 (NG2), prevent vascular leakage by stabilizing ECs [7, 9]. In the blood-brain barrier (BBB), pericytes regulate vascular permeability, and their loss can contribute to BBB disruption [10, 11]. Lipopolysaccharide (LPS)-induced microvascular dysfunction and mortality have been associated with pericyte loss; however, this loss is not caused by apoptosis [12]. In a recent study, a novel type of regulated cell death, pyroptosis, has been reported [13, 14]. Pyroptosis, an inflammatory form of programmed cell death, is dependent on caspases 1, 4, 5, and 11 and is accompanied by the release of proinflammatory cytokines [15]. Recent studies indicate that inhibition of pyroptosis improved sepsis outcomes in animal models [16, 17]. However, the role of pyroptosis in pericyte loss remains unknown.
The transcription factor Foxd1 belongs to human forkhead-box (FOX) gene family and has been related to different key biological processes including retina development and embryo implantation [18]. Deletion of Foxd1 leads to abnormal lung development and is lethal due to failure of nephrogenesis [19]. Nonarteriolar or nonglomerular Foxd1-lineage cells can migrate to and invest into capillary walls and regulate capillary lumen diameter, vessel stability, and permeability [20]. Furthermore, most of Foxd-1-derived lung cells are pericytes because they express pericyte markers PDGFRβ and NG2 [19]. Therefore, Foxd1 Fli-1 Td tomato mice were used in the in vivo studies to determine lung pericyte density.
Friend leukemia virus integration 1 (Fli-1) belongs to the ETS transcription factor family. Fli-1 regulates a wide spectrum of biological processes including cancer development, fibrosis, vasculopathy, and inflammation [21–26]. Fli-1 is expressed in ECs, macrophages, B cells, and T cells, and it regulates expression of several important cytokines including monocyte chemoattractant protein-1 (MCP-1), interleukin (IL)-6, and granulocyte colony-stimulating factor (G-CSF) [22, 23, 27–31]. A recent study further suggested that (1) Fli-1 is a key regulator of inflammation in ECs [21] and (2) Fli-1 deficiency promotes proliferation and cell survival of ECs [32].
This constellation of findings led us to explore the role of pericytes in the microvascular dysfunction of sepsis. We hypothesized that sepsis would result in pericyte loss through pyroptosis and be regulated by Fli-1. Furthermore, we hypothesized that Fli-1-regulated pericyte loss contributes to the vascular dysfunction of sepsis.
MATERIALS AND METHODS
Mouse Lung Pericytes Isolation, Culture, and Stimulation
Mouse lung pericytes were isolated as described previously [19, 33]. In brief, single-cell preparations from whole lung digests were expanded, negatively selected by CD31, CD45, and CD326 magnetic beads (Miltenyi Biotec Inc., Auburn, CA), and positively selected by PDGFRβ magnetic beads (Miltenyi Biotec Inc.). PDGFRβ-positive lung pericytes were cultured in pericyte medium (ScienCell Research Laboratories, Carlsbad, CA) supplemented with pericyte growth supplement, 2% fetal bovine serum, and 1% penicillin/streptomycin (ScienCell Research Laboratories). Pericytes (up to 3 passages) were seeded in 12-well plates and incubated with LPS (100 ng/mL) for 6 and 24 hours. Protein and mRNA levels of Fli-1 were determined by Western blot and real-time polymerase chain reaction (PCR), respectively. Pericytes were transfected with Fli-1-specific small interfering ribonucleic acid (siRNA) or scrambled siRNA by HiPerFect Transfection Reagent (QIAGEN, Germantown, MD) for 24 hours, followed by stimulation with LPS (100 ng/mL) for another 24 hours. Total RNA and supernatant were collected for further analyses. For ex vivo studies determining apoptosis, pyroptosis, and necrosis markers in lung pericytes by Western blot, the cells were freshly used without in vitro culture.
Cecal Ligation and Puncture-Induced Sepsis and Survival Study
C57BL/6J mice (7–8 weeks old) were housed in a pathogen-free environment. All procedures were complied with the standards for care and use of animal subjects as stated in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Resources, National Academy of Sciences, Bethesda, MD). The protocol for all animal studies was approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. All surgery was performed under anesthesia. Cecal ligation and puncture was performed as described previously [34]. In brief, the cecum was ligated at the colon juncture and punctured twice with a 22-gauge needle. All animals were fluid-resuscitated subcutaneously with sterile normal saline. Sham operation was performed in the same way as CLP but without ligation and puncture of the cecum.
Generation of Pericyte-Specific Fli-1 Knockout Mice
Foxd1-derived pericyte Fli-1 knockout mice were generated by crossing Foxd1tm1(GFP/cre)Amc (Foxd1 Cre, Stock No: 012463; The Jackson Laboratory) mice with Fli-1flox/flox mice. Pericyte Fli-1 knockout mice and littermate controls (7–8 weeks old) were subjected to CLP as described, and survival (n = 15 mice per group) was monitored for 7 days. Lung and kidney permeability was determined by Evans blue dye at 24 hours post-CLP [35]. Lung and kidney tissues were also collected at 12, 24, and 48 hours post-CLP for NG2 protein levels. In brief, the mice were sacrificed under anesthesia and perfused via the heart. The lung and kidney tissues were collected and their weights were measured. Equal weight tissues of each group were lysed with ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer (Cell Signaling, Danvers, MA) and used for Western blot analysis. In separate experiments, lung pericytes were isolated at 24 hours post-CLP, and total RNA and protein were collected for real-time PCR and Western blot, respectively. Foxd1 Fli-1 Td tomato (Foxd1 Fli-1 Td) mice were generated by crossing Foxd1 Fli-1 mice with the universal Cre-dependent reporter mice B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J ([Td] stock no. 012463; The Jackson Laboratory). Mice (7–8 weeks old) were subjected to sham or CLP. Lung tissues were collected at 12, 24, and 48 hours post-CLP for immunohistochemistry staining.
Mouse genotyping was performed by PCR using Terra PCR Direct Polymerase Mix (Clontech). Polymerase chain reaction was used to detect fragments of the wild-type (WT) Fli-1 and Fli-1flox/flox allele, as previously described [36]. The primers for Fli-1 PCR were as follows: forward, 5’-TAGTGACTCAGCCTTAACTCTC-3’ and reverse 5’-GAGTGTTGCCCTGCTCTCTAC-3’. A 250-base pair (bp) fragment indicated the presence of the WT allele, and a 490-bp fragment was amplified from the mutant allele. The primers for Cre PCR were as follows: forward, 5’-TGC CAC GAC CAA GTG ACA GCA ATG-3’ and reverse 5’-AGA GAC GGA AAT CCA TCG CTC-3’. A 400-bp fragment indicated the presence of the cre allele. The primers for Td tomato PCR were as follows: WT forward 5’-AAG GGA GCT GCA GTG GAG TA-3’ and WT reverse 5’-CCG AAA ATC TGT GGG AAG TC-3’. Mutant forward 5’-CTG TTC CTG TAC GGC ATG G-3’ and mutant reverse 5’-GGC ATT AAA GCA GCG TAT CC-3’. A 279-bp fragment indicated the presence of the WT allele, and a 196-bp fragment was amplified from the mutant allele.
Immunohistochemistry Staining and Confocal Microscopy
Lung tissues from Foxd1 Td mice and Foxd1 Fli-1 Td mice were fixed with 10% buffered formalin in phosphate-buffered saline and dehydrated. Frozen tissues were cut into 8-µm sections. The sections were stained with primary antibody against CD-31 (1:200; BD Biosciences, Franklin Lakes, NJ) followed by Alexa Fluor 488 Goat anti-rat IgG (H+L) Secondary Antibody (Thermo Fisher Scientific, Rockford, IL) and 4’,6-diamidino-2-phenylindole staining reagent (Thermo Fisher Scientific), as previously described [37]. Image capture and processing were performed using Leica TCS SP5 confocal microscopy (Leica Microsystems Inc., Buffalo Grove, IL). Fluorescence density of pericytes and ECs at 6 random fields per mice were analyzed with NIH ImageJ software to determine pericyte density and pericytes/EC coverage. The observer was blinded to the experimental groups.
Cytokine/Chemokine Assay
Mouse cytokine/chemokine production in the supernatants of lung pericytes was determined by Mouse Cytoline/Chemoline Array 31-Plex (Eve Technologies, Calgary, Canada).
Real-Time Reverse-Transcription Polymerase Chain Reaction
Total RNA was extracted from pericytes with RNeasy plus mini kit (QIAGEN). Complementary deoxyribonucleic acid (cDNA) was synthesized with High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was performed by Prism 7300 Real-Time PCR System (Applied Biosystems) using SYBR Green PCR Kit (QIAGEN) in a final reaction volume of 25 µL with each primer (QIAGEN). Data were analyzed with 2−ΔΔCt value calculation using glyceraldehyde 3-phosphate dehydrogenase for normalization.
Western Blot Analysis
Lung and kidney tissues or isolated lung pericytes were lysed with ice-cold RIPA lysis buffer (Cell Signaling). Western blot was performed as described previously [38]. All lysed samples were kept on ice for 30 minutes and centrifuged for 10 minutes at 4°C at 12000 ×g. The supernatant was collected and stored at −20°C until further analysis. Cell lysates were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. The membranes were blocked with 7% milk in TBST (20 mM Tris, 500 mM NaCl, and 0.1% Tween 20) for 1 hour. After washing with TBST twice, membranes were incubated with primary antibody overnight at 4°C. Fli-1 primary antibody was provided by Dr. Xiankui Zhang (Medical University of South Carolina). Primary antibodies α-tubulin and NG2 were from Cell Signaling. Caspase-3, caspase-1, and RIPK3 primary antibodies were from Novus Biologicals. The membranes were washed twice with TBST and incubated with horseradish peroxidase-conjugated secondary antibody in blocking buffer for 1 hour. After washing 3 times with TBST, immunoreactive bands were visualized by incubation with ECL plus detection reagents (GE Healthcare, Waukesha, WI). The densitometry of bands was quantified with Image J2 software.
Data Analyses
Data are expressed as means ± standard error of the mean. Statistical significance was determined by (1) analysis of variance with Fisher’s probable least-squares difference test and (2) Student’s t test or log-rank (Mantel-Cox) test using GraphPad Prism software. A value of P < .05 was considered statistically significant.
RESULTS
Cecal Ligation and Puncture Induces Lung and Kidney Pericyte Loss in Mice
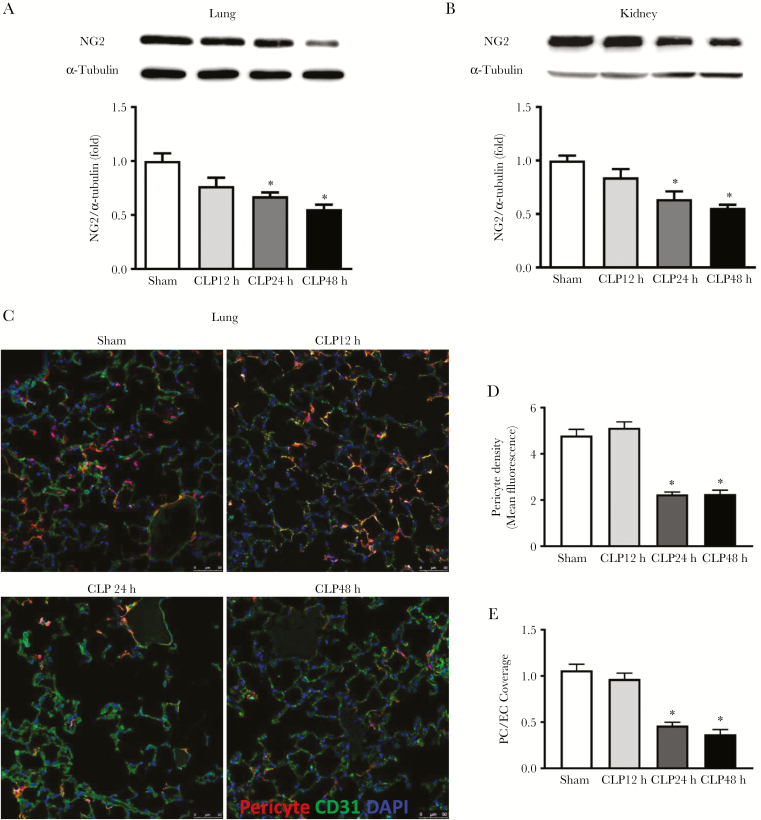
Lung and kidney injury are common complications of sepsis. Mice were subjected to sham or CLP, and lung and kidney tissues were collected at 12, 24, and 48 hours after surgery to determine pericyte viability. Lung and kidney protein levels of the pericyte marker NG2 were significantly decreased at 24 and 48 hours in the CLP group (lung: 32.6% ± 7.8% and 44.5% ± 4.0% reduction, respectively, P < .05; kidney: 36.1% ± 7.1% and 44.1% ± 2.8% reduction, respectively, P < .05) compared with the sham group (Figure 1A and B). Foxd1 Td tomato mice, which labeled Foxd1-derived pericytes with Td tomato reporter, were subjected to sham or CLP. Pericyte density and pericyte/EC coverage were also markedly reduced in the lungs of septic mice at 24 and 48 hours post-CLP surgery (Figure 1C–E).
Figure 1.
The effect of cecal ligation and puncture (CLP) on lung and kidney pericyte loss in mice. C57BL/6J and Foxd1 Td tomato mice were subjected to sham or CLP. Lung and kidney tissues were collected at 12, 24, and 48 hours post-CLP surgery. Lung (A) and kidney (B) tissue neural/glial antigen 2 (NG2) protein levels were determined by Western blot (n = 5 mice per group for sham and CLP 12-hour group; n = 4 mice for CLP 24-hour group; and n = 6 mice for CLP 48-hour group), and lung pericytes and endothelial cells (ECs) were visualized by confocal microscopy (C). Pericytes were labeled red, CD-31-positive ECs were labeled green, and nuclei were labeled blue by 4’,6-diamidino-2-phenylindole (DAPI). Scale bars are 50 μM. Images were taken at 6 random fields per mice, and pericytes density (D) and pericytes/EC coverage (E) were analyzed with ImageJ software (n = 3 mice for sham group and n = 4 mice per group for CLP groups). Data are expressed as means ± standard error. *, P < .05 compared with sham group by analysis of variance with Fisher’s probable least-squares difference test.
Fli-1 Is Up-Regulated in Lung Pericytes During Sepsis
To study whether the transcriptional factor Fli-1 plays a role in lung pericyte loss, we determined the expression levels of Fli-1 in lung pericytes in vivo and ex vivo. Fli-1 mRNA and protein levels were significantly increased (8.3 ± 0.9-fold and 2.1 ± 0.2-fold, respectively, P < .05) in the lung pericytes of septic mice at 24 hours post-CLP compared with those of sham-operated mice (Figure 2A and B). Lung pericytes isolated from control mice were treated with LPS for 6 and 24 hours. Upon LPS stimulation, both mRNA and protein levels of Fli-1 were significantly increased (1.4 ± 0.1-fold and 2.3 ± 0.5-fold, respectively, P < .05) (Figure 2C and D).
Figure 2.
The effect of cecal ligation and puncture (CLP) and lipopolysaccharide (LPS) on Fli-1 expression levels in lung pericytes. C57BL/6J mice were subjected to sham or severe CLP. Lung pericytes were isolated at 24 hours post-CLP. Messenger ribonucleic acid (mRNA) (A) and protein (B) levels of Fli-1 in lung pericytes were determined (n = 3–6 mice per group). *, P < .05 compared with sham group by unpaired Student t test. Lung pericytes were isolated from normal C57BL/6J mice and stimulated with LPS (100 ng/mL) for 6 and 24 hours. The mRNA (C) and protein (D) levels of Fli-1 were determined (n = 3–4). Data are expressed as means ± standard error. *, P < .05 compared with control group by analysis of variance with Fisher’s probable least-squares difference test.
Knockout of Fli-1 Attenuates Cecal Ligation and Puncture-Induced Lung and Kidney Pericyte Loss, Vascular Leak, and Mortality
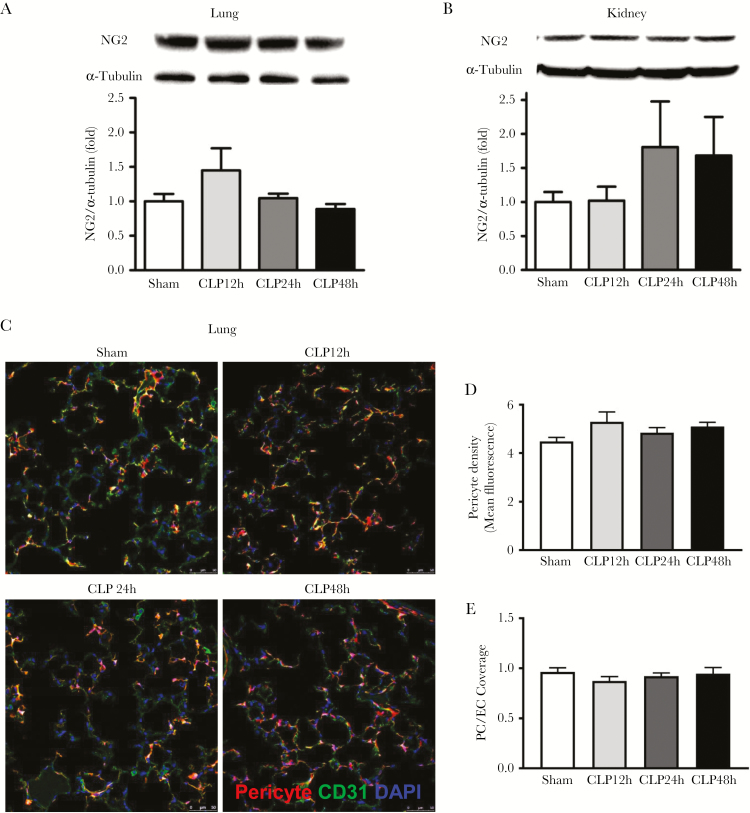
To further determine whether Fli-1 regulates lung and kidney pericyte loss during sepsis, pericyte Fli-1 knockout mice were used. Knockout of Fli-1 in pericytes was confirmed by Western blot analysis (Supplementary Figure S1). Pericyte Fli-1 knockout restored lung and kidney NG2 protein levels in septic mice at 24 and 48 hours post-CLP surgery (P > .05) (Figure 3A and B) and rescued lung pericyte density and pericytes/EC coverage in septic mice (P > .05) (Figure 3C–E). In addition, knockout of Fli-1 significantly reduced lung and kidney vascular leak at 24 hours post-CLP surgery (39.1% ± 6.5% reduction for lung and 40.1% ± 5.0% reduction for kidney, P < .05) (Figure 4A and B) and improved survival in septic mice (40% improvement, P < .05) (Figure 4C).
Figure 3.
Pericyte Fli-1 knockout reverses cecal ligation and puncture (CLP)-induced lung and kidney pericyte loss. Foxd1 FLi-1 KO mice and Foxd1 Fli-1 Td tomato mice were subjected to sham or CLP. Lung and kidney tissues were collected at 12, 24, and 48 hours post-CLP surgery. Lung (A) and kidney (B) tissue neural/glial antigen 2 (NG2) protein levels were determined by Western blot (n = 4 mice for CLP 48-hour group, and n = 5 mice per group for the other groups), and lung pericytes and endothelial cells (ECs) were visualized by confocal microscopy (C). Pericytes were labeled red, CD-31-positive ECs were labeled green, and nuclei were labeled blue by 4’,6-diamidino-2-phenylindole (DAPI). Scale bars are 50 μM. Images were taken at 6 random fields per mice, and pericytes density (D) and pericytes/EC coverage (E) were analyzed with ImageJ software (n = 3 mice for sham group, and n = 4 mice per group for CLP groups).
Figure 4.
Pericyte Fli-1 knockout (KO) reverses cecal ligation and puncture (CLP)-induced lung and kidney vascular leakage and mortality. Wild-type (WT) and Foxd1 Fli-1 KO mice were subjected to sham or CLP. Lung (A) and (B) kidney vascular leakage (n = 4 mice per group) and mice survival ([C] n = 15 mice per group) were determined. Survival of mice was monitored for 7 days. Mice numbers in each group were labeled as survived/total mice. Data are expressed as means ± standard error. *, P < .05 compared with WT sham group; #, P < .05 compared with WT CLP group by analysis of variance with Fisher’s probable least-squares difference test. Survival was analyzed by log-rank (Mantel-Cox) test.
Fli-1 Regulates Lipopolysacharide-Induced Inflammatory Response in Lung Pericytes
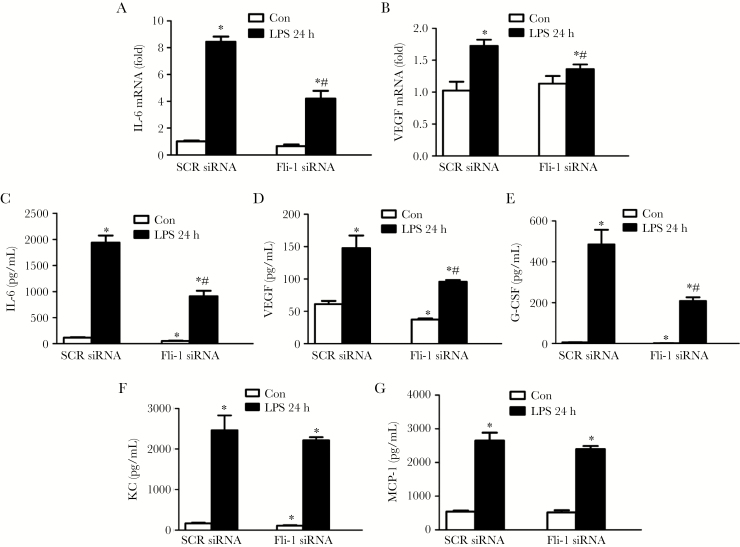
To examine the role of Fli-1 in LPS-induced lung pericyte activation, we knocked down Fli-1 using Fli-1 siRNA. Real-time PCR confirmed significant Fli-1 knockdown (Supplementary Figure S2). Exposure of lung pericytes to LPS significantly increased both mRNA and protein levels of IL-6 (8.4 ± 0.4-fold and 16.7 ± 1.2-fold, respectively, P < .05) and vascular endothelial growth factor ([VEGF] 1.7 ± 0.1-fold and 2.4 ± 0.3-fold, respectively; P < .05), which were reversed in cells transfected with Fli-1 siRNA compared with scrambled siRNA (Figure 5A–D). In addition, disrupted Fli-1 expression blocked LPS-induced G-CSF production (57.1% ± 3.8% reduction, P < .05) but had no effect on keratinocyte chemoattractant and MCP-1 protein levels in supernatants of lung pericytes (P > .05) (Figure 5E–G).
Figure 5.
Fli-1 regulates inflammatory responses in lung pericytes. Lung pericytes were isolated from C57BL/6J mice and transfected with Fli-1 small interfering ribonucleic acid (siRNA) or scrambled siRNA. Then, pericytes were further stimulated with lipopolysaccharide (LPS) (100 ng/mL) for 24 hours. The messenger RNA (mRNA) levels of interleukin (IL)-6 (A) and vascular endothelial growth factor (VEGF) (B) were measured. Protein levels of IL-6 (C), VEGF (D), granulocyte colony-stimulating factor (G-CSF) (E), keratinocyte chemoattractant (KC) (F), and monocyte chemoattractant protein-1 (MCP-1) (G) in the supernatant were also determined. N = 3 independent experiments. Data are expressed as means ± standard error. *, P < .05 compared with scrambled siRNA control group; #, P < .05 compared with scrambled siRNA LPS group by analysis of variance with Fisher’s probable least-squares difference test. Abbreviation: SCR, scramble.
Fli-1 Regulates Cecal Ligation and Puncture-Induced Pyroptosis of Lung Pericytes In Vivo
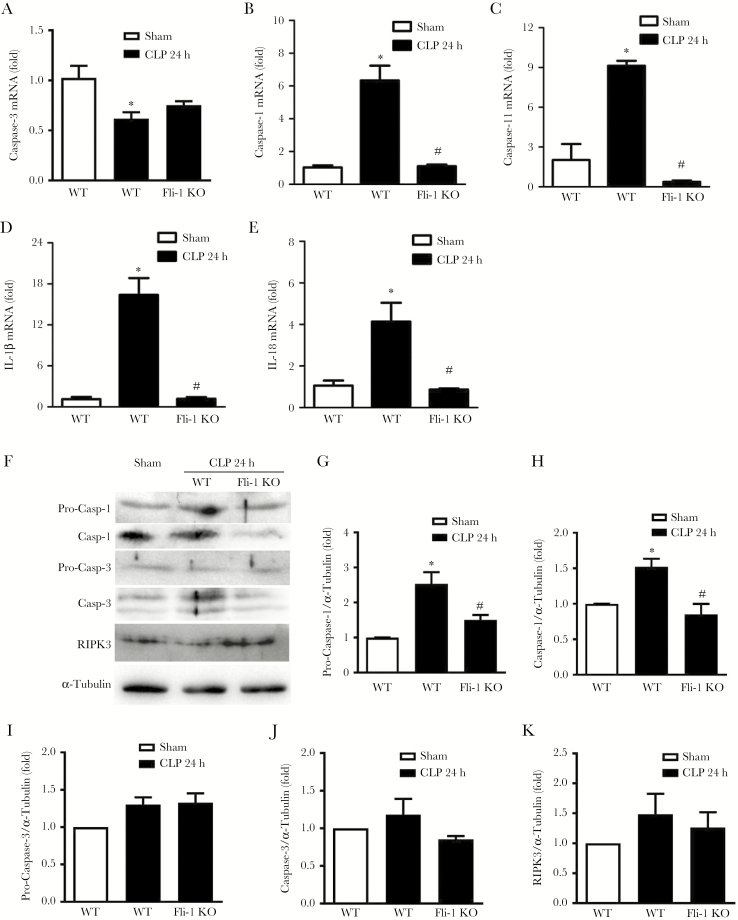
Lung pericytes are decreased by both LPS- [12] or CLP-induced sepsis. Thus, we investigated the possible mechanisms underlying the reduction of lung pericytes during sepsis. Expression levels of the apoptosis marker caspase-3, necrosis marker RIPK3, and pyroptosis markers caspase-1 and caspase-11 were determined by real-time PCR and/or Western blot. The expression levels of caspase-1, caspase-11, IL-1β, and IL-18 but not caspase-3 were significantly increased (6.3 ± 0.9-fold for caspase-1, 9.1 ± 0.7-fold for caspase-11, 16.4 ± 2.5-fold for IL-1β, and 4.1 ± 0.9-fold for IL-18, respectively; P < .05) in lung pericytes during sepsis (Figure 6A–E). However, knockout of Fli-1 in pericytes significantly attenuated CLP-induced caspase-1, caspase-11, IL-1β, and IL-18 mRNA levels (Figure 6B–E). Protein levels of procaspase-1 and activated caspase-1, but not pro-caspase-3, activated caspase-3, or RIPK3, were up-regulated in lung pericytes during sepsis (2.5 ± 0.3-fold for pro-caspase-1 and 1.5 ± 0.1-fold for activated caspase-1, respectively; P < .05), which was abrogated in Fli-1 pericyte knockout mice (Figure 6F–K).
Figure 6.
Fli-1 governs cecal ligation and puncture (CLP)-induced lung pericyte pyroptosis. Lung pericytes were isolated from sham wild-type (WT) mice or septic WT and pericyte Fli-1 knockout mice at 24 hours post-CLP surgery. The messenger ribonucleic acid (mRNA) levels of caspase-3 (A), caspase-1 (B), caspase-11 (C), interleukin (IL)-1β (D), and IL-18 (E) in lung pericytes were measured. Protein levels (F) of pro-caspase-1 (G), active caspase-1 (H), pro-caspase-3 (I), active caspase-3 (J), and RIPK3 (K) were also determined. N = 3 independent experiments. Data are expressed as means ± standard error from 12 mice per group (4 mice were used to get enough cells for each group at each time for 1 data point). *, P < .05 compared with sham group; #, P < .05 compared with WT CLP group by analysis of variance with Fisher’s probable least-squares difference test.
DISCUSSION
This study demonstrates several novel and important discoveries. First, sepsis leads to pericyte loss in the microvasculature of the lung and the kidney, 2 organs prone to microvascular dysfunction and injury during sepsis. Second, pericyte Fli-1 levels are elevated in response to sepsis, and knockdown of Fli-1 protects against pericyte loss and vascular dysfunction. Finally, Fli-1 governs pericyte loss in sepsis by facilitating pyroptosis. Collectively, these findings reveal a protective role of pericytes in the microvascular dysfunction of sepsis and a critical role of Fli-1 in pericyte survival. Thus, Fli-1 may be a novel target for treating sepsis in humans.
Increasingly, microvascular dysfunction and vascular leakage are recognized as important features of severe sepsis and septic shock. Decreased capillary blood flow has been observed in sepsis [39, 40], whereas disruption of the endothelial vascular barrier resulting in microvascular leakage and multiple organ failure is a hallmark of the disease [4, 41, 42]. Pericytes regulate microvascular integrity by supporting ECs through local contact [11]. Lipopolysaccharide induces pericyte loss, which is accompanied by a significant increase in vascular leakage in the heart and lung [12, 43]. Consistent with this, we found that CLP-induced sepsis led to significant lung and kidney pericyte loss, which is, in part, attributed to increased Fli-1 leading to pyroptosis. Thus, therapies to protect pericytes from pyroptosis, perhaps through targeted disruption of Fli-1, may protect organs from microvascular dysfunction and improve sepsis outcomes. Further efforts to support pericyte homeostasis should be an area of future research.
Sepsis is also characterized by an overwhelming inflammatory response, which is, in part, driven by up-regulated cytokines and chemokines production. Fli-1 belongs to the Ets transcription factor family and regulates a variety of cellular processes, including the inflammatory response [22]. Recent studies have highlighted the critical role of Fli-1 in regulating inflammation as evidenced by its modulation of the expression of several important cytokines and chemokines including MCP-1, IL-6, G-CSF, and chemokine C-X-C motif ligand 2 (CXCL2) through direct binding to their respective promoters [21–23, 27]. We demonstrated that Fli-1 is a key regulator of the pericyte inflammatory response. Inhibition of Fli-1 expression by transfecting Fli-1-specific siRNA into lung pericytes blocked LPS-induced G-CSF, IL-6, and VEGF production (Figure 5). Both G-CSF and IL-6 are proinflammatory cytokines with known relevance to sepsis [44–46]. Furthermore, VEGF is a known vascular permeability factor with elevated circulating levels in sepsis [47] and an established causative role in sepsis-related lung injury [48]. Therefore, activated lung pericytes may contribute to both lung inflammatory response and vascular dysfunction via release of cytokines, chemokines, and VEGF, and Fli-1 may again be an important regulator of this process.
In addition to Fli-1’s direct impact on cytokine and chemokine transcription, its modulation of pyroptosis may further influence local inflammation and vascular leak. We found that pericyte pyrotposis, but not apoptosis or necrosis, contributed to lung pericyte loss in CLP-induced sepsis. Unlike apoptosis, pyroptosis leads to plasma membrane rupture and release of inflammatory cytokines, which can induce an inflammatory response in neighboring cells [15, 17]. These findings suggest that pericyte pyroptosis may be partially responsible for pericyte-mediated lung inflammation in LPS-treated mice [33] via released inflammatory mediators. Previous work has demonstrated that inhibition of pyroptosis reduces inflammatory cytokine production, attenuates liver injury, and improves survival in CLP-induced septic mice [16, 17]. Therefore, inhibition of pyroptosis may have a protective effect on lung pericyte loss and vascular dysfunction during sepsis, which needs further investigation. Our findings further demonstrated that pericyte Fli-1 knockout significantly attenuated CLP-induced lung pericyte pyroptosis. Thus, Fli-1 may represent a pivotal nexus for vascular inflammation in sepsis through its direct regulation of cytokine and chemokine production as well as its governance of pericyte pyroptosis. Zeng et al [12] found that mice treated with LPS significantly reduced the expression of Sirt3, Hif-2α, and Notch3 in the lung, and this signaling pathway is critical for LPS-induced lung pericyte loss. Our current findings showed that (1) Fli-1 is increased in lung pericytes during CLP-induced murine sepsis and (2) Fli-1 pericyte deletion attenuated lung pericyte loss. Thus, we further determined the expression levels of Sirt3, Hif-2a, and Notch3 in lung pericytes during CLP-induced sepsis. Our data showed that CLP significantly decreased Sirt3, Hif-2α, and Notch3 expression in lung pericytes; however, Fli-1 knockout had no significant effect on the expression of those genes (data not shown).
There are several limitations to our study. Although we used NG2 expression as a surrogate for pericytes cell number, we cannot rule out that sepsis caused decreased expression of NG2 in pericytes. However, confocal microscopy data showed that pericyte numbers were significantly reduced during sepsis. Due to the heterogeneity of the pericyte population, there is debate as to the definition of pericytes’ phenotypic characteristics, as well as their origin [49, 50]. The common markers expressed are PDGFRβ and NG2. We characterized the transcription factor Foxd1-derived pericytes in the lung tissues, and we demonstrated that most of the cells are PDGFRβ and NG2 positive [19]. Thus, Foxd1-derived pericyte Fli-1 KO mice were used in the in vivo studies.
CONCLUSIONS
In conclusion, our composite findings confirmed the critical importance of pericytes in vascular barrier integrity in sepsis and demonstrated a novel role of Fli-1 in CLP-induced lung and kidney pericyte loss and vascular dysfunction. Signaling pathways reducing Fli-1 expression need to be further explored as potential novel therapeutic approaches for sepsis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was funded in part by National Institute of General Medical Sciences grants 1R01GM113995 (to H. F.), 1K23HL135263-01A1 (to A. J. G.), UL1TR001450 (to P. V. H.), and R01 HL133751 (L. M. S.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Dolin HH, Papadimos TJ, Stepkowski S, Chen X, Pan ZK. A novel combination of biomarkers to herald the onset of sepsis prior to the manifestation of symptoms. Shock 2017; 49:364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 2002; 166:98–104. [DOI] [PubMed] [Google Scholar]
- 4. De Backer D, Orbegozo Cortes D, Donadello K, Vincent JL. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence 2014; 5:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou Y, Li P, Goodwin AJ, et al. Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol Ther 2018; 26:1375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Díaz-Flores L, Gutiérrez R, Madrid JF, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 2009; 24:909–69. [DOI] [PubMed] [Google Scholar]
- 7. Kottke MA, Walters TJ. Where’s the leak in vascular barriers? A review. Shock 2016; 46:20–36. [DOI] [PubMed] [Google Scholar]
- 8. Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 2005; 97:512–23. [DOI] [PubMed] [Google Scholar]
- 9. Dominguez E, Raoul W, Calippe B, et al. Experimental branch retinal vein occlusion induces upstream pericyte loss and vascular destabilization. PLoS One 2015; 10:e0132644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Armulik A, Genové G, Mäe M, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468:557–61. [DOI] [PubMed] [Google Scholar]
- 11. Nishioku T, Dohgu S, Takata F, et al. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol 2009; 29:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeng H, He X, Tuo QH, Liao DF, Zhang GQ, Chen JX. LPS causes pericyte loss and microvascular dysfunction via disruption of Sirt3/angiopoietins/Tie-2 and HIF-2alpha/Notch3 pathways. Sci Rep 2016; 6:20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 2009; 7:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu J, Jiang Y, Wang J, et al. Macrophage endocytosis of high-mobility group box 1 triggers pyroptosis. Cell Death Differ 2014; 21:1229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aglietti RA, Dueber EC. Recent insights into the molecular mechanisms underlying pyroptosis and gasdermin family functions. Trends Immunol 2017; 38:261–71. [DOI] [PubMed] [Google Scholar]
- 16. Chen YL, Xu G, Liang X, et al. Inhibition of hepatic cells pyroptosis attenuates CLP-induced acute liver injury. Am J Transl Res 2016; 8:5685–95. [PMC free article] [PubMed] [Google Scholar]
- 17. Hu Z, Murakami T, Suzuki K, et al. Antimicrobial cathelicidin peptide LL-37 inhibits the pyroptosis of macrophages and improves the survival of polybacterial septic mice. Int Immunol 2016; 28:245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quintero-Ronderos P, Laissue P. The multisystemic functions of FOXD1 in development and disease. J Mol Med 2018. doi: 10.1007/s00109-018-1665-2 [DOI] [PubMed] [Google Scholar]
- 19. Hung C, Linn G, Chow YH, et al. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 2013; 188:820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 2014; 124:2299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lou N, Lennard Richard ML, Yu J, Kindy M, Zhang XK. The Fli-1 transcription factor is a critical regulator for controlling the expression of chemokine C-X-C motif ligand 2 (CXCL2). Mol Immunol 2017; 81:59–66. [DOI] [PubMed] [Google Scholar]
- 22. Sato S, Lennard Richard M, Brandon D, et al. A critical role of the transcription factor fli-1 in murine lupus development by regulation of interleukin-6 expression. Arthritis Rheumatol 2014; 66:3436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suzuki E, Karam E, Williams S, Watson DK, Gilkeson G, Zhang XK. Fli-1 transcription factor affects glomerulonephritis development by regulating expression of monocyte chemoattractant protein-1 in endothelial cells in the kidney. Clin Immunol 2012; 145:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Akamata K, Asano Y, Yamashita T, et al. Endothelin receptor blockade ameliorates vascular fragility in endothelial cell-specific Fli-1-knockout mice by increasing Fli-1 DNA binding ability. Arthritis Rheumatol 2015; 67:1335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Theisen ER, Pishas KI, Saund RS, Lessnick SL. Therapeutic opportunities in Ewing sarcoma: EWS-FLI inhibition via LSD1 targeting. Oncotarget 2016; 7:17616–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takahashi T, Asano Y, Sugawara K, et al. Epithelial Fli1 deficiency drives systemic autoimmunity and fibrosis: possible roles in scleroderma. Int J Clin Exp Med 2017; 214:1129–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lennard Richard ML, Brandon D, Lou N, et al. Acetylation impacts Fli-1-driven regulation of granulocyte colony stimulating factor. Eur J Immunol 2016; 46:2322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao P, Yuan M, Ma X, et al. Transcription factor Fli-1 positively regulates lipopolysaccharide-induced interleukin-27 production in macrophages. Mol Immunol 2016; 71:184–91. [DOI] [PubMed] [Google Scholar]
- 29. Zhang XK, Watson DK. The FLI-1 transcription factor is a short-lived phosphoprotein in T cells. J Biochem 2005; 137:297–302. [DOI] [PubMed] [Google Scholar]
- 30. Zhang XK, Moussa O, LaRue A, et al. The transcription factor Fli-1 modulates marginal zone and follicular B cell development in mice. J Immunol 2008; 181:1644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richard EM, Thiyagarajan T, Bunni MA, et al. Reducing FLI1 levels in the MRL/lpr lupus mouse model impacts T cell function by modulating glycosphingolipid metabolism. PLoS One 2013; 8:e75175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Toyama T, Asano Y, Miyagawa T, et al. The impact of transcription factor Fli1 deficiency on the regulation of angiogenesis. Exp Dermatol 2017; 26:912–8. [DOI] [PubMed] [Google Scholar]
- 33. Hung CF, Mittelsteadt KL, Brauer R, et al. Lung pericyte-like cells are functional interstitial immune sentinel cells. Am J Physiol Lung Cell Mol Physiol 2017; 312:L556–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fan H, Goodwin AJ, Chang E, et al. Endothelial progenitor cells and a stromal cell-derived factor-1alpha analogue synergistically improve survival in sepsis. Am J Respir Crit Care Med 2014; 189:1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Breithaupt-Faloppa AC, Breithaupt-Faloppa AC, Fantozzi ET, et al. Protective effect of estradiol on acute lung inflammation induced by an intestinal ischemic insult is dependent on nitric oxide. Shock 2013; 40:203–9. [DOI] [PubMed] [Google Scholar]
- 36. Asano Y, Stawski L, Hant F, et al. Endothelial Fli1 deficiency impairs vascular homeostasis: a role in scleroderma vasculopathy. Am J Pathol 2010; 176:1983–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li P, Bledsoe G, Yang ZR, Fan H, Chao L, Chao J. Human kallistatin administration reduces organ injury and improves survival in a mouse model of polymicrobial sepsis. Immunology 2014; 142:216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li P, Cook JA, Gilkeson GS, et al. Increased expression of beta-arrestin 1 and 2 in murine models of rheumatoid arthritis: isoform specific regulation of inflammation. Mol Immunol 2011; 49:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Farquhar I, Martin CM, Lam C, Potter R, Ellis CG, Sibbald WJ. Decreased capillary density in vivo in bowel mucosa of rats with normotensive sepsis. J Surg Res 1996; 61:190–6. [DOI] [PubMed] [Google Scholar]
- 40. Wafa K, Lehmann C, Wagner L, Drzymulski I, Wegner A, Pavlovic D. Desmopressin improves intestinal functional capillary density and decreases leukocyte activation in experimental endotoxemia. Microvasc Res 2015; 97:98–104. [DOI] [PubMed] [Google Scholar]
- 41. Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med 2011; 3:88ps25. [DOI] [PubMed] [Google Scholar]
- 42. Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med 2003; 31:2316–23. [DOI] [PubMed] [Google Scholar]
- 43. Ziegler T, Horstkotte J, Schwab C, et al. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J Clin Invest 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reilly JP, Anderson BJ, Hudock KM, et al. Neutropenic sepsis is associated with distinct clinical and biological characteristics: a cohort study of severe sepsis. Crit Care 2016; 20:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lloyd AR, Biragyn A, Johnston JA, et al. Granulocyte-colony stimulating factor and lipopolysaccharide regulate the expression of interleukin 8 receptors on polymorphonuclear leukocytes. J Biol Chem 1995; 270:28188–92. [DOI] [PubMed] [Google Scholar]
- 46. Pettilä V, Hynninen M, Takkunen O, Kuusela P, Valtonen M. Predictive value of procalcitonin and interleukin 6 in critically ill patients with suspected sepsis. Intensive Care Med 2002; 28:1220–5. [DOI] [PubMed] [Google Scholar]
- 47. van der Flier M, van Leeuwen HJ, van Kessel KP, Kimpen JL, Hoepelman AI, Geelen SP. Plasma vascular endothelial growth factor in severe sepsis. Shock 2005; 23:35–8. [DOI] [PubMed] [Google Scholar]
- 48. Kaner RJ, Ladetto JV, Singh R, Fukuda N, Matthay MA, Crystal RG. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol 2000; 22:657–64. [DOI] [PubMed] [Google Scholar]
- 49. Ferland-McCollough D, Slater S, Richard J, Reni C, Mangialardi G. Pericytes, an overlooked player in vascular pathobiology. Pharmacol Ther 2017; 171:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shi X, Zhang W, Yin L, Chilian WM, Krieger J, Zhang P. Vascular precursor cells in tissue injury repair. Transl Res 2017; 184:77–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.