We identified 67 posttreatment HIV controllers with sustained HIV remission after antiretroviral treatment interruption. Posttreatment control was more commonly identified amongst early treated individuals, frequently characterized by early transient viral rebound and heterogeneous durability of HIV remission.
Keywords: HIV, treatment interruption, posttreatment controller, HIV rebound, viral decay
Abstract
Background
HIV posttreatment controllers are rare individuals who start antiretroviral therapy (ART), but maintain HIV suppression after treatment interruption. The frequency of posttreatment control and posttreatment interruption viral dynamics have not been well characterized.
Methods
Posttreatment controllers were identified from 14 studies and defined as individuals who underwent treatment interruption with viral loads ≤400 copies/mL at two-thirds or more of time points for ≥24 weeks. Viral load and CD4+ cell dynamics were compared between posttreatment controllers and noncontrollers.
Results
Of the 67 posttreatment controllers identified, 38 initiated ART during early HIV infection. Posttreatment controllers were more frequently identified in those treated during early versus chronic infection (13% vs 4%, P < .001). In posttreatment controllers with weekly viral load monitoring, 45% had a peak posttreatment interruption viral load of ≥1000 copies/mL and 33% had a peak viral load ≥10000 copies/mL. Of posttreatment controllers, 55% maintained HIV control for 2 years, with approximately 20% maintaining control for ≥5 years.
Conclusions
Posttreatment control was more commonly identified amongst early treated individuals, frequently characterized by early transient viral rebound and heterogeneous durability of HIV remission. These results may provide mechanistic insights and have implications for the design of trials aimed at achieving HIV remission.
One of the highest priorities of the human immunodeficiency virus (HIV) field is the search for therapies that induce sustained antiretroviral therapy (ART)-free HIV remission. While discontinuation of ART leads to rapid viral rebound in the vast majority of individuals [1], a small subset can maintain control of HIV replication and provide evidence that natural control of HIV replication after an initial course of ART is possible [2–4]. However, the study of these posttreatment controllers has been hindered by how few of these individuals have been identified to date. This is due to a combination of factors, including (1) in clinical practice, patients are strongly discouraged from interrupting ART, (2) there are few trials involving a treatment interruption, and (3) within treatment interruption studies, the frequency of posttreatment control is low and their detection is hindered by early ART resumption.
Given the rarity of posttreatment controllers at a given clinical center or trial, the true frequency of this phenomenon has been difficult to ascertain, especially given the significant heterogeneity in both the study populations and posttreatment controller definitions [2–11]. The most comprehensive evaluation of posttreatment controllers to date has been the French VISCONTI cohort of 14 individuals [6], but this analysis was limited by the small size and the lack of participants treated during chronic HIV infection. In the Control of HIV after Antiretroviral Medication Pause (CHAMP) study, we report 67 posttreatment controllers identified through 14 treatment interruption studies involving more than 700 participants. This represents the largest number of posttreatment controllers reported to date and the results provide an estimated posttreatment controller frequency in both early and chronic-treated individuals, the range of posttreatment interruption viral load peaks, subsequent viral decay rates, and the durability of viral remission over time. As more clinical trials are conducted to test strategies for inducing post-ART HIV remission, treatment interruption will increasingly be employed to demonstrate their efficacy in delaying HIV rebound, reducing viral set points, and producing posttreatment controllers. Understanding the frequency of posttreatment control and their posttreatment interruption viral dynamics may provide mechanistic insights and has implications for the design of trials aimed at achieving HIV remission.
METHODS
Study Design and Participants
The CHAMP study includes participants from 8 AIDS Clinical Trials Group (ACTG) studies (ACTG 371 [12], A5024 [13], A5068 [14], A5102 [15], A5130 [16], A5170 [17], A5187 [18], and A5197 [19]), the Montreal Primary HIV Infection Cohort (Montreal PIC) [20], the Seattle Primary Infection Program (SeaPIP) [21], the University of California San Diego Primary Infection Cohort (UCSD PIC) [7], a National Institutes of Health (NIH) therapeutic vaccine trial [9], the University of California San Francisco (UCSF) OPTIONS study [22], and the Ragon HIV Controllers cohort (Supplementary Figure 1) [23]. Posttreatment controllers were defined as individuals who remained off ART for ≥24 weeks posttreatment interruption and maintained viral loads ≤400 copies/mL for at least two-thirds of the time points. Viral loads >400 HIV-1 RNA copies/mL were acceptable if the participant was subsequently able to suppress to ≤400 HIV-1 RNA copies/mL and maintained virologic control through week 24 posttreatment interruption. Early treated posttreatment controllers were identified from the following studies: ACTG 371, A5187, SeaPIP, Montreal PIC, UCSF OPTIONS, and the NIH study (Supplementary Table 1) [9, 12, 18, 21, 22, 24]. The posttreatment controller frequency for early treated participants was calculated for the 148 participants of ACTG 371, A5187, SeaPIP, and the NIH study. Posttreatment controller frequency for participants who initiated ART during chronic HIV infection was calculated for the 460 participants of A5024, A5068, A5102, A5130, A5170, and A5197. Additional details on the study cohorts and calculation of posttreatment controller frequency can be found in the Supplementary Methods.
Data and Statistical Analyses
Viral load and CD4+ cell dynamics were compared between posttreatment controllers and noncontrollers. Noncontrollers were ACTG participants who did not receive any immunologic interventions (eg, therapeutic vaccines), were virologically suppressed at the time of treatment interruption, and did not meet the posttreatment controller criteria after treatment interruption. The CD4+ cell decline analysis was performed for all participants who had ≥3 CD4+ count determinations during the first 24 weeks posttreatment interruption, at least 1 of which must be within 4 weeks of week 24. CD4+ cell slope was calculated with a linear regression equation. Early viral load peak was defined as the highest documented viral load during the first 24 weeks posttreatment interruption. Posttreatment controllers with viral load data 1 week prior to and after the viral load peak were defined as having weekly measurements. Viral decay rates for posttreatment controllers with an early viral load peak ≥1000 HIV-1 RNA copies/mL were calculated with the following formula: (Ln [peak viral load] − Ln [subsequent viral load]) / days in between. Viral decay rates for the posttreatment controllers were compared to the decay rates of 3 comparison groups: (1) noncontrollers from the ACTG treatment interruption studies; (2) untreated acutely infected participants from a published viral dynamics analysis [25] and from the UCSD PIC [26]; and (3) the phase 1 and 2 viral decay rates of individuals from 2 ACTG studies (A5160s and A5166s) initiating first-line nonnucleoside reverse transcriptase inhibitor (NNRTI)-based ART [27, 28]. Viral decay rate analysis for the posttreatment controllers, noncontrollers, and untreated acutely infected participants was limited to participants with available viral load within 1–2 weeks of the viral load peak to match the duration of first-phase viral decay found in NNRTI-treated individuals.
At each year posttreatment interruption, the point estimates of the proportion of posttreatment controllers who maintained viral control was calculated as follows: (N with viral control) / (N with viral control + N with documented loss of viral control before that time point). In the later years, a smaller number of participants had available viral load data and the uncertainty around the point estimates is depicted by an upper and lower bound. The upper bound is calculated by assuming that all participants who did not have viral load data through that time point continued to suppress HIV and the lower bound assumed that they had all lost viral control.
Wilcoxon-Rank sum tests were used for comparing continuous data and Fisher exact test was used to compare the posttreatment controller frequency of those initiating ART during early versus chronic infection.
RESULTS
A total of 67 posttreatment controllers were identified from the 14 studies enrolling over 700 participants, including 38 who were treated during early HIV infection and 25 who were treated during chronic infection (Supplementary Figure 2). Four individuals from the Ragon Controller studies with early ART initiation but incomplete laboratory records were categorized as having an “ambiguous” timing of ART initiation. There were no significant differences in the characteristics of those treated during early versus chronic infection with the exception that early treated individuals were more likely to receive a protease inhibitor-based regimen and received a shorter duration of ART prior to the treatment interruption (Table 1). The median duration of documented viral suppression after ART discontinuation was 89 weeks (Q1, Q3: 44, 174 weeks). Posttreatment controllers were more commonly identified in those treated during early versus chronic infection (13% vs 4%, P < .001). Including early treated participants from the UCSF OPTIONS and UCSD PIC cohorts, the lower and upper bounds of posttreatment controller frequency was 11% and 14%, respectively. Incorporating the chronic-treated participants from the UCSF OPTIONS study had no effect on the posttreatment controller estimate. Posttreatment controllers treated during early infection had slightly lower pre-ART viral load than noncontrollers (posttreatment controllers vs noncontrollers: 4.7 vs 4.9 log10 HIV-1 RNA copies/mL, P = .09).
Table 1.
Demographic Characteristics of Posttreatment Controllers Categorized by ART Initiation During Early or Chronic HIV Infection
| Characteristics | All (n = 67) |
Early Treated (n = 38) |
Chronic Treated (n = 25) |
P Value |
|---|---|---|---|---|
| Median age at treatment interruption, y (Q1, Q3) | 41 (35,47) | 38 (33,46) | 42 (39,49) | .12 |
| Sex, % | ||||
| Male | 81 | 84 | 76 | .52 |
| Female | 19 | 16 | 24 | |
| Race, % | ||||
| Black | 25 | 16 | 40 | .08 |
| White | 69 | 79 | 52 | |
| Hispanic | 4 | 3 | 8 | |
| More than 1 race | 1 | 3 | 0 | |
| Median duration of ART, weeks (Q1, Q3) | 195 (60,330) | 121 (53,242) | 289 (207,331) | <.001 |
| Combination therapy regimen, % | ||||
| NNRTI | 34 | 16 | 54 | .01 |
| PI | 58 | 70 | 46 | |
| INSTI | 3 | 5 | 0 | |
| Multiple | 5 | 8 | 0 | |
| Study, % | ||||
| AIDS Clinical Trials Group | 46 | 32 | 76 | |
| Montreal Primary HIV Infection Cohort | 9 | 16 | 0 | |
| Seattle Primary Infection Program | 4 | 8 | 0 | |
| Ragon HIV Controllers Cohort | 22 | 13 | 24 | |
| UCSF Options Cohort | 12 | 21 | 0 | |
| NIH | 6 | 11 | 0 | |
Four individuals have unclear timing of ART and thus are not included in either the early or chronic-treated groups.
Abbreviations: ART, antiretroviral therapy; INSTI, integrase strand-transfer inhibitor; NIH, National Institutes of Health; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; Q, quartile; UCSF, University of California San Francisco.
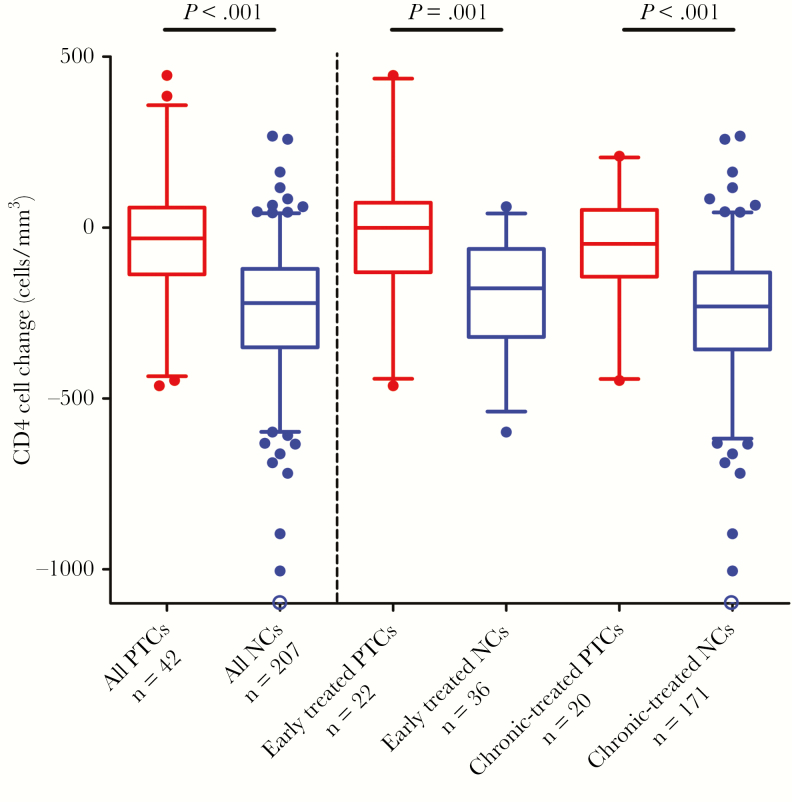
We evaluated CD4+ cell decline over the first 24 weeks of the treatment interruption. The median CD4+ cell counts prior to treatment interruption was 882 cells/mm3 for posttreatment controllers and 825 cells/mm3 for noncontrollers (P = .7). In the first 24 weeks after ART discontinuation, CD4+ levels were generally preserved in the posttreatment controllers, but declined in the noncontrollers (posttreatment controllers vs noncontrollers: −32 vs −221 CD4+ cells/mm3, P < .001; Figure 1). These findings were mirrored both in the early and chronic-treated participants.
Figure 1.
Changes in CD4+ cells in the initial 24 weeks of treatment interruption in posttreatment controllers (PTCs) versus noncontrollers (NCs). Boxes depict median and interquartile range; whiskers show the 5th and 95th percentile. Dots represent all data not contained within the whiskers. The open blue circle depicts a value of −2163 cells/mm3.
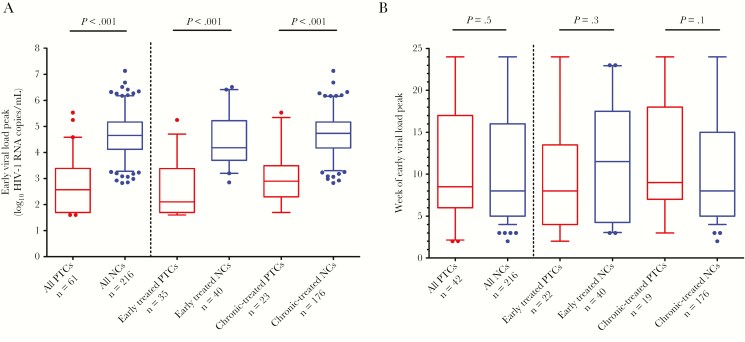
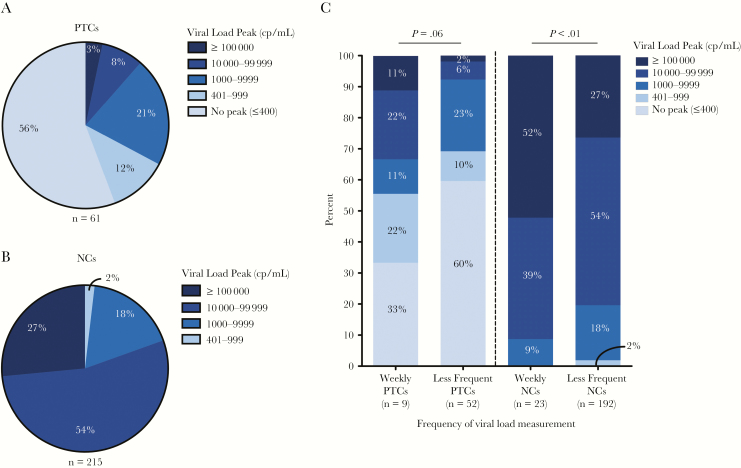
After the treatment interruption, a subset of posttreatment controllers had transient viral rebound in the first 24 weeks of treatment interruption before regaining viral control. The median viral load peak was lower for posttreatment controllers than noncontrollers (posttreatment controllers vs noncontrollers: median 2.6 log10 HIV-1 RNA copies/mL vs 4.7 log10 HIV-1 RNA copies/mL, P < .001; Figure 2A). This difference was consistent for individuals treated during early or chronic infection. In participants with detectable viral loads, no significant difference was measured for the time to peak viral load for posttreatment controllers versus noncontrollers (9 vs 8 weeks posttreatment interruption; Figure 2B). Amongst all posttreatment controllers, approximately 90% had a peak viremia <10000 HIV-1 RNA copies/mL and 70% had peak viral loads <1000 HIV-1 RNA copies/mL (Figure 3A). In contrast, 98% of noncontrollers had a peak viremia ≥1000 HIV-1 RNA copies/mL and 80% had a peak viral load ≥10000 HIV-1 RNA copies/mL (Figure 3B). As there were differences in the follow-up schedule among clinical studies, we evaluated the effect of viral load testing frequency on the magnitude of the viral load peaks. We compared the documented viral load peaks in participants who were undergoing weekly viral load monitoring (n = 9) compared to those with less frequent viral load monitoring (n = 52). Higher viral load peaks were more commonly observed in those with more frequent (ie, weekly) viral load monitoring. For instance, a greater proportion of posttreatment controllers with weekly measurements had viral load peaks ≥10000 HIV-1 RNA copies/mL versus those with less frequent viral load testing (33% vs 8%, P = .06; Figure 3C). When viral loads were measured on a weekly basis, 45% of the posttreatment controllers had viral load peaks ≥1000 HIV-1 RNA copies/mL versus 31% amongst posttreatment controllers with less frequent viral load sampling, although this comparison was not statistically significant. A similar trend of higher viral load peaks was also observed for the noncontrollers with more frequent posttreatment interruption viral load monitoring (Figure 3C).
Figure 2.
Highest viral load in posttreatment controllers (PTCs) versus noncontrollers (NCs) over the first 24 weeks of treatment interruption. A, Early viral load peak levels in PTCs versus NCs, also categorized by timing of ART initiation. B, The week of treatment interruption at which an early detectable viral load peak occurred. Boxes depict median and interquartile range; whiskers show the 5th and 95th percentile. Dots represent all data not contained within the whiskers. PTCs with ambiguous timing of ART start were excluded from the analysis that categorized PTCs by early versus chronic ART initiation (3 and 1 participants for A and B, respectively).
Figure 3.
Early viral load peaks during the first 24 weeks of treatment interruption. Proportion of posttreatment controllers (PTCs) (A) and posttreatment noncontrollers (NCs) (B) categorized by viral load peak. C, Proportion of viral load peaks in the PTCs and NCs for participants with weekly versus less frequent viral load measurements. P value represents Fishers exact test of PTCs with viral load peak ≥10000 HIV-1 RNA copies/mL with weekly viral load measurement versus less frequent monitoring. For NCs, the comparison is for the proportion with viral load peak ≥100000 HIV-1 RNA copies/mL. Abbreviation: Cp/mL, HIV-1 RNA copies/mL.
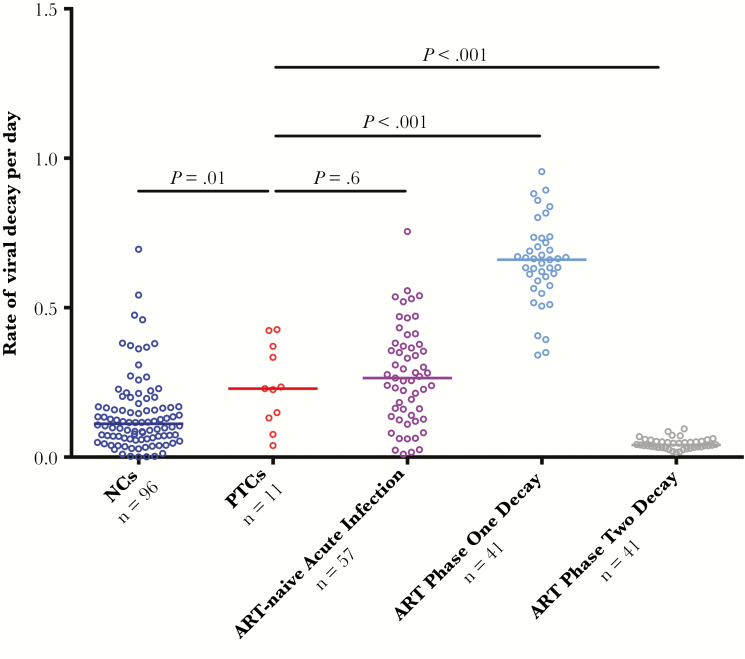
For posttreatment controllers with a viral load peak ≥1000 HIV-1 RNA copies/mL and subsequent viral load measurement 1–2 weeks later (Supplementary Figure 3), we calculated the rate of viral decay and compared it to the rates for 3 different groups: (1) noncontrollers after treatment interruption, (2) untreated viral decay rates during acute infection, and (3) phase 1 and 2 decay rates after ART initiation. Phase 1 viral load decay represents a steep decline in viral load during the first 2 weeks of ART while phase 2 decay reflects a slower subsequent viral load decrease [27, 28]. Overall, posttreatment controllers had a median decrease of 1.2 log10 HIV-1 RNA copies/mL over the subsequent 1–2 weeks. The ART-free viral decay of posttreatment controllers was found to be 0.23 per day, similar to the viral decay observed during untreated acute infection [25, 26] and significantly faster than that seen in the noncontrollers after treatment interruption (posttreatment controllers vs noncontrollers: 0.23 vs 0.11, P < .01; Figure 4). The viral decay in posttreatment controllers was also compared to that of NNRTI-based ART-naive individuals initiating an NNRTI-based regimen [27, 28]. Posttreatment controllers had a slower viral decay compared to the phase 1 decay after ART initiation (0.23 vs 0.66, P < .001) and faster than phase 2 decay after ART initiation (0.23 vs 0.04, P < .001).
Figure 4.
Rate of viral load decay in posttreatment controllers (PTCs) versus 3 comparator groups. Viral decay rates per day were compared between PTCs versus 3 comparator groups: (1) posttreatment noncontrollers (NCs) after treatment interruption, (2) antiretroviral therapy (ART)-naive participants during natural acute infection, and (3) phase 1 and 2 decay rates in participants initiating first-line nonnucleoside reverse transcriptase inhibitor-based ART from 2 previously published ACTG trials [27, 28].
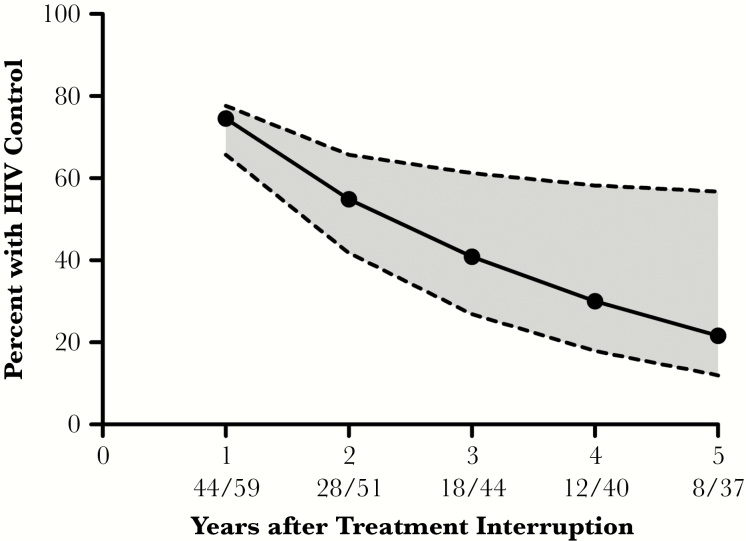
We also assessed the durability of posttreatment control in the first 5 years after treatment interruption. The proportion of posttreatment controllers who remained virologically suppressed in years 1–5 were 75%, 55%, 41%, 30%, and 22%, respectively (Figure 5). Of note, there was a high degree of uncertainty in the point estimates for durability of viral control in the later years due to an increasingly limited number of participants with available viral load data. There were no significant differences in the durability of posttreatment control between posttreatment controllers when categorized by the timing of ART initiation or by pretreatment interruption ART drug class (Supplementary Figure 4). Two posttreatment controllers, both treated during early infection, maintained documented viral control for more than 10 years after treatment interruption. One of these posttreatment controllers had a pre-ART viral load of 196000 copies/mL and initiated ART 5 weeks after diagnosis of infection. Pre-ART viral loads were not available for the other posttreatment controller.
Figure 5.
Durability of viral control. The solid line depicts the point estimates for the proportion of posttreatment controllers (PTCs) who maintained viral control at each time point. In the numbers under the X axis, the numerator represents PTCs who maintained viral control and the denominator are all PTCs with available data through this time point or were known to have lost viral control prior to this time point. Uncertainty around the point estimates is depicted by an upper and lower bound (dotted lines). For each time point, the upper bound is calculated by assuming that all participants who did not have virologic data maintained HIV control and the lower bound assumed that they had all lost viral control.
Amongst ACTG trial participants, 8 of the 19 (42%) chronically treated posttreatment controllers were identified from the A5068 study. The frequency of posttreatment control was 10% in A5068 versus 3% for all other ACTG studies enrolling chronic-treated participants (P = .01; Supplementary Figure 5A). A5068 was a 4-arm study evaluating the impact of an ALVAC-HIV vCP1452 therapeutic vaccine with or without multiple structured treatment interruptions [14]. The structured treatment interruptions involved 2 short treatment interruptions lasting 4–6 weeks, each followed by 16 weeks of ART, and a final longer period of treatment interruption. In A5068, the majority of posttreatment controllers (6 out of 8) were identified in the 2 study arms that included the structured treatment interruptions. Of those who underwent structured treatment interruptions, 15% eventually became posttreatment controllers as compared to only 5% of A5068 participants who underwent a single treatment interruption (Supplementary Figure 5B).
DISCUSSION
The presence of individuals who can maintain HIV suppression after discontinuing ART provides hope that the goal of sustained HIV remission is possible. However, as few patients undergo intensively monitored treatment discontinuations, the frequency of this phenomenon has been challenging to quantify. In published studies to date, a wide range of posttreatment controller frequencies have been reported, ranging from 0% to 26% of those undergoing treatment interruption [2–9]. These differences are likely influenced by factors such as small study sizes and heterogeneity, both in the timing of ART initiation and the precise posttreatment controller definitions. In addition, almost all of the previously reported posttreatment controllers have been individuals who initiated ART during acute/early infection [3, 4, 6, 9]. Posttreatment control in individuals treated during chronic infection has rarely been reported [8, 29] and this phenotype has not been well characterized. We performed a pooled analysis of over 700 participants of treatment interruption studies and found that 13% of those treated during early infection met our posttreatment controller definition, which was significantly higher than the 4% frequency of chronic-treated posttreatment controllers. This finding represents another benefit of early ART initiation and suggests that patients treated during early HIV infection may have a lower barrier to achieving HIV remission and may be a priority for clinical studies of HIV eradication strategies.
Prolonged ART interruptions in noncontrollers were associated with clinical events in the SMART study [30], but the extent of increased risk for posttreatment controllers is unclear. In ART-naive spontaneous controllers, CD4+ cell loss and clinical disease progression are frequently observed [31]. We found that posttreatment controllers maintained a stable CD4+ cell count over the first 24 weeks of treatment interruption, unlike noncontrollers, who lost a median of 221 CD4+ cells. Additional studies are needed to assess levels of systemic inflammation and whether this portends future morbidity given the increased immune activation observed in spontaneous controllers [32].
The evaluation of new therapies to achieve an ART-free remission of HIV infection will require demonstration of efficacy through ART interruption studies. However, these trials entail some potential risks, including possible clinical symptoms (eg, acute retroviral syndrome), immune damage, selection of HIV drug resistance, and HIV transmission to partners [33]. To prevent exposure of participants to extended periods of elevated viremia, modern treatment interruption trials often use the time to viral rebound as the primary outcome and ART is restarted once the viral rebound threshold has been reached. However, the optimal design of these studies is controversial, especially the viral load threshold at which participants would restart ART. A lower viral load threshold for reinitiating ART minimizes participant risk while a higher viral rebound threshold may allow more time for a robust HIV-specific immune response to be mounted and may identify more instances of posttreatment control. In this analysis, we show that the viral load threshold at which participants of treatment interruption trials restart ART may have a dramatic effect on the frequency of posttreatment controller identification as many posttreatment controllers demonstrate a transient elevated viremia prior to subsequent sustained virologic control. With weekly viral load monitoring, treatment interruption trials that restart ART at the 1000 HIV-1 RNA copy/mL threshold will miss almost half of the posttreatment controllers, while trials that use a 10000 HIV-1 RNA copy/mL threshold will miss a third of posttreatment controllers. These results may be helpful to guide investigators as they weigh the risks and benefits of different study designs for future treatment interruption studies.
While nearly half of posttreatment controllers demonstrated viral load peaks above 1000 HIV-1 RNA copies/mL, we observed a relatively rapid subsequent decline in viremia with a median decrease of 1.2 log10 HIV-1 RNA copies/mL over the subsequent 2 weeks. The rate of viral decay in these individuals was compared to the HIV decay rate after peak viremia in noncontrollers, individuals during untreated acute infection, and those initiating first-line NNRTI-based ART. We found that the viral decay rate in posttreatment controllers was significantly faster than that of noncontrollers and similar to the viral decay rate observed after peak viremia in untreated acute HIV infection. Compared to individuals initiating first-line NNRTI-based ART, the viral decay rate of posttreatment controllers was slower than phase 1 decay rates, but faster than phase 2 decay rates. During untreated acute HIV infection, the decline in viral load after peak viremia coincides with the development of HIV-specific cell-mediated immunity [34]. The differences in viral decay between posttreatment controllers and noncontrollers after treatment interruption may reflect a more robust cell-mediated immune response in the posttreatment controllers, although the rate of viral decay is also likely influenced by other factors, including viral fitness, availability of target cells, humoral immunity, and other host antiviral responses.
The results also show that posttreatment control is not always durable, with approximately half of individuals maintaining viral control at 2 years posttreatment interruption and 1 in 5 posttreatment controllers able to sustain HIV remission for at least 5 years posttreatment interruption. However, there was substantial uncertainty in the point estimates for durability of viral control given the increasingly limited number of participants with available data over time. This was primarily due to variability in the follow-up period for the various studies. These results show that routine longitudinal monitoring for HIV rebound is indicated for the posttreatment controllers. Additional studies are needed to also assess the mechanisms of HIV suppression in posttreatment controllers and the causes of eventual virologic failure.
Interestingly, we noted that the frequencies of posttreatment control were not uniform across studies, especially amongst those enrolling participants treated in chronic infection. Specifically, we found that a greater percentage of A5068 participants were found to be posttreatment controllers. In A5068, participants were randomized to receive continued ART, two 4 to 6-week cycles of structured treatment interruptions, ALVAC-HIV vCP1452 therapeutic vaccine, or therapeutic vaccine plus structured treatment interruptions [14]. All participants underwent a subsequent prolonged treatment interruption. The increased frequency of posttreatment control was especially evident in those participants who were randomized to the study arms that included the sequential structured treatment interruptions, where the rates of posttreatment control rivaled that of participants treated during early infection. To date, there has not been a therapeutic vaccine strategy that has been shown to effectively induce sustained HIV remission. The “autovaccination” hypothesis proposes that viral reactivation during structured treatment interruptions may stimulate effective HIV-specific responses to the individual’s viral antigens. This approach has yielded more limited success in other studies with varying structured treatment interruption protocols and study populations [35, 36], but should be explored further as a potential strategy for achieving HIV remission.
This study has several limitations. First, we did not have pre-ART viral loads for all participants, especially those who were treated during chronic infection and we cannot rule out the presence of spontaneous HIV controllers. Second, the frequency of posttreatment control is highly dependent on the posttreatment controller definition, including the viral rebound threshold and minimum duration of control. For instance, if the definition of posttreatment control was modified to include only participants who maintained viral suppression for ≥90% of the posttreatment interruption time points, the frequency of posttreatment control would be reduced to 8% of early treated and 2% of chronic-treated participants. We included posttreatment controllers who maintained viral remission for at least 24 weeks, which is a shorter time frame than some other studies [6]. This criterion excluded the vast majority of participants of treatment interruption trials as median to viral rebound is approximately 3–4 weeks [1]. Importantly, this definition also allowed us to take a broader view of the spectrum of posttreatment control and to assess the durability of posttreatment control over time, an important characteristic that could not be studied in the timeframe of previous trials.
This paper represents a major collaborative effort in the field and for the first time provides the estimated frequency of posttreatment control in both early and chronic-treated individuals, viral rebound dynamics after treatment interruption, and the rate of loss of viral remission over time. Our understanding of how posttreatment controllers achieve sustained HIV remission remains incomplete. A concerted international effort is needed to identify posttreatment controllers and to study the determinants of posttreatment HIV control.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments. We thank the participants, staff, and investigators who contributed to the included studies.
Financial support. This work was supported by the Harvard University Center for AIDS Research (CFAR) (National Institute of Allergy and Infectious Diseases grant number 5P30AI060354-08 to J. Z. L. and R. T. G); National Institutes of Health (NIH) (grant numbers AI125109 to J. Z. L., AI106039 to S. J. L., UM1 AI068634 to Statistical and Data Management Center of the AIDS Clinical Trials Group, UM1 AI068636 to AIDS Clinical Trials Group, UM1AI069419 to Cornell Clinical Trials Unit, UL1RR024996 to Cornell Clinical and Translational Science Center, subcontract from UM1 AI106701 to the Harvard Virology Support Laboratory and J. Z. L., AI100665 and AI036214 to D. Smith, AI111806 and AI125026 to A. C. C., and AI 27757 to University of Washington/Fred Hutch Center for AIDS Research, AI127966 to S. D., U01 AI41531 to F. M. H. Other support was provided by the Foundation for AIDS Research (amfAR) Institute for HIV Cure Research (grant number amfAR 109301); Fonds de Recherche en Santé du Québec SIDA Maladies Infectieuses; and Canadian Institute of Health Research (grant number 385806).
Potential conflicts of interest. J. Z. L. has received research support and consulted for Gilead Sciences and Merck. R. T. G. has received educational grants from Gilead, Merck, and ViiV. D. R. K. serves as a consultant to and/or has received research support from Gilead, Janssen, Merck, and ViiV. P. V. chairs a Data Safety Monitoring Board for Merck. J.-P. R. received research support and consulted for Gilead, Merck, AbbVie, and ViiV. S. J. L. has received research support from Gilead and has consulted for GlaxoSmithKline. D. M. has consulted for Merck, and owns common stock in Gilead. D. Smith has consulted for Merck and AIDS Healthcare Foundation. D. Skiest has received research support from Gilead, ViiV, and GlaxoSmithKline. A. C. C. is a member of a Data Safety Monitoring Board for Merck and has received research support from Bristol-Myers-Squibb. S. D. has received grant support from Gilead, Merck and ViiV, has consulted for Janssen Pharmaceuticals, and is a member of the scientific advisory board from Enochian and Bryologyx. Other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Li JZ, Etemad B, Ahmed H, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 2016; 30:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goujard C, Girault I, Rouzioux C, et al. ; ANRS CO6 PRIMO Study Group. HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy. Antivir Ther 2012; 17:1001–9. [DOI] [PubMed] [Google Scholar]
- 3. Lodi S, Meyer L, Kelleher AD, et al. Immunovirologic control 24 months after interruption of antiretroviral therapy initiated close to HIV seroconversion. Arch Intern Med 2012; 172:1252–5. [DOI] [PubMed] [Google Scholar]
- 4. Maenza J, Tapia K, Holte S, et al. How often does treatment of primary HIV lead to post-treatment control?Antivir Ther 2015; 20:855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hocqueloux L, Prazuck T, Avettand-Fenoel V, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS 2010; 24:1598–601. [DOI] [PubMed] [Google Scholar]
- 6. Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. ; ANRS VISCONTI Study Group. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gianella S, Anderson CM, Richman DD, Smith DM, Little SJ. No evidence of posttreatment control after early initiation of antiretroviral therapy. AIDS 2015; 29:2093–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Assoumou L, Weiss L, Piketty C, et al. ; ANRS 116 SALTO Study Group. A low HIV-DNA level in peripheral blood mononuclear cells at antiretroviral treatment interruption predicts a higher probability of maintaining viral control. AIDS 2015; 29:2003–7. [DOI] [PubMed] [Google Scholar]
- 9. Sneller MC, Justement JS, Gittens KR, et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med 2017; 9:pii: eaan8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin GE, Gossez M, Williams JP, et al. ; the SPARTAC Trial Investigators. Post-treatment control or treated controllers? Viral remission in treated and untreated primary HIV infection. AIDS 2017; 31:477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wen Y, Li JZ. Post-treatment HIV controllers or spontaneous controllers in disguise?AIDS 2017; 31:587–9. [DOI] [PubMed] [Google Scholar]
- 12. Volberding P, Demeter L, Bosch RJ, et al. ; ACTG 371 Team. Antiretroviral therapy in acute and recent HIV infection: a prospective multicenter stratified trial of intentionally interrupted treatment. AIDS 2009; 23:1987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kilby JM, Bucy RP, Mildvan D, et al. ; Adult AIDS Clinical Trials Group A5024 Protocol Team. A randomized, partially blinded phase 2 trial of antiretroviral therapy, HIV-specific immunizations, and interleukin-2 cycles to promote efficient control of viral replication (ACTG A5024). J Infect Dis 2006; 194:1672–6. [DOI] [PubMed] [Google Scholar]
- 14. Jacobson JM, Pat Bucy R, Spritzler J, et al. ; National Institute of Allergy and Infectious Diseases-AIDS Clinical Trials Group 5068 Protocol Team. Evidence that intermittent structured treatment interruption, but not immunization with ALVAC-HIV vCP1452, promotes host control of HIV replication: the results of AIDS Clinical Trials Group 5068. J Infect Dis 2006; 194:623–32. [DOI] [PubMed] [Google Scholar]
- 15. Henry K, Katzenstein D, Cherng DW, et al. ; A5102 Study Team of the AIDS Clinical Trials Group. A pilot study evaluating time to CD4 T-cell count <350 cells/mm(3) after treatment interruption following antiretroviral therapy +/- interleukin 2: results of ACTG A5102. J Acquir Immune Defic Syndr 2006; 42:140–8. [DOI] [PubMed] [Google Scholar]
- 16. Gandhi RT, O’Neill D, Bosch RJ, et al. ; AIDS Clinical Trials Group A5130 Team. A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine 2009; 27:6088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skiest DJ, Su Z, Havlir DV, et al. ; AIDS Clinical Trials Group 5170 Study Team. Interruption of antiretroviral treatment in HIV-infected patients with preserved immune function is associated with a low rate of clinical progression: a prospective study by AIDS Clinical Trials Group 5170. J Infect Dis 2007; 195:1426–36. [DOI] [PubMed] [Google Scholar]
- 18. Rosenberg ES, Graham BS, Chan ES, et al. ; AIDS Clinical Trials Group A5187 Team. Safety and immunogenicity of therapeutic DNA vaccination in individuals treated with antiretroviral therapy during acute/early HIV-1 infection. PLoS One 2010; 5:e10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schooley RT, Spritzler J, Wang H, et al. ; AIDS Clinical Trials Group 5197 Study Team. AIDS clinical trials group 5197: a placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J Infect Dis 2010; 202:705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Routy JP, Vanhems P, Rouleau D, et al. Comparison of clinical features of acute HIV-1 infection in patients infected sexually or through injection drug use. J Acquir Immune Defic Syndr 2000; 24:425–32. [PubMed] [Google Scholar]
- 21. Stekler JD, Wellman R, Holte S, et al. Are there benefits to starting antiretroviral therapy during primary HIV infection? Conclusions from the Seattle Primary Infection Cohort vary by control group. Int J STD AIDS 2012; 23:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jain V, Liegler T, Vittinghoff E, et al. Transmitted drug resistance in persons with acute/early HIV-1 in San Francisco, 2002-2009. PLoS One 2010; 5:e15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pereyra F, Jia X, McLaren PJ, et al. ; International HIV Controllers Study. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2010; 330:1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehraj V, Cox J, Lebouche B, et al. Socio-economic status and time trends associated with early ART initiation following primary HIV infection in Montreal, Canada: 1996 to 2015. J Int AIDS Soc 2018; 21:e25034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stafford MA, Corey L, Cao Y, Daar ES, Ho DD, Perelson AS. Modeling plasma virus concentration during primary HIV infection. J Theor Biol 2000; 203:285–301. [DOI] [PubMed] [Google Scholar]
- 26. Little SJ, McLean AR, Spina CA, Richman DD, Havlir DV. Viral dynamics of acute HIV-1 infection. J Exp Med 1999; 190:841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haubrich RH, Riddler SA, Ribaudo H, et al. ; AIDS Clinical Trials Group (ACTG) A5160 and A5142 Study Teams. Initial viral decay to assess the relative antiretroviral potency of protease inhibitor-sparing, nonnucleoside reverse transcriptase inhibitor-sparing, and nucleoside reverse transcriptase inhibitor-sparing regimens for first-line therapy of HIV infection. AIDS 2011; 25:2269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuritzkes DR, Ribaudo HJ, Squires KE, et al. ; ACTG A5166s Protocol Team. Plasma HIV-1 RNA dynamics in antiretroviral-naive subjects receiving either triple-nucleoside or efavirenz-containing regimens: ACTG A5166s. J Infect Dis 2007; 195:1169–76. [DOI] [PubMed] [Google Scholar]
- 29. Maggiolo F, Di Filippo E, Comi L, Callegaro A. Post-treatment controllers after treatment interruption in chronically HIV-infected patients. AIDS 2018; 32:623–8. [DOI] [PubMed] [Google Scholar]
- 30. El-Sadr WM, Lundgren JD, Neaton JD, et al. ; Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–96. [DOI] [PubMed] [Google Scholar]
- 31. Leon A, Perez I, Ruiz-Mateos E, et al. ; EC and Immune Pathogenesis Working group of the Spanish AIDS Research Network. Rate and predictors of progression in elite and viremic HIV-1 controllers. AIDS 2016; 30:1209–20. [DOI] [PubMed] [Google Scholar]
- 32. Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008; 197:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li JZ, Smith DM, Mellors JW. The need for treatment interruption studies and biomarker identification in the search for an HIV cure. AIDS 2015; 29:1429–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koup RA, Safrit JT, Cao Y, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol 1994; 68:4650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fagard C, Oxenius A, Günthard H, et al. ; Swiss HIV Cohort Study. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch Intern Med 2003; 163:1220–6. [DOI] [PubMed] [Google Scholar]
- 36. Kaufmann DE, Lichterfeld M, Altfeld M, et al. Limited durability of viral control following treated acute HIV infection. PLoS Med 2004; 1:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.