Abstract
Significance: Heme binds to and serves as a cofactor for a myriad of proteins that are involved in diverse biological processes. Hemoproteins also exhibit varying modes of heme binding, suggesting that the protein environment contributes to the functional versatility of this prosthetic group. The subject of this review is a subset of hemoproteins that contain at least one heme regulatory motif (HRM), which is a short sequence containing a Cys-Pro core that, in many cases, binds heme with the Cys acting as an axial ligand.
Recent Advances: As more details about HRM-containing proteins are uncovered, some underlying commonalities are emerging, including a role in regulating protein stability. Further, the cysteines of some HRMs have been shown to form disulfide bonds. Because the cysteines must be in the reduced, dithiol form to act as a heme axial ligand, heme binds at these sites in a redox-regulated manner, as demonstrated for heme oxygenase-2 (HO2) and Rev-erbβ.
Critical Issues: HRM-containing proteins have wide variations in heme affinity, utilize different axial ligand schemes, and exhibit differences in the ability to act as a redox sensor—all while having a wide variety of biological functions. Here, we highlight HO2 and Rev-erbβ to illustrate the similarities and differences between two hemoproteins that contain HRMs acting as redox sensors.
Future Directions: HRMs acting as redox sensors may be applicable to other HRM-containing proteins as many contain multiple HRMs and/or other cysteine residues, which may become more evident as the functional significance of HRMs is probed in additional proteins.
Keywords: : heme, heme regulatory motif, disulfide, redox regulation, heme oxygenase, nuclear receptor
Introduction
The heme regulatory motif (HRM) is a short sequence containing a Cys-Pro core that, in many cases, binds heme with the Cys acting as an axial ligand. HRMs are found in a diverse set of proteins that have a wide variety of biological functions, but the study of HRMs began in earnest only relatively recently. In 1989, the DNA sequence of heme activator protein 1 (Hap-1), a yeast transcription activator, revealed a short sequence (Lys/Arg-Cys-Pro-Val/Ile-Asp-His) repeated six times over an ∼200 amino acid section of the protein that modulated DNA binding in response to heme (67). Then, in 1993, three copies of a similar sequence (Arg/Ser-Cys-Pro-Val/Ile-Leu-Ala) were identified by Lathrop and Timko in the precursor protein sequence of both the nonspecific (ALAS1) and erythroid-specific (ALAS2) forms of δ-aminolevulinic acid synthase. Two copies are located in the presequence for mitochondrial translocation that is proteolytically removed on import to form the mature protein, and mutation of both Cys residues to Ser in those copies eliminated the heme-dependent inhibition of mitochondrial transport of mouse ALAS2 (52). Thus, the name “heme regulatory motif” was bestowed on the short sequence by the authors of the report, and they additionally noted the presence of HRMs in sequences of catalase and hemopexin. Two years later, Zhang and Guarente also referred to the short sequences in Hap-1 as HRMs and identified HRMs in heme-regulated eIF2α kinase (HRI), heme lyase, and heme oxygenase-2 (HO2) (105).
The Zhang and Guarente report was much more defining to the field than simply cementing a name for the motif. First, an alignment of the sequences around the Cys-Pro dipeptide showed a tendency for a basic residue before the Cys and a hydrophobic residue after Pro (105), helping to define an HRM. Second, the report provided spectroscopic evidence of direct heme binding to a short peptide (Ala-Lys-Arg-Cys-Pro-Val-Asp-His-Thr-Met) corresponding to the consensus of the six HRMs of Hap-1 (105). Finally, based on mutational studies (Cys to Ala and Pro to Ala) of Hap-1, it was proposed that Cys acts as an axial ligand to Fe3+-heme and that Pro plays a structural role, possibly by exposing Cys for ligation (105). Thus, the study included several key factors in initially defining what an HRM is and how the motifs would be studied in the future.
The report also demonstrated that not all HRMs are created equal. The authors identified a seventh HRM (HRM7) in Hap-1 located near the activation domain closer to the C-terminus of the protein (the first six HRMs are located near the N-terminal DNA-binding domain) and demonstrated that it is HRM7 that modulates the activation of Hap-1 in response to heme (105), a finding that was confirmed by later studies that pointed to only a minor role of the other HRMs despite the ability of the peptide to bind heme (28, 33). Thus, given these findings on Hap-1 and ALAS2 demonstrating that HRMs even in the same protein can function differently, it became clear, and is one of the themes in this review, how important it is to characterize the function of the heme-binding event and to assess (as quantitatively as possible) heme binding to a designated HRM.
The functions assigned to HRMs
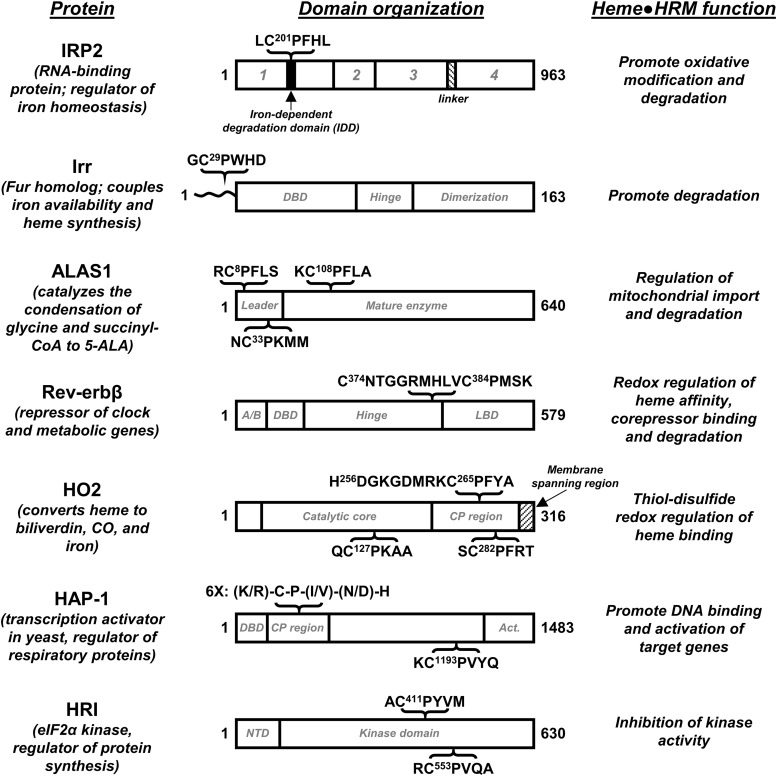
Assigning a function to a particular HRM is of great interest to those who study HRM-containing proteins. There does not appear to be one common function assignable to the HRMs—and it is believed that some HRMs have no functional relevance—so each HRM must be investigated thoroughly. Further, proteins that contain HRMs are varied, including transcription factors and enzymes involved in many different processes. Thus, the field includes a wide variety of approaches to study HRM-containing proteins, which has, in turn, led to the uncovering of a variety of effects of heme binding to an HRM (Fig. 1).
FIG. 1.
HRMs regulate the activity of enzymes, transcription factors, and an RNA regulatory protein. The modular structure of HRM-containing proteins is shown, along with the context and sequence of the HRMs. Domain organization (1–4) of IRP2 is based on its homology to aconitase (41, 74). Similarly, Irr is homologous to the Ferric Uptake Regulator (Fur) from Pseudomonas aeruginosa, and its domains are tentatively assigned as such; the N-terminal region of Irr that is distinct from Fur and contains the HRM is shown as a wavy line (29). The sequence of Rev-erbβ containing the HRM and redox-active Cys374 is shown; the A/B domain is hypervariable among members of the NR superfamily. For HO2, the sequence encompassing the heme ligand, His256 is shown in context to the nearby HRM. Hap-1 domain organization is based on functional studies (67); Act. refers to the domain that imparts transcription activation activity. NTD, or N-terminal domain of HRI is important for regulating quaternary structure (62). Delineation of domain boundaries are approximations. ALAS1, aminolevulinic acid synthase-1 nonspecific; Hap-1, heme activator protein-1; HO2, heme oxygenase isoform 2; HRM, heme regulatory motif; IRP2, iron regulatory protein 2; Irr, bacterial iron response regulator.
Despite a common function not having been assigned to HRMs, there are several examples of HRMs promoting heme-dependent protein degradation. The nuclear receptor (NR) Rev-erbβ, which represses the transcription of genes involved in regulating circadian rhythms and is described in detail later, is degraded in a heme-dependent manner (9). Heme binding to human Period-2 targets the protein for degradation, likely through the ubiquitin-proteasome pathway, and prevents formation of the heterodimeric complex between period-2 and human cryptochrome 1 that is involved in the regulation of circadian rhythms (94). Heme binding to tumor suppressor protein and metabolic regulator, p53, interferes with the ability of the protein to interact with DNA (14) (Fig. 2) and facilitates its nuclear export for cytosolic degradation (79). Similarly, the ability of Bach1, which regulates the expression of heme oxygenase-1 (HO1), to bind to DNA is significantly decreased on heme binding, which also targets Bach1 for nuclear export, polyubiquitination, and degradation (65, 82, 85, 103). Bradyrhizobium japonicum iron response regulator (Irr) regulates iron homeostasis and metabolism by acting as a repressor under low iron conditions and, under high iron conditions, is degraded on heme binding, allowing transcription to proceed (69, 97). Irr binds heme at both an HRM and a His-cluster site (His-His-His sequence) (39), and the protein appears to be degraded in a mechanism involving both sites that results in oxidative modification of the protein (46, 95, 96). ALAS1, the nonspecific form of δ-aminolevulinic acid synthase that catalyzes the first step of heme biosynthesis, contains three HRMs: two in the presequence involved in mitochondrial import (HRM1 and HRM2) and one near the N-terminus of the mature protein (HRM3) (52). HRM1 and HRM3 of rat ALAS1 were shown through mutagenesis studies to be critical for the heme-dependent inhibition of mitochondrial import (63). In addition, HRM3 of human ALAS1 was recently shown to be required for the heme-dependent formation of an ALAS1/ClpXP complex as well as for the oxidative modification of ALAS1 that triggers recruitment of LONP1, an ATP-dependent protease (48). Heme-dependent degradation on heme binding to HRMs has also been proposed for iron regulatory protein 2 (IRP2), a regulator of iron metabolism (38), and arginyl-transferase, which conjugates arginine to the N-terminus of proteins as a part of the N-end rule of protein degradation (35).
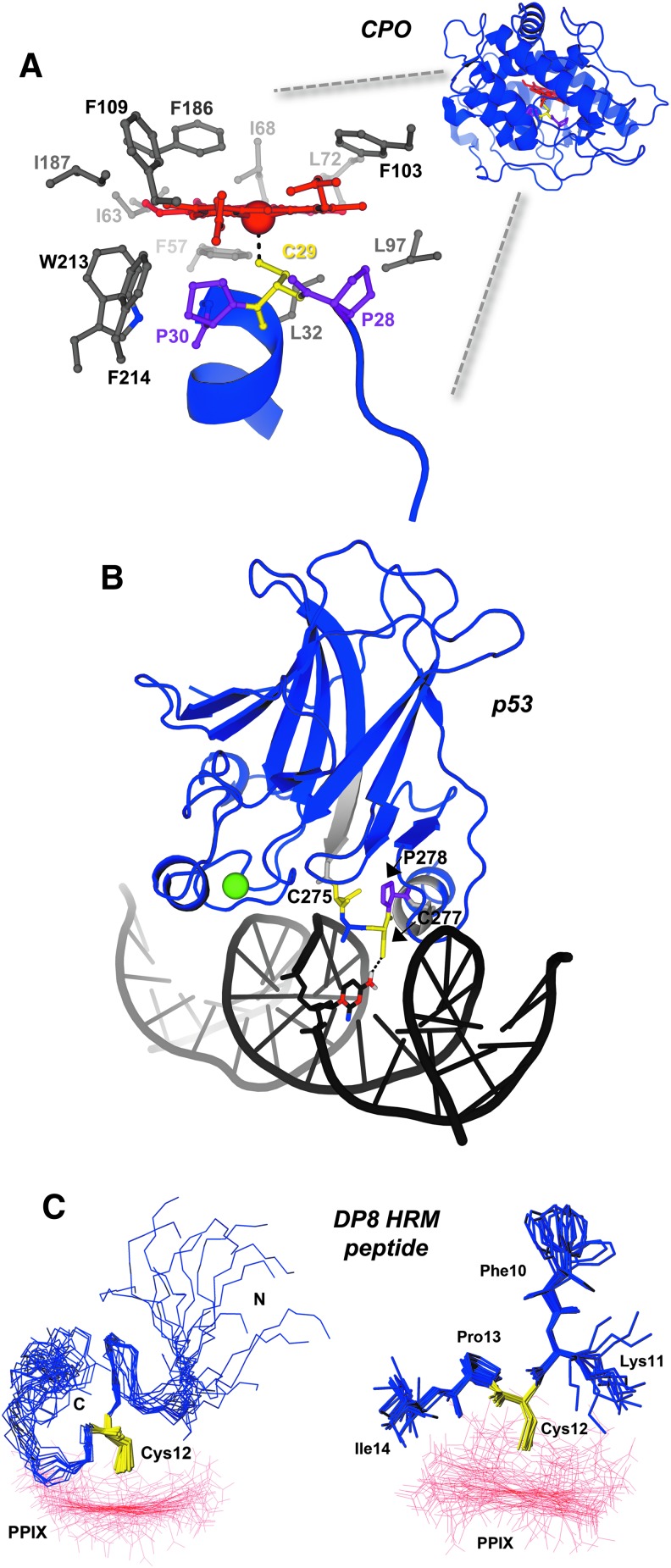
FIG. 2.
HRMs provide unique structural determinants to proteins and enzymes. (A) The overall structure of chloroperoxidase (PDB: 2ciw) shows that the HRM and Fe3+-heme cofactor are buried within the enzyme. A close-up view of the active site highlights the unique Pro-Cys-Pro HRM of CPO (prolines are shown as purple sticks, cysteine as yellow sticks), which positions the axial thiolate for heme ligation. Nonpolar residues within 4 Å of heme are shown as gray sticks. (B) p53, a transcription factor and tumor suppressor, is shown to be complexed with its cognate DNA promoter element (PDB: 1tsr). Residues flanking the HRM are shown as light gray cartoons to highlight the region, and a structural zinc atom is shown as a green sphere. (C) The NMR structure of an HRM-containing peptide from DP8 in complex with Ga3+-PPIX. Left panel, the 15 structures of lowest target function are shown with the peptide backbone as blue sticks, the HRM Cys12 in yellow sticks, and Ga3+-PPIX as red lines; the right panel depicts the inflexibility of the Cys-Pro core versus the side chains of surrounding residues. The structure serves as an excellent model demonstrating how the HRM Pro residue positions C-terminal residues away from heme, while poising the Cys-thiolate to act as a heme axial ligand. The structure of an IL-36α-derived HRM peptide in complex with Ga3+-PPIX recapitulates many of the features observed with the DP8 peptide (6). Figures in (C) are reprinted (adapted) with permission from Kühl et al. (50). Copyright (2013) American Chemical Society. CPO, chloroperoxidase; DP8, dipeptidyl peptidase 8; IL-36α, interleukin-36α; NMR, nuclear magnetic resonance; PPIX, protoporphyrin IX.
In contrast to the examples provided earlier of HRMs participating in heme-dependent protein degradation are the HRM-containing proteins Hap-1 and HRI. Hap-1, briefly discussed earlier as one of the proteins in which HRMs were initially identified, activates the transcription of genes involved in respiration and the control of oxidative damage in yeast in response to heme. Heme binding to HRM7 of Hap-1 promotes the transition of a high-molecular-weight complex containing Hap-1 and several other proteins to a Hap-1 homodimer that binds DNA with a high affinity (106). Recently, it has been shown that Hap-1 represses transcription of its own gene and several others in the absence of heme (32, 34), making the HRM of Hap-1 an important sensor of heme levels in yeast. HRI also appears to be a sensor of intracellular heme levels. However, heme binding to HRI at an HRM promotes the transition from an active to an inactive state. It is when reticulocytes are heme deficient that HRI (without heme bound to the HRM) autophosphorylates and, subsequently, phosphorylates eIF2α to downregulate protein synthesis, particularly globin synthesis (12, 37, 62).
In addition to the examples listed earlier, there are many more HRM-containing proteins in which the function of heme binding is unclear. For example, in contrast to what Lanthrop and Timko reported on the function of HRMs in mouse ALAS2 (52), a subsequent study showed no effect of heme on mitochondrial import of rat ALAS2 (63); thus, the relevance of the HRM is questioned. In addition, there are examples of proteins for which the function of the HRM remains elusive: HO2, which will be described in detail later; stanniocalcin-2, a glycoprotein shown to interact with HO1 (42); and DiGeorge critical region 8 (DGCR8), a protein involved in the first step of microRNA processing that appears to bind heme at a Pro-Cys (rather than Cys-Pro) site (19). Lastly, using a combinatorial peptide library approach, several other proteins were recently identified as having potential HRMs that may bind heme (6, 49, 50, 77). The authors used high-throughput screening to identify heme-binding peptides, and their sequences were then searched against protein sequence databases to identify proteins that may bind heme. Dipeptidyl peptidase 8 [(DP8), a member of the serine proteases (104)], FeoB [a bacterial ferrous iron transport protein (44)], and interleukin-36α [(IL-36α), a cytokine that induces inflammatory responses (21)] were, among other proteins, identified. The results suggest that not all HRM-containing proteins have even been identified, so the full extent of neither the capabilities of HRMs nor the physiological links between heme levels and metabolic pathways is fully understood.
Heme binding to HRMs
A critical point in assigning a function to an HRM-containing protein is to show direct heme binding to the HRM. Providing evidence of this HRM-heme interaction subsequently eliminates the possibility of indirect heme regulation and, particularly in the case of proteins such as Hap-1 (105) and Bach1 (65) that contain multiple HRMs, helps to distinguish responsive from unresponsive HRMs. Although X-ray crystal structures of only a few of the HRM-containing proteins (and their heme-bound forms) have been reported (Fig. 2), heme and heme-bound species lend themselves well to a variety of spectroscopic methods.
The classic model of the heme-HRM interaction includes Fe3+ of the heme in a penta-coordinated state with Cys acting an axial ligand. A shift in the absorbance of Fe3+-heme in aqueous solution from ∼385 nm to ∼370 nm in the presence of the HRM-containing peptide or protein is characteristic of Fe3+-heme binding to an HRM in this manner (105). Such complexes have an electron paramagnetic resonance (EPR) signal at g ∼ 6, although the signal is often broadened as compared with the signal for free Fe3+-heme in solution (39, 77), as shown later in the UV-vis and EPR spectra of some specific HRMs. Other spectroscopic methods such as resonance Raman (39, 50, 77), electron nuclear double resonance (ENDOR) (2), extended X-ray absorption fine structure (EXAFS) (2), and X-ray absorption (XAS) (2) have demonstrated Cys ligation of heme at HRMs and assisted in the characterization of the heme-HRM interaction.
Structural data available for HRM-containing peptides and proteins provide insights into how heme binds at these sites. Chloroperoxidase (CPO) is a versatile enzyme that uses heme to catalyze a variety of oxidative reactions: halogenation, dehydrogenation (heme peroxidase-like activity), hydrogen peroxide decomposition (catalase-like activity), and oxygen insertion (cytochrome P450-like activity). The structure of CPO shows the heme bound within an 8-helix bundle; the proximal side resembles cytochrome P450 and provides an axial thiolate (cysteine) ligand, whereas the distal side contains polar residues that form the peroxide-binding site, like other peroxidases (Fig. 2) (84). The Cys ligand in CPO resides within an unusual Pro-Cys-Pro motif on a helix that is nearly perpendicular to the heme plane, and the two prolines position the Cys for ligation to the heme. The only access to the Fe3+-heme is in the distal face, allowing only small organic substrates to interact with an iron-linked oxygen atom, accounting for its P450-like reaction. Another example of an HRM in a structured region is the Cys275-X-Cys277-Pro motif of the transcription factor, p53, which lies at the interface of the protein and its cognate DNA element (14) (Fig. 2). Cys277 of the HRM accepts a hydrogen bond from a nearby cytosine, but it is also responsible for binding heme along with neighboring C275 (14, 79). High heme levels drive binding to Cys275 and Cys277 and cause p53 dissociation from DNA, likely by disrupting the DNA:p53 hydrogen bond network.
In contrast, a number of HRMs are within disordered regions, as represented by the nuclear magnetic resonance (NMR) structures of two peptides spanning the HRM-containing regions of DP8 (Fig. 2) and IL-36α (6, 50). UV-vis, EPR, and resonance Raman spectroscopies demonstrated that both a 23-mer peptide (SGGLPAPSDFKCPIKEEEIAITSG) spanning residues S495-G517 of DP8 and a 9-mer peptide (SEGGCPLIL) spanning the HRM of IL-36α bound Fe3+-heme in a penta-coordinated state, with Cys acting as an axial ligand (6, 50, 77). Using gallium(III) protoporphyrin IX (PPIX), a diamagnetic Fe3+-heme mimic (5), the authors were able to show that structural definition is introduced on Ga(III) PPIX binding only to the central amino acids around Cys and that Pro positions the Cys for axial ligation while positioning the C-terminal residues away from Ga(III) PPIX for both DP8 and IL-36α peptides (6, 50). The surface binding of Ga(III) PPIX to the peptides, in contrast to the more structured and less solvent-exposed Fe3+-heme-binding pocket of CPO (Fig. 2), is consistent with HRMs being found in unstructured regions, such as in HO2 (2).
The HRM of Rev-erbβ is also in a flexible unstructured loop, but, in contrast to the structures of CPO and the DP8 peptide, His and Cys act as axial ligands to Fe3+-heme (Fig. 3) (66). Although the structure will be discussed in greater detail later, it demonstrates that Fe3+-heme bound to HRMs can also be hexa-coordinated. In fact, a growing number of proteins have been shown to bind Fe3+-heme at their HRM as a hexa-coordinated complex with Cys and an additional protein residue, usually His, acting as axial ligands. In addition to Rev-erbβ, HRI (37, 62) and HO2 (20) also bind Fe3+-heme with Cys and His ligands. These proteins have characteristic absorbance at ∼420 nm and a low-spin rhombic EPR signal with g-values of 2.5, 2.3, and 1.9. Resonance Raman has also been shown to be useful in distinguishing penta- and hexa-coordination (39, 50).
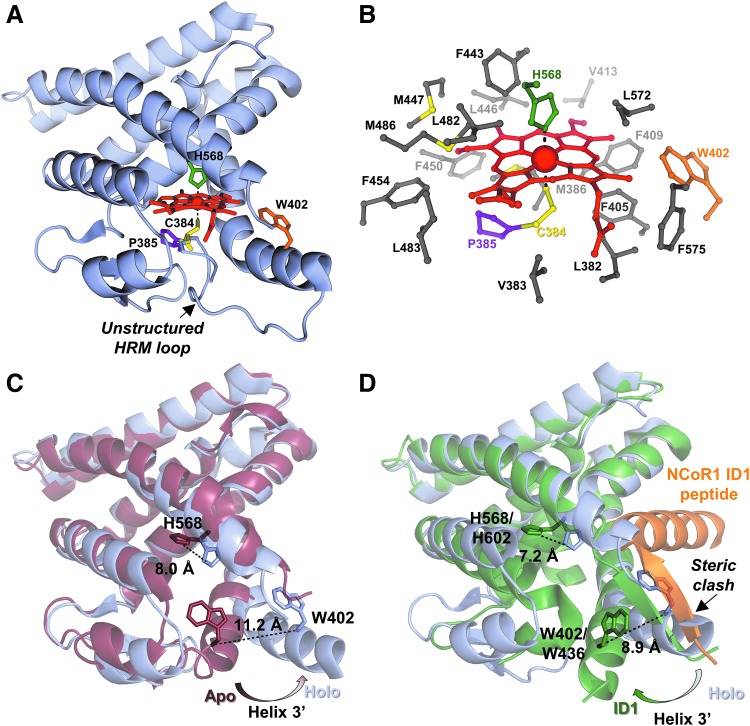
FIG. 3.
Structural insight into HRM-mediated redox regulation of heme binding to nuclear receptor, Rev-erbβ. (A) Structure of the Rev-erbβ LBD in complex with Fe3+-heme (holo-LBD, PDB: 3cqv). Heme binds in a 6-coordinate, low-spin complex with His (H568, green sticks) and Cys (C384, yellow sticks) axial ligands. C384 and P385 (purple sticks) comprise an HRM core that resides on a flexible, unstructured loop. W402 (orange sticks) provides hydrophobic contacts to heme, and its fluorescence is quenched on heme binding (unpublished observations). (B) The Rev-erbβ heme pocket is highly hydrophobic with nonpolar residues within 4 Å of heme shown as gray sticks (66). (C) Heme binding induces conformational changes of the LBD. Overlay of apo-LBD (dark red; PDB: 2v0v) and holo-LBD (light blue) structures aligned in Pymol; heme and the flexible HRM-harboring loop from the holo-LBD structure are omitted for clarity. The H3' helix swings out to accommodate the porphyrin ring, leading to a 11.2 Å shift of the W402 α-carbon. Further, conformational changes in helix 11 cause the τ-nitrogen of H568 to shift 8.0 Å, and assume its role as a Fe3+-heme ligand. (D) Pymol structural alignment of holo-LBD (light blue) versus apo-Rev-erbα LBD (dark green) in complex with an NCoR1 ID1 peptide (orange), PDB: 3n00; again, heme and the HRM loop are omitted for clarity. An anti-parallel beta sheet formed between an unraveled portion of Rev-erbα helix 11 and the NCoR1 peptide exhibits steric clash with helix H3' in the holo-LBD structure. Further, the heme axial ligand, H602 in Rev-erbα pivots away from the heme-binding pocket, suggesting that heme and NCoR1 would compete for different Rev-erb conformers. ID, interaction domain; LBD, ligand-binding domain; NCoR1, nuclear receptor compressor 1.
Although spectroscopy may simplify the assignment of the ligands as Cys and His, actually identifying the His residue can be a challenge. In each of the three examples—Rev-erbβ, HRI, and HO2—the His is located far outside of the Cys-Pro sequence motif of the HRM. Further, likely due to the surface binding of Fe3+-heme to some HRMs rather than in a more defined binding pocket, Fe3+-heme binds to some HRM-containing proteins in multiple conformations. For example, Fe3+-heme bound to the HRM of Irr is in a mixture of penta- and hexa-coordinated states (39), complicating the assignment of ligands. A mixture of states was also observed for several peptides used in recent combinatorial peptide library studies (6, 49, 50, 77). For instance, an Fe3+-heme-bound peptide, TPILCPFHL, based on the sequence of IRP2 displayed UV-vis absorbance, EPR, and resonance Raman spectra consistent with a mixture of penta- and hexa-coordinated Fe3+-heme. The NMR structure of the Ga(III) PPIX-bound peptide is in the penta-coordinated state, but by the admission of the authors, there is insufficient distance between the Cys and His to form a hexa-coordinated species unless a second peptide were present (6). The histidine in this peptide corresponds to His204 in the protein, and this residue is not involved in Fe3+-heme binding to Cys201 of the HRM. Rather, His204 is postulated to be necessary for protein degradation and the binding of Fe2+-heme (38). Indeed, the spacing between the HRMs and the His residues that act as axial ligands in proteins such as Rev-erbβ, HRI, and HO2 would be difficult to mimic with short peptides.
Although most of the HRMs that bind Fe3+-heme in the hexa-coordinated state use Cys and His as axial ligands, one example of a protein that does not is DGCR8. As mentioned earlier, the sequence of the HRM of DGCR8 is Pro-Cys, rather than Cys-Pro. Spectroscopic studies have concluded that DGCR8 binds Fe3+-heme with an unusual ligand arrangement of two Cys axial ligands (3). Fe3+-heme is postulated to bind at a dimer interface with the Cys of an HRM from each monomer acting as an axial ligand. Two Cys ligands are not likely to be a common ligation environment for heme, but the example of DGCR8 is given here to emphasize that since not all HRM-containing proteins have been characterized, protein residues other than His can be the sixth ligand to hexa-coordinated Fe3+-heme bound to HRMs.
HRMs and redox chemistry
This review focuses on Rev-erbβ and HO2, which have been extensively studied in our laboratory, leading to characterization of the function and the various modes of heme binding to HRMs. In both Rev-erbβ and HO2, the HRMs act as redox sensors in which the Cys residues form disulfide bonds, altering the affinity of the HRM for heme. Although this has thus far only been noted for Rev-erbβ and HO2, many HRM-containing proteins contain multiple HRMs and/or other Cys that may be available to form disulfide bonds. Therefore, by highlighting these two very different proteins, we hope to contribute to a more general understanding of HRMs and their potential link to the redox state of the cell.
Rev-Erb
Regulation of circadian rhythms by heme
Mammals are entrained to the diurnal cycle through light perception, a signal that is transduced via the optic nerve to the suprachiasmatic nucleus (SCN), a small region of the brain located at the optic chiasm (73, 76). Within the SCN, a set of core molecular clock proteins synchronize cellular processes through activation and repression of gene transcription. At the apex of the molecular clock are the Bmal1 and CLOCK genes, whose protein products form a heterodimeric complex that binds to the promoters of clock-controlled genes to activate transcription. Among those genes activated are Period 1, 2, and 3, Cryptochrome 1 and 2, and Rev-erbα and Rev-erbβ. Period (Per) and Cryptochrome (Cry) proteins form a complex that inhibits the activator function of Bmal1•CLOCK, whereas Rev-erbs repress the transcription of Bmal1 and CLOCK genes. Together, Per, Cry, and Rev-erbs comprise the negative limb of the molecular clock that completes the feedback loop required for circadian rhythm maintenance (47).
The interplay of heme with the molecular clock is emerging as an important signaling axis. For example, heme biosynthesis and intracellular heme levels are entrained to the clock through regulation of ALAS1 by the Bmal1•NPAS2 (a forebrain CLOCK homolog) heterodimer (43). Significantly, NPAS2 is also a hemoprotein that binds carbon monoxide (CO), which further regulates its DNA-binding activity (17). On the negative limb of the circadian oscillator, Per2 contains an HRM that binds Fe3+-heme and promotes its degradation (94). Further, Per2 expression is regulated by p53, another HRM-containing transcription factor and metabolic regulator as discussed earlier (61). Rev-erbα and β isoforms also contain a redox-sensitive HRM that regulates heme binding, corepressor recruitment, and degradation (9, 11, 27). Because heme plays an integral role in energy production and metabolism, controlled degradation of Per2, Rev-erbs, and p53 through heme binding to their HRMs effectively couples those processes to the molecular clock. In the next section, we will discuss in detail the heme-dependent activities of Rev-erbs, and how they relate to circadian rhythm maintenance and entrainment of metabolic, inflammatory, and differentiation pathways.
Heme binding to Rev-erbα and Rev-erbβ indirectly facilitates corepressor recruitment
Rev-erbs are transcription factors that belong to the NR superfamily, which includes receptors of thyroid, estrogen, and testosterone hormones. In the ligand-bound state, many NRs recruit transcription coactivator complexes with histone acetyltransferase activity that ultimately leads to target gene activation (25). Rev-erbs are unique to the superfamily in that they lack a structural determinant required for coactivator binding, rendering them as repressors of transcription (93). The α and β isoforms share >70% amino acid identity in their DNA- and ligand-binding domains (LBD), and they are highly redundant in terms of circadian expression patterns and their target genes (8, 13, 18). Rev-erbs repress transcription either by direct competition for promoter elements with the transcription activator, RAR-related orphan receptor (RORα), or by interacting with nuclear receptor corepressor (NCoR1) (1, 8, 13). NCoR1 is a 270 kDa protein that acts as a scaffold bringing together NRs and histone deacetylase complexes that enhance repression (40). As discussed earlier, Rev-erbs have been well studied in their role as circadian factors, but they also repress the transcription of key genes involved in glucose and lipid metabolism, inflammatory responses, differentiation, and body temperature regulation, entraining these processes to the clock (23, 24, 72, 88, 91, 102).
In 2007, two independent groups identified heme as the endogenous ligand for Rev-erbs (70, 102). Estimates of Rev-erbα/β affinity for Fe3+-heme using isothermal titration calorimetry (ITC) were between 0.35 and 3.52 μM (59, 70). Diminution of cellular heme levels with the ALA-dehydratase inhibitor, succinylacetone, reversed Rev-erbα-dependent repression of target genes by disrupting the interaction of Rev-erbα with NCoR1 (70, 102). There is also evidence that heme binding to both α and β isoforms promotes their degradation likely through a ubiquitin-dependent pathway involving the E3-ligases, Arf-bp1, and Myc-bp2 (9, 51, 100). Based on these observations, a model was proposed in which Rev-erbs rapidly equilibrate with the regulatory heme (RH) pool so that when heme levels are high, Rev-erbs exist as holoproteins bound to NCoR1 and repress target gene transcription in a fine balance with their own degradation; controlling transcription output via degradation has also been observed with the thyroid hormone receptor (15). Presumably, low heme levels occlude binding to Rev-erbs based on the high Kd of the complex, leading to NCoR1 dissociation and relief of repression (Fig. 4). A potential problem with this model is that the apparent weak affinity for heme (μM Kd) coupled with very low free (or exchangeable) cellular heme levels (<100 nM) would be inconsistent with the proposed (70) role of Rev-erbs as sensors of intracellular heme. The results of other in vitro studies indicated that the simple heme-based recruitment model was incomplete.
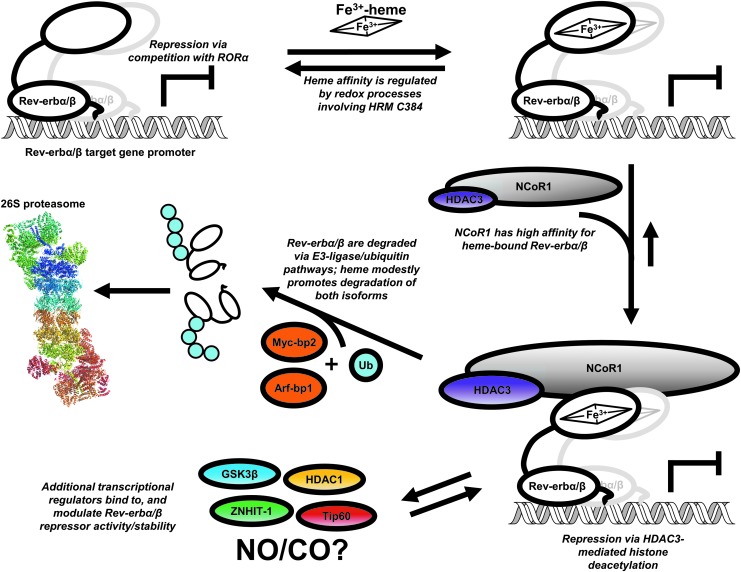
FIG. 4.
Heme-dependent repression and degradation of Rev-erbα/β. One mode of Rev-erbα/β repression is through competition with RORα for binding to its response element (ROR-RE) within the promoters of target genes. Rev-erbs can bind as monomers and homodimers to Rev-REs, although recent studies suggest that they may also form heterodimers (8, 13, 31). Although Fe3+-heme binds to Rev-erbβ with high affinity (Kd ≤0.1 nM), redox processes that cause dissociation of the Fe3+-heme axial thiolate ligand (including thiol-disulfide interconversion and Fe3+- to Fe2+-heme reduction) cause Kd to increase to ≥14 nM (11). Since the concentration of RH in the nucleus is ≤2.5 nM (30), loss of the HRM heme axial thiolate would cause dissociation of heme. Coimmunoprecipitation studies show that the NCoR1-HDAC3 complex has a high affinity for heme-bound Rev-erbα/β (70, 102); once bound, the complex leads to target gene repression via histone deacetylation. Additional regulators of Rev-erbβ repressor activity have been identified, including Tip60, an acetyl transferase, HDAC1, a histone deacetylase, and ZNHIT-1, a zinc-finger containing protein; GSK3β has also been shown to regulate the stability of the α-isoform (see main text for references); whether heme plays a role in regulating interactions of these proteins with Rev-erbs is unknown. Gaseous signaling molecules, NO and CO may also regulate the repressor function of Rev-erbs by binding to heme; however, additional studies are required to support this hypothesis. Although heme facilitates interaction of NCoR1 with Rev-erbs, it also appears to promote proteasomal degradation of both isoforms through a ubiquitin-dependent pathway involving E3-ligases Arf-bp1 and Myc-bp2. CO, carbon monoxide; HDAC1, histone deacetylase 1; NO, nitric oxide; RH, regulatory heme; RORα, RAR-related orphan receptor α.
Despite congruity among the cellular results described earlier, structural and in vitro studies provided evidence contrary to the heme-dependent NCoR1 recruitment model. The structure of the Fe3+-heme•Rev-erbβ complex shows heme bound in a six-coordinate system with axial ligands H568 and C384; the latter is provided by an HRM found on a flexible loop that is not present in the structure of the apoprotein (Fig. 3A, C) (66). The heme pocket is highly hydrophobic, stabilizing the nonpolar character of the porphyrin ring (Fig. 3B). Significant conformational changes are associated with heme binding to the apoprotein, namely a large shift of helix 3′ that opens the pocket to accommodate heme, a pivot of H568 to assume its position as a heme axial ligand, and the appearance of the disordered HRM loop that provides C384 (Fig. 3C) (93). More importantly, comparing the structures of the Rev-erbβ holoprotein to apoRev-erbα in complex with an NCoR1 peptide reveals a significant steric clash between helix 3 in the holoprotein structure and an anti-parallel beta sheet formed between helix 11 of Rev-erbα and the NCoR1 peptide (Fig. 3D) (68). The axial His residue, H602 in Rev-erbα, also swings out and away from the heme pocket in the NCoR1 structure, suggesting that heme and NCoR1 would not bind simultaneously to the LBD. Several in vitro studies supported the structural data and demonstrated that heme and cobalt-PPIX effectively compete with NCoR1 peptides for binding to purified Rev-erbα/β LBDs (59, 70, 102).
A potential caveat to the structural and in vitro studies was the use of truncated Rev-erbα/β LBDs and NCoR1 peptides that may not accurately represent the interactions between full-length proteins. For instance, the C-terminus of NCoR1 contains three NR interaction domains (IDs), two of which are known to associate with Rev-erbα (45); thus, the use of a single ID peptide for binding and structural studies may not be sufficient to promote cooperative interactions between different IDs on a single polypeptide. Thus, it seemed likely that contacts among the Rev-erb N-terminus, DNA-binding domain, and hinge region may impart unique biochemical properties and heme-dependent activities unobservable in experiments utilizing isolated LBDs.
To discover whether the in vitro and cellular experiments would coincide if we used the full-length Rev-erb instead of the LBD alone, we developed strategies to purify full-length Rev-erbβ (FLRev-erbβ) and an NCoR1 construct (instead of the short peptide) containing all three IDs (9). First, we found through the use of electromobility shift assays and UV-vis spectroscopy that heme and NCoR1, indeed, form a ternary complex with Rev-erbβ under equilibrium conditions, indicating the LBD is flexible in solution and can accommodate both ligands. However, heme was not required for the interaction of NCoR1 with the FLRev-erbβ•DNA complex in a purified system and heme does not promote NCoR1 binding when present at stoichiometric levels with respect to Rev-erbβ. In stark contrast to those results, heme is required for coimmunoprecipitation of endogenous NCoR1 from HEK 293 cell extracts with Rev-erbβ. Further, wild-type recombinant FLRev-erbβ co-precipitated with NCoR1, however a variant in which the His axial ligand is substituted with Phe (H568F), which exhibits a micromolar Kd for heme binding, is incapable of interacting with NCoR1. Similar results were previously observed with Rev-erbα and the cognate H602F variant, indicating that heme is essential for the interaction of both Rev-erb isoforms with NCoR1 (102). Thus, we hypothesize that an unidentified cellular component present in soluble extracts promotes the heme-dependent NCoR1 recruitment to Rev-erbs. Numerous candidates are worth investigating, especially those proteins shown to modulate Rev-erbα and β stability or repressor activity, including glycogen synthase kinase 3β, which phosphorylates Rev-erbα promoting its stability (101); Tip60, an acetylase, and histone deacetylase 1 (HDAC1) that regulate Rev-erbβ repressor activity by adding, or removing an acetyl group from an RXKK motif (90); and ZNHIT-1, a zinc-finger protein that interacts with Rev-erbβ at the apo-CIII promoter, inhibiting its repressor activity (89). Heme-dependent interaction between any of these proteins and Rev-erb has not been investigated (Fig. 4).
An alternative hypothesis to explain the missing link between heme binding and NCoR1 recruitment is that diatomic gaseous signaling molecules, primarily nitric oxide (NO) and CO, could regulate Rev-erbα/β repressor activity. Both the truncated Rev-erbβ LBD and FLRev-erbβ bind CO with low nanomolar Kds, but CO appears to have no effect on repressor activity in a cellular system (9, 27, 66). NO, on the other hand, has been shown to inhibit Rev-erbα/β repressor activity in cultured cells (66), but those results have yet to be validated in vivo, or the mechanistic details revealed. For example, does NO binding cause dissociation of NCoR1? What is the affinity of Fe2+-heme•Rev-erbα/β for NO, and is that value consistent with the intracellular concentrations of NO? Does NO have secondary effects such as nitrosylation of Rev-erb zinc-finger thiolates as has been observed with other NRs (10)? In sum, additional studies are required to clarify the role, if any, of diatomic gases in Rev-erb regulation of target genes.
UV-vis spectrophotometric titrations revealed that Fe3+- and Fe2+-heme bind FLRev-erbβ with at least one order of magnitude higher affinity (low nanomolar Kds) than those early estimates using ITC. Further, based on competition experiments, dissociation of Fe3+-heme from the FLRev-erbβ•heme complex was too slow to allow rapid equilibration of Rev-erbβ with RH (10−6 s−1 = <1 day−1), negating its role as a sensor of intracellular Fe3+-heme (9). If so, does the HRM serve any regulatory function other than to bind heme and poise the receptor for interaction with NCoR1? In the next section, we discuss results suggesting that Rev-erbs may exploit the redox properties of the HRM cysteine thiolate and Fe3+-/Fe2+-heme couple to regulate heme affinity and corepressor recruitment.
Role of heme dissociation in redox signaling by Rev-erbβ
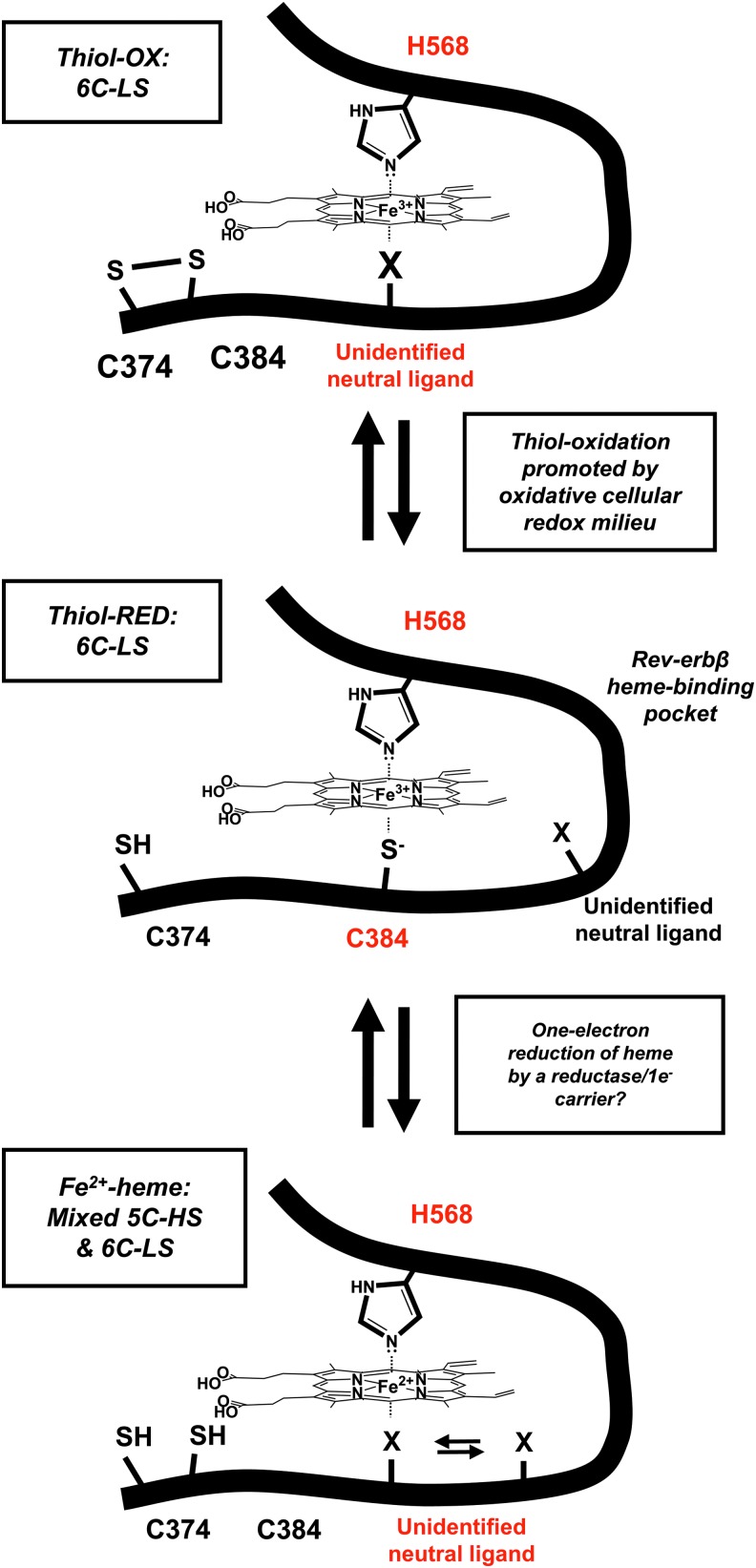
Early biophysical studies of Rev-erbβ described UV-vis, resonance Raman, EPR, and magnetic circular dichroism (MCD) spectra consistent with a low-spin 6-coordinate His/Cys ligated Fe3+-heme, as observed in the crystal structure shown in Figure 3A (58). However, reduction by dithionite to Fe2+-heme leads to dissociation of the C384 axial thiolate and its replacement by an exchangeable neutral ligand (Fig. 5). Since the loop containing the HRM is largely disordered and is not present in the structure of the apoprotein, we assume that dissociation of C384 from heme would result in significant conformational changes. UV-vis equilibrium titrations suggested that Rev-erbβ binds both Fe2+- and Fe3+-heme with similar low nanomolar affinity (9, 27). However, as discussed later, most equilibrium techniques vastly underestimate heme affinities and Fe2+-heme may not remain bound under the limiting heme concentrations in the cell. Several important questions require investigation to determine whether Fe3+/Fe2+-heme switching plays a role in regulating Rev-erb activity. For instance, what is the midpoint potential of the Fe3+/Fe2+-heme•Rev-erb couple, and what is the redox state of heme bound to Rev-erbs in the cell? Is the Fe3+/Fe2+-heme switch sensitive to changes in intracellular redox poise? If it is, what one-electron carrier or reductase is responsible for mediating Rev-erbα/β reduction?
FIG. 5.
Rev-erbβ undergoes redox-mediated heme ligand switching. The thiol-reduced form of the Rev-erbβ LBD (thiol-RED; C374 and C384 exist as thiols/thiolates) binds Fe3+-heme in a 6C-LS complex with H568 and C384 axial ligands and a Kd of ∼100 pM. Under oxidizing conditions, C384 forms a disulfide with neighboring C374 (thiol-OX), leading to a 6C-LS system where an unidentified neutral ligand assumes the position once occupied by the thiolate. Thiol-OX has a Kd for Fe3+-heme of ≥14 nM, well above the nuclear RH level of ≤2.5 nM, suggesting that thiol-OX would exist as an apoprotein in the nucleus. Our working hypothesis is that C374–C384 thiol-disulfide interconversion is in equilibrium with the GSH:GSSG or cysteine:cystine couples and reflects the redox poise of the nucleus. Similarly, the one-electron reduction of Fe3+- to Fe2+-heme is accompanied by dissociation of the HRM heme axial thiolate, leading to a mixed 5C-HS/6C-LS system where an unidentified neutral ligand (presumably the same ligand involved in the thiol-RED/OX ligand switch) is loosely associated with heme. Although it is unclear what heme redox state is relevant to Rev-erbβ in the cell, it is intriguing to consider one-electron reductants that could interface with Rev-erbβ, such as ferredoxins or cytochrome P450 reductase, although nuclear localization of the reductant is presumably required. 5C-HS, five-coordinate high spin; 6C-LS, six-coordinate low-spin.
Another potential redox switch regulating Rev-erbβ repressor activity involves thiol-disulfide interconversion of the HRM cysteine. Under oxidizing conditions, C384 forms a disulfide bond with neighboring C374, causing an unidentified neutral ligand to assume the position of C384 in the Fe3+-heme complex (Fig. 5) (27). The Kd of the thiol-oxidized protein for Fe3+-heme (117 nM) is approximately five-fold higher than that for the thiol-reduced form (23 nM), suggesting that thiol-disulfide interplay could regulate Rev-erbβ heme occupancy. Further, the switch occurs in Escherichia coli cells overproducing the Rev-erbβ LBD, which exhibit a whole-cell EPR spectrum consistent with that of a thiol-reduced, six-coordinate low-spin Fe3+-heme; then, treatment of cells with the thiol oxidant, diamide, yields a spectrum indicative of a mixture of thiol-reduced/thiol-oxidized species. These data support the hypothesis that the Rev-erbβ HRM is redox active and that the disulfide bond forms under oxidizing conditions. Future work should focus on whether the Rev-erbβ thiol-disulfide rheostat occurs in mammalian cells in the context of the full-length protein and whether it interfaces with cellular redox buffers, for example, the glutathione:glutathione disulfide and dehydroascorbic acid:ascorbic acid systems that are under circadian control (92). Because Rev-erbα/β protein levels are tightly regulated by the transcription/translation feedback cycle of the molecular clock, redox regulation might represent an additional mechanism of controlling Rev-erb repressor activity.
Transient kinetic experiments support the hypothesis that the HRM in Rev-erbβ is a redox sensor
A caveat of our early estimates of Kd for the Fe3+/Fe2+-heme complexes with FLRev-erbβ and thiol-reduced/oxidized LBDs was the use of equilibrium titrations. Because Kds for these complexes are in the low nanomolar range, UV-vis spectrophotometric titrations performed with micromolar concentrations of heme and protein yield typical, “dog-leg” curves that sharply saturate at 1:1 stoichiometry of heme and protein. Under such conditions, the concentration of free ligand cannot be assumed to equal the total concentration since most of the ligand is protein bound below a 1:1 ratio. The quadratic equation accounts for the concentrations of total versus free heme in such titrations but is limited in that the accuracy of the Kd is dependent on the sharpness of the curve; below a 10 nanomolar Kd, the standard error of the fit becomes rather large. To circumvent these issues, we used a kinetic approach, analyzing individual rate constants for the association (kon) and dissociation (koff) of heme and Rev-erbβ, ultimately establishing accurate Kds using the basic expression, Kd = koff/kon. As detailed in our recent manuscript (11), we utilized a unique buffer system containing caffeine, which has been shown to dissociate heme dimers and oligomers that usually complicate measurements of kon using stopped-flow techniques because of their first-order, rate-limiting dissociation.
Heme association to Rev-erbβ was complex and proceeded through several different kinetic steps. However, the initial binding phase, which made up >50% of total amplitude change, was hyperbolic with respect to protein concentration, indicative of a two-step binding mechanism (81). Similar hyperbolic dependence of kobs on protein concentration has been observed in other heme:hemoprotein systems, suggesting that they have similar mechanisms of heme association (20, 54, 64). Interestingly, the kon values for Fe3+-heme binding to wild-type protein, a C384A variant lacking the HRM cysteine thiolate, and the thiol-oxidized form were between 3.6 × 105 M−1 s−1 and 4.1 × 105 M−1 s−1, indicating that loss of the axial thiolate has a negligible influence on heme binding. Fe2+-heme association, however, proceeded with a second-order rate constant of kon = 2.7 × 106 M−1 s−1, demonstrating that the heme redox state is an important determinant of kon.
The measurement of kon was relatively straightforward, whereas heme dissociation was biphasic, that is, affording two koff values. For these experiments, one follows heme dissociation from Rev-erbβ by monitoring formation of the complex between heme and the H64Y/V68F myoglobin variant, which exhibits a unique UV-vis spectrum. For wild-type Rev-erbβ, off-rates were between 10−4–10−5 s−1, with a Kd range of 104–639 pM; the slow kinetic phase predominated and made up >80% of the total amplitude, indicating that most of the bound heme dissociates with a Kd of ∼100 pM. In contrast, the Fe3+-heme off-rates from thiol-oxidized and C384A proteins were between 10−2 and 10−3 s−1, giving Kd ranges of 14–50 nM and 4–32 nM, respectively. Further, the koff values of Fe2+-heme from wild-type protein were 1.4 s−1–6.5 × 10−2 s−1, yielding a Kd range of 24–519 nM. Thus, dissociation of the axial thiolate via either redox process (oxidation of the HRM thiolate to a disulfide or heme reduction) significantly lowers Rev-erbβ heme affinity. In other words, Rev-erbβ exhibits features of a redox sensor rather than a direct heme sensor. This hypothesis gains momentum given coimmunoprecipitation results demonstrating that the C384A variant of FLRev-erbβ loses >70% NCoR1 binding capacity with respect to wild-type protein, corroborating the importance of the HRM cysteine in stabilizing both heme and NCoR1 interactions.
These redox sensor-related results take on an even greater impact in the context with recent work by Reddi and colleagues describing the accurate determination of intracellular RH levels in Saccharomyces cerevisiae (30). In that study, fluorescent heme sensors were targeted to different subcellular organelles and RH levels were determined by using elegant normalization techniques that defined fluorescence emission ratios (with respect to an internal fluorescence standard) of the sensors in heme-free and heme-replete conditions. RH in the cytosol was found to be 20–40 nM, whereas heme levels are kept at a minimum (<2.5 nM) in the mitochondria and nucleus. RH in the nucleus and mitochondria is likely maintained at these extremely low levels to limit the amount of free heme available to participate in Fenton reactions, leading to damaged DNA or respiratory chain elements. A complicating factor is that the heme sensor employed is insensitive to Fe3+-heme at the low concentrations present in the cell; thus, the aforementioned values are reflective of the Fe2+-heme RH pool, although Fe3+-heme RH is also likely to exist due to varying ligand conformations with glutathione or protein side chains in the cellular milieu. In any case, those values provided in the aforementioned study lay the groundwork for discussions on the likelihood of heme:protein interactions with an RH pool in the low nanomolar range. Since the majority of the thiol-reduced, wild-type Rev-erbβ population binds Fe3+-heme with a Kd of 0.1 nM, it would predominantly exist as holoprotein with <2.5 nM RH in the nucleus. However, thiol oxidation or heme reduction drives the Kd above 14 nM. Because these values lie on the upper end of the RH estimation, Rev-erbβ may exist as an apoprotein under those conditions. Another hypothesis is that Rev-erbs might bind Fe2+-heme after being translated in the cytosol, with heme then oxidizing to Fe3+-heme to “lock in” a particular conformation of the protein. Regardless of this, the initial estimates of Rev-erbα/β affinity of 0.35–3.52 μM with ITC are problematic in that they suggest the protein would exist within the cell in the apo form exclusively (59, 70). Our results underscore the importance of using highly sensitive spectroscopic and kinetic studies to investigate the molecular mechanism of heme-dependent NCoR1 recruitment, and the possibility of Rev-erbs acting as redox sensors either through the Fe3+/Fe2+-heme or the thiol-disulfide couple (11).
Heme Oxygenase-2
HO2 is an HRM-containing protein
A variety of mechanisms are involved in the regulation of heme homeostasis to ensure that heme levels are maintained at concentrations that are high enough for cellular processes but low enough to avoid the cytotoxic effects of heme. One such mechanism involves heme degradation, a pathway in which heme is converted to biliverdin, and biliverdin is, subsequently, converted to the more readily excreted product bilirubin (55). The first step is rate limiting and is catalyzed by HO, the only enzyme in mammals known to degrade heme.
HO2, one of the two major isoforms of HO identified in mammals, contains three HRMs (Fig. 1). One HRM, centered at Cys127-Pro128 in human HO2, is located in the α-helical catalytic core region, consisting of residues 28-248 where heme binds and undergoes conversion to biliverdin. The other two HRMs, centered at Cys265-Pro266 and Cys282-Pro283, are located in an unstructured region of the protein between the catalytic core and the membrane-spanning region that tethers the protein to the endoplasmic reticulum. HO2 was among the proteins first identified as having an HRM (105), and it was immediately thereafter noted that the presence of HRMs in HO2 distinguished it from HO1, which contains no cysteines (60). HO1 is an inducible protein expressed in most tissues but with particularly high levels in the liver and spleen, whereas HO2 is constitutively expressed and has been primarily identified in the brain and testes (55). However, the two proteins convert heme to biliverdin with similar catalytic efficiencies (56) and share a high level of homology (55% identical and 76% similar for the human proteins). A comparison of the structures of HO2 and HO1 illustrates how similar the two proteins are in the catalytic core region, consisting of residues 1-230 in human HO1 (4, 78). However, the HO2 and HO1 sequences diverge significantly between the catalytic core and the membrane-spanning region where the HRMs of HO2 are located, a region for which there is little structural information. As the HRMs appear to be the major difference between the two proteins, attention has been given to the heme-binding properties and the function of the HRMs of HO2 in an effort to understand why nature has selected a new form of HO that catalyzes the same reaction with a similar catalytic efficiency as the ancient form (HO1).
Heme binding to the HRMs of HO2
To infer that any differences between HO2 and HO1 are linked to the presence of HRMs in HO2, heme binding to the HRMs needed to be confirmed. An initial study of rat HO2 (60) demonstrated that adding heme to a 10-mer peptide spanning one of the HRMs (Val-Arg-Lys-Cys264-Pro-Phe-Tyr-Ala-Ala-Gln) results in a dramatic color change, suggesting heme ligation. A more subtle color change was observed for a peptide spanning the other C-terminal HRM (Gly-Ser-Asn-Cys281-Pro-Phe-Arg-Thr-Ala-Met). Further, subtle differences in the absorbance spectra of a Cys264Ala/Cys281Ala variant of rat HO2 as compared with the wild-type led to the hypothesis that the wild-type was binding ∼2 more equivalents of heme per monomer of protein than the variant, suggesting that the HRMs were involved in binding heme independently of heme binding to the catalytic core (60). The results were slightly ambiguous but enough to pique the curiosity of others.
A significant finding in the further characterization of the HRMs was the discovery of a disulfide bond involving the cysteines in the HRMs of the soluble form of human HO2 (HO2sol), which spans residues 1-288, thus eliminating residues 289-316, which form the membrane-spanning region. Cys265 and Cys282 were shown to form a disulfide bond by the 5,5′-dithiobis(nitrobenzoic acid) (DTNB) assay and by monitoring alkylation of the cysteine residues using mass spectrometry (99). A later study using 13C-Cys-labeled HO2sol and two-dimensional NMR also confirmed disulfide bond formation (86). Further, through thiol trapping experiments using the isotope-coded affinity tag technique (53), the thiols were shown to be 60–70% reduced in vivo under normal growth conditions (98). The dithiol/disulfide ratio also changed to reflect changes in the cellular redox state, with 81–87% of thiols reduced under reducing conditions and 86–89% of thiols in the disulfide state under oxidizing conditions. The midpoint potential for the thiol/disulfide conversion, measured by thiol trapping and fluorescence trapping, was calculated to be −200 mV, which is near the ambient cellular redox potential (71). Thus, the thiol/disulfide conversion appears to be physiologically relevant and to link HO2 to the cellular redox state.
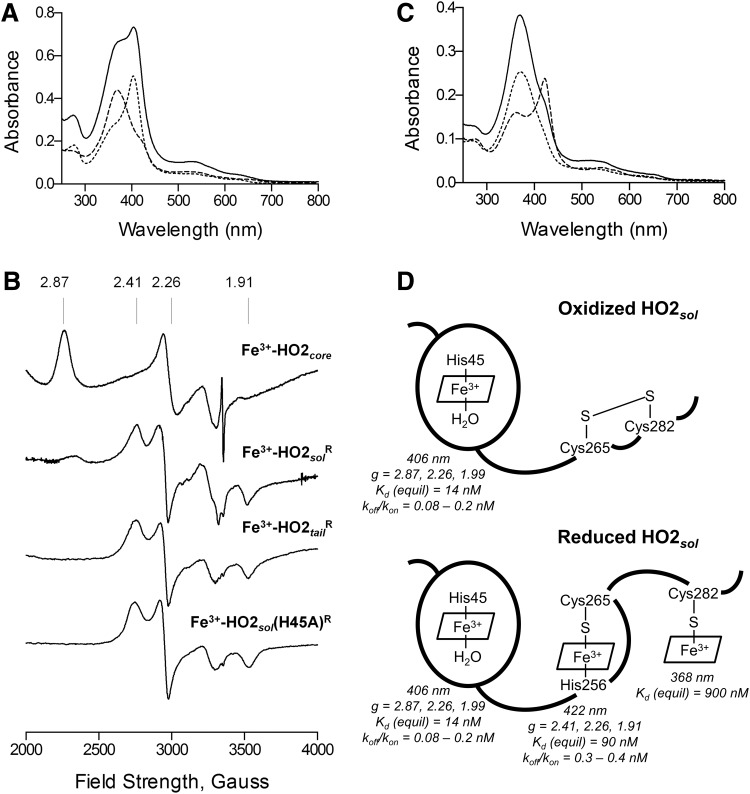
The added significance of the thiol/disulfide conversion in HO2 is that a disulfide bond necessarily limits the ability of Cys265 and Cys282 to coordinate Fe3+-heme. Indeed, no thiol coordination of Fe3+-heme was observed with fully oxidized (disulfide state) as-purified HO2sol (99), and its spectral features were identical to those of a truncated form of HO2 (HO2core), which spans residues 1-248 and lacks the C-terminal HRMs (20, 99). Titration of oxidized HO2sol (HO2solO) or HO2core with Fe3+-heme resulted in an increase at 406 nm that saturated at 1:1 protein to Fe3+-heme and an EPR signal (signal X) with g values at 2.87, 2.26, and 1.99 (Fig. 6). These results are consistent with His/aquo axial ligation to the Fe3+-heme in the catalytic core, including the imidazole of a conserved His (His45 in human HO2) and a solvent water, which interacts with a glycine-rich helix (4).
FIG. 6.
Spectral characterization of HO2 in the Fe3+-heme-bound forms. (A) Absorbance spectra of 5 μM Fe3+-heme-bound forms of HO2core (…), HO2tailR ( ), and HO2solR (—) in 50 mM Tris (pH 8.0) and 50 mM KCl at 20°C. (B) EPR spectra of the Fe3+-heme-bound forms of HO2core, HO2solR, HO2tailR, and the His45Ala variant of HO2solR. The g values are indicated above the spectra. (C) Absorbance spectra of 5 μM Fe3+-heme-bound forms of HO2tailR (—), HO2tail(C282A)R (
), and HO2solR (—) in 50 mM Tris (pH 8.0) and 50 mM KCl at 20°C. (B) EPR spectra of the Fe3+-heme-bound forms of HO2core, HO2solR, HO2tailR, and the His45Ala variant of HO2solR. The g values are indicated above the spectra. (C) Absorbance spectra of 5 μM Fe3+-heme-bound forms of HO2tailR (—), HO2tail(C282A)R ( ), and HO2tail(C265A)R (…) in 50 mM Tris (pH 8.0) and 50 mM KCl at 20°C. (D) A model of heme binding to the oxidized form of HO2sol, which has a single heme-binding site due to the participation of Cys265 and Cys282 in a disulfide bond, and the reduced form of HO2sol, which has three heme-binding sites. Below each heme-binding site are the characteristics of that site, including the maximum absorbance, the g-values, the Kd values obtained by equilibrium titrations [Kd (equil)], and the koff/kon values obtained by kinetic measurements. The figures in (A–C) are reprinted (adapted) with permission from Fleischhacker et al. (20). Copyright (2015) American Chemical Society. EPR, electron paramagnetic resonance; HO2core, HO2 spanning residues 1-248; HO2R, HO2 in the disulfide bond-reduced form; HO2sol, HO2 spanning residues 1-288; HO2tail, HO2 spanning residues 213-288. EPR, electron paramagnetic resonance; HO2core, HO2 spanning residues 1-248; HO2R, HO2 in the disulfide bond-reduced form; HO2sol, HO2 spanning residues 1-288; HO2tail, HO2 spanning residues 213-288.
), and HO2tail(C265A)R (…) in 50 mM Tris (pH 8.0) and 50 mM KCl at 20°C. (D) A model of heme binding to the oxidized form of HO2sol, which has a single heme-binding site due to the participation of Cys265 and Cys282 in a disulfide bond, and the reduced form of HO2sol, which has three heme-binding sites. Below each heme-binding site are the characteristics of that site, including the maximum absorbance, the g-values, the Kd values obtained by equilibrium titrations [Kd (equil)], and the koff/kon values obtained by kinetic measurements. The figures in (A–C) are reprinted (adapted) with permission from Fleischhacker et al. (20). Copyright (2015) American Chemical Society. EPR, electron paramagnetic resonance; HO2core, HO2 spanning residues 1-248; HO2R, HO2 in the disulfide bond-reduced form; HO2sol, HO2 spanning residues 1-288; HO2tail, HO2 spanning residues 213-288. EPR, electron paramagnetic resonance; HO2core, HO2 spanning residues 1-248; HO2R, HO2 in the disulfide bond-reduced form; HO2sol, HO2 spanning residues 1-288; HO2tail, HO2 spanning residues 213-288.
Surprisingly, on reduction of the disulfide bond of HO2 and subsequent incubation with Fe3+-heme, the EPR spectrum of HO2solR (reduced HO2sol) included both signal X and a second signal (signal Y) with g values at 2.41, 2.26, and 1.91, which are characteristic of six-coordinate Fe3+-heme with Cys/His ligation (20, 99) (Fig. 6). Thiol ligation had never been observed for any form of HO and is a feature characteristic of an entirely different class of hemoproteins, for example, cytochrome P450 (26) and some regulatory hemoproteins (37, 71). Various results in different groups indicated that only one heme was bound per HO2 monomer (86, 99). Thus, to account for signal Y, a “fishhook” mechanism was initially proposed in which a Cys from an HRM (likely Cys265 based on mutational studies) displaces an axial ligand at Fe3+-heme bound to the catalytic site on reduction of the disulfide bond, resulting in His45/Cys265 ligation of Fe3+-heme (99). However, problems with this proposal began to mount, favoring a model (Fig. 6D) in which Fe3+-heme binding to Cys residues in the HRMs is independent of binding to His45 at the catalytic core (20). For example, signal Y was observed in forms of HO2 in which His45 is not available to ligate heme, as in, the His45Ala variant of HO2solR and HO2 in the disulfide bond-reduced form, spanning residues 213-288 (HO2tailR), which lacks the heme-binding site in the catalytic core (20). Rather, 14N and 15N Davies ENDOR spectroscopic experiments showed that His256 is an axial ligand to the heme giving rise to signal Y (20). Indeed, the absorbance spectra of the Fe3+-heme-bound forms of HO2tailR and HO2core could account for all of the features observed for HO2solR (Fig. 6A, B); for example, the absorbance band at 422 nm is typical for Cys/His ligated Fe3+-heme (37, 58, 62) and could be assigned to Cys265/His256 ligation (as well as accounting for the shoulder in the spectrum of HO2solR), whereas the broad band at 368 nm could be assigned to the Cys282 site (20, 105). Quantification of Fe3+-heme bound to the various HO2 forms by pyridine hemochrome assay (20) also demonstrated that the thiol-reduced HO2 has three independent Fe3+-heme-binding sites.
Various spectroscopic techniques were used to probe for the thiol-coordinated Fe3+-heme in HO2. MCD spectra of Fe3+-heme-bound human HO2 and the Cys variants suggested that a six-coordinate, Cys265-ligated Fe3+-heme is formed as a minor species on reduction of the disulfide bond (22); Fe K-edge EXAFS and XAS data supported thiol coordination by both Cys265 and Cys282, although Cys265 appeared to bind with a somewhat higher affinity than Cys282 to the Fe3+ center (2); and 1H and 13C ENDOR experiments using reduced, Fe3+-heme-bound HO2 that had been 2H-Cys or 13C-Cys labeled conclusively demonstrated Fe3+-heme coordination at the HRMs (2). Thus, collectively these various lines of enquiry unambiguously showed that the reduced state of HO2 contains three hemes (Fig. 6D): one at the catalytic core (His45/aquo) and two additional heme-binding sites at the C-terminal HRMs: Cys265/His256 and Cys282. Regardless of this, one study proposed that the HRMs of HO2 do not stably coordinate to Fe3+-heme (86); however, in that study involving equilibrium titrations, Fe3+-heme was added to HO2 in the presence of excess tris(2-carboxyethyl)phosphine, and phosphines are reasonably strong Fe3+-heme ligands (57, 75, 83).
Kinetics of heme binding to HO2
Spectral data suggested that the three binding sites of HO2 had varying affinities for Fe3+-heme. At less than saturating concentrations of Fe3+-heme, the spectra were dominated by features associated with Fe3+-heme binding at the His45/aquo site, suggesting that the catalytic core had a higher affinity for Fe3+-heme than the HRMs. Further, Fe K-edge EXAFS and XANES data suggested that Cys265 had a higher affinity for Fe3+-heme than Cys282 (2). However, Fe3+-heme Kd values were difficult to ascertain from equilibrium titrations of HO2solR due to the complexity of the UV-vis spectra. Thus, equilibrium titrations of the truncated forms of HO2 were performed and revealed Kd values of 14 nM for HO2core (Fe3+-heme binding to the His45/aquo site), 90 nM for HO2tail(C282A)R (Cys265/His256 site), and 900 nM for HO2tail(C265A)R (Cys282 site) (20), confirming that the three sites have varying affinities for Fe3+-heme.
However, as discussed earlier for Rev-erbβ, equilibrium titrations may not give the most accurate Kd values. Instead, individual rate constants for the association (kon) and dissociation (koff) of Fe3+-heme provided a more detailed view of binding. Further, this kinetic approach permitted the observation of Fe3+-heme binding to some of the sites in HO2solR. The rate constants were very similar for the sites in the truncated proteins as the comparable site in HO2solR, indicating that the truncated proteins are good models for the full-length protein. Calculation of Kd values using the simple equation Kd = koff/kon revealed Kd values of 0.08–0.2 nM for Fe3+-heme binding to the His45/aquo site and 0.3–0.4 nM for Fe3+-heme binding to the Cys265/His256 site (20). The kon value could not be determined for Fe3+-heme binding to the Cys282 site, but estimates would suggest a value of ∼1 nM. Values obtained by kinetic measurements follow the same trend as equilibrium titrations, that is, the His45/aquo site has a higher affinity than Cys265/His256, which, in turn, has a higher affinity than Cys282. However, the lower Kd values are on par with RH levels in the cell, in contrast to the values obtained by equilibrium titrations that would have suggested that the HRMs only bind Fe3+-heme under conditions in which heme levels were elevated. Therefore, the redox state of the cell, by influencing the thiol/disfulfide conversion of Cys265 and Cys282, appears to be more relevant to Fe3+-heme binding to the HRMs of HO2 than previously thought, suggesting that the HRMs function as redox sensors.
Functional significance of HRMs in HO2
HRMs appear to place HO2 in a unique position at which the redox state of the cell and regulation of heme and iron homeostasis intersect. However, the exact nature of their function remains unclear. Repeated experiments have demonstrated that the steady-state activity of HO2 is not affected by substitution of any of the Cys to Ala or by exclusion of the cysteine residues by truncation of the protein (16, 20, 60, 99). A more detailed investigation of heme hydroxylation, the initial steps in heme degradation, by HO2 using a combination of radiolytic cryoreduction/annealing with UV–vis and EPR spectroscopy demonstrates that there is no involvement of the HRMs in the catalytic cycle of HO2 (16). In addition, the HRMs can bind but not oxidize heme as no steady-state activity was observed with HO2tailR (16). HO2 and HO1 also appear to bind cytochrome P450 reductase, which provides electrons for the HO reaction, with the same corresponding residues (80), further suggesting that the degradation of heme proceeds in the catalytic core independently of the HRMs. HRMs are also not involved in the specific activation of HO2 by menadione analogs or its specific inhibition by clemizole analogs (87) nor do they participate in binding of the heme mimic Zn(II) PPIX, an HO inhibitor (36). Thus, further investigation is needed to detail the functional significance of heme binding to HRMs in a redox-regulated manner.
Conclusions and Future Directions
Here, we have highlighted HO2 and Rev-erbβ to illustrate the similarities and differences between two hemoproteins that both contain HRMs. HRMs of both proteins appear to act as redox sensors in that the participation of the HRM cysteine(s) in a thiol/disulfide switch alters affinity for heme. Therefore, future studies of HRM-containing proteins should include experiments that probe for disulfide bonds and/or differences in heme binding under oxidizing and reducing conditions. HRMs acting as redox sensors may be applicable to other HRM-containing proteins as many contain multiple HRMs and/or other cysteine residues. Further, the affinity for heme at the HRMs, based on the kinetic approach used for HO2 and Rev-erbβ, may be lower than previously proposed, suggesting that the functional significance of the HRMs may be greater than expected. The survey of HRM-containing proteins (see the Introduction section and Fig. 1) indicates that HRMs play a role in regulating protein stability in a significant number of proteins, many of which are involved in regulating circadian rhythm and metabolic pathways (Rev-erbs, p53, and Per2), suggesting that it may be advisable to probe more HRMs for this function. Thus, the study of HRMs, in general, is a growing field with many interesting questions yet to be answered.
Abbreviations Used
- ALAS1 and ALAS2
aminolevulinic acid synthase-1 nonspecific, and erythroid-specific aminolevulinic acid synthase-2
- CO
carbon monoxide
- CPO
chloroperoxidase
- Cry
cryptochrome protein
- DGCR8
DiGeorge critical region
- DP8
dipeptidyl peptidase 8
- ENDOR
electron nuclear double resonance
- EPR
electron paramagnetic resonance
- EXAFS
extended X-ray absorption fine structure
- FLRev-erbβ
full-length Rev-erbβ
- Hap-1
heme activator protein-1
- HDAC1
histone deacetylase 1
- HO or HO1 or HO2
heme oxygenase and heme oxygenase isoform 1 or 2
- HO2core
HO2 spanning residues 1-248
- HO2R
HO2 in the disulfide bond-reduced form
- HO2sol
HO2 spanning residues 1-288
- HO2tail
HO2 spanning residues 213-288
- HRI
heme-regulated inhibitor, or heme-regulated eIF2α kinase
- HRM
heme regulatory motif
- ID
interaction domain
- IL-36α
interleukin-36α
- IRP2
iron regulatory protein 2
- Irr
bacterial iron response regulator
- ITC
isothermal titration calorimetry
- LBD
ligand-binding domain
- MCD
magnetic circular dichroism
- NCoR1
nuclear receptor corepressor 1
- NMR
nuclear magnetic resonance
- NO
nitric oxide
- NR
nuclear receptor
- Per
period protein
- PPIX
protoporphyrin IX
- RH
regulatory heme
- RORα
RAR-related orphan receptor α
- SCN
suprachiasmatic nucleus
- XAS
X-ray absorption
Acknowledgments
This work has been funded by NIH (R21HL089837, R01-GM123513, and R01-HL102662A) to SWR, F32HL114150 to ELC and by the University of Michigan Biomedical Research Council (to SWR).
References
- 1.Akashi M. and Takumi T. The orphan nuclear receptor RORα regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol 12: 441–448, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bagai I, Sarangi R, Fleischhacker AS, Sharma A, Hoffman BM, Zuiderweg ERP, and Ragsdale SW. Spectroscopic studies reveal that the heme regulatory motifs of heme oxygenase-2 are dynamically disordered and exhibit redox-dependent interaction with heme. Biochemistry 54: 2693–2708, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr I, Smith AT, Senturia R, Chen YQ, Scheidemantle BD, Burstyn JN, and Guo F. DiGeorge critical region 8 (DGCR8) is a double-cysteine-ligated heme protein. J Biol Chem 286: 16716–16725, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchetti CM, Yi L, Ragsdale SW, and Phillips GN., Jr Comparison of apo- and heme-bound crystal structures of a truncated human heme oxygenase-2. J Biol Chem 282: 37624–37631, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohle DS, Dodd EL, Pinter TBJ, and Stillman MJ. Soluble diamagnetic model for malaria pigment: coordination chemistry of gallium(III)protoporphyrin-IX. Inorg Chem 51: 10747–10761, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Brewitz HH, Kühl T, Goradia N, Galler K, Popp J, Neugebauer U, Ohlenschlager O, and Imhof D. Role of the chemical environment beyond the coordination site: structural insight into Fe-III protoporphyrin binding to cysteine-based heme-regulatory protein motifs. Chembiochem 16: 2216–2224, 2015 [DOI] [PubMed] [Google Scholar]
- 7.This reference has been deleted
- 8.Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, Jager J, and Lazar MA. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev 26: 657–667, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter EL, Gupta N, and Ragsdale SW. High affinity heme binding to a heme regulatory motif on the nuclear receptor Rev-erbβ leads to its degradation and indirectly regulates its interaction with nuclear receptor corepressor. J Biol Chem 291: 2196–2222, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter EL. and Ragsdale SW. Modulation of nuclear receptor function by cellular redox poise. J Inorg Biochem 133: 92–103, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter EL, Ramirez Y, and Ragsdale SW. The heme-regulatory motif of nuclear receptor Rev-erbβ is a key mediator of heme and redox signaling in circadian rhythm maintenance and metabolism. J Biol Chem 292: 11280–11299, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JJ. and London IM. Regulation of protein-synthesis by heme-regulated EIF-2α kinase. Trends Biochem Sci 20: 105–108, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong L-W, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, and Evans RM. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485: 123–127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho Y, Gorina S, Jeffrey P, and Pavletich N. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science 265: 346–355, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Dace A, Zhao L, Park KS, Furuno T, Takamura N, Nakanishi M, West BL, Hanover JA, and Cheng S-Y. Hormone binding induces rapid proteasome-mediated degradation of thyroid hormone receptors. Proc Natl Acad Sci U S A 97: 8985–8990, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davydov R, Fleischhacker AS, Bagai I, Hoffman BM, and Ragsdale SW. Comparison of the mechanisms of heme hydroxylation by heme oxygenases-1 and-2: kinetic and cryoreduction studies. Biochemistry 55: 62–68, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dioum EM, Rutter J, Tuckerman JR, Gonzalez G, Gilles-Gonzalez M-A, and McKnight SL. NPAS2: a gas-responsive transcription factor. Science 298: 2385–2387, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Dumas B, Harding HP, Choi HS, Lehmann KA, Chung M, Lazar MA, and Moore DD. A new orphan member of the nuclear hormone receptor superfamily closely related to Rev-Erb. Mol Endocrinol 8: 996–1005, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Faller M, Matsunaga M, Yin S, Loo JA, and Guo F. Heme is involved in microRNA processing. Nat Struct Mol Biol 14: 23–29, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Fleischhacker AS, Sharma A, Choi M, Spencer AM, Bagai I, Hoffman BM, and Ragsdale SW. The C-terminal heme regulatory motifs of heme oxygenase-2 are redox-regulated heme binding sites. Biochemistry 54: 2709–2718, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabay C. and Towne JE. Regulation and function of interleukin-36 cytokines in homeostasis and pathological conditions. J Leukoc Biol 97: 645–652, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Gardner JD, Yi L, Ragsdale SW, and Brunold TC. Spectroscopic insights into axial ligation and active-site H-bonding in substrate-bound human heme oxygenase-2. J Biol Inorg Chem 15: 1117–1127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerhart-Hines Z, Feng D, Emmett MJ, Everett LJ, Loro E, Briggs ER, Bugge A, Hou C, Ferrara C, Seale P, Pryma DA, Khurana TS, and Lazar MA. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature 503: 410–413, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, and Loudon ASI. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A 109: 582–587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glass CK. and Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14: 121–141, 2000 [PubMed] [Google Scholar]
- 26.Guengerich FP. New trends in cytochrome P450 research at the half-century mark. J Biol Chem 288: 17063–17064, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta N. and Ragsdale SW. Thiol-disulfide redox dependence of heme binding and heme ligand switching in nuclear hormone receptor Rev-erbβ. J Biol Chem 286: 4392–4403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hach A, Hon T, and Zhang L. A new class of repression modules is critical for heme regulation of the yeast transcriptional activator Hap1. Mol Cell Biol 19: 4324–4333, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamza I, Chauhan S, Hassett R, and O'Brian MR. The bacterial Irr protein is required for coordination of heme biosynthesis with iron availability. J Biol Chem 273: 21669–21674, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Hanna DA, Harvey RM, Martinez-Guzman O, Yuan X, Chandrasekharan B, Raju G, Outten FW, Hamza I, and Reddi AR. Heme dynamics and trafficking factors revealed by genetically encoded fluorescent heme sensors. Proc Natl Acad Sci U S A 113: 7539–7544, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harding HP. and Lazar MA. The monomer-binding orphan receptor Rev-Erb represses transcription as a dimer on a novel direct repeat. Mol Cell Biol 15: 4791–4802, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hickman MJ. and Winston F. Heme levels switch the function of Hap1 of Saccharomyces cerevisiae between transcriptional activator and transcriptional repressor. Mol Cell Biol 27: 7414–7424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hon T, Hach A, Lee HC, Cheng T, and Zhang L. Functional analysis of heme regulatory elements of the transcriptional activator Hap1. Biochem Biophys Res Commun 273: 584–591, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Hon T, Lee HC, Hu ZZ, Iyer VR, and Zhang L. The heme activator protein Hap1 represses transcription by a heme-independent mechanism in Saccharomyces cerevisiae. Genetics 169: 1343–1352, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu RG, Wang HQ, Xia ZX, and Varshavsky A. The N-end rule pathway is a sensor of heme. Proc Natl Acad Sci U S A 105: 76–81, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang TJ, McCoubrey WK, and Maines MD. Heme oxygenase-2 interaction with metalloporphyrins: function of heme regulatory motifs. Antioxid Redox Signal 3: 685–696, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Igarashi J, Sato A, Kitagawa T, Yoshimura T, Yamauchi S, Sagami I, and Shimizu T. Activation of heme-regulated eukaryotic initiation factor 2α kinase by nitric oxide is induced by the formation of a five-coordinate NO-heme complex: optical absorption, electron spin resonance, and resonance Raman spectral studies. J Biol Chem 279: 15752–15762, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa H, Kato M, Hori H, Ishimori K, Kirisako T, Tokunaga F, and Iwai K. Involvement of heme regulatory motif in heme-mediated ubiquitination and degradation of IRP2. Mol Cell 19: 171–181, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Ishikawa H, Nakagaki M, Bamba A, Uchida T, Hori H, O'Brian MR, Iwai K, and Ishimori K. Unusual heme binding in the bacterial iron response regulator protein: spectral characterization of heme binding to the heme regulatory motif. Biochemistry 50: 1016–1022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishizuka T. and Lazar MA. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol Cell Biol 23: 5122–5131, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwai K, Klausner RD, and Rouault TA. Requirements for iron-regulated degradation of the RNA binding protein, iron regulatory protein 2. EMBO J 14: 5350–5357, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang J, Westberg JA, and Andersson LC. Stanniocalcin 2, forms a complex with heme oxygenase 1, binds hemin and is a heat shock protein. Biochem Biophys Res Commun 421: 274–279, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Kaasik K. and Chi Lee C. Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature 430: 467–471, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Kammler M, Schon C, and Hantke K. Characterization of the ferrous iron uptake system of Escherichia coli. J Bacteriol 175: 6212–6219, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JY, Son YL, Kim J-S, and Lee YC. Molecular determinants required for selective interactions between the thyroid hormone receptor homodimer and the nuclear receptor corepressor N-CoR. J Mol Biol 396: 747–760, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Kitatsuji C, Izumi K, Nambu S, Kurogochi M, Uchida T, Nishimura SI, Iwai K, O'Brian MR, Ikeda-Saito M, and Ishimori K. Protein oxidation mediated by heme-induced active site conversion specific for heme-regulated transcription factor, iron response regulator. Sci Rep 6: 18703, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ko CH. and Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet 15: R271–R277, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Kubota Y, Nomura K, Katoh Y, Yamashita R, Kaneko K, and Furuyama K. Novel mechanisms for heme-dependent degradation of ALAS1 protein as a component of negative feedback regulation of heme biosynthesis. J Biol Chem 291: 20516–20529, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kühl T, Sahoo N, Nikolajski M, Schlott B, Heinemann SH, and Imhof D. Determination of hemin-binding characteristics of proteins by a combinatorial peptide library approach. Chembiochem 12: 2846–2855, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Kühl T, Wißbrock A, Goradia N, Sahoo N, Galler K, Neugebauer U, Popp J, Heinemann SH, Ohlenschläger O, and Imhof D. Analysis of Fe(III) heme binding to cysteine-containing heme-regulatory motifs in proteins. ACS Chem Biol 8: 1785–1793, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Kumar N, Solt LA, Wang Y, Rogers PM, Bhattacharyya G, Kamenecka TM, Stayrook KR, Crumbley C, Floyd ZE, Gimble JM, Griffin PR, and Burris TP. Regulation of adipogenesis by natural and synthetic REV-ERB ligands. Endocrinology 151: 3015–3025, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lathrop JT. and Timko MP. Regulation by heme of mitochondrial protein-transport through a conserved amino-acid motif. Science 259: 522–525, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Leichert LI, Gehrke F, Gudiseva HV, Blackwell T, Ilbert M, Walker AK, Strahler JR, Andrews PC, and Jakob U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc Natl Acad Sci U S A 105: 8197–8202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu M, Tanaka WN, Zhu H, Xie G, Dooley DM, and Lei B. Direct hemin transfer from IsdA to IsdC in the iron-regulated surface determinant (Isd) heme acquisition system of Staphylococcus aureus. J Biol Chem 283: 6668–6676, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 37: 517–554, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Maines MD, Trakshel GM, and Kutty RK. Characterization of two constitutive forms of rat-liver microsomal heme oxygenase: only one molecular-species of the enzyme is inducible. J Biol Chem 261: 411–419, 1986 [PubMed] [Google Scholar]
- 57.Mansuy D, Duppel W, Ruf HH, and Ullrich V. Phosphines as ligand to microsomal cytochrome-P450. Hoppe Seylers Z Physiol Chem 355: 1341–1349, 1974 [DOI] [PubMed] [Google Scholar]
- 58.Marvin KA, Reinking JL, Lee AJ, Pardee K, Krause HM, and Burstyn JN. Nuclear receptors Homo sapiens Rev-erbβ and Drosophila melanogaster E75 are thiolate-ligated heme proteins which undergo redox-mediated ligand switching and bind CO and NO. Biochemistry 48: 7056–7071, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matta-Camacho E, Banerjee S, Hughes TS, Solt LA, Wang Y, Burris TP, and Kojetin DJ. Structure of REV-ERBβ ligand-binding domain bound to a porphyrin antagonist. J Biol Chem 289: 20054–20066, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCoubrey WK, Huang TJ, and Maines MD. Heme oxygenase-2 is a hemoprotein and binds heme through heme regulatory motifs that are not involved in heme catalysis. J Biol Chem 272: 12568–12574, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Miki T, Matsumoto T, Zhao Z, and Lee CC. p53 regulates Period2 expression and the circadian clock. Nat Commun 4: 2444, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miksanova M, Igarashi J, Minami M, Sagami I, Yamauchi S, Kurokawa H, and Shimizu T. Characterization of heme-regulated eIF2α kinase: roles of the N-terminal domain in the oligomeric state, heme binding, catalysis, and inhibition. Biochemistry 45: 9894–9905, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Munakata H, Sun JY, Yoshida K, Nakatani T, Honda E, Hayakawa S, Furuyama K, and Hayashi N. Role of the heme regulatory motif in the heme-mediated inhibition of mitochondrial import of 5-aminolevulinate synthase. J Biochem 136: 233–238, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Nygaard TK, Blouin GC, Liu M, Fukumura M, Olson JS, Fabian M, Dooley DM, and Lei B. The mechanism of direct heme transfer from the streptococcal cell surface protein Shp to HtsA of the HtsABC transporter. J Biol Chem 281: 20761–20771, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogawa K, Sun J, Taketani S, Nakajima O, Nishitani C, Sassa S, Hayashi N, Yamamoto M, Shibahara S, Fujita H, and Igarashi K. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO J 20: 2835–2843, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pardee KI, Xu X, Reinking J, Schuetz A, Dong A, Liu S, Zhang R, Tiefenbach J, Lajoie G, Plotnikov AN, Botchkarev A, Krause HM, and Edwards A. The structural basis of gas-responsive transcription by the human nuclear hormone receptor REV-ERBβ. PLoS Biol 7: e1000043, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pfeifer K, Kim K-S, Kogan S, and Guarente L. Functional dissection and sequence of yeast HAP1 activator. Cell 56: 291–301, 1989 [DOI] [PubMed] [Google Scholar]
- 68.Phelan CA, Gampe RT, Lambert MH, Parks DJ, Montana V, Bynum J, Broderick TM, Hu X, Williams SP, Nolte RT, and Lazar MA. Structure of Rev-erbα bound to N-CoR reveals a unique mechanism of nuclear receptor-co-repressor interaction. Nat Struct Mol Biol 17: 808–814, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qi Z, Hamza I. and O'Brian MR. Heme is an effector molecule for iron-dependent degradation of the bacterial iron response regulator (Irr) protein. Proc Natl Acad Sci U S A 96: 13056–13061, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, and Rastinejad F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat Struct Mol Biol 14: 1207–1213, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ragsdale SW. and Yi L. Thiol/disulfide redox switches in the regulation of heme binding to proteins. Antioxid Redox Signal 14: 1039–1047, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raspé E, Duez H, Mansén A, Fontaine C, Fiévet C, Fruchart J-C, Vennström B, and Staels B. Identification of Rev-erbα as a physiological repressor of apoC-III gene transcription. J Lipid Res 43: 2172–2179, 2002 [DOI] [PubMed] [Google Scholar]
- 73.Reppert SM. and Weaver DR. Coordination of circadian timing in mammals. Nature 418: 935–941, 2002 [DOI] [PubMed] [Google Scholar]
- 74.Robbins AH. and Stout CD. Structure of activated aconitase: formation of the [4Fe-4S] cluster in the crystal. Proc Natl Acad Sci U S A 86: 3639–3643, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruf HH. and Wende P. Hyperporphyrin spectra of ferric dimercaptide hemin complexes: models for ferric cytochrome P450-thiol complexes. J Am Chem Soc 99: 5499–5500, 1977 [DOI] [PubMed] [Google Scholar]
- 76.Sancar A. Regulation of the mammalian circadian clock by cryptochrome. J Biol Chem 279: 34079–34082, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Schubert E, Florin N, Duthie F, Brewitz HH, Kühl T, Imhof D, Hagelueken G, and Schiemann O. Spectroscopic studies on peptides and proteins with cysteine-containing heme regulatory motifs (HRM). J Inorg Biochem 148: 49–56, 2015 [DOI] [PubMed] [Google Scholar]
- 78.Schuller DJ, Wilks A, de Montellano PRO, and Poulos TL. Crystal structure of human heme oxygenase-l. Nat Struct Biol 6: 860–867, 1999 [DOI] [PubMed] [Google Scholar]
- 79.Shen J, Sheng X, Chang Z, Wu Q, Wang S, Xuan Z, Li D, Wu Y, Shang Y, Kong X, Yu L, Li L, Ruan K, Hu H, Huang Y, Hui L, Xie D, Wang F, and Hu R. Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function. Cell Rep 7: 180–193, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spencer ALM, Bagai I, Becker DF, Zuiderweg ERP, and Ragsdale SW. Protein/protein interactions in the mammalian heme degradation pathway: heme oxygenase-2, cytochrome P450 reductase, and biliverdin reductase. J Biol Chem 289: 29836–29858, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strickland S, Palmer G, and Massey V. Determination of dissociation constants and specific rate constants of enzyme-substrate (or protein-ligand) interactions from rapid reaction kinetic data. J Biol Chem 250: 4048–4052, 1975 [PubMed] [Google Scholar]
- 82.Sun JY, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, and Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J 21: 5216–5224, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun SF, Sono M, and Dawson JH. Mono- and bis-phosphine-ligated H93G myoglobin: spectral models for ferrous-phosphine and ferrous-CO cytochrome P450. J Inorg Biochem 127: 238–245, 2013 [DOI] [PubMed] [Google Scholar]
- 84.Sundaramoorthy M, Terner J, and Poulos TL. The crystal structure of chloroperoxidase: a heme peroxidase-cytochrome P450 functional hybrid. Structure 3: 1367–1377, 1995 [DOI] [PubMed] [Google Scholar]
- 85.Suzuki H, Tashiro S, Hira S, Sun JY, Yamazaki C, Zenke Y, Ikeda-Saito M, Yoshida M, and Igarashi K. Heme regulates gene expression by triggering Crm1-dependent nuclear export of Bach1. EMBO J 23: 2544–2553, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Varfaj F, Lampe JN, and de Montellano PRO. Role of cysteine residues in heme binding to human heme oxygenase-2 elucidated by two-dimensional NMR spectroscopy. J Biol Chem 287: 35181–35191, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vukomanovic D, Rahman MN, Maines MD, Ozolins TRS, Szarek WA, Jia ZJ, and Nakatsu K. Cysteine-independent activation/inhibition of heme oxygenase-2. Med Gas Res 6: 10–13, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang J. and Lazar MA. Bifunctional role of Rev-erbα in adipocyte differentiation. Mol Cell Biol 28: 2213–2220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang J, Li Y, Zhang M, Liu Z, Wu C, Yuan H, Li Y-Y, Zhao X, and Lu H. A zinc finger HIT domain-containing protein, ZNHIT-1, interacts with orphan nuclear hormone receptor Rev-erbβ and removes Rev-erbβ-induced inhibition of apoCIII transcription. FEBS J 274: 5370–5381, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Wang J, Liu N, Liu Z, Li Y, Song C, Yuan H, Li Y-y, Zhao X, and Lu H. The orphan nuclear receptor Rev-erbβ recruits Tip60 and HDAC1 to regulate apolipoprotein CIII promoter. Biochim Biophys Acta 1783: 224–236, 2008 [DOI] [PubMed] [Google Scholar]
- 91.Wang J, Yin L, and Lazar MA. The orphan nuclear receptor Rev-erbα regulates circadian expression of plasminogen activator inhibitor type 1. J Biol Chem 281: 33842–33848, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Wang TA, Yu YV, Govindaiah G, Ye X, Artinian L, Coleman TP, Sweedler JV, Cox CL, and Gillette MU. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science 337: 839–842, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Woo E-J, Jeong DG, Lim M-Y, Kim SJ, Kim K-J, Yoon S-M, Park B-C, and Ryu SE. Structural insight into the constitutive repression function of the nuclear receptor Rev-erbβ. J Mol Biol 373: 735–744, 2007 [DOI] [PubMed] [Google Scholar]
- 94.Yang J, Kim KD, Lucas A, Drahos KE, Santos CS, Mury SP, Capelluto DGS, and Finkielstein CV. A novel heme-regulatory motif mediates heme-dependent degradation of the circadian factor Period 2. Mol Cell Biol 28: 4697–4711, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang J, Panek HR, and O'Brian MR. Oxidative stress promotes degradation of the Irr protein to regulate haem biosynthesis in Bradyrhizobium japonicum. Mol Microbiol 60: 209–218, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Yang JH, Ishimori K, and O'Brian MR. Two heme binding sites are involved in the regulated degradation of the bacterial iron response regulator (Irr) protein. J Biol Chem 280: 7671–7676, 2005 [DOI] [PubMed] [Google Scholar]
- 97.Yang JH, Sangwan I, Lindemann A, Hauser F, Hennecke H, Fischer HM, and O'Brian MR. Bradyrhizobium japonicum senses iron through the status of haem to regulate iron homeostasis and metabolism. Mol Microbiol 60: 427–437, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yi L, Jenkins PM, Leichert LI, Jakob U, Martens JR, and Ragsdale SW. Heme regulatory motifs in heme oxygenase-2 form a thiol/disulfide redox switch that responds to the cellular redox state. J Biol Chem 284: 20556–20561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yi L. and Ragsdale SW. Evidence that the heme regulatory motifs in heme oxygenase-2 serve as a thiol/disulfide redox switch regulating heme binding. J Biol Chem 282: 21056–21067, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yin L, Joshi S, Wu N, Tong X, and Lazar MA. E3 ligases Arf-bp1 and Pam mediate lithium-stimulated degradation of the circadian heme receptor Rev-erbα. Proc Natl Acad Sci U S A 107: 11614–11619, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yin L, Wang J, Klein PS, and Lazar MA. Nuclear receptor Rev-erbα is a critical lithium-sensitive component of the circadian clock. Science 311: 1002–1005, 2006 [DOI] [PubMed] [Google Scholar]
- 102.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, and Lazar MA. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science 318: 1786–1789, 2007 [DOI] [PubMed] [Google Scholar]
- 103.Zenke-Kawasaki Y, Dohi Y, Katoh Y, Ikura T, Ikura M, Asahara T, Tokunaga F, Iwai K, and Igarashi K. Heme induces ubiquitination and degradation of the transcription factor Bach1. Mol Cell Biol 27: 6962–6971, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang H, Chen YQ, Keane FM, and Gorrell MD. Advances in understanding the expression and function of dipeptidyl peptidase 8 and 9. Mol Cancer Res 11: 1487–1496, 2013 [DOI] [PubMed] [Google Scholar]
- 105.Zhang L. and Guarente L. Heme binds to a short sequence that serves a regulatory function in diverse proteins. EMBO J 14: 313–320, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang L. and Hach A. Molecular mechanism of heme signalling in yeast: the transcriptional activator Hap1 serves as the key mediator. Cell Mol Life Sci 56: 415–426, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]