Abstract
Tuberculosis, caused by Mycobacterium tuberculosis, remains a leading cause of morbidity and mortality globally, with nearly 10.4 million new cases of incidence and over 1.7 million deaths annually. Drug-resistant M. tuberculosis strains, especially multidrug-resistant or extensively drug-resistant strains, have further intensified the problem associated with tuberculosis control. Host-directed therapy is a promising alternative for tuberculosis control. IL-32 is increasingly recognized as an important host molecule against tuberculosis. In this review, we highlight the proinflammatory properties of IL-32 and the mode of action of IL-32 in mycobacterial infections to inspire the development of novel immunity-based countermeasures and host-directed therapies against tuberculosis.
1. Introduction
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), latently infected one-third of the global population. TB is a global public health threat, with 10.4 million new cases and 1.7 million TB-associated deaths reported worldwide in 2016. New classes of effective anti-TB antibiotics are urgently needed [1] largely due to the occurrence of drug-resistant M. tuberculosis. Six hundred thousand new cases are rifampin resistant, including four hundred and ninety thousand patients exhibiting multidrug-resistant infection (http://www.who.int/tb/publications/global_report/en/). Host-directed therapy is a promising direction for the treatment of TB. Interleukin-32 (IL-32), originally called NK cell transcript 4 (NK4), can be produced by human NK and T cells stimulated with IL-2 [2]. IL-32 is a pleiotropic cytokine that can induce proinflammatory cytokines such as TNF-α and IL-1β via activation of NF-κB and p38 MAPK signaling [3]. IL-32 is primarily found only in primates [3, 4]; in humans, this gene is located on chromosome 16p13.3 and consists of eight exons [3, 5]. The presence of IL-32 mRNA in both immune and nonimmune tissues and cells, including NK cells, T cells, dendritic cells, endothelial cells, and epithelial cells [6, 7], suggests that this gene has multiple functions [7–10], such as inflammatory response [3], apoptosis [11], cell death [12], differentiation [8, 9], and in the pathogenesis of inflammatory disorders, including rheumatoid arthritis [13, 14], allergic rhinitis [15, 16], neuromyelitis optica [17], inflammatory bowel disease [18], chronic rhinosinusitis [19], osteoporosis [20], atherosclerosis [21], cardiovascular diseases [22], pulmonary diseases [23], Crohn's disease [24], Behçet's disease [25], hidradenitis suppurativa [26], cancer [27], and myeloid leukemia [28]. IL-32, as a proinflammatory cytokine, has been extensively studied [29], and the mechanisms of action and functions of IL-32 during bacterial and viral infection as well as in cancer have been reviewed [30–32]. IL-32 plays protective roles in multiple infectious diseases, such as HIV [33–35], influenza [36], cytomegalovirus [37], HBV [38, 39], Leishmania braziliensis [40, 41], Mycobacterium avium [42], and M. tuberculosis [43, 44] infection. In this review, we highlight the immunomodulatory effects and signaling pathways of IL-32 during mycobacterial infection.
2. The Isoforms and Secretion of IL-32
Many cytokines have multiple splicing isoforms. IL-17, IL-15, and vascular endothelial growth factor (VEGF) as well as IL-32 possess differently spliced isoforms. IL-15 has two alternatively spliced isoforms with identical biological properties but distinct modes of regulation and expression patterns [45]. There are nine alternatively spliced isoforms of IL-32 in the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/), namely, IL-32α, IL-32β, IL-32γ, IL-32δ, IL-32ε, IL-32ζ, IL-32η, IL-32θ, and IL-32s, generated by alternative mRNA splicing [46]. These isoforms interact with each other to control their biological activities [46]. IL-32 isoforms IL-32δ and IL-32β can interact. IL-32δ interacts with IL-32β and inhibits IL-32β-induced production of IL-10 [47]. The sequence of IL-32β is similar to that of IL-32γ which is spliced into IL-32β in different cell lines, such as THP-1, HeLa, and human synovial fibroblast cells [48, 49]. IL-32α is frequently observed in the cytosol but not in the culture supernatants of epithelial cells, including primary keratinocytes, intestinal epithelial cell lines, and colonic subepithelial myofibroblasts [18, 50, 51]. IL-32α specifically binds to proteinase-3 with high affinity, and this binding is independent of enzyme activity [52]. IL-32α has been reported to interact with PKCε and STAT3 [53] and with focal adhesion kinase 1 (FAK1) and integrins [54]. IL-32β and IL-32γ can induce caspase-8- and caspase-3-dependent apoptosis [54, 55]. IL-32β interacts with C/EBPα and PKCδ, culminating in increased IL-10 production [56]. IL-32γ, without exon deletions, is the most active isoform [46, 57].
The secretion of IL-32 isoforms remains to be investigated. IL-32γ possesses an N-terminal hydrophobic signal peptide, which is a typical feature of secreted cytokines. IL-32 is expressed in peripheral blood mononuclear cells (PBMCs) by LPS stimulation or M. tuberculosis infection, instead of Staphylococcus aureus and Candida albicans [58]. The IL-32α isoform was detected as an intracellular fraction, whereas the IL-32β isoform was found in the cell culture supernatant of Cos7 cells under transient transfection [3]. However, when performing transient transfection of IL-32β into bovine aortic vascular endothelial cells (BAVECs), IL-32β was found mainly in the cytosol and localized in the endoplasmic reticulum [6]. In addition, IL-32β was detected in the supernatant derived from the cytoplasm of apoptotic T cells but not secreted in anti-CD3 antibody-activated human T cells [12]. However, IL-32 can bind to the RGD motif of integrin, and IL-32 isoforms contain predicted tyrosine sulfation sites, which are prevalent in secreted proteins [2, 5, 59]. In HT-29 cells stimulated with TNF-α and IFN-γ, IL-32 was associated with membrane vesicles, and the release of IL-32 depended on exosome-like vesicle release mechanisms [60]. Therefore, IL-32 may be secreted via a nonclassical protein secretion pathway, similar to IL-33 and HMGB1, without typical signal peptides and are released via ER/Golgi-independent means [60, 61].
3. The Cellular Source and Expression of IL-32
IL-32 does not share homology with known cytokines. IL-32 expression has been detected in multiple human tissues and organs, including spleen, thymus, leukocytes, lungs, heart, placenta, liver, muscle, kidneys, pancreas, prostate, small intestine, colon, and brain [3]. The IL-32 mRNA is highly expressed in immune cells, and IL-32 expression has also been detected in nonimmune tissues and cells [6, 55, 62]. NK cells [2, 3, 63], monocytes/macrophages [3, 62, 64], dendritic cells (DCs) from PBMCs [58, 62, 65], neutrophils [66], T lymphocytes [62], epithelial cells [67], endothelial cells [68], fibroblasts [69], and hepatocytes [64] can express IL-32. IL-32 is also expressed and released in both cancer and noncancer cell lines, including the HepG2 human cancer cell line [3, 70], A549 cells [71, 72], pancreatic cancer cell lines such as MIA PaCa-2, PANC-1, and BxPC-3 [73, 74], the human hepatoma cell line Huh-7.5 [64], cervical cancer cells and tissues [75], the HEK293T cell line [34, 57], the HT-29 human colon cell line [60], the human colon neuroendocrine LCC-18 cell line [34], human colonic subepithelial myofibroblasts [51], human primary keratinocytes [50], synovial cells and fibroblast-like synoviocytes (FLS) [14, 69], and the marrow stromal cell lines HS-5 and HS-27A [76].
Four major isoforms (IL-32α, IL-32β, IL-32γ, and IL-32δ) were found in IL-2-stimulated human NK cells [3]. IL-32β, IL-32ε, and IL-32ζ were isolated from activated T cells [12], and IL-32s expression was first observed in Jurkat human leukemia T cells [70]. IL-32ε, IL-32ζ, IL-32θ, and IL-32s are also found in T cells, and the IL-32β isoform is mainly expressed in activated T cells [2, 12, 46]. IL-32θ and IL-32s were identified from monocyte-derived dendritic cells purified from human PBMCs and Jurkat T cells via 5′ RACE [46]. The function of different IL-32 isoforms in different cell types was summarized in Table 1. IL-32 mRNA levels increased after stimulation with Con A and monoclonal antibodies against CD3 and CD28 [62]. TNF-α reciprocally induced the expression of IL-32 mRNA in monocyte-derived dendritic cells, T cells, and synovial fibroblasts [62]. Intracellular IL-32 is constitutively expressed in human umbilical vein endothelial cells (HUVECs). The IL-32α and IL-32γ isoforms are the most prominently expressed IL-32 mRNAs in unstimulated endothelial cells [6, 60, 68, 77], while TNF-α and IL-1β induced the expression of IL-32β in endothelial cells [4]. Studies have shown that GM-CSF induces the expression of the IL-32α, IL-32β, IL-32γ, and IL-32δ isoforms in a caspase-1-dependent manner in eosinophils [15, 16]. Synovial fibroblasts isolated from patients with rheumatoid arthritis express IL-32γ after stimulation with IL-1β and TNF-α [48]. TNF-α can also promote the expression of the IL-32α, IL-32β, IL-32δ, and IL-32γ isoforms by activating the Syk/PKCδ/JNK/c-Jun signaling pathway [69]. The cell or tissue-specific expression patterns and functions of each isoform of IL-32 remain to be determined.
Table 1.
The function of IL-32 isoforms in different cell type.
| Cell type | IL-32 isoform | Targets | Function | Reference |
|---|---|---|---|---|
| U937 and monocyte-derived DCs | IL-32β | Increase in IL-10 production | Anti-inflammatory effects | [65] |
| Tumor cells | IL-32β | Decrease IL-1β, IL-6, TNF-α, and increase IL-10 production | Tumor growth | [78] |
| Myeloid cells and U937 cells | IL-32β | Increase in IL-10 production | Anti-inflammatory effects | [56] |
| Eosinophils | IL-32γ | Induces production of IL-6, TNF-α, IL-8, and VEGF | Inflammation of allergic rhinitis | [15] |
| Eosinophils | IL-32γ | Induces IL-1β, TNF-α, CXCL8, CCL3, CCL4, CD18, and ICAM-1 | Interacts with NOD1 or NOD2; PR3 activation | [79] |
| Monocytes or monocyte-derived macrophages | IL-32γ | TNF-a, IL-1b, IL-6, GROa/CXCL1, and MCP-1/CCL2, IL-10, and IL-1ra | Activation of ERK1/2, Akt, and Fyn signaling pathways | [80] |
| PBMC | IL-32α/β | TNF-α, IL-6 | — | [57] |
| Murine macrophage | IL-32α/β | TNF-α, CXCL2 | — | |
| THP-1 and RAW264.7 | IL-32α/β | TNF-α, IL-8, and, CXCL2 | — | [3, 62] |
| THP-1 cells | IL-32γ | Induces TNF-a, IL-1b, IL-8, and IL-6 | Activation of p38, caspase-1 and NF-κB pathways | [16] |
| THP-1 cells | IL-32γ | TNF-α, IL-23, CXCL1, CXCL8, and IL-1β | PI3K/Akt/P300/NF-κB signaling pathways | [81] |
| Endothelial cells | IL-32α/β/ε | ICAM-1, IL-1α, IL-8, and IL-6 | Vascular inflammation | [68] |
| PBMC/precursors | IL-32α | Activates Akt, JNK, ERK1/2, and NF-κB pathways | Cell differentiation | [10] |
| Murine DC | IL-32γ | Suppresses the production of CCL5 | Driving acquired immunity | [82] |
| Murine bone marrow–derived DCs | IL-32γ | IL-6 and IL-12 | Driving acquired immunity | [83] |
| PBMCs, CD4+ T cells, CD163+ macrophages, Treg cells, and DCs | IL-32γ | IDO and ILT4 | Immunosuppression | [35] |
| Monocyte-derived macrophages | IL-32γ | Induce cathelicidin and β-defensin 2 (DEFB4) | Microbicidal activity | [84] |
| PBMC | IL-32γ | IFNλ1 | Antiviral activity | [85] |
| T cells, epithelial cells, THP-1, and tumor cells | IL-32γ/β | Caspase-3, Caspase-8 | Cell apoptosis | [12, 27] |
| THP-1 cells | IL-32θ | Suppresses the production of CCL5 | Modulators of inflammation | [86] |
| THP-1 cells | IL-32θ | Decreases TNF-α | p38 and NF-κB signaling pathways | [28] |
4. The Function of IL-32 in the Activation of Signaling Pathways
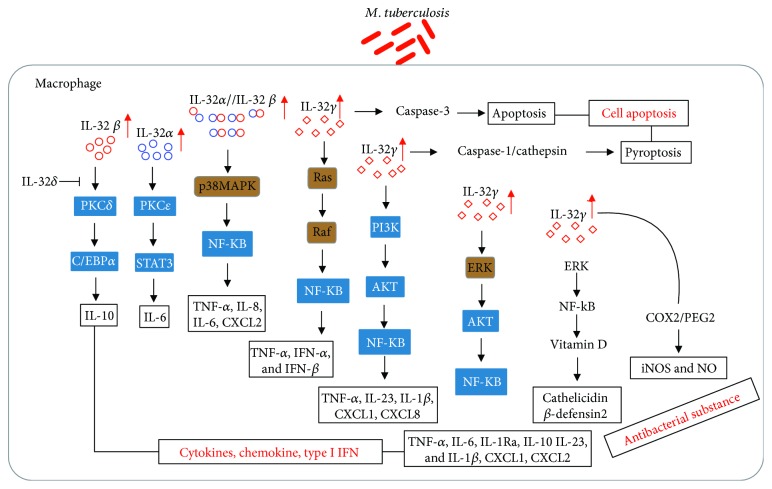
Although proinflammatory activities are key features of IL-32 and are enhanced by the different IL-32 isoforms, which induce the expression of cytokines such as TNF-α [3], IL-1β [87], IL-6 [53], IL-8 [88], and COX-2 [75], the mechanism of IL-32-based signaling remains unknown. The potential signaling pathways of macrophages induced by IL-32 are summarized in Figure 1. IL-32α, IL-32β, and IL-32γ are the main isoforms of IL-32 and have been shown to enhance the inflammatory response, suggesting that IL-32 can mediate diverse responses by interacting with different signaling molecules [53, 54, 56]. Intracellular IL-32α interacts with PKCε and STAT3, leading to phosphorylation of STAT3 and induction of IL-6 production after PMA stimulation [53]. Induction of TNF-α by IL-32α is mediated by phosphorylation of inhibitor kappaB (IkB) and ERK1/2 [89], NF-κB activation, and p38 MAPK phosphorylation in macrophage cell lines such as THP-1 and RAW264.7 [3]. Both IL-32α and IL-32β induce the expression of TNF-α, IL-8, and CXCL2 in THP-1 and RAW264.7 cells [3, 62] and induce the expression of TNF-α and CXCL2 in peritoneal murine macrophages [57]. Treatment of THP-1 cells with IL-32γ induced TNF-α, IL-6, IL-1β, and IL-8 expression via activation of the p38, caspase-1, and NF-κB pathways [16]. In addition, IL-32γ-stimulated monocytes and macrophages, such as THP-1-derived macrophages and monocyte-derived macrophages, induce the expression of TNF-α, IL-1β, IL-6, CXCL1, and CXCL2 along with IL-1Ra and IL-10 via the ERK1/2 and Akt signaling pathways [80]. Moreover, IL-32γ triggers the production of TNF-α, IL-1β, IL-23, CXCL1, and CXCL8 via the PI3K/Akt/P300/NF-κB signaling pathway [81]. PR3 cleaves IL-32α and increases the activity of IL-32, which subsequently activates PAR2 and triggers the TRIF and Ras/Raf pathways, resulting in increased type I IFN (IFN-α and IFN-β) and TNF-α production [90]. However, IL-32 isoforms can reduce cellular inflammation [47, 65]. IL-32δ inhibits the binding of IL-32β to PKCδ, resulting in decreased IL-10 production [47]. In monocyte-derived DCs and human macrophages, endogenous IL-32β promotes IL-10 expression, resulting in decreased expression of proinflammatory cytokines, such as IL-12, TNF-α, and IL-1β [65]. IL-32β promotes IL-10 production via interaction with PKCδ, which phosphorylates C/EBPa, an inhibitor that binds to the IL-10 promoter [56]. Moreover, low-severity arthritis was observed in a human IL-32β transgenic mouse model [91]. In summary, IL-32 regulates the expression of inflammatory cytokines.
Figure 1.
Endogenous IL-32-induced signaling pathway activation in macrophages and the potential roles of this pathway in M. tuberculosis infection.
5. IL-32 Regulates the Expression of MicroRNAs
IL-32 isoforms were shown to induce inflammation by regulating the expression of microRNAs [20, 37, 92, 93]. The expression of IL-32 is activated by human cytomegalovirus infection and functionally downregulated by hcmv-miR-UL112-1 [37]. MiR-23b-3p directly targets and induces the expression of PTEN, resulting in reduction in PI3-kinase, total Akt, and IL-32 levels [93]. IL-32α promotes the expression of the atheroprotective-associated genes Timp3 and Reck by downregulating the Rprd2-Dgcr8/Ddx5-Dicer1 biogenesis axis downstream of microRNA-205 [92]. Overexpression of human IL-32γ in transgenic mice led to increased bone formation, reduced bone loss with advancing age, and high osteogenic capacity of osteoblasts by upregulation of microRNA-29α [20]. Therefore, IL-32 is a novel protective cytokine that acts against mycobacterial infection. Elucidating the complex interactions between the IL-32 isoforms, microRNA-based regulation of the isoforms and the function of IL-32 will provide novel insight into the novel mechanism of the protective roles of IL-32 in multiple diseases.
6. The Function of IL-32 in Mycobacterial Infection
M. tuberculosis, the causative agent of human TB, can subvert host immune defenses to promote its own intracellular survival. Infection of human macrophages or PBMCs with M. tuberculosis H37Rv induced IL-32 production [11, 58], suggesting a role for IL-32 in the control of M. tuberculosis infection. M. tuberculosis and Mycobacterium bovis induced the release of IL-32 from PBMCs via IFN-γ, which was produced after caspase-1-activated IL-18 release [58]. Silencing of endogenous IL-32 in differentiated THP-1 human macrophages significantly decreased TNF-α, IL-1β, and IL-8 production and simultaneously increased the M. tuberculosis burden in infected macrophages [11].
The antimycobacterial effect of IL-32 may be partly due to enhanced cell apoptosis in infected macrophages. IL-32γ is a potent inducer of apoptosis; both IL-32γ and IL-32β can induce caspase-3- and caspase-8-dependent apoptosis [12, 27]. Endogenous IL-32 mediated M. tuberculosis-induced apoptosis of macrophages, suggesting that apoptosis of infected macrophages is a mechanism to protect against mycobacterial infection. IL-32γ decreased the M. tuberculosis burden within macrophages via classic caspase-3-mediated apoptosis [11] and caspase-1- or lysosomal-cathepsin-mediated apoptosis [94]. Our previous study showed that M. tuberculosis PE/PPE (Pro(P)-Glu(E) and Pro(P)-Pro(P)-Glu(E)) family antigen PPE32 induced ER-stress-mediated cell apoptosis via the stimulation of IL-32 production [95]. In addition, IL-32 serves as a mediator of IFNγ-vitamin D-related antimicrobial activity and a marker for latent TB infection (LTBI), as determined via the mining of TB transcriptomic datasets [96]. IL-32γ was also found to be associated with the vitamin D antimicrobial pathway in human macrophages [84]. IFN-γ-induced IL-32γ increases the expression of the vitamin D receptor, leading to the expression of cathelicidin and β-defensin 2 (DEFB4), which are potent antimicrobial peptides that act against intracellular infection in macrophages [84]. IFN-γ treatment activates the production of NO in macrophages, which is the main microbicidal molecule involved in the control of M. tuberculosis infection [97]. Human THP-1 cells express iNOS and produce NO after differentiation into macrophages by treatment with IL-32γ [98]. The production of reactive oxygen species (ROS) is required to induce the microbicidal activity mediated by vitamin D and cathelicidin, and cathelicidin enhances the production of ROS and proinflammatory cytokines, such as TNF-α, IL-8, and IL-6 [99]. M. tuberculosis-induced GM-CSF can promote NO production and phagolysosomal fusion against M. tuberculosis infection [100, 101]. GM-CSF might kill intracellular M. tuberculosis via induction of IL-32 as GM-CSF increases the expression of IL-32 in other cell types [15, 16]. In summary, IL-32γ is a protective molecule that enhances the microbicidal activity of macrophages against M. tuberculosis via increased apoptosis and pyroptosis, and antimicrobial peptides induced by vitamin D and GM-CSF are involved in protection against M. tuberculosis infection (Figure 1).
IL-32, lacking sequence homology with known cytokine families, is a novel proinflammatory cytokine [3]. The expression of IL-32 was increased in patients with M. avium infection [42]. IL-32γ significantly reduced the intracellular survival of M. avium in human monocyte-derived macrophages [42]. Moreover, the expression of endogenous IL-32 and NOD2 was increased in patients with the restrictive tuberculoid form of leprosy, which is caused by Mycobacterium leprae infection [102], suggesting that both NOD2 and IL-32 are associated with leprosy. IL-32 expression was increased in surgically resected lungs of active TB patients, particularly in airway epithelial cells and granuloma macrophages [43], suggesting a protective role of IL-32 against in vivo M. tuberculosis infection. However, there was a decrease in the protective response of IL-32γ against M. tuberculosis at later time points of infection as IL-32γ mRNA is spliced into IL-32β, leading to increased levels of IL-10-expressing macrophages or DCs in the lungs [43].
Acknowledgments
This work was supported by the National Natural Science Foundation (81701979, 81371851, 81071316 and 81601740), the general program of Chongqing Science and Technology Commission (cstc2017jcyjA0560), the Venture & Innovation Support Program for Chongqing Overseas Returnees (cx2017106), the National Megaprojects for Key Infectious Diseases (2008ZX10003-006 and 2008ZX10003-001), the Applied Basic Research Program of Science & Technology Department of Sichuan Province (2018JY0108), and the Doctoral Scientific Research Foundation of Neijiang Normal University.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Matteelli A., Migliori G. B., Cirillo D., Centis R., Girard E., Raviglione M. Multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis: epidemiology and control. Expert Review of Anti-Infective Therapy. 2007;5(5):857–871. doi: 10.1586/14787210.5.5.857. [DOI] [PubMed] [Google Scholar]
- 2.Dahl C. A., Schall R. P., He H. L., Cairns J. S. Identification of a novel gene expressed in activated natural killer cells and T cells. Journal of Immunology. 1992;148(2):597–603. [PubMed] [Google Scholar]
- 3.Kim S. H., Han S. Y., Azam T., Yoon D. Y., Dinarello C. A. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22(1):131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi H., Huang J., Ye F., Shyr Y., Blackwell T. S., Lin P. C. Interleukin-32beta propagates vascular inflammation and exacerbates sepsis in a mouse model. PLoS One. 2010;5(3, article e9458) doi: 10.1371/journal.pone.0009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q., Carroll H. P., Gadina M. The newest interleukins: recent additions to the ever-growing cytokine family. Vitamins and Hormones. 2006;74:207–228. doi: 10.1016/S0083-6729(06)74008-0. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi H., Lin P. C. Molecular characterization of IL-32 in human endothelial cells. Cytokine. 2009;46(3):351–358. doi: 10.1016/j.cyto.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimoto K. P., Laust A. K., Nelson E. L. A human dendritic cell subset receptive to the Venezuelan equine encephalitis virus-derived replicon particle constitutively expresses IL-32. Journal of Immunology. 2008;181(6):4010–4018. doi: 10.4049/jimmunol.181.6.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim Y. G., Lee C. K., Oh J. S., Kim S. H., Kim K. A., Yoo B. Effect of interleukin-32gamma on differentiation of osteoclasts from CD14+ monocytes. Arthritis and Rheumatism. 2010;62(2):515–523. doi: 10.1002/art.27197. [DOI] [PubMed] [Google Scholar]
- 9.Netea M. G., Lewis E. C., Azam T., et al. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(9):3515–3520. doi: 10.1073/pnas.0712381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mabilleau G., Sabokbar A. Interleukin-32 promotes osteoclast differentiation but not osteoclast activation. PLoS One. 2009;4(1, article e4173) doi: 10.1371/journal.pone.0004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai X., Kim S. H., Azam T., et al. IL-32 is a host protective cytokine against Mycobacterium tuberculosis in differentiated THP-1 human macrophages. Journal of Immunology. 2010;184(7):3830–3840. doi: 10.4049/jimmunol.0901913. [DOI] [PubMed] [Google Scholar]
- 12.Goda C., Kanaji T., Kanaji S., et al. Involvement of IL-32 in activation-induced cell death in T cells. International Immunology. 2006;18(2):233–240. doi: 10.1093/intimm/dxh339. [DOI] [PubMed] [Google Scholar]
- 13.Joosten L. A. B., Netea M. G., Kim S. H., et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(9):3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsaleh G., Sparsa L., Chatelus E., et al. Innate immunity triggers IL-32 expression by fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Research & Therapy. 2010;12(4):p. R135. doi: 10.1186/ar3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong H. J., Shin S. Y., Oh H. A., Kim M. H., Cho J. S., Kim H. M. IL-32 up-regulation is associated with inflammatory cytokine production in allergic rhinitis. The Journal of Pathology. 2011;224(4):553–563. doi: 10.1002/path.2899. [DOI] [PubMed] [Google Scholar]
- 16.Nam S. Y., Oh H. A., Choi Y., Park K. Y., Kim H. M., Jeong H. J. Inhibition of IL-32 signaling by bamboo salt decreases pro-inflammatory responses in cellular models of allergic rhinitis. Journal of Medicinal Food. 2014;17(9):939–948. doi: 10.1089/jmf.2013.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H., Wang K., Wang C., Xu F., Qiu W., Hu X. Increased plasma interleukin-32 expression in patients with neuromyelitis optica. Journal of Clinical Immunology. 2013;33(3):666–670. doi: 10.1007/s10875-012-9837-2. [DOI] [PubMed] [Google Scholar]
- 18.Shioya M., Nishida A., Yagi Y., et al. Epithelial overexpression of interleukin-32α in inflammatory bowel disease. Clinical and Experimental Immunology. 2007;149(3):480–486. doi: 10.1111/j.1365-2249.2007.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keswani A., Chustz R. T., Suh L., et al. Differential expression of interleukin-32 in chronic rhinosinusitis with and without nasal polyps. Allergy. 2012;67(1):25–32. doi: 10.1111/j.1398-9995.2011.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee E. J., Kim S. M., Choi B., et al. Interleukin-32 gamma stimulates bone formation by increasing miR-29a in osteoblastic cells and prevents the development of osteoporosis. Scientific Reports. 2017;7(1):p. 40240. doi: 10.1038/srep40240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Z., Dong A., Feng Z., Li J. Interleukin-32 promotes lipid accumulation through inhibition of cholesterol efflux. Experimental and Therapeutic Medicine. 2017;14(2):947–952. doi: 10.3892/etm.2017.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damen M. S. M. A., Popa C. D., Netea M. G., Dinarello C. A., Joosten L. A. B. Interleukin-32 in chronic inflammatory conditions is associated with a higher risk of cardiovascular diseases. Atherosclerosis. 2017;264:83–91. doi: 10.1016/j.atherosclerosis.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese F., Baraldo S., Bazzan E., et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2008;178(9):894–901. doi: 10.1164/rccm.200804-646OC. [DOI] [PubMed] [Google Scholar]
- 24.Netea M. G., Azam T., Ferwerda G., et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(45):16309–16314. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha Y. J., Park J. S., Kang M. I., Lee S. K., Park Y. B., Lee S. W. Increased serum interleukin-32 levels in patients with Behçet’s disease. International Journal of Rheumatic Diseases. 2017:1–8. doi: 10.1111/1756-185X.13072. [DOI] [PubMed] [Google Scholar]
- 26.Thomi R., Yerly D., Yawalkar N., Simon D., Schlapbach C., Hunger R. E. Interleukin-32 is highly expressed in lesions of hidradenitis suppurativa. British Journal of Dermatology. 2017;177(5):1358–1366. doi: 10.1111/bjd.15458. [DOI] [PubMed] [Google Scholar]
- 27.Heinhuis B., Plantinga T. S., Semango G., et al. Alternatively spliced isoforms of IL-32 differentially influence cell death pathways in cancer cell lines. Carcinogenesis. 2016;37(2):197–205. doi: 10.1093/carcin/bgv172. [DOI] [PubMed] [Google Scholar]
- 28.Kim M. S., Kang J. W., Jeon J. S., et al. IL-32θ gene expression in acute myeloid leukemia suppresses TNF-α production. Oncotarget. 2015;6(38):40747–40761. doi: 10.18632/oncotarget.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felaco P., Castellani M. L., de Lutiis M. A., et al. IL-32: a newly-discovered proinflammatory cytokine. Journal of Biological Regulators and Homeostatic Agents. 2009;23(3):141–147. [PubMed] [Google Scholar]
- 30.Hong J. T., Son D. J., Lee C. K., Yoon D. Y., Lee D. H., Park M. H. Interleukin 32, inflammation and cancer. Pharmacology & Therapeutics. 2017;174:127–137. doi: 10.1016/j.pharmthera.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro-Dias F., Saar Gomes R., de Lima Silva L. L., dos Santos J. C., Joosten L. A. B. Interleukin 32: a novel player in the control of infectious diseases. Journal of Leukocyte Biology. 2017;101(1):39–52. doi: 10.1189/jlb.4RU0416-175RR. [DOI] [PubMed] [Google Scholar]
- 32.Bae S., Kang D., Hong J., et al. Characterizing antiviral mechanism of interleukin-32 and a circulating soluble isoform in viral infection. Cytokine. 2012;58(1):79–86. doi: 10.1016/j.cyto.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 33.Nold M. F., Nold-Petry C. A., Pott G. B., et al. Endogenous IL-32 controls cytokine and HIV-1 production. Journal of Immunology. 2008;181(1):557–565. doi: 10.4049/jimmunol.181.1.557. [DOI] [PubMed] [Google Scholar]
- 34.Rasool S. T., Tang H., Wu J., et al. Increased level of IL-32 during human immunodeficiency virus infection suppresses HIV replication. Immunology Letters. 2008;117(2):161–167. doi: 10.1016/j.imlet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Smith A. J., Toledo C. M., Wietgrefe S. W., et al. The immunosuppressive role of IL-32 in lymphatic tissue during HIV-1 infection. Journal of Immunology. 2011;186(11):6576–6584. doi: 10.4049/jimmunol.1100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W., Yang F., Liu Y., et al. Negative feedback regulation of IL-32 production by iNOS activation in response to dsRNA or influenza virus infection. European Journal of Immunology. 2009;39(4):1019–1024. doi: 10.1002/eji.200838885. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y., Qi Y., Ma Y., et al. The expression of interleukin-32 is activated by human cytomegalovirus infection and down regulated by hcmv-miR-UL112-1. Virology Journal. 2013;10(1):p. 51. doi: 10.1186/1743-422X-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhuang G. L., Li G. H., Qu Z. J., Kuang J. Y. Interleukin-32 expression in serum of patients with HBV-related liver failure and its significance. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2013;27(4):247–249. [PubMed] [Google Scholar]
- 39.Cao H., Pan X. F., Zhang K., Shu X., Li G. Interleukin-32 expression is induced by hepatitis B virus. Zhonghua Gan Zang Bing Za Zhi. 2013;21(6):442–445. doi: 10.3760/cma.j.issn.1007-3418.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Gomes R. S., Silva M. V. T., dos Santos J. C., et al. IL-32γ promotes the healing of murine cutaneous lesions caused by Leishmania braziliensis infection in contrast to Leishmania amazonensis. Parasites & Vectors. 2017;10(1):p. 336. doi: 10.1186/s13071-017-2268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.dos Santos J. C., Heinhuis B., Gomes R. S., et al. Cytokines and microbicidal molecules regulated by IL-32 in THP-1-derived human macrophages infected with New World Leishmania species. PLoS Neglected Tropical Diseases. 2017;11(2, article e0005413) doi: 10.1371/journal.pntd.0005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bai X., Ovrutsky A. R., Kartalija M., et al. IL-32 expression in the airway epithelial cells of patients with Mycobacterium avium complex lung disease. International Immunology. 2011;23(11):679–691. doi: 10.1093/intimm/dxr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai X., Shang S., Henao-Tamayo M., et al. Human IL-32 expression protects mice against a hypervirulent strain of Mycobacterium tuberculosis. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(16):5111–5116. doi: 10.1073/pnas.1424302112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai X., Dinarello C. A., Chan E. D. The role of interleukin-32 against tuberculosis. Cytokine. 2015;76(2):585–587. doi: 10.1016/j.cyto.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Tagaya Y., Kurys G., Thies T. A., et al. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14444–14449. doi: 10.1073/pnas.94.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang J. W., Park Y. S., Lee D. H., et al. Interaction network mapping among IL-32 isoforms. Biochimie. 2014;101:248–251. doi: 10.1016/j.biochi.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Kang J. W., Park Y. S., Lee D. H., et al. Interleukin-32δ interacts with IL-32β and inhibits IL-32β-mediated IL-10 production. FEBS Letters. 2013;587(23):3776–3781. doi: 10.1016/j.febslet.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Heinhuis B., Koenders M. I., van de Loo F. A., Netea M. G., van den Berg W. B., Joosten L. A. B. Inflammation-dependent secretion and splicing of IL-32{gamma} in rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(12):4962–4967. doi: 10.1073/pnas.1016005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinhuis B., Koenders M. I., van Riel P. L., et al. Tumour necrosis factor alpha-driven IL-32 expression in rheumatoid arthritis synovial tissue amplifies an inflammatory cascade. Annals of the Rheumatic Diseases. 2011;70(4):660–667. doi: 10.1136/ard.2010.139196. [DOI] [PubMed] [Google Scholar]
- 50.Meyer N., Zimmermann M., Bürgler S., et al. IL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitis. Journal of Allergy and Clinical Immunology. 2010;125(4):858–865.e10. doi: 10.1016/j.jaci.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 51.Yagi Y., Andoh A., Imaeda H., et al. Interleukin-32α expression in human colonic subepithelial myofibroblasts. International Journal of Molecular Medicine. 2011;27(2):263–268. doi: 10.3892/ijmm.2010.575. [DOI] [PubMed] [Google Scholar]
- 52.Novick D., Rubinstein M., Azam T., Rabinkov A., Dinarello C. A., Kim S. H. Proteinase 3 is an IL-32 binding protein. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(9):3316–3321. doi: 10.1073/pnas.0511206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang J. W., Park Y. S., Lee D. H., et al. Intracellular interaction of interleukin (IL)-32α with protein kinase Cϵ (PKCϵ) and STAT3 protein augments IL-6 production in THP-1 promonocytic cells. Journal of Biological Chemistry. 2012;287(42):35556–35564. doi: 10.1074/jbc.M112.400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinhuis B., Koenders M. I., van den Berg W. B., Netea M. G., Dinarello C. A., Joosten L. A. B. Interleukin 32 (IL-32) contains a typical α-helix bundle structure that resembles focal adhesion targeting region of focal adhesion kinase-1. The Journal of Biological Chemistry. 2012;287(8):5733–5743. doi: 10.1074/jbc.M111.288290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heinhuis B., Netea M. G., van den Berg W. B., Dinarello C. A., Joosten L. A. B. Interleukin-32: a predominantly intracellular proinflammatory mediator that controls cell activation and cell death. Cytokine. 2012;60(2):321–327. doi: 10.1016/j.cyto.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Kang J. W., Park Y. S., Kim M. S., et al. Interleukin (IL)-32β-mediated CCAAT/enhancer-binding protein α (C/EBPα) phosphorylation by protein kinase Cδ (PKCδ) abrogates the inhibitory effect of C/EBPα on IL-10 production. Journal of Biological Chemistry. 2013;288(33):23650–23658. doi: 10.1074/jbc.M113.465575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi J. D., Bae S. Y., Hong J. W., et al. Identification of the most active interleukin-32 isoform. Immunology. 2009;126(4):535–542. doi: 10.1111/j.1365-2567.2008.02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Netea M. G., Azam T., Lewis E. C., et al. Mycobacterium tuberculosis induces interleukin-32 production through a caspase- 1/il-18/interferon-γ-dependent mechanism. PLoS Medicine. 2006;3(8, article e277) doi: 10.1371/journal.pmed.0030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monigatti F., Hekking B., Steen H. Protein sulfation analysis—a primer. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2006;1764(12):1904–1913. doi: 10.1016/j.bbapap.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Hasegawa H., Thomas H. J., Schooley K., Born T. L. Native IL-32 is released from intestinal epithelial cells via a non-classical secretory pathway as a membrane-associated protein. Cytokine. 2011;53(1):74–83. doi: 10.1016/j.cyto.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Nickel W. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. European Journal of Biochemistry. 2003;270(10):2109–2119. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- 62.Shoda H., Fujio K., Yamaguchi Y., et al. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Research & Therapy. 2006;8(6):p. R166. doi: 10.1186/ar2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gorvel L., Korenfeld D., Tung T., Klechevsky E. dendritic cell–derived il-32α: a novel inhibitory cytokine of NK cell function. The Journal of Immunology. 2017;199(4):1290–1300. doi: 10.4049/jimmunol.1601477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moschen A. R., Fritz T., Clouston A. D., et al. Interleukin-32: a new proinflammatory cytokine involved in hepatitis C virus-related liver inflammation and fibrosis. Hepatology. 2011;53(6):1819–1829. doi: 10.1002/hep.24285. [DOI] [PubMed] [Google Scholar]
- 65.Kang J. W., Choi S. C., Cho M. C., et al. A proinflammatory cytokine interleukin-32beta promotes the production of an anti-inflammatory cytokine interleukin-10. Immunology. 2009;128(1Part2):e532–e540. doi: 10.1111/j.1365-2567.2008.03025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radom-Aizik S., Zaldivar F., Jr, Leu S. Y., Galassetti P., Cooper D. M. Effects of 30 min of aerobic exercise on gene expression in human neutrophils. Journal of Applied Physiology. 2008;104(1):236–243. doi: 10.1152/japplphysiol.00872.2007. [DOI] [PubMed] [Google Scholar]
- 67.Ota K., Kawaguchi M., Fujita J., et al. Synthetic double-stranded RNA induces interleukin-32 in bronchial epithelial cells. Experimental Lung Research. 2015;41(6):335–343. doi: 10.3109/01902148.2015.1033569. [DOI] [PubMed] [Google Scholar]
- 68.Nold-Petry C. A., Nold M. F., Zepp J. A., Kim S. H., Voelkel N. F., Dinarello C. A. IL-32-dependent effects of IL-1beta on endothelial cell functions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(10):3883–3888. doi: 10.1073/pnas.0813334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mun S. H., Kim J. W., Nah S. S., et al. Tumor necrosis factor alpha-induced interleukin-32 is positively regulated via the Syk/protein kinase Cdelta/JNK pathway in rheumatoid synovial fibroblasts. Arthritis and Rheumatism. 2009;60(3):678–685. doi: 10.1002/art.24299. [DOI] [PubMed] [Google Scholar]
- 70.Ko N. Y., Chang S. H., Lee J. H., et al. Unique expression of a small IL-32 protein in the Jurkat leukemic T cell line. Cytokine. 2008;42(1):121–127. doi: 10.1016/j.cyto.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Li W., Liu Y., Mukhtar M. M., et al. Activation of interleukin-32 pro-inflammatory pathway in response to influenza A virus infection. PLoS One. 2008;3(4, article e1985) doi: 10.1371/journal.pone.0001985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li W., Sun W., Liu L., et al. IL-32: a host proinflammatory factor against influenza viral replication is upregulated by aberrant epigenetic modifications during influenza A virus infection. The Journal of Immunology. 2010;185(9):5056–5065. doi: 10.4049/jimmunol.0902667. [DOI] [PubMed] [Google Scholar]
- 73.Nishida A., Andoh A., Inatomi O., Fujiyama Y. Interleukin-32 expression in the pancreas. Journal of Biological Chemistry. 2009;284(26):17868–17876. doi: 10.1074/jbc.M900368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishida A., Andoh A., Shioya M., Kim-Mitsuyama S., Takayanagi A., Fujiyama Y. Phosphatidylinositol 3-kinase/Akt signaling mediates interleukin-32alpha induction in human pancreatic periacinar myofibroblasts. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2008;294(3):G831–G838. doi: 10.1152/ajpgi.00535.2007. [DOI] [PubMed] [Google Scholar]
- 75.Lee S., Kim J. H., Kim H., et al. Activation of the interleukin-32 pro-inflammatory pathway in response to human papillomavirus infection and over-expression of interleukin-32 controls the expression of the human papillomavirus oncogene. Immunology. 2011;132(3):410–420. doi: 10.1111/j.1365-2567.2010.03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marcondes A. M., Mhyre A. J., Stirewalt D. L., Kim S. H., Dinarello C. A., Deeg H. J. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(8):2865–2870. doi: 10.1073/pnas.0712391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho K. S., Park S. H., Joo S. H., Kim S. H., Shin C. Y. The effects of IL-32 on the inflammatory activation of cultured rat primary astrocytes. Biochemical and Biophysical Research Communications. 2010;402(1):48–53. doi: 10.1016/j.bbrc.2010.09.099. [DOI] [PubMed] [Google Scholar]
- 78.Yun H. M., Oh J. H., Shim J. H., et al. Antitumor activity of IL-32β through the activation of lymphocytes, and the inactivation of NF-κB and STAT3 signals. Cell Death & Disease. 2013;4(5, article e640) doi: 10.1038/cddis.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wong C. K., Dong J., Lam C. W. K. Molecular mechanisms regulating the synergism between IL-32γ and NOD for the activation of eosinophils. Journal of Leukocyte Biology. 2014;95(4):631–642. doi: 10.1189/jlb.0813452. [DOI] [PubMed] [Google Scholar]
- 80.Choi K. Y. G., Napper S., Mookherjee N. Human cathelicidin LL-37 and its derivative IG-19 regulate interleukin-32-induced inflammation. Immunology. 2014;143(1):68–80. doi: 10.1111/imm.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turner-Brannen E., Choi K. Y. G., Arsenault R., el-Gabalawy H., Napper S., Mookherjee N. Inflammatory cytokines IL-32 and IL-17 have common signaling intermediates despite differential dependence on TNF-receptor 1. Journal of Immunology. 2011;186(12):7127–7135. doi: 10.4049/jimmunol.1002306. [DOI] [PubMed] [Google Scholar]
- 82.Son M. H., Jung M. Y., Choi S., Cho D., Kim T. S. IL-32γ induces chemotaxis of activated T cells via dendritic cell-derived CCL5. Biochemical and Biophysical Research Communications. 2014;450(1):30–35. doi: 10.1016/j.bbrc.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 83.Jung M. Y., Son M. H., Kim S. H., Cho D., Kim T. S. IL-32gamma induces the maturation of dendritic cells with Th1- and Th17-polarizing ability through enhanced IL-12 and IL-6 production. Journal of Immunology. 2011;186(12):6848–6859. doi: 10.4049/jimmunol.1003996. [DOI] [PubMed] [Google Scholar]
- 84.Montoya D., Inkeles M. S., Liu P. T., et al. IL-32 is a molecular marker of a host defense network in human tuberculosis. Science Translational Medicine. 2014;6(250, article 250ra114) doi: 10.1126/scitranslmed.3009546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Y., Xie J., Xu X., et al. Inducible interleukin 32 (IL-32) exerts extensive antiviral function via selective stimulation of interferon λ1 (IFN-λ1) Journal of Biological Chemistry. 2013;288(29):20927–20941. doi: 10.1074/jbc.M112.440115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bak Y., Kang J. W., Kim M. S., et al. IL-32θ downregulates CCL5 expression through its interaction with PKCδ and STAT3. Cellular Signalling. 2014;26(12):3007–3015. doi: 10.1016/j.cellsig.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 87.Hong J., Bae S., Kang Y., et al. Suppressing IL-32 in monocytes impairs the induction of the proinflammatory cytokines TNFalpha and IL-1beta. Cytokine. 2010;49(2):171–176. doi: 10.1016/j.cyto.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 88.Jaekal J., Jhun H., Hong J., et al. Cloning and characterization of bovine interleukin-32 beta isoform. Veterinary Immunology and Immunopathology. 2010;137(1-2):166–171. doi: 10.1016/j.vetimm.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 89.Nakayama M., Niki Y., Kawasaki T., et al. Enhanced susceptibility to lipopolysaccharide-induced arthritis and endotoxin shock in interleukin-32 alpha transgenic mice through induction of tumor necrosis factor alpha. Arthritis Research & Therapy. 2012;14(3):p. R120. doi: 10.1186/ar3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakayama M., Niki Y., Kawasaki T., et al. IL-32-PAR2 axis is an innate immunity sensor providing alternative signaling for LPS-TRIF axis. Scientific Reports. 2013;3(1):p. 2960. doi: 10.1038/srep02960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park M. H., Yoon D. Y., Ban J. O., et al. Decreased severity of collagen antibody and lipopolysaccharide-induced arthritis in human IL-32β overexpressed transgenic mice. Oncotarget. 2015;6(36):38566–38577. doi: 10.18632/oncotarget.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Son D. J., Jung Y. Y., Seo Y. S., et al. Interleukin-32α inhibits endothelial inflammation, vascular smooth muscle cell activation, and atherosclerosis by upregulating Timp3 and Reck through suppressing microRNA-205 biogenesis. Theranostics. 2017;7(8):2186–2203. doi: 10.7150/thno.18407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zaman M. S., Thamminana S., Shahryari V., et al. Inhibition of PTEN gene expression by oncogenic miR-23b-3p in renal cancer. PLoS One. 2012;7(11, article e50203) doi: 10.1371/journal.pone.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bai X., Kinney W. H., Su W. L., et al. Caspase-3-independent apoptotic pathways contribute to interleukin-32γ-mediated control of Mycobacterium tuberculosis infection in THP-1 cells. BMC Microbiology. 2015;15(1):p. 39. doi: 10.1186/s12866-015-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deng W., Yang W., Zeng J., Abdalla A. E., Xie J. Mycobacterium tuberculosis PPE32 promotes cytokines production and host cell apoptosis through caspase cascade accompanying with enhanced ER stress response. Oncotarget. 2016;7(41):67347–67359. doi: 10.18632/oncotarget.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deffur A., Wilkinson R. J., Coussens A. K. Tricks to translating TB transcriptomics. Annals of Translational Medicine. 2015;3(Supplement 1):p. S43. doi: 10.3978/j.issn.2305-5839.2015.04.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bloom B. R., Modlin R. L. Mechanisms of defense against intracellular pathogens mediated by human macrophages. Microbiology Spectrum. 2016;4(3) doi: 10.1128/microbiolspec.MCHD-0006-2015. [DOI] [PubMed] [Google Scholar]
- 98.Sato K., Akaki T., Tomioka H. Differential potentiation of anti-mycobacterial activity and reactive nitrogen intermediate-producing ability of murine peritoneal macrophages activated by interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α) Clinical and Experimental Immunology. 1998;112(1):63–68. doi: 10.1046/j.1365-2249.1998.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang C. S., Shin D. M., Kim K. H., et al. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. Journal of Immunology. 2009;182(6):3696–3705. doi: 10.4049/jimmunol.0802217. [DOI] [PubMed] [Google Scholar]
- 100.Cho J. E., Park S., Lee H., Cho S. N., Kim Y. S. Mycobacterium tuberculosis-induced expression of granulocyte-macrophage colony stimulating factor is mediated by PI3-K/MEK1/p38 MAPK signaling pathway. BMB Reports. 2013;46(4):213–218. doi: 10.5483/BMBRep.2013.46.4.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pasula R., Azad A. K., Gardner J. C., Schlesinger L. S., McCormack F. X. Keratinocyte growth factor administration attenuates murine pulmonary mycobacterium tuberculosis infection through granulocyte-macrophage colony-stimulating factor (GM-CSF)-dependent macrophage activation and phagolysosome fusion. The Journal of Biological Chemistry. 2015;290(11):7151–7159. doi: 10.1074/jbc.M114.591891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schenk M., Krutzik S. R., Sieling P. A., et al. NOD2 triggers an interleukin-32-dependent human dendritic cell program in leprosy. Nature Medicine. 2012;18(4):555–563. doi: 10.1038/nm.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]