Abstract
Background
Current evidence indicates that statins increase the risk of new onset diabetes mellitus (NOD) and also deteriorate the glycemic control in patients with known diabetes mellitus (DM) after high-dose statin therapy.
Aims
The aim of this review was to explore the effect of atorvastatin in causing NOD or deteriorating glycemic control in patients with DM.
Methods
Two independent reviewers conducted the literature search, through PubMed database searching for articles published in English until April 2015, and only primary studies were included.
Results
Of the 919 articles identified in our original search, 33 met the criteria for this review encompassing 1,951,113 participants. Twenty articles examined dysregulation of DM due to atorvastatin. Half of them showed that there was no significant change in glycemic control in patients treated with atorvastatin. Other studies showed that fasting plasma glucose and HbA1c levels were increased by atorvastatin. Thirteen articles examined if atorvastatin causes NOD. The majority of these articles showed that patients who used atorvastatin had a higher dose-dependent risk of developing NOD.
Conclusion
This systematic review suggests that there is an association between atorvastatin treatment and NOD. Moreover, it showed that atorvastatin in high dose causes worsening of the glycemic control in patients with DM.
1. Introduction
Dyslipidemia is a primary well-established independent risk factor for cardiovascular disease [1]. An effective treatment, the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (also known as statins) are proven to lower low-density lipoprotein (LDL) cholesterol levels in patients with hypercholesterolemia [2].
Multiple prospective studies have showed the cardioprotective and antioxidant effects of statins, which have widely and for many decades been used for that purpose [3, 4]. LDL-cholesterol levels remain the principal target for lipid modification and statin therapy as the main treatment of achieving LDL goal attainment. The beneficial effect of statins in both primary and secondary prevention of cardiovascular events by lowering LDL-cholesterol concentrations has been documented among patients with or without diabetes [5, 6].
Diabetes mellitus is a growing public health problem that is approaching epidemic proportions globally, and it is also related with increased cardiovascular risk. In adults aged over 40 with diabetes mellitus, according to the American Diabetes Association (ADA) [7] and ACC/AHA guidelines [8], statin treatment is recommended, while it should also be considered for those less than 40 years old based on their risk profile.
Statin therapy is associated with significant reduction in cardiovascular endpoints; however, concerns have been raised over the use of statins and an increased risk of diabetes. Several statins are now available, with different potencies and drug interactions such as atorvastatin, pitavastatin, simvastatin, and rosuvastatin. However, their influence on insulin levels and insulin resistance has not been clarified yet. There are some theories suggesting a potential risk of developing new onset diabetes mellitus (NOD) [9] or a risk of deteriorating the glycemic control in patients with diabetes after high-dose statin therapy [10]. This risk is seemingly elevated with the use of atorvastatin [11]. Therefore, many clinical trials [12–14] have investigated the possible association between atorvastatin and new onset diabetes or dysregulation of already existing diabetes as well as the underlying mechanisms. It has also been reported that some groups with special characteristics, such as postmenopausal women [15] and renal allograft recipients [16], are in particular danger. On the other hand, few studies have demonstrated that atorvastatin did not worsen insulin sensitivity in patients with diabetes [17, 18], whereas one study suggested that patients treated with atorvastatin may be at a lower risk of developing new onset diabetes [14].
The aim of this review was to look systematically into the current literature and carefully collect and analyse results to explore the potential effect of atorvastatin in both causing new onset diabetes and deterioration of glycemic control in patients with known diabetes.
2. Methods
2.1. Literature Search
This review has adopted the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A systematic search strategy was followed. PubMed database was used to search for publications of interest using as keywords “atorvastatin AND diabetes.” Eligible studies were primary studies of every design (observational studies, cross-sectional, cohort, case studies, case series, clinical trials, etc.) published in English until 30/04/2015 (date of last search). Secondary studies (reviews, letters, and meta-analyses) as well as studies published in languages other than English were excluded. Two reviewers working independently conducted the literature search.
2.2. Data Collection and Synthesis
The titles of studies, which were considered for retrieval, were recorded on a form and then were classified on an inclusion and exclusion search diary. All the articles that came up but were irrelevant or were secondary research were excluded. Studies were selected for retrieval after two independent reviewers had collected titles and abstracts identified in electronic searches. The results of the two reviewers were compared by a third independent reviewer, and any differences of opinion were resolved by discussion. The corresponding authors were contacted on account of missing data. The included studies were grouped and presented in Summary Tables featuring key points of each study; the following data were collected: first author surname, study name, year of publication, study design, country, number of total population (percentage of male/female), total population age (Mean-Standard Deviation-Median range), Quantitative results (HR, p) of the study findings, diabetes status of the participants, and evidence of association between atorvastatin use and new onset diabetes mellitus or increased risk for worsening of glycemic control. Outcome measures of included studies were organized and then analysed cumulatively. Given the lack of primary data, a narrative form of synthesis was adopted as a way of expressing and synthesizing the results of the eligible studies (i.e., numerical data expressed as weighted means whenever possible). No further statistical analysis was possible.
3. Results
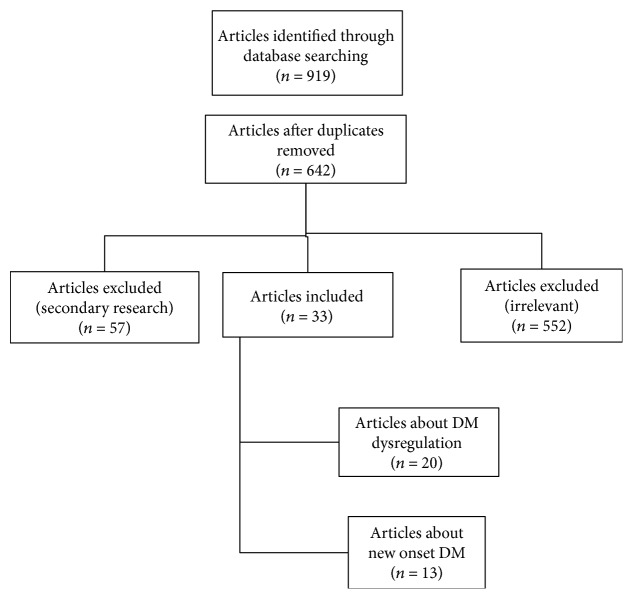
In total, 919 articles were identified through database searching, which were reduced to 642 articles after removing duplicates. In all, 33 articles were eligible for the review, following exclusions. Eighteen clinical trials [1–3, 5, 9, 10, 13, 17, 19–28], 14 cohort studies [11, 12, 14–16, 18, 29–36], and one case control study [37] involving a total of 1,951,113 participants were included in the current review. A relevant flow chart was constructed to detail the number of papers retrieved and the steps undertaken (Figure 1).
Figure 1.
Flowchart of the systematic review process.
It should be noted that the primary endpoints of the included studies were cardiovascular disease, LDL-cholesterol levels, HDL-cholesterol levels, or other outcomes such as serum triglyceride levels, apolipoprotein B, serum dehydroepiandrosterone sulfate levels, and C-reactive protein levels. However, all the included studies reported evidence of glycemic control or the incidence of new onset diabetes as secondary endpoints. The proportion of females in the studies ranged between 0% and 100%. More specifically, the total female population was 1,197,855–1,197,955 (approximately 1,197,900) (61.4%). Three studies had only females [15, 25, 37] and 2 studies had only males [28, 34]. In the majority of studies, females were almost as many as males.
The mean average age of the subjects by study (for which age data were available) ranged between 44 and 74.9 years. Thirteen studies included only patients with type 2 diabetes mellitus [2, 3, 10, 11, 17–19, 21, 27–30, 33], while 3 studies included only patients with type 1 diabetes mellitus [13, 20, 24]. The average time since diagnosis of diabetes ranged between 4 and 26 years. The participants had been followed for an average of 4 months to 5 years (weighted average, 3.6 years).
Of note, Koh et al. [9] compared the effect of atorvastatin at doses of 10 mg, 20 mg, 40 mg, and 80 mg. Fasting plasma insulin (mean changes: 25%, 42%, 31%, and 45%) and HbA1c levels (2%, 5%, 5%, and 5%) were increased by atorvastatin 10, 20, 40, and 80 mg when contrast with either baseline (all p < 0.05 by paired t-test) or placebo (p = 0.009 for insulin and p = 0.008 for HbA1c by ANOVA). Atorvastatin 10, 20, 40, and 80 mg declined insulin sensitivity (1%, 3%, 3%, and 4%, respectively) when compared with either baseline (p = 0.312, p = 0.008, p < 0.001, and p = 0.008, respectively, by paired t-test) or placebo (p = 0.033 by ANOVA).
Baseline characteristics of the enrolled participants were generally similar between the groups in each study. Information regarding the included studies is presented in Tables 1 and 2.
Table 1.
Atorvastatin use and new onset diabetes mellitus.
| Study author, year | Design | Study location | Total population (% F) | Total population: age, mean (SD), median (range/IQR) | Duration | Comparison groups | Risk of developing NOD (statin users vs nonstatin users) |
|---|---|---|---|---|---|---|---|
| Waters et al., 2011 [4, 22, 38, 95] | 3 clinical trials | TNT: worldwide | TNT: 7595 (17%) | TNT: 60.6 (8.9), NA |
TNT: 4.9 years | TNT: atorvastatin 10 mg and atorvastatin 80 mg | TNT: HR: 1.10 (0.94–1.29), p = 0.22 |
| IDEAL: northern Europe | IDEAL: 7461 (19%) | IDEAL: 61.5 (9.5), NA | IDEAL: 4.8 years | IDEAL: atorvastatin 80 mg and simvastatin 20 mg | IDEAL: HR: 1.19, (0.99–1.44), p = 0.072 | ||
| SPARCL: worldwide | SPARCL: 3803 (41%) | SPARCL: 62.5 (11.6), NA | SPARCL: 4.9 years | SPARCL: nonstatin users and atorvastatin 80 mg | SPARCL: HR: 1.34, (1.05–1.71), p = 0.018 | ||
|
| |||||||
| Waters et al., 2013 [1, 4, 95] | 2 clinical trials | TNT: worldwide | 15,056 (18%) | 61.1 (9.2), NA | TNT: 4.9 years | TNT: atorvastatin 10 mg and atorvastatin 80 mg | 0–1 risk factors: HR: 0.97 (0.77–1.22) |
| IDEAL: northern Europe | [TNT: 7595, IDEAL: 7461] | IDEAL: 4.8 years | IDEAL: atorvastatin 80 mg and simvastatin 20 to 40 mg | 2 to 4 risk factors: HR: 1.24 (1.08–1.42), p = 0.0027 | |||
|
| |||||||
| Sever et al., 2003 [26] | Clinical trial | UK, Ireland, Nordic countries | 10,305 (19%) | 63 (NA), NA (40–79) | 3.3 years | Atorvastatin 10 mg and nonstatin users | HR: 1.15 (0.91–1.44), p = 0.2493 |
|
| |||||||
| Chen et al., 2013 [37] | Case control | Taiwan | 11,715 (100%) | NA, NA | 2 years | Atorvastatin users and nonstatin users | Adj. OR: 2.80 (1.74–4.49), p < 0.001 |
|
| |||||||
| Ma et al., 2012 [14] | Retrospective cohort study | Taiwan | 15,637 (NA) | 74.9 (6.3), NA | 5.5 years | Atorvastatin users and nonstatin users | Adj. HR: 0.77 (0.72–0.83), p < 0.0001 |
|
| |||||||
| Ma et al., 2012 [31] | Retrospective cohort study | Taiwan | 16,027 (54%) | 59.9 (18.7), NA (20–84) | 3.5 years | Atorvastatin users and nonstatin users | Users vs non users: Adj. HR: 1.29 (1.16–1.44), p < 0.0001 |
| Among users: Adj. HR: 1.15 (0.96–1.35), p = 0.5465 | |||||||
|
| |||||||
| Carter et al., 2013 [12] | Retrospective cohort study | Canada | 471,250 (54%) | NA, 73 (69–78) |
12.5 years | Atorvastatin users and pravastatin users | All users: Adj. HR: 1.22 (1.15–1.29) Primary prevention users: 1.20 (1.10–1.30) Secondary prevention users: 1.25 (1.16–1.34) |
|
| |||||||
| Cho et al., 2015 [35] | Retrospective cohort study | Korea | 3680 (52.01%) | NA, NA | 62.6 (15.3) months | Atorvastatin users and simvastatin users | Adj. HR = 1.52(0.72–3.21), p = 0.268 |
|
| |||||||
| Zaharan et al., 2013 [32] | Retrospective cohort study | Ireland | 1,235,671 (61%) | NA, NA | 8.5 years | Atorvastatin users and nonstatin users | Adj. HR: 1.25 (1.21–1.28), p < 0.0001 |
|
| |||||||
| Choe et al., 2014 [16] | Cohort study | Korea | 394 (42%) | NA, NA | 5 years | Atorvastatin users and nonstatin users | Adj. HR: 3.76 (2.22–6.40), p = 0.001 |
| Culver et al., 2012 [15] | Cohort study | USA | 153,840 (100%) | 63.17 (7.25) NA (50–79) |
NA | Atorvastatin users and nonstatin users | Adj. HR: 1.61 (1.26–2.06) |
|
| |||||||
| Cederberg et al., 2015 [34] | Cohort study | Finland | 8749 (0%) | 57 (7) 57 (45–73) |
5.9 years | Atorvastatin users and nonstatin users | Adj. HR: 1.21 (1.04–1.40) |
| Atorvastatin users (20 or 40 mg) and nonstatin users | HR: 1.37 (1.14–1.65) | ||||||
|
| |||||||
| Park et al., 2015 [36] | Cohort study | Korea | Initial: 3566 (49.41%), after PSM adjustment: 818 (49.14%) | NA, NA | 3 years | Atorvastatin users (10 or 20 mg) and nonstatin users | OR: 1.99 (1.00–3.98), p = 0.050 |
Variables are expressed as absolute numbers, percentages, mean ± SD, and median (IQR). NOD: new onset diabetes mellitus; NA: not available; PSM: propensity score matching analysis; Adj. HR: adjusted hazard ratio.
Table 2.
Atorvastatin use and dysregulation of diabetes mellitus.
| Study author, year | Design | Study location | Total population (% F) | Total population: age, mean (SD), median (range/IQR) | Comparison groups | DM type | Duration of study | Quantitative results related to atorvastatin |
|---|---|---|---|---|---|---|---|---|
| Tam et al., 2010 [19] | Clinical trial | China | 80 (NA) | NA, NA | Atorvastatin and placebo | T2DM | 6 months | FPG (mmol/L) (vs baseline) Placebo: 7.95 ± 1.99 → 7.56 ± 2.06, ns Atorvastatin: 7.91 ± 2.10 → 8.03 ± 2.48, ns |
| HbA1c (%) [mmol/mol] (vs baseline) Placebo: 8.0 ± 1.1 [64 ± 11] → 8.0 ± 1.2 [64 ± 10], ns Atorvastatin: 7.8 ± 1.2 [62 ± 10] → 8.2 ± 1.4 [66 ± 8], ns | ||||||||
| Placebo vs atorvastatin: ns | ||||||||
|
| ||||||||
| Mandosi et al., 2010 [2] | Clinical trial | Italy | 22 (23%) | 60.8 (7.1), NA | All atorvastatin 20 mg users | T2DM | 8 weeks | From baseline HbA1c (%) [mmol/mol]: 7.6 ± 1.1 [60 ± 11] → 7.6 ± 0.9 [60 ± 14], p = 0.52 FPG (mmol/L): 8.6 ± 2.2 → 9.1 ± 1.9, p = 0.36 |
|
| ||||||||
| Koh et al., 2010 [9] | Clinical trial | Korea | 213 (50%) | NA, NA | Atorvastatin 10 mg, 20 mg, 40 mg, 80 mg, and placebo | T2DM & without diabetes | 2 months | HbA1c (%) [mmol/mol] Placebo: 5.8 ± 0.5 [40 ± 18] → 5.8 ± 0.6 [40 ± 17], ns 10 mg: 5.8 ± 0.6 [40 ± 17] → 6.0 ± 0.6 [42 ± 17] (p < 0.001 vs baseline) 20 mg: 5.9 ± 0.8 [41 ± 15] → 6.2 ± 0.9 [44 ± 14] (p < 0.001 vs baseline, p < 0.05 vs placebo) 40 mg: 6.1 ± 0.8 [43 ± 15] → 6.4 ± 1.0 [46 ± 13] (p < 0.01 vs baseline, p < 0.05 vs placebo) 80 mg: 6.1 ± 0.8 [43 ± 15] → 6.4 ± 1.1 [46 ± 11] (p < 0.05 vs baseline, p < 0.05 vs placebo) |
| Global ANOVA: p = 0.008 | ||||||||
|
| ||||||||
| Tehrani et al., 2010 [20] | Clinical trial | Sweden | 20 (NA) | NA 44 (39–61) |
Atorvastatin 80 mg and placebo | T1DM | 2 months | HbA1c (%) [mmol/mol] Placebo: 7.6 ± 0.9 [60 ± 14] → 7.3 ± 0.8 [56 ± 15] Atorva 80 mg: 7.5 ± 0.9 [58 ± 14] → 7.8 ± 1.1 [62 ± 11], p < 0.01 |
| Atorva vs placebo: p < 0.001 | ||||||||
|
| ||||||||
| Rutter et al., 2011 [21] | Clinical trial | UK | 119 (17%) | 64 (10) NA |
Atorvastatin 80 mg and atorvastatin 10 mg | T2DM | 2.1 years | HbA1c (%) [mmol/mol] (vs baseline) Atorva 10 mg vs 80 mg: 0.3 [2.4] (0.1–0.5) [0.4–4.3], p = 0.017 |
| Tehrani et al., 2013 [13] | Clinical trial | Sweden | 20 (50%) | NA 44 (39–61) |
Atorvastatin 80 mg and placebo | T1DM | 2 months | HbA1c (%) [mmol/mol] Atorvastatin treatment period: 7.5 ± 0.9 [58 ± 14] → 7.8 ± 1.1 [62 ± 11], p = 0.008 Atorvastatin vs placebo: p < 0.001 |
|
| ||||||||
| Grigoropoulou et al., 2014 [18] | Clinical trial | Greece | 79 (61%) | NA NA (45–75) |
Atorvastatin 10 mg and control | T2DM | 12 months | HbA1c (%) [mmol/mol], (0 → 3mo → 6mo → 12mo) Atorvastatin: 6.7 ± 0.8 [50 ± 15] → 6.7 ± 0.7 [50 ± 16] → 6.8 ± 0.8 [51 ± 15] → 6.9 ± 0.7 [52 ± 16], p = 0.09 Control: 7.0 ± 0.7 [53 ± 16] → 6.8 ± 0.6 [51 ± 17] → 6.9 ± 0.7 [52 ± 16] → 7.0 ± 0.7 [53 ± 16], p = 0.06 Atorvastatin vs control: p = 0.26 |
| FPG (mg/dL), (0 → 3mo → 6mo → 12mo) Atorvastatin: 141.2 ± 33.3 → 138.1 ± 27.5 → 136.2 ± 29.8 → 138.3 ± 29.8, p = 0.76 Control: 153.9 ± 50.6 → 142.1 ± 54.3 → 142.7 ± 42.2 → 145.6 ± 35.5, p = 0.36 Atorvastatin vs control: p = 0.21 | ||||||||
|
| ||||||||
| Thongtang et al., 2011 [23] | Clinical trial | USA | 272 (49%) | NA, NA | Atorvastatin 80 mg, rosuvastatin 40 mg | T2DM & without diabetes | 6 weeks | HbA1c (%) [mmol/mol] Mean differences vs baseline: Atorvastatin: +0.1 [22] (−2.6–9.3) [−52–78] |
|
| ||||||||
| Martin et al., 2011 [24] | Clinical trial | Germany | 89 (40%) | 30 (NA), NA (18–39) | Atorvastatin 80 mg and placebo | T1DM | 18 months | HbA1c (%) [mmol/mol] (baseline → 6mo → 18mo) Atorvastatin vs baseline: 7.8 [62] → 6.6 [49] → 6.8 [51], p < 0.001 |
| Atorvastatin vs placebo: ns | ||||||||
|
| ||||||||
| Simsek et al., 2012 [10] | Clinical trial | Netherlands | 263 (54%) | 60 (10), NA | Atorvastatin and rosuvastatin | T2DM | 24 weeks | HbA1c (%) [mmol/mol] 18w vs baseline: Atorva 80 mg: 7.4 ± 1.0 [57 ± 13] → 7.7 ± 1.3 [61 ± 9], p = 0.003 HbA1c 6w vs baseline: Atorva 20 mg: 7.4 ± 1.0 [57 ± 13] → 7.5 ± 1.1 [58 ± 11], ns |
| Mean FPG (mmol/L) 18w vs baseline: Atorva 80 mg: 8.7 ± 2.4 → 9.0 ± 3.0, ns Mean FPG (mmol/L) 6w vs baseline: Atorva 20 mg: 8.7 ± 2.4 → 9.5 ± 3.0, p = 0.002 | ||||||||
|
| ||||||||
| Puurunen et al., 2013 [25] | Clinical trial | Finland | 28 (100%) | NA NA (29–50) |
Atorvastatin 20 mg and placebo | Without diabetes | 6 months | FPG, mmol/L (0 → 3mo → 6mo) Atorva: 5.5 ± 0.3 → 5.5 ± 0.4 → 5.5 ± 0.4, p = 0.763 Placebo: 5.3 ± 0.3 → 5.3 ± 0.3 → 5.0 ± 0.5, p = 0.076 Atorva vs placebo (6mo): p = 0.007 |
|
| ||||||||
| Chu et al., 2008 [17] | Clinical trial | Taiwan | 29 (50%) | 60.0 (2.2) NA (18–80) |
Atorvastatin 10 mg, 20 mg, 40 mg | T2DM | 12 weeks | HbA1c (%) [mmol/mol] 10 mg: 7.6 ± 0.4 [60 ± 19] → 7.4 ± 0.3 [57 ± 20], ns |
| 20 mg: 8.2 ± 0.3 [66 ± 20] → 8.0 ± 0.3 [64 ± 20], ns | ||||||||
| 40 mg: 8.3 ± 0.3 [67 ± 20] → 8.7 ± 0.5 [72 ± 18], ns | ||||||||
|
| ||||||||
| Goyal et al., 2014 [27] | Clinical trial | India | 43 (37.21%) | NA, NA | Atorvastatin 10 mg and placebo | T2DM | 12 weeks | HbA1c, (%) [mmol/mol] (vs baseline) Atorva: 7.6 ± 0.9 [60 ± 14] → 7.6 ± 1.4 [60 ± 8], p = 0.92 Placebo: 7.9 ± 0.9 [63 ± 14] → 7.4 ± 1.8 [57 ± 4], p = 0.25 |
| FPG (mmol/L) (vs baseline) Atorva: 7.44 ± 1.52 → 7.99 ± 2.18, p = 0.18 Placebo: 8.18 ± 1.86 → 8.16 ± 3.28, p = 0.95 | ||||||||
|
| ||||||||
| Ogawa et al., 2014 [3] | Clinical trial | Japan | 1018 (54.22%) | 66.4 (11.1) NA |
Atorvastatin 10 mg and rosuvastatin 5 mg | T2DM | 12 months | HbA1c (%) [mmol/mol] (0 → 6mo → 12mo) Atorva: 6.4 [46] → 6.5 [48] → 6.5 [48], ns |
| Glu (mg/dL) (0 → 6mo → 12mo) Atorva: 118.8 → 126.0 → 122.8, p < 0.001 | ||||||||
| Sadeghi et al., 2014 [5] | Clinical trial | Iran | 140 (55%) | NA, NA | Atorvastatin 40 mg and atorvastatin 20 mg | Without diabetes | 3 months | HbA1c (%) [mmol/mol] (0 → 3mo): Atorva 40 mg: 5.5 ± 0.6 [37 ± 17] → 5.9 ± 0.6 [41 ± 17], p < 0.001 Atorva 20 mg: 5.5 ± 0.7 [37 ± 16] → 5.5 ± 0.6 [37 ± 17], p = 0.442 Atorva 40 mg vs 20 mg: p < 0.001 |
| FPG (mg/dL) (0 → 3mo): Atorva 40 mg: 85.67 ± 13.59 → 99.86 ± 16.22, p < 0.001 Atorva 20 mg: 84.63 ± 26.26 → 85.21 ± 14.19, p = 0.656 Atorva 40 mg vs 20 mg: p < 0.001 | ||||||||
|
| ||||||||
| Black et al., 2014 [28] | Clinical trial | UK | 13 (0%) | 61.3 (2.5), NA (35–70) | Atorvastatin 10 mg and fenofibrate 267 mg | T2DM | 12 weeks | HbA1c (%) [mmol/mol] (0 → 12w) Atorva 10 mg: 7.0 ± 0.2 [53 ± 21] → 7.1 ± 0.3 [54 ± 20], ns Fenofibrate 267 mg: 7.1 ± 0.2 [54 ± 21] → 7.0 ± 0.3 [53 ± 20], ns Atorva10 mg vs fenofibrate 267 mg: p = 0.52 |
| FPG (mmol/L) (0 → 12w) Atorva 10 mg: 7.6 ± 0.3 → 8.4 ± 0.5, ns Fenofibrate 267 mg: 8.1 ± 0.5 → 7.7 ± 0.5, ns Atorva 10 mg vs fenofibrate 267 mg: p = 0.07 | ||||||||
|
| ||||||||
| Tanaka, 2011 [29] | Study 1: prospective cohort study | Japan | 114 (49%) | NA, NA | Study 1: (statin-naïve and other statin users) all switched to atorvastatin 10 mg | T2DM | Study 1: 3 months | HbA1c (%) [mmol/mol] Study 1 (vs baseline) Statin-naïve: 7.6 ± 1.1 [60 ± 11] → 7.5 ± 0.9 [58 ± 14], ns Statin users: 7.1 ± 1.1 [54 ± 11] → 7.1 ± 1.0 [54 ± 13], ns |
| Study 2: retrospective cohort study | Study 2: all atorvastatin users | Study 2: 3 years | Study 2 (vs baseline) Baseline: 7.8 ± 1.5 [62 ± 7] → 1y: 7.6 ± 1.4 [60 ± 8], p < 0.05 → 2y: 7.4 ± 1.5 [57 ± 7], p < 0.01 → 3y: 7.5 ± 1.5 [58 ± 7], p < 0.01 |
|||||
| Yamakawa et al., 2008 [11] | Retrospective cohort study | Japan | 279 (54%) | NA, NA | Atorvastatin 10 mg Pitavastatin 2 mg Pravastatin 10 mg |
T2DM | 3 months | HbA1c (%) [mmol/mol] Atorva: 7.0 ± 1.1 [53 ± 11] → 7.4 ± 1.2 [57 ± 10], p < 0.001 |
| Glu (mg/dL) Atorva: 147 ± 51 → 176 ± 69, p < 0.001. | ||||||||
|
| ||||||||
| Takano et al., 2006 [33] | Retrospective cohort study | Japan | 154 (56%) | NA, NA | Atorvastatin 10 mg, pravastatin 10 mg | T2DM | 3 months | HbA1c (%) [mmol/mol] Atorva: 6.8 ± 0.9 [51 ± 14] → 7.2 ± 1.1 [55 ± 11], p < 0.001 |
| Glu (mg/dL) Atorva: 147 ± 50 → 177 ± 70, p < 0.001 | ||||||||
|
| ||||||||
| Shinozaki et al., 2012 [30] | Cohort study | Japan | 1173 (54%) | NA, NA (65–84) | Atorvastatin and atorvastatin-untreated | T2DM | 6 years | HbA1c (%) (vs baseline) Atorvastatin treatment period: 0.06% (−0.08%–0.21%, p = 0.38) Atorvastatin vs untreated: 1.69 (0.42–6.84) |
Variables are expressed as absolute numbers, percentages, mean ± SD, and median (IQR). NA: not available; NS: no significance; DM: diabetes mellitus; T2DM: type 2 diabetes mellitus; T1DM: type 1 diabetes mellitus; FPG: fasting plasma glucose; HbA1c: haemoglobin A1c; Glu: glucose; ANOVA: analysis of variance; Mo: months; W: weeks.
The mean baseline HbA1c across the studies (weighted average) was 7.2% (56 mmol/mol) in the statin group and 7.3% (57 mmol/mol) in the control group. At the end of the studies, the mean HbA1c was 7.4% (57 mmol/mol) in the statin group and 7.2% (55 mmol/mol) in the control group. The mean baseline fasting glucose across the studies (weighted average) was 7.28 mmol/L in the atorvastatin group and 7.49 mmol/L in the control group. At the end of the studies, the mean fasting glucose was 7.84 mmol/L in the atorvastatin group and 7.20 mmol/L in the control group.
3.1. New Onset Diabetes Mellitus
Thirteen articles [1, 12, 14–16, 22, 26, 31, 32, 34–37], 3 of which were clinical trials, examined the association between atorvastatin use and NOD. Patients who used atorvastatin had a higher risk of developing NOD. Also, the results of the majority of studies indicated that the risk of diabetes was dose dependent for atorvastatin. The majority of the articles and all the clinical trials showed that high-dose, compared with lower-dose, atorvastatin increased the risk of NOD. Waters et al. [22] noted that in the TNT clinical trial the HR was 1.10 between those who took atorvastatin 10 mg and 80 mg. Further, the absolute rates of new onset T2DM were 6.40% in the group who took atorvastatin and 6.06% in the placebo group according to the SPARCL clinical trial. The SPARCL clinical trial was a multicenter, double blind, parallel-group, randomized, placebo-controlled trial which randomized patients with prior stroke or transient ischemic attack (TIA) but without known heart disease [38].
However, in one study, the low dose of atorvastatin was related to a small increased risk of NOD (OR: 1.99, 95% CI: 1.00–3.98, p value 0.050) [36].
Moreover, statin medication use in postmenopausal women was shown to be associated with an increased risk for DM [15].
3.2. Dysregulation of Glycemic Control
Twenty articles examined if deterioration of diabetes mellitus is associated with the use of atorvastatin [2, 3, 5, 9–11, 13, 17–21, 23–25, 27–30, 33], 15 of which were clinical trials. Worsening of glycemic control was examined by measuring parameters related to glucose level such as fasting plasma glucose and HbA1c.
Ten studies showed that there was no significant change in glycemic control in patients treated with atorvastatin [2, 3, 17–19, 24, 27–30].
On the other hand, in 8 studies, atorvastatin use tended to be an independent predictor of increasing HbA1c levels and/or fasting plasma glucose levels (p < 0.001) [9–11, 13, 20, 21, 23, 33].
In addition, it was noticed that fasting plasma glucose and HbA1c increased in the group that received higher doses of atorvastatin [5].
The rates of new onset T2DM were higher in groups that took higher dose of atorvastatin. The absolute rates of new onset T2DM were 9.24% in the group who took 80 mg atorvastatin and 8.11% in the group who took 10 mg atorvastatin according to the TNT clinical trial [22]. Among clinical trials, the majority of studies indicated that deregulation of diabetes mellitus (expressed mostly with increased HbA1c levels) was more frequent in the statin group than in the placebo group.
4. Discussion
Evidence from randomized clinical trials suggests that the benefits from preventing cardiovascular disease and mortality with statins overweigh the risk of new onset diabetes mellitus [39]. Nevertheless, in patients at low risk for cardiovascular implications, lipid-lowering therapy with statins should be carefully used, and lifestyle changes along with close blood glucose levels monitoring should constitute the first line of treatment [40]. Therefore, it is absolutely necessary for clinical doctors to evaluate the positive and the potential negative effects of statin therapy, taking into consideration the unique characteristics of each patient.
However, according to several studies, the risk of developing NOD varies with different types of statins. Patients who are treated with pravastatin and pitavastatin are in lower risk for adverse effects [41] than those treated with lipophilic statins, such as atorvastatin [11]. Furthermore, the dose of atorvastatin plays an important role. Higher dosage and more intensive treatment are associated with greater incidence of NOD [42]. It should also be noted that older patients, with impaired fasting glucose prior to the use of statins and with other characteristics of the metabolic syndrome, face a greater risk for diabetogenicity [43].
Not many systematic reviews or meta-analyses investigating the association of atorvastatin with NOD and glycemic control dysregulation have been published recently. More specifically, the latest review [44] examining this association, published in 2017, referred only to the correlation between different statins and new onset diabetes mellitus and did not provide detailed data concerning atorvastatin. Also, four previous meta-analyses [45–48] and one review [49] included data concerning all different statins and NOD, but there were no data about statins deteriorating the glycemic control in patients with preexisting diabetes. One meta-analysis [50] and one review [51] underlined only the detrimental effect of atorvastatin, among other statins, on the glycemic control in diabetic patients, unlike our review which is mainly concentrated on the effect of atorvastatin specifically on both NOD and deterioration of the glycemic control.
4.1. New Onset Diabetes Mellitus
In this review, we found that there is an association between atorvastatin use and new onset diabetes (NOD). The diabetogenic effect is more significant with high dose of atorvastatin [1, 32, 34, 37], although new data from a most recent cohort study suggest that low-dose atorvastatin (10–20 mg) consists a risk factor for NOD [36], though results were based on a relatively small number of participants (N = 818). A recent study investigated whether there is association between statin use and new onset diabetes in postmenopausal women who took part in the Women's Health Initiative. It was revealed that all statins increase the risk of type 2 diabetes mellitus. Particularly, atorvastatin was associated with 61% increased risk of diabetes [15]. One cohort study demonstrated that, in comparison with pravastatin, patients treated with atorvastatin faced a 22% increase in the risk of new onset diabetes [12]. Another analysis, which collated data from 3 different clinical trials (TNT, SPARCL, and IDEAL) on atorvastatin, suggested that atorvastatin at the maximum dose (80 mg) increased the risk for new onset diabetes by 34%, and this was more obvious in the SPARCL trial [22]. Later results from the same clinical trials indicated that the risk for new onset diabetes for patients with 2–4 risk factors was elevated by 24% with high-dose atorvastatin, while there was no diabetogenic effect on patients with 0–1 risk factor [1]. It should also be mentioned that atorvastatin treatment caused dysglycemia (impaired fasting glucose and diabetes mellitus) in a high percentage of renal allograft recipients as indicated by a recent cohort study [16]. However, a retrospective cohort study has shown that atorvastatin had a neutral effect on new onset diabetes, which is dose-response [31], and another study reported that not only does atorvastatin not elevate the risk of diabetes but also may have a protective effect for elderly hypertensive and dyslipidaemic patients [14]. A potential explanation for this may be that the participants had median age of 74.9 years, and there is no record of the dosage of atorvastatin used during the study. No association between atorvastatin and new onset diabetes was demonstrated by one study [26], but this may be attributed to the fact that the lowest dose of atorvastatin (10 mg) was used for this study.
4.2. Dysregulation of Glycemic Control
As far as glycemic control is concerned, this review has shown that high dose of atorvastatin is associated with the deterioration and worsening of glucose homeostasis [13, 21, 23, 33]. A clinical trial published in 2010 reported that atorvastatin 10 mg compared to atorvastatin 80 mg increased fasting plasma glucose (FPG) by 25% and 45%, respectively, and HbA1c by 2% and 5%, respectively [9]. The influence of high-dose atorvastatin on glycemic control was also demonstrated by 2 different studies, which reported a significant increase (0.3%) of HbA1c compared to baseline along with no alteration in FPG levels [10, 20].
No significant changes in glycemic control between the atorvastatin group and the control group were found by a clinical trial in China [19], but this was a low-quality study (follow-up of 6 months in population of 80 patients and the exact dose of atorvastatin used was not reported). Furthermore, a mediocre increase (0.06%) of HbA1c was documented for patients treated with atorvastatin compared to the control group according to a recent study; however, no data regarding the dosage of atorvastatin were available [30]. Another study that has also shown no difference in the glycemic control is a clinical trial from Greece, which included only 79 participants who were treated with a low dose (10 mg) of atorvastatin [18]. A neutral effect of atorvastatin 10 mg was also reported by 3 more studies [3, 27, 28]. Finally, 2 clinical studies which used atorvastatin 20 mg [2, 25] and one with atorvastatin groups of 10-20-40 mg, respectively [17, 25], showed no association.
A positive effect on HbA1c levels was suggested by one study investigating the effects of atorvastatin in renal function of patients with diabetes [29]. This retrospective cohort study showed that HbA1c was significantly decreased at 1, 2, and 3 years. There was no information about the dosage of atorvastatin. In a different trial, a nonsignificant decrease of HbA1c was reported in patients treated with atorvastatin high dose (80 mg) compared to placebo [24].
4.3. Mechanisms
Lipid-lowering drugs intervene with glucose control and insulin sensitivity in many different ways. Mainly, HbA1c and FPG are affected. Although the precise underlying pathogenetic mechanisms that lead to the development of diabetes are not yet scientifically proven, there are some studies which suggest that the intervention of statins in the Mevalonate path and hence in the isoprenoids' synthesis is connected with the deterioration of glycemic control [9]. Statin treatment also results in the downregulation of glucose transporter 4 (GLUT4) in adipocytes, which causes insulin resistance [52]. Furthermore, statins decrease insulin secretion via the decrease of glucose-dependent intracellular calcium concentration [53] and via the inhibition of ubiquinone (CoQ10), which leads to reduction of ATP in pancreatic β-cells [54]. These effects are more intense with lipophilic statins like atorvastatin than with hydrophilic statins like pravastatin [55]. Other plausible mechanisms, including genetic factors, have also been explored but with no concrete evidence yet.
4.4. Drugs That May Have a Metabolic Effect on Insulin Resistance and on the Development of Diabetes
Several classes of antidiabetic drugs are available. Each category has a distinct pathophysiological mechanism of action and consequently a different effect regarding insulin resistance and β-cell pancreatic function.
Indicatively, metformin inhibits hepatic gluconeogenesis and also improves insulin sensitivity via activation of AMP-activated protein kinase (AMPK) signalling and reducing cAMP (cyclic adenosine monophosphate) levels. Moreover, metformin is associated with microbiome modification of the gastrointestinal tract and entails an increase of incretin (glucagon-like peptide-1, GLP-1) secretion and glucose utilization [56].
Pioglitazone binds and activates the peroxisome proliferator-activated receptor (PPAR) γ leading to metabolic changes concerning carbohydrate and lipid metabolism. It is associated with an increase in tissue sensitivity to insulin and subsequently an enhanced glucose uptake in the skeletal muscle and adipose tissue. Also, it causes a reduction in hepatic glucose production and an increase in hepatic glucose uptake. It may stimulate β-cell insulin production and may have beneficial effects both on endothelial and pancreatic β-cells [57–59].
Sulfonylureas stimulate the endogenous secretion of insulin from pancreatic β-cells by inhibiting the ATP-sensitive K-channels. Therefore, sulfonylureas exert their effects only when residual β-cells exist [60, 61].
Reduced incretin levels may play a part in the pathogenesis of T2DM. Incretin-based therapies, dipeptidyl peptidase-4 (DPP-4) inhibitors, and GLP-1 receptor agonists (GLP-1RAs) affect glucose control via pleiotropic mechanisms and play a significant role in glucose homeostasis.
GLP-1RAs enhance glucose-dependent insulin secretion, delay gastric emptying, and reduce food intake and postprandial glucagon secretion [62].
DPP4 inhibitors delay the inactivation of incretin hormones, also resulting in increased insulin synthesis and decreased glucagon levels in a glucose-dependent manner [63].
According to clinical and preclinical study results, incretin-based therapies may have a beneficial effect on hepatic steatosis and steatohepatitis, inhibit intestinal lipoprotein production, enhance β-cell function, and produce multiple biological actions in peripheral tissues [63, 64].
On the other hand, various nondiabetic drugs seem to play a crucial role in insulin sensitivity and endothelial dysfunction [65]. Several experimental evidence suggests that overactivity of renin-angiotensin-aldosterone system (RAAS), namely angiotensin II, interferes with insulin resistance and glucose metabolism [66]. Thus, drugs with RAAS blockade activity may interact with the skeletal muscle, adipose tissue, or pancreas contributing to altered glucose metabolism and insulin sensitivity.
It has been shown that angiotensin II interferes with insulin metabolic signalling, induces insulin resistance, and impairs insulin-stimulated glucose disposal. It inhibits insulin receptor substrates 1 and 2 (IRS-1, IRS-2), enhances serine phosphorylation affecting the PI3K (phosphoinositide 3-kinase) pathway, impairs the insulin-mediated vasodilation, and also reduces the ability of IRS-1 to interact with the activated insulin receptor [67, 68].
It has also been proposed that angiotensin II is associated with the functional impairment of pancreatic β-cells through inflammation, attenuation of islet fibrosis, and oxidative damage of the pancreas. The metabolic stress, as well as the dedifferentiated status of β-cells induced by angiotensin II, has also been associated with pancreatic β-cell failure and the potential progressive development of T2DM [69–71].
Angiotensin II receptor blockers (ARBs) are drugs that block the action of angiotensin II by selectively inhibiting its binding to angiotensin II receptors on the muscles surrounding blood vessels.
According to some studies, ARB medications improved insulin sensitivity [72–74] while others showed an enhancement in the early phase of insulin secretion, a significant effect possibly attributed to the recovery of pancreatic β-cell function [75, 76].
ACE (angiotensin-converting-enzyme) inhibitors are also involved in the renin-angiotensin axis. It has been proposed that ACE inhibitors promote glycemia and glucose tolerance probably though preservation of β-cell function [77], improvement of insulin sensitivity (via activation of bradykinin-nitric oxide pathway) [78], anti-inflammatory processes [79], and multiple other underlying mechanisms [80, 81].
It is evident that apart from their antihypertensive effect, both ARBs and ACE inhibitors may exert beneficial effects on lipid and carbohydrate metabolism and insulin resistance [66, 82, 83], which may explain the possible protective role of these medications partially [84–86]. Furthermore, existing evidence demonstrates a potential protective role of RAAS inhibitors in new onset of T2DM [86–88].
Fibrates are a class of hypolipidemic agents that exert their effects through activation of PPARs, namely PPARα, which modulate carbohydrate and lipid metabolism and adipose tissue differentiation. According to several studies, including patients with T2DM, fibrates and especially fenofibrate improved lipidemic parameters, insulin resistance, and glycemic control [89–91]. Moreover, a further investigation is proposed to examine the potential protective role of fibrates in preventing T2DM [92].
Additionally, other lipid-lowering drugs such as ANGPTL3 antisense oligonucleotides are also associated with an improvement of insulin sensitivity [93].
Taking into account all the aforementioned, it seems essential to clarify the underlying pathophysiologic mechanisms of action for each drug, their impact on insulin resistance and on the overall glucose homeostasis especially in patients with T2DM or in patients at high risk of NOD who also receive statin therapy [65].
4.5. Strengths and Limitations
This systematic review includes approximately 2,000,000 participants in total from all studies, contributing to a large cohort. In addition, a comprehensive literature search was followed, as well as bias protection methods such as three independent reviewers.
Of note, our data were extracted from more recent clinical studies, the latest of which conducted in 2015, in contrast to aforementioned review studies. Two reviews [41, 94], with clinical trials until 2009 and 2013, respectively, found no association between atorvastatin and NOD as well as atorvastatin and deterioration of the glycemic control. The review by Kostapanos et al. [94] included a very small number of subjects and mainly low doses of atorvastatin, while the review by Naci et al. [41] investigated only the incidence of NOD in trials that were not designed for this purpose and had no information about the doses of atorvastatin used.
However, the limitations of the review should be acknowledged. Firstly, the medical status of each participant (e.g., coexisting diseases) and the concomitant medications (especially the glucose-lowering agents or other drugs with metabolic impact, as already mentioned) were not taken into account, since data were not consistently available. Furthermore, only clinical studies published in PubMed were included, thus results from nonindexed trials are missing. All studies not published in the English language were excluded. Further, about a third (13 of 33) of the included studies used observational designs. Finally, most of the studies were not designed to investigate the association between atorvastatin and new onset diabetes or dysregulation of existing diabetes mellitus, but their primary designation purpose was different, contributing to the heterogeneity of the results.
5. Conclusions
Our findings suggest that there is association between atorvastatin treatment and new onset diabetes mellitus. It was also demonstrated that atorvastatin causes worsening of glycemic control in patients with known diabetes but only in maximum dose and not in lower doses. Nevertheless, serious consideration needs to be placed on the cost-benefit ratio and the potential importance of these adverse effects of atorvastatin when compared to the scientifically and clinically observed beneficial effects of statins on cardiovascular risk. Given the wide availability of statins and relevant databases, more studies using routine clinical data are required to be conducted, on wider homogeneous populations of participants and with larger periods of follow-up, in order to clarify the real association between statin therapy and the development of new onset diabetes mellitus. Moreover, the impact of other coadministrated drugs on insulin resistance and glucose homeostasis should be taken into deep consideration.
Hence, our review underlines the existence of a significant yet not so thoroughly investigated issue, which is the development of NOD and also the potential deterioration of the glycemic control by atorvastatin in patients with diabetes.
Abbreviations
- NOD:
New onset diabetes mellitus
- HMG-CoA:
3-Hydroxy-3-methylglutaryl coenzyme A
- LDL:
Low-density lipoprotein cholesterol
- HDL:
High-density lipoprotein cholesterol
- HR:
Hazard ratio
- SD:
Standard deviation
- IQR:
Interquartile range
- OR:
Odds ratio
- NA:
Not available
- NS:
No significance
- DM:
Diabetes mellitus
- T2DM:
Type 2 diabetes mellitus
- T1DM:
Type 1 diabetes mellitus
- FPG:
Fasting plasma glucose
- HbA1c:
Haemoglobin A1c
- Glu:
Glucose
- ANOVA:
Analysis of variance
- Y:
Years
- Mo:
Months
- W:
Weeks
- PSM:
Propensity score matching analysis
- ATORVA:
Atorvastatin
- ADA:
American Diabetes Association
- PRISMA:
Preferred reporting items for systematic reviews and meta-analyses
- ACC/AHA:
American College of Cardiology and the American Heart Association
- AMPK:
AMP-activated protein kinase
- cAMP:
Cyclic adenosine monophosphate
- GLP-1:
Glucagon-like peptide-1
- GLP-1RAs:
GLP-1 receptor agonists
- DPP-4:
Dipeptidyl peptidase-4
- RAAS:
Renin-angiotensin-aldosterone system
- IRS-1:
Insulin receptor substrates 1
- IRS-2:
Insulin receptor substrates 2
- ARBs:
Angiotensin II receptor blockers
- ACE:
Angiotensin-converting-enzyme
- PPARs:
Peroxisome proliferator-activated receptors.
Additional Points
Key points. Atorvastatin may cause dysregulation of glycemic control in patients with diabetes. Association between atorvastatin treatment and new onset diabetes mellitus. Deterioration of glycemic control is increased after high-dose atorvastatin therapy. Association of new onset diabetes mellitus with high-dose atorvastatin therapy. More studies are required to clarify the underlying mechanisms and the real association between statin therapy and the development of new onset diabetes mellitus.
Conflicts of Interest
The authors declare that they have no conflicts of interest concerning this article.
Authors' Contributions
AA and AK contributed to the conception of the study and designed the study. AA, ES, and KA were involved in the literature research and collection and assembly of the data. AA and AK led on reaching consensus on study inclusion and data extraction. ES, KA, and AA wrote the manuscript. AK and AA participated in editing and revising the manuscript. All authors read and approved the final manuscript.
References
- 1.Waters D. D., Ho J. E., Boekholdt S. M., et al. Cardiovascular event reduction versus new-onset diabetes during atorvastatin therapy: effect of baseline risk factors for diabetes. Journal of the American College of Cardiology. 2013;61(2):148–152. doi: 10.1016/j.jacc.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 2.Mandosi E., Fallarino M., Gatti A., et al. Atorvastatin downregulates monocyte CD36 expression, nuclear NFkappaB and TNFalpha levels in type 2 diabetes. Journal of Atherosclerosis and Thrombosis. 2010;17(6):539–545. doi: 10.5551/jat.2956. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa H., Matsui K., Saito Y., et al. Differences between rosuvastatin and atorvastatin in lipid-lowering action and effect on glucose metabolism in Japanese hypercholesterolemic patients with concurrent diabetes. Lipid-lowering with highly potent statins in hyperlipidemia with type 2 diabetes patients (LISTEN) study. Circulation Journal. 2014;78(10):2512–2515. doi: 10.1253/circj.cj-14-0810. [DOI] [PubMed] [Google Scholar]
- 4.LaRosa J. C., Grundy S. M., Waters D. D., et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. The New England Journal of Medicine. 2005;352(14):1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 5.Sadeghi R., Asadpour-Piranfar M., Asadollahi M., Taherkhani M., Baseri F. The effects of different doses of atorvastatin on serum lipid profile, glycemic control, and liver enzymes in patients with ischemic cerebrovascular accident. ARYA Atherosclerosis. 2014;10(6):298–304. [PMC free article] [PubMed] [Google Scholar]
- 6.Arca M. Atorvastatin efficacy in the prevention of cardiovascular events in patients with diabetes mellitus and/or metabolic syndrome. Drugs. 2007;67(Supplement 1):43–54. doi: 10.2165/00003495-200767001-00005. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. 8. Cardiovascular disease and risk management. Diabetes Care. 2016;38(Supplement 1):S49–S57. doi: 10.2337/dc15-S011. [DOI] [PubMed] [Google Scholar]
- 8.Stone N. J., Robinson J. G., Lichtenstein A. H., et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25) Supplement 2:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 9.Koh K. K., Quon M. J., Han S. H., Lee Y., Kim S. J., Shin E. K. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. Journal of the American College of Cardiology. 2010;55(12):1209–1216. doi: 10.1016/j.jacc.2009.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simsek S., Wolffenbuttel B. H., Schalkwijk C. G. Effects of rosuvastatin and atorvastatin on glycaemic control in type 2 diabetes---the CORALL study. Diabetic Medicine. 2012;29(5):628–631. doi: 10.1111/j.1464-5491.2011.03553.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamakawa T., Takano T., Tanaka S., Kadonosono K., Terauchi Y. Influence of pitavastatin on glucose tolerance in patients with type 2 diabetes mellitus. Journal of Atherosclerosis and Thrombosis. 2008;15(5):269–275. doi: 10.5551/jat.E562. [DOI] [PubMed] [Google Scholar]
- 12.Carter A. A., Gomes T., Camacho X., Juurlink D. N., Shah B. R., Mamdani M. M. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346, article f2610 doi: 10.1136/bmj.f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tehrani S., Mobarrez F., Lins P. E., Adamson U., Wallén H. N., Jörneskog G. Impaired endothelium-dependent skin microvascular function during high-dose atorvastatin treatment in patients with type 1 diabetes. Diabetes & Vascular Disease Research. 2013;10(6):483–488. doi: 10.1177/1479164113491275. [DOI] [PubMed] [Google Scholar]
- 14.Ma T., Chang M. H., Tien L., Liou Y. S., Jong G. P. The long-term effect of statins on the risk of new-onset diabetes mellitus in elderly Taiwanese patients with hypertension and dyslipidaemia: a retrospective longitudinal cohort study. Drugs & Aging. 2012;29(1):45–51. doi: 10.2165/11597250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Culver A. L., Ockene I. S., Balasubramanian R., et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Archives of Internal Medicine. 2012;172(2):144–152. doi: 10.1001/archinternmed.2011.625. [DOI] [PubMed] [Google Scholar]
- 16.Choe E. Y., Wang H. J., Kwon O., et al. HMG CoA reductase inhibitor treatment induces dysglycemia in renal allograft recipients. Transplantation. 2014;97(4):419–425. doi: 10.1097/01.TP.0000437427.04733.ad. [DOI] [PubMed] [Google Scholar]
- 17.Chu C. H., Lee J. K., Lam H. C., et al. Atorvastatin does not affect insulin sensitivity and the adiponectin or leptin levels in hyperlipidemic type 2 diabetes. Journal of Endocrinological Investigation. 2008;31(1):42–47. doi: 10.1007/BF03345565. [DOI] [PubMed] [Google Scholar]
- 18.Grigoropoulou P., Eleftheriadou I., Zoupas C., et al. Effect of atorvastatin on baroreflex sensitivity in subjects with type 2 diabetes and dyslipidaemia. Diabetes & Vascular Disease Research. 2013;11(1):26–33. doi: 10.1177/1479164113508293. [DOI] [PubMed] [Google Scholar]
- 19.Tam H. L., Shiu S. W. M., Wong Y., Chow W. S., Betteridge D. J., Tan K. C. B. Effects of atorvastatin on serum soluble receptors for advanced glycation end-products in type 2 diabetes. Atherosclerosis. 2010;209(1):173–177. doi: 10.1016/j.atherosclerosis.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Tehrani S., Mobarrez F., Antovic A., et al. Atorvastatin has antithrombotic effects in patients with type 1 diabetes and dyslipidemia. Thrombosis Research. 2010;126(3):e225–e231. doi: 10.1016/j.thromres.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Rutter M. K., Prais H. R., Charlton-Menys V., et al. Protection Against Nephropathy in Diabetes with Atorvastatin (PANDA): a randomized double-blind placebo-controlled trial of high- vs. low-dose atorvastatin(1) Diabetic Medicine. 2011;28(1):100–108. doi: 10.1111/j.1464-5491.2010.03139.x. [DOI] [PubMed] [Google Scholar]
- 22.Waters D. D., Ho J. E., DeMicco D. A., et al. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. Journal of the American College of Cardiology. 2011;57(14):1535–1545. doi: 10.1016/j.jacc.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 23.Thongtang N., Ai M., Otokozawa S., et al. Effects of maximal atorvastatin and rosuvastatin treatment on markers of glucose homeostasis and inflammation. The American Journal of Cardiology. 2011;107(3):387–392. doi: 10.1016/j.amjcard.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin S., Herder C., Schloot N. C., et al. Residual beta cell function in newly diagnosed type 1 diabetes after treatment with atorvastatin: the randomized DIATOR trial. PLoS One. 2011;6(3, article e17554) doi: 10.1371/journal.pone.0017554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puurunen J., Piltonen T., Puukka K., et al. Statin therapy worsens insulin sensitivity in women with polycystic ovary syndrome (PCOS): a prospective, randomized, double-blind, placebo-controlled study. The Journal of Clinical Endocrinology and Metabolism. 2013;98(12):4798–4807. doi: 10.1210/jc.2013-2674. [DOI] [PubMed] [Google Scholar]
- 26.Sever P. S., Dahlöf B., Poulter N. R., et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 27.Goyal A., Singh S., Tandon N., Gupta N., Gupta Y. K. Effect of atorvastatin on pancreatic beta-cell function and insulin resistance in type 2 diabetes mellitus patients: a randomized pilot study. Canadian Journal of Diabetes. 2014;38(6):466–472. doi: 10.1016/j.jcjd.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Black R. N. A., Ennis C. N., Young I. S., Hunter S. J., Atkinson A. B., Bell P. M. The peroxisome proliferator-activated receptor alpha agonist fenofibrate has no effect on insulin sensitivity compared to atorvastatin in type 2 diabetes mellitus; a randomised, double-blind controlled trial. Journal of Diabetes and its Complications. 2014;28(3):323–327. doi: 10.1016/j.jdiacomp.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka M. Beneficial effect of atorvastatin on renal function in patients with type 2 diabetes. The Journal of International Medical Research. 2011;39(4):1504–1512. doi: 10.1177/147323001103900440. [DOI] [PubMed] [Google Scholar]
- 30.Shinozaki T., Matsuyama Y., Iimuro S., et al. Effective prevention of cardiovascular disease and diabetes-related events with atorvastatin in Japanese elderly patients with type 2 diabetes mellitus: adjusting for treatment changes using a marginal structural proportional hazards model and a rank-preserving structural failure time model. Geriatrics & Gerontology International. 2012;12:88–102. doi: 10.1111/j.1447-0594.2011.00816.x. [DOI] [PubMed] [Google Scholar]
- 31.Ma T., Tien L., Fang C. L., Liou Y. S., Jong G. P. Statins and new-onset diabetes: a retrospective longitudinal cohort study. Clinical Therapeutics. 2012;34(9):1977–1983. doi: 10.1016/j.clinthera.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Zaharan N. L., Williams D., Bennett K. Statins and risk of treated incident diabetes in a primary care population. British Journal of Clinical Pharmacology. 2013;75(4):1118–1124. doi: 10.1111/j.1365-2125.2012.04403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takano T., Yamakawa T., Takahashi M., Kimura M., Okamura A. Influences of statins on glucose tolerance in patients with type 2 diabetes mellitus. Journal of Atherosclerosis and Thrombosis. 2006;13(2):95–100. doi: 10.5551/jat.13.95. [DOI] [PubMed] [Google Scholar]
- 34.Cederberg H., Stančáková A., Yaluri N., Modi S., Kuusisto J., Laakso M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: a 6 year follow-up study of the METSIM cohort. Diabetologia. 2015;58(5):1109–1117. doi: 10.1007/s00125-015-3528-5. [DOI] [PubMed] [Google Scholar]
- 35.Cho Y., Choe E., Lee Y. H., et al. Risk of diabetes in patients treated with HMG-CoA reductase inhibitors. Metabolism. 2015;64(4):482–488. doi: 10.1016/j.metabol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Park J. Y., Rha S. W., Choi B., et al. Impact of low dose atorvastatin on development of new-onset diabetes mellitus in Asian population: three-year clinical outcomes. International Journal of Cardiology. 2015;184:502–506. doi: 10.1016/j.ijcard.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 37.Chen C. W., Chen T. C., Huang K. Y., Chou P., Chen P. F., Lee C. C. Differential impact of statin on new-onset diabetes in different age groups: a population-based case-control study in women from an asian country. PLoS One. 2013;8(8, article e71817) doi: 10.1371/journal.pone.0071817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amarenco P., Bogousslavsky J., Callahan A., 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. The New England Journal of Medicine. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 39.Ridker P. M., Pradhan A., MacFadyen J. G., Libby P., Glynn R. J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380(9841):565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chogtu B., Magazine R., Bairy K. L. Statin use and risk of diabetes mellitus. World Journal of Diabetes. 2015;6(2):352–357. doi: 10.4239/wjd.v6.i2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naci H., Brugts J., Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circulation. Cardiovascular Quality and Outcomes. 2013;6(4):390–399. doi: 10.1161/CIRCOUTCOMES.111.000071. [DOI] [PubMed] [Google Scholar]
- 42.Preiss D., Seshasai S. R., Welsh P., et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 43.Zafrir B., Jain M. Lipid-lowering therapies, glucose control and incident diabetes: evidence, mechanisms and clinical implications. Cardiovascular Drugs and Therapy. 2014;28(4):361–377. doi: 10.1007/s10557-014-6534-9. [DOI] [PubMed] [Google Scholar]
- 44.Laakso M., Kuusisto J. Diabetes secondary to treatment with statins. Current Diabetes Reports. 2017;17(2):p. 10. doi: 10.1007/s11892-017-0837-8. [DOI] [PubMed] [Google Scholar]
- 45.Koh K. K., Sakuma I., Quon M. J. Differential metabolic effects of distinct statins. Atherosclerosis. 2011;215(1):1–8. doi: 10.1016/j.atherosclerosis.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 46.Rahal A. J., ElMallah A. I., Poushuju R. J., Itani R. Do statins really cause diabetes? A meta-analysis of major randomized controlled clinical trials. Saudi Medical Journal. 2016;37(10):1051–1060. doi: 10.15537/smj.2016.10.16078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thakker D., Nair S., Pagada A., Jamdade V., Malik A. Statin use and the risk of developing diabetes: a network meta-analysis. Pharmacoepidemiology and Drug Safety. 2016;25(10):1131–1149. doi: 10.1002/pds.4020. [DOI] [PubMed] [Google Scholar]
- 48.Navarese E. P., Buffon A., Andreotti F., et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. The American Journal of Cardiology. 2013;111(8):1123–1130. doi: 10.1016/j.amjcard.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 49.Ray K. Statin diabetogenicity: guidance for clinicians. Cardiovascular Diabetology. 2013;12(Supplement 1):p. S3. doi: 10.1186/1475-2840-12-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y., Yuan Y., Cai R. R., et al. Statin therapy on glycaemic control in type 2 diabetes: a meta-analysis. Expert Opinion on Pharmacotherapy. 2013;14(12):1575–1584. doi: 10.1517/14656566.2013.810210. [DOI] [PubMed] [Google Scholar]
- 51.Sasaki J., Iwashita M., Kono S. Statins: beneficial or adverse for glucose metabolism. Journal of Atherosclerosis and Thrombosis. 2006;13(3):123–129. doi: 10.5551/jat.13.123. [DOI] [PubMed] [Google Scholar]
- 52.Nakata M., Nagasaka S., Kusaka I., Matsuoka H., Ishibashi S., Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia. 2006;49(8):1881–1892. doi: 10.1007/s00125-006-0269-5. [DOI] [PubMed] [Google Scholar]
- 53.Yada T., Nakata M., Shiraishi T., Kakei M. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet beta-cells. British Journal of Pharmacology. 1999;126(5):1205–1213. doi: 10.1038/sj.bjp.0702397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mabuchi H., Higashikata T., Kawashiri M., et al. Reduction of serum ubiquinol-10 and ubiquinone-10 levels by atorvastatin in hypercholesterolemic patients. Journal of Atherosclerosis and Thrombosis. 2005;12(2):111–119. doi: 10.5551/jat.12.111. [DOI] [PubMed] [Google Scholar]
- 55.Ishikawa M., Namiki A., Kubota T., et al. Effect of pravastatin and atorvastatin on glucose metabolism in nondiabetic patients with hypercholesterolemia. Internal Medicine. 2006;45(2):51–55. doi: 10.2169/internalmedicine.45.1476. [DOI] [PubMed] [Google Scholar]
- 56.Rena G., Hardie D. G., Pearson E. R. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin J., Zeng H., Yang O., Kong J. The endothelial protective effects of pioglitazone on insulin resistance in endothelial cells. Clinical Laboratory. 2014;60(7):1129–1134. doi: 10.7754/clin.lab.2013.130713. [DOI] [PubMed] [Google Scholar]
- 58.Smith U. Pioglitazone: mechanism of action. International Journal of Clinical Practice. 2001;121:13–18. [PubMed] [Google Scholar]
- 59.Kimura T., Kaneto H., Shimoda M., et al. Protective effects of pioglitazone and/or liraglutide on pancreatic β-cells in db/db mice: comparison of their effects between in an early and advanced stage of diabetes. Molecular and Cellular Endocrinology. 2015;400:78–89. doi: 10.1016/j.mce.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 60.Sola D., Rossi L., Schianca G. P. C., et al. Sulfonylureas and their use in clinical practice. Archives of Medical Science. 2015;11(4):840–848. doi: 10.5114/aoms.2015.53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khunti K., Chatterjee S., Gerstein H. C., Zoungas S., Davies M. J. Do sulphonylureas still have a place in clinical practice? The Lancet Diabetes and Endocrinology. 2018;6(10):821–832. doi: 10.1016/S2213-8587(18)30025-1. [DOI] [PubMed] [Google Scholar]
- 62.Drucker D. J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metabolism. 2018;27(4):740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Mulvihill E. E., Drucker D. J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocrine Reviews. 2014;35(6):992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalra S. Glucagon-like peptide-1 receptors agonists (GLP1 RA) The Journal of the Pakistan Medical Association. 2013;63(10):1312–1315. [PubMed] [Google Scholar]
- 65.Cho K. I., Sakuma I., Sohn I. S., Hayashi T., Shimada K., Koh K. K. Best treatment strategies with statins to maximize the cardiometabolic benefits. Circulation Journal. 2018;82(4):937–943. doi: 10.1253/circj.CJ-17-1445. [DOI] [PubMed] [Google Scholar]
- 66.Underwood P. C., Adler G. K. The renin angiotensin aldosterone system and insulin resistance in humans. Current Hypertension Reports. 2013;15(1):59–70. doi: 10.1007/s11906-012-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andreozzi F., Laratta E., Sciacqua A., Perticone F., Sesti G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circulation Research. 2004;94(9):1211–1218. doi: 10.1161/01.RES.0000126501.34994.96. [DOI] [PubMed] [Google Scholar]
- 68.Izawa Y., Yoshizumi M., Fujita Y., et al. ERK1/2 activation by angiotensin II inhibits insulin-induced glucose uptake in vascular smooth muscle cells. Experimental Cell Research. 2005;308(2):291–299. doi: 10.1016/j.yexcr.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 69.Tikellis C., Wookey P. J., Candido R., Andrikopoulos S., Thomas M. C., Cooper M. E. Improved islet morphology after blockade of the renin–angiotensin system in the ZDF rat. Diabetes. 2004;53(4):989–997. doi: 10.2337/diabetes.53.4.989. [DOI] [PubMed] [Google Scholar]
- 70.Weir G. C., Aguayo-Mazzucato C., Bonner-Weir S. β-cell dedifferentiation in diabetes is important, but what is it? Islets. 2013;5(5):233–237. doi: 10.4161/isl.27494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen H., Zhou W., Ruan Y., et al. Reversal of angiotensin ll-induced β-cell dedifferentiation via inhibition of NF-κb signaling. Molecular Medicine. 2018;24(1):p. 43. doi: 10.1186/s10020-018-0044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takagi H., Umemoto T. Telmisartan improves insulin sensitivity: a meta-analysis of randomized head-to-head trials. International Journal of Cardiology. 2012;156(1):92–96. doi: 10.1016/j.ijcard.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 73.Paolisso G., Tagliamonte M. R., Gambardella A., et al. Losartan mediated improvement in insulin action is mainly due to an increase in non-oxidative glucose metabolism and blood flow in insulin-resistant hypertensive patients. Journal of Human Hypertension. 1997;11(5):307–312. doi: 10.1038/sj.jhh.1000434. [DOI] [PubMed] [Google Scholar]
- 74.Moan A., Høieggen A., Nordby G., Eide I. K., Kjeldsen S. E. Effects of losartan on insulin sensitivity in severe hypertension: connections through sympathetic nervous system activity? Journal of Human Hypertension. 1995;9(Supplement 5):S45–S50. [PubMed] [Google Scholar]
- 75.Suzuki K., Nakagawa O., Aizawa Y. Improved early-phase insulin response after candesartan treatment in hypertensive patients with impaired glucose tolerance. Clinical and Experimental Hypertension. 2008;30(5):309–314. doi: 10.1080/10641960802269927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perlstein T. S., Henry R. R., Mather K. J., et al. Effect of angiotensin receptor blockade on insulin sensitivity and endothelial function in abdominally obese hypertensive patients with impaired fasting glucose. Clinical Science. 2012;122(4):193–202. doi: 10.1042/CS20110284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ko S. H., Kwon H. S., Kim S. R., et al. Ramipril treatment suppresses islet fibrosis in Otsuka Long–Evans Tokushima fatty rats. Biochemical and Biophysical Research Communications. 2004;316(1):114–122. doi: 10.1016/j.bbrc.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 78.Shiuchi T., Cui T. X., Wu L., et al. ACE inhibitor improves insulin resistance in diabetic mouse via bradykinin and NO. Hypertension. 2002;40(3):329–334. doi: 10.1161/01.HYP.0000028979.98877.0C. [DOI] [PubMed] [Google Scholar]
- 79.Singh B., Mourya A., Sah S. P., Kumar A. Protective effect of losartan and ramipril against stress induced insulin resistance and related complications: anti-inflammatory mechanisms. European Journal of Pharmacology. 2017;801:54–61. doi: 10.1016/j.ejphar.2017.02.050. [DOI] [PubMed] [Google Scholar]
- 80.Weisinger R. S., Stanley T. K., Begg D. P., Weisinger H. S., Spark K. J., Jois M. Angiotensin converting enzyme inhibition lowers body weight and improves glucose tolerance in C57BL/6J mice maintained on a high fat diet. Physiology & Behavior. 2009;98(1-2):192–197. doi: 10.1016/j.physbeh.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 81.Das U. N. Renin–angiotensin–aldosterone system in insulin resistance and metabolic syndrome. Journal of Translational Internal Medicine. 2016;4(2):66–72. doi: 10.1515/jtim-2016-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Putnam K., Shoemaker R., Yiannikouris F., Cassis L. A. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. American Journal of Physiology. Heart and Circulatory Physiology. 2012;302(6):H1219–H1230. doi: 10.1152/ajpheart.00796.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bitkin Eda Ç., Mehmet B., Necati T., et al. Effects of ACE inhibitors on insulin resistance and lipid profile in children with metabolic syndrome. Journal of Clinical Research in Pediatric Endocrinology. 2013;5(3):164–169. doi: 10.4274/Jcrpe.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gillespie E. L., White C. M., Kardas M., Lindberg M., Coleman C. I. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care. 2005;28(9):2261–2266. doi: 10.2337/diacare.28.9.2261. [DOI] [PubMed] [Google Scholar]
- 85.McMurray J. J., Holman R. R., Haffner S. M., et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. The New England Journal of Medicine. 2010;362(16):1477–1490. doi: 10.1056/NEJMoa1001121. [DOI] [PubMed] [Google Scholar]
- 86.Solski L. V., Longyhore D. S. Prevention of type 2 diabetes mellitus with angiotensin-converting-enzyme inhibitors. American Journal of Health-System Pharmacy. 2008;65(10):935–940. doi: 10.2146/ajhp070388. [DOI] [PubMed] [Google Scholar]
- 87.Abuissa H., Jones P. G., Marso S. P., O’Keefe J. H., Jr Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. Journal of the American College of Cardiology. 2005;46(5):821–826. doi: 10.1016/j.jacc.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 88.Prisant L. M. Preventing type II diabetes mellitus. Journal of Clinical Pharmacology. 2004;44(4):406–413. doi: 10.1177/0091270004263018. [DOI] [PubMed] [Google Scholar]
- 89.Haluzík M. M., Haluzík M. PPAR-alpha and insulin sensitivity. Physiological Research. 2006;55(2):115–122. doi: 10.33549/physiolres.930744. [DOI] [PubMed] [Google Scholar]
- 90.Damci T., Tatliagac S., Osar Z., Ilkova H. Fenofibrate treatment is associated with better glycemic control and lower serum leptin and insulin levels in type 2 diabetic patients with hypertriglyceridemia. European Journal of Internal Medicine. 2003;14(6):357–360. doi: 10.1016/S0953-6205(03)90001-X. [DOI] [PubMed] [Google Scholar]
- 91.Chan S. M. H., Sun R. Q., Zeng X. Y., et al. Activation of PPARα ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress. Diabetes. 2013;62(6):2095–2105. doi: 10.2337/db12-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elkeles R. Fibrates: old drugs with a new role in type 2 diabetes prevention? The British Journal of Diabetes & Vascular Disease. 2011;11(1):4–9. doi: 10.1177/1474651410397245. [DOI] [Google Scholar]
- 93.Graham M. J., Lee R. G., Brandt T. A., et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. The New England Journal of Medicine. 2017;377(3):222–232. doi: 10.1056/NEJMoa1701329. [DOI] [PubMed] [Google Scholar]
- 94.Kostapanos M. S., Liamis G. L., Milionis H. J., Elisaf M. S. Do statins beneficially or adversely affect glucose homeostasis? Current Vascular Pharmacology. 2010;999(999):1–20. doi: 10.2174/1570210205072741611. [DOI] [PubMed] [Google Scholar]
- 95.Pedersen T. R., Faergeman O., Kastelein J. J., et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the ideal study: a randomized controlled trial–correction. JAMA. 2005;294(19):2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]