Abstract
The high energy cost of walking in individuals with cerebral palsy (CP) contributes significantly to reduced mobility and quality of life. The purpose of this study was to develop and clinically evaluate an untethered ankle exoskeleton with the ability to reduce the metabolic cost of walking in children and young adults with gait pathology from CP. We designed a battery-powered device consisting of an actuator-and-control module worn above the waist with a Bowden cable transmission used to provide torque to pulleys aligned with the ankle. Special consideration was made to minimize adding mass to the body, particularly distal portions of the lower-extremity. The exoskeleton provided plantar-flexor assistance during the mid-tolate stance phase, controlled using a real-time control algorithm and embedded sensors. We conducted a device feasibility and a pilot clinical evaluation study with five individuals with CP ages five through thirty years old. Participants completed an average of 130 minutes of exoskeleton-assisted walking practice. We observed a 19 ± 5% improvement in the metabolic cost of transport (p = 0.011) during walking with untethered exoskeleton assistance compared to how participants walked normally. These preliminary findings support the future investigation of powered ankle assistance for improving mobility in this patient population.
Keywords: Cerebral palsy (CP), metabolic cost, exoskeleton, walking, wearable robotics, rehabilitation robotics
I. INTRODUCTION
PATHOLOGICAL gait patterns reduce quality of life by drastically increasing the effort and metabolic energy required to walk. Improving walking efficiency remains a critical challenge for clinicians and therapists treating children and young adults with gait disorders, particularly those caused by cerebral palsy (CP), which is the most common cause of pediatric physical disability [1]. The energy cost of walking can be more than two-to-three times greater in individuals with CP compared to their unimpaired peers [2]. A strong negative relationship between the energy cost of walking and amount of physical activity in individuals with CP [3] underscores the need for new strategies to drastically improve walking economy. The call for new methods to improve mobility and increase activity levels [4] has been made to help mitigate the loss of walking ability that afflicts a significant portion of this patient population in adulthood [5].
Interventions aimed at improving mobility typically focus on treating the ankle joint due to its critical role during walking, acting to stabilize, support, and, most importantly, propel the body [6]. Indeed, reduced ankle performance during walking in those with CP is suggested as a primary contributor to reduced walking efficiency [7] and debilitating gait patterns [8]. Individuals with CP typically exhibit an overly flexed walking posture [9] that worsens with age and can culminate in the inability to ambulate independently [10]. Ankle-foot-orthoses (AFOs), which function by restricting ankle dorsiflexion during the stance phase of walking, show mild to moderate effectiveness for eliciting clinically-relevant improvements in gait mechanics [11] and energetics [12]. Orthopedic surgery [13], muscle injections [14], and physical therapy [15], can improve ankle function, but complete correction is not achieved, and long-term gait and motor deficits usually remain.
Assistive devices that can significantly reduce walking effort for individuals with gait impairments would likely contribute to increased quality of life through improved and prolonged independent mobility. Increased physical activity facilitated by improved mobility may be beneficial from a rehabilitation perspective because increased repetitions of functional locomotor tasks can benefit neuromuscular health. Powered assistance provided to the ankle joint has been shown to reduce the metabolic cost of walking in unimpaired adults [16]–[21], and recently, tethered powered assistance was shown to improve walking economy in stroke victims [22]; a tethered system is one in which not all of the components needed to provide assistance are worn on the user. Whether the device is tethered or untethered is a critical distinction because carrying the added mass of the power supply and actuators often negates the metabolic benefit of powered assistance [23]. To the best of our knowledge, no studies have reported improvements in the metabolic cost of walking using an untethered exoskeleton in individuals with neurological gait impairment, including individuals with CP. Of the two untethered exoskeletons that demonstrated the ability to reduce the energy cost of transport in healthy adults [18], [19], neither were optimally designed for children and adolescents with gait impairments. They were sized for adults and either rely on unimpaired ankle motion for the storage and return of elastic energy or do not offer the fine-tune controllability necessary to treat pathological gait deficits.
The overarching goal of this study was to design and clinically evaluate a battery-powered ankle exoskeleton specifically intended to improve walking economy in children and young adults with neuromotor impairment. We constrained our design to be battery-powered and untethered, minimally cumbersome with regards to both physical profile and added mass, able to provide finely-tuned personalized assistance, and to allow for rapid customized fabrication. Our primary clinical objective was to evaluate the ability of the untethered ankle exoskeleton to improve the energy cost of walking. We completed a multi-visit pilot clinical study to determine how untethered bilateral powered ankle assistance affects the mechanics and energetics of walking in a preliminary cohort of children and young adults with CP. To provide insight for future studies, we also evaluated the sensitivity of metabolic cost of transport to active and passive properties of the assistive device.
II. METHODS
A. Mechanical Design
Several design criteria have been identified for a device to meet the inherent challenges. First, the device must accommodate a range of body sizes, requiring interchangeable actuators to provide appropriate assistance with minimal metabolic detriment caused by adding mass to the body. Second, the device must accommodate reduced ankle function during walking, including reduced plantar-flexion, which limits the potential storage and return of elastic energy. Third, the device must accommodate heterogeneous gait deficits, including drop-foot, inter-limb asymmetry, and highly variable spatiotemporal characteristics that make controlling the timing of assistance difficult.
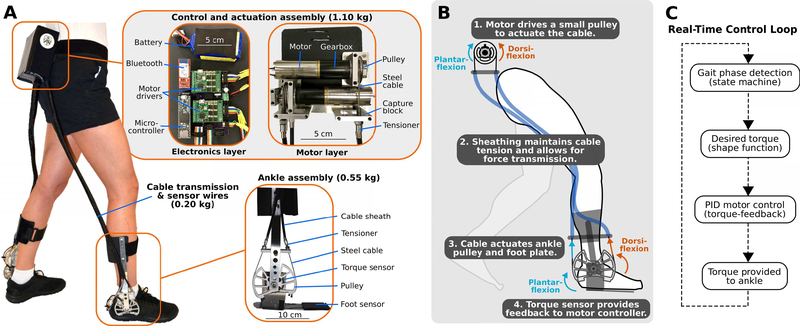
Our ankle exoskeleton consisted of an actuator-and-control module, Bowden cable transmission, and bilateral shank and foot assemblies (Fig. 1A). Our design was inspired from prior designs utilizing Bowden cables, like tethered emulators [24] and similar to a soft exosuit [22]. Our actuator-and-control module, which was worn on the torso, contained brushless DC motors, electronics, and battery. Torque was transferred from pulleys mounted on the DC motors to pulleys mounted on the ankle assemblies via the Bowden cables (Fig. 1B). The ankle joint of the exoskeleton operated as a simple sagittal-plane revolute joint, allowing both plantar-flexion and dorsi-flexion.
Fig. 1. Overview of a novel untethered ankle exoskeleton designed to improve walking economy in children and adults with CP.
(A) The lightweight device incorporates a waist or torso mounted control and actuation assembly (top inset), Bowden cable transmission, and an ankle assembly (bottom inset). (B) Schematic depiction of how the device provides ankle plantar-flexor and dorsi-flexor assistance from the waist-mounted actuation assembly. (C) The real-time control loop for exoskeleton operation.
Because our target demographic encompassed a wide range of body masses, we created a modular design whereby two different sized actuators could be implemented depending on the mass of the participant. For the smaller motor assembly design, we used a 24 V, 90 W motor with 89:1 integrated planetary gearbox (EC-4pole, Maxon). For the larger motor assembly, we used a 24 V, 120 W motor with 111:1 integrated planetary gearbox (EC-4pole, Maxon). The gear reduction from each gearbox combined with a 3:1 gear reduction from the pulleys to obtain a total gear reduction of 267:1 for the small assembly, allowing for up to 12 Nm of torque, and a 333:1 for the large motor assembly, allowing for up to 20 Nm of torque. This design allowed for 0.30 Nm·kg−1 of plantar-flexor assistance for individuals up to 66.7 kg. Despite the high gear reductions, exoskeleton operation was relatively quiet with tuned control gains.
The configuration of our exoskeleton allowed us to place motors and battery close to the body’s center of mass, the location that minimizes the metabolic cost of walking when mass is added to the body [25]. The custom shank and foot assemblies consisted of aluminum sheet-metal insoles, torque sensors, torque transmission pulleys, Bowden cable attachment points, aluminum bar-stock lateral supports, and plastic-molded calf attachments. Because it was necessary to create custom exoskeletons for each participant, material selection was based on cost as well as ease of fabrication. We implemented a single lateral support to minimize profile and mass added to the body. The farthest distance the exoskeleton extended outward from the body was 7 cm. The assembled exoskeleton, including the battery, had a mass of 1.85 kg for the smaller assembly and 2.20 kg for the larger assembly; greater than 65% of the total mass was located above the waist for both. The achieved exoskeleton mass included a battery with sufficient capacity for greater than 30 minutes of assisted walking at the torque setpoint for all five participants in this study. A limitation of the mechanical design was that material selection was not optimized. Aluminum was utilized for this feasibility study due to ease of fabrication, an initial design priority, considering each participant required custom components. In the future, materials with greater strength-to-density ratio, such as carbon fiber, will likely be selected. Additionally, we will make the mechanical design more appropriate for use at home by addressing jagged features, exposed wires, and durability concerns. Exoskeleton bill of materials and CAD files are located in Supplemental Material.
B. Electronics & Control
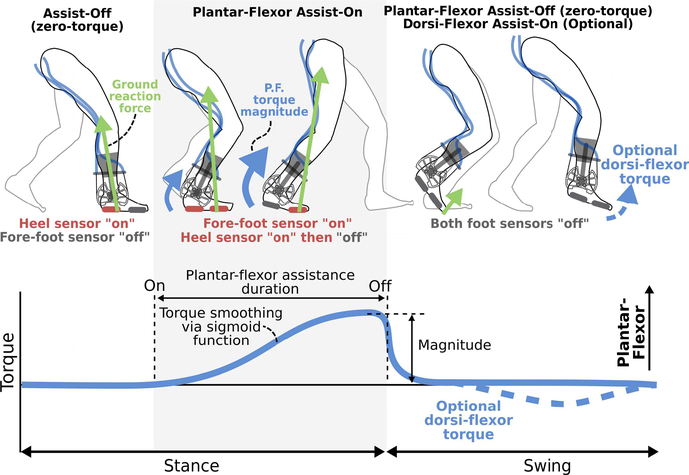
We implemented a finite-state-machine (Fig. 1C) to control the on-off timing of ankle assistance across the gait cycle. Our state machine included Plantar-Flexor Assist-On and Assist-Off states, with an optional Dorsi-Flexor Assist-On state (Fig. 2). We utilized a force-sensitive-resistor placed under the ball of the foot to govern the transition to and from the Plantar-Flexor Assist-On state. This sensor location takes advantage of how, during walking, the foot’s center of pressure centralizes around the distal end of the metatarsal bones (i.e. under the “ball” of the foot) when the ankle plantar-flexor muscles are most active [26]. If dorsi-flexor assistance was required during the swing phase to treat drop-foot, as assessed via repeated observation of toe-drag by the participant, we utilized a force-sensitive-resistor placed under the heel to govern the transition to the Assist-Off state in early stance.
Fig. 2. Timing of exoskeleton assistance.
Foot sensors are used for gait phase detection via a finite state machine. The Plantar-Flexor Assist-On state triggers the application of a biologically-synergistic torque profile via a shaping function. Dorsi-flexor assistance is optional during the swing phase.
We controlled motor torque output via a proportional-integral-derivative feedback controller using a torque sensor (TRT-500, Transducer Techniques) placed in-line with each exoskeleton ankle joint; the exoskeleton ankle joint, and therefore the torque sensor, were subsequently aligned with each biological ankle joint. We sought to control the torque signal such that it matched the general characteristic (timing and shape) of the biological ankle moment [27], [28]. There-fore, when assistance was turned on (Plantar-Flexor Assist-On state), the instantaneous torque profile (Torque(t)) was smoothed from a square wave (supplemental Fig. 1) using a simple sigmoid function (Equation 1), as follows:
| 1 |
where Torqueset represents the peak torque setpoint, t represents each time step, and smoothwas the tunable parameter that influenced the torque rate of change.
Our real-time control scheme (Fig. 1C), operating at 1 kHz, was implemented on a 32-bit ARM microprocessor (Teensy 3.6, TJRC). A custom printed circuit board integrated the microcontroller, motor servocontrollers (ESCON 50/5, Maxon), sensor connectors, signal processing components, and Bluetooth module. The system was powered by a 0.14 kg 22.2V 910 mAh Lithium Polymer (Li-Po) battery (Eflite 6S). We created a graphical user interface (GUI) in Matlab to remotely operate the exoskeleton via Bluetooth. Our GUI displayed and recorded data from the exoskeleton and allowed us to calibrate the finite-state-machine thresholds and adjust the operating parameters of the exoskeleton (e.g. sensor calibration, torque setpoint, torque smoothing function, etc.) during online operation. Design considerations and customization procedure for our ankle exoskeleton controller are included in the supplemental material. Exoskeleton embedded control code and PCB design files are located in Supplemental Material.
C. Participants & Experimental Evaluation
Five individuals diagnosed with gait deficits from CP participated in this cohort study (Table I). To maximize the applicability of this initial small-sample study to the wider CP population, we recruited participants across a wide age range (5–35 years old), body masses (15.7–51.1 kg), and level of impairment (Gross Motor Function Classification System level I-III). Clinical characterization of each participant’s ankle function is presented in Supplemental Table I. We received informed written consent from each adult participant, or from a parent or legal guardian if the participant was a minor. Inclusion criteria included diagnosis of CP, a minimum of 10° of passive ankle plantar-flexion, age between five and seventy-five years old, the ability to walk on a treadmill for five minutes with or without hand-held assistance, and the ability to understand and follow simple directions. Exclusion criteria included orthopaedic surgery within six months of participation and any health condition other than CP that would affect participant safety.
Table I.
Participant Information
| Participant | Age (yrs) | Gender | Height (m) | Body Mass (kg) | GMFCSa Level | MASb (L/R) | Baseline Condition | Number of Visits |
|---|---|---|---|---|---|---|---|---|
| PI | 7 | M | 1.32 | 26.9 | I | 2/2 | Shoe Inserts | 8 |
| P2 | 22 | F | 1.47 | 45.3 | III | 3/3 | AFOs | 9 |
| P3 | 5 | M | 1.03 | 15.7 | II | 2/2 | AFOs | 10c |
| P4 | 30 | M | 1.70 | 51.0 | I | 0/0 | Shod | 5 |
| P5 | 12 | M | 1.39 | 34.8 | I | 3/1 | Shod | 9 |
GMFCS: Gross Motor Function Classification System.
MAS: Modified Ashworth Scale for the ankle plantar-flexors for the left and right limbs.
Two visits resulted in less than 10 minutes of walking due to noncompliance from this 5-year-old participant.
The Northern Arizona University Institutional Review Board granted approval for this study (#986744). Participants completed an evaluation and assessment visit, between four and ten exoskeleton training visits, and a final data collection visit. The number of training visits completed by each participant was not predetermined due to variable participant walking ability and attention span. Instead, we specified a minimum exoskeleton gait training duration of 90 minutes and developed customized schedules and visit allocations based on ease of acclimation to powered assistance, attention levels, and walking capacity of each participant.
On the first visit, a physical therapist conducted a physical exam and neurological assessment, and technicians took lower-extremity measurements for custom exoskeleton fabrication. During subsequent training visits, participants practiced walking with the exoskeleton on the treadmill as the research team tuned the exoskeleton torque parameters. Initially, the magnitude of assistance was slowly increased from zero to approximately 0.15 Nm·kg−1. As participants accommodated to assistance, the torque was increased up to a maximum of 0.35 Nm·kg−1 and subsequently refined. A detailed description of the tuning procedure is included in supplemental material. Participants walked for as long as they could up to twenty-five minutes per day. Seated breaks lasting 5–10 minutes were provided upon request or after a maximum of 10 minutes of walking. The final visit was scheduled following 90 minutes of practice and when the research team collectively agreed that a suitable level of personalized assistance was identified. Suitability was assessed visually and based on the criteria that the participant could quickly reach a steady-state walking pattern. On average, participants completed 130 minutes of training with the exoskeleton (Table II). One participant (P5) struggled to acclimate to exoskeleton assistance and required 208 minutes of training with the device. The other participants required between 108 to 114 minutes.
Table II.
Experimental Walking and Exoskeleton Information
| Participant | Exo Walk Time (min) | Gait Speed (m/s) | Torque RMSE (Nm•kg−1)b |
Exoskeleton Mass (% Body Mass) | Peak Torquea (Nm•kg−1) |
||
|---|---|---|---|---|---|---|---|
| Exo-Assisted | Zero-T orque-Ctrl | Possible | Provided | ||||
| PI | 113.6 | 0.60 | 0.054 | 0.015 | 6.9 | 0.44 | 0.29 |
| P2 | 108.4 | 0.55 | 0.035 | 0.017 | 4.1 | 0.40 | 0.24 |
| P3 | 107.7 | 0.65 | 0.044 | 0.036 | 11.8 | 0.76 | 0.25 |
| P4 | 112.1 | 1.10 | 0.045 | 0.021 | 4.3 | 0.39 | 0.24 |
| P5 | 208.1 | 0.95 | 0.030 | 0.015 | 5.3 | 0.35 | 0.26 |
Nominal possible torque represents the peak amount of torque capable of being provided by the exoskeleton. Nominal provided torque represents the setpoint used during the trial that resulted in the lowest metabolic cost of transport.
Torque RMSE reports the root-mean-squared error between the desired torque and the measured torque.
On the final visit, participants completed walking trials under the following walking conditions: baseline walking, which replicated the participant’s everyday walking condition and included AFOs if they were prescribed from a primary physician; Exo-Assisted walking, which entailed wearing the exoskeleton with subject-specific powered assistance; and finally, Zero-Torque-Ctrl, which entailed wearing the exoskeleton while it was controlled to produce zero-torque, seeking to remove the effects of cable friction and motor inertia. The zero-torque condition was included to evaluate the metabolic cost of walking while wearing mass of the exoskeleton. When time and participant willingness allowed, participants completed the Exo-Assisted walking condition for multiple torque magnitudes to allow us to explore the effects of varying assistance on metabolic cost of transport. We had one participant whose baseline condition involved walking with AFOs complete a shod (shoes only) trial to allow comparison to a completely unassisted condition.
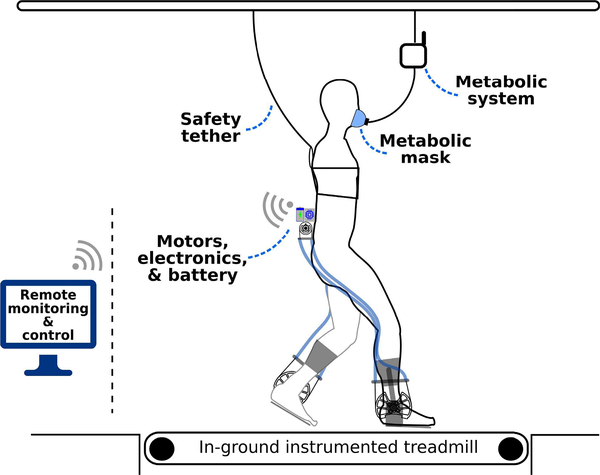
Each walking trial analyzed in this study took place on an in-ground treadmill (Bertec) and lasted for five minutes (Fig. 3). A safety tether was attached to each participant for all walking conditions. Preferred treadmill walking speed was determined during the baseline condition by sequentially increasing and decreasing the belt velocity until the participant consistently identified their preference.
Fig. 3. Schematic of the experimental setup.
The exoskeleton operated completely untethered (wireless and battery powered) for all exoskeleton walking trials. The exoskeleton was remotely operated via Bluetooth from a laptop computer, which also recorded the experimental exoskeleton data. Rate of oxygen consumption and carbon dioxide production were measured from a portable metabolic indirect calorimetry system.
Baseline walking trials were randomized to take place at the beginning or end of each visit to minimize transitions between the exoskeleton trials. The order of the exoskeleton trials (e.g. Exo-Assisted, Zero-Torque-Ctrl) was also randomized (Supplemental Table II). Walking trials were separated by a seated rest break, each lasting at least five minutes; participants provided verbal indication when they were ready to begin the next trial. The most impaired participant (P2, GMFCS III) required the use of a hand support to complete all walking conditions. To ensure similarity of weight-bearing across conditions, we used a tension load cell (Futek) to instrument a handle that was attached to a rope (supplemental Fig. 2); the handle supported 6.9 ± 1.6% body-weight during the baseline trial and 6.2 ± 1.6% during the Exo-Assisted trial.
We recorded each participant’s rate of oxygen consumption and carbon dioxide production during each walking trial using a wireless portable metabolic measurement system (Cosmed, K5). The portable metabolic backpack module was suspended so that the participant was not loaded with additional mass. A pediatric perceived exertion scale (PCERT) [29] was administered following each walking trial by the same member of the research team (within and across participants). At the end of the final visit the same researcher asked each participant if they preferred walking how they do normally or with exoskeleton assistance.
D. Data Processing & Statistical Analysis
Metabolic data were processed using Brockway’s standard equation [30]. First, we calculated the net metabolic cost (W) during each walking condition by subtracting the metabolic cost of quiet standing[31]. Next, to account for the heterogeneous body sizes and different gait speeds, we computed the body-mass-normalized metabolic energy required to walk a unit distance (metabolic cost of transport, J·kg−1·m−1) by dividing the net metabolic rate (W) by the participant’s body mass (kg) and walking speed (m·s−1) [32]. Exoskeleton torque data were normalized to percent gait cycle and averaged; reported data are from the final minute of each walking trial.
We used paired two-tailed t tests to determine if metabolic cost of transport was affected by walking with exoskeleton assistance compared to baseline. To assist with the design of future intervention protocols, 2nd order polynomial regression was used to assess the relationship between the magnitude of assistance provided during Exo-Assisted walking and change in metabolic cost of transport; non-linear regression was used based on prior relevant findings [18]. To inform the selection of potential candidates for future clinical trials, linear regression was used to assess the relationship between relative body mass and the change in metabolic cost of walking under the Zero-Torque-Ctrl condition. To account for the multiple statistical comparisons performed in this study, significance was sequentially adjusted from α < 0.05 to α < 0.0167 via the Holm-Bonferroni approach to control the family-wise type one error rate. We tested that data in each comparison were normally distributed using the small-sample Lillifors correction of the Kolmogorov-Smirnov test; all tests confirmed normality. Mean ± standard error (SE) are presented in the text for group-level comparisons.
III. RESULTS
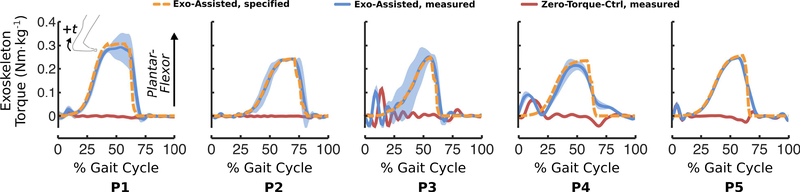
Our primary design objective was the ability to fabricate custom exoskeletons that were less than 10% of each participant’s body mass while providing at least 0.30 Nm·kg−1 of plantar-flexion assistance (See Design Considerations in Supplemental Material). We achieved this design goal with one exception; the exoskeleton for the lightest and youngest participant (P3, 15.7 kg, 5-years-old) was 11.8% of their body mass (Table II). We assessed the reliability of the motor controller by quantifying the root-mean-square-error (RMSE) between measured and desired ankle torque (Fig. 4, Table II). The average RMSE was 0.042 ± 0.004 Nm·kg−1 for the Exo-Assisted trials and 0.021 ± 0.004 Nm·kg−1 for the Zero-Torque-Ctrl trials. Measured analysis of battery performance during walking revealed a 31.7-minute capacity for the largest absolute Exo-Assisted torque setpoint across the five participants; the rate of discharge was 28.4 mAh per minute during walking with bilateral 12 Nm of plantar-flexor assistance.
Fig. 4. Exoskeleton torque during walking.
Experimentally-measured exoskeleton torque applied to each participant’s right limb during Exo-Assisted (blue) and Zero-Torque-Ctrl (red) conditions. Shading depicts ± 1.0 standard deviation. The specified (desired) torque for the Exo-Assisted condition is shown by the orange dashed line. The desired torque for the Zero-Torque-Ctrl condition was zero. A small amount of non-zero torque was observed for the Zero-Torque-Ctrl condition for some participants at intervals in the gait cycle when the ankle angular velocity exceeded the controllable speed of the motor at the implemented gear reduction. Data from each participant’s left limb are presented in Supplemental Fig. 1. The large standard deviation for the torque and power output for P3 was a result of this participant’s variable gait pattern, which elicited variable onset of assistance.
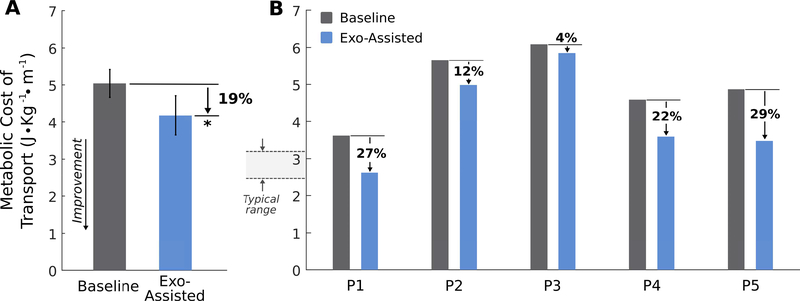
On average, the net metabolic cost of transport (J·kg−1·m−1) decreased by 18.7 ± 4.8 % (paired t test, p = 0.011) during walking with exoskeleton assistance compared to baseline (Fig. 5A). On an individual level, net metabolic cost of transport decreased for all participants during walking with exoskeleton assistance, ranging 4–29% for the best-case trials (Fig. 5B). The average amount of ankle plantar-flexor assistance from the Exo-Assisted walking trial that resulted in each participant’s lowest metabolic cost of transport was 0.26 ± 0.01 Nm·kg−1(Table II). Ground reaction forces during Exo-Assisted and baseline walking are reported in Supplemental Fig. 2.
Fig. 5. Reduction in metabolic cost of transport during walking with untethered exoskeleton assistance.
(A) Group-average metabolic cost of transport during walking with battery-powered plantar-flexor assistance (blue) compared to baseline walking (gray) (paired t test, p = 0.011). Error bars depict SE. ∗ indicates significance. (B) Individual results of the reduction in metabolic cost of transport across the five participants. Presented data are from the trial that resulted in the largest reduction in metabolic cost of transport for participants that walked with multiple magnitudes of assistance. The “Typical range” indicates the range of net metabolic cost of transport values reported for unimpaired children and adults from 5 years old to adulthood [32]; baseline metabolic cost of transport for our participants was 80% greater than in unimpaired individuals, on average.
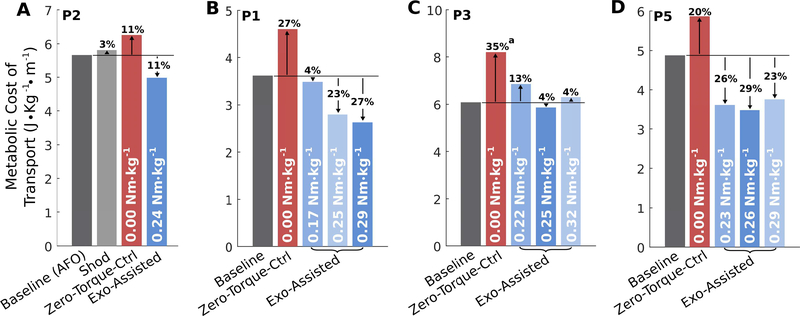
One participant (P2) received 0.07 Nm·kg−1 of left ankle dorsi-flexion assistance to address persistent drop-foot (Supplemental Fig. 1). One participant (P2) completed baseline (using prescribed AFOs), shod, and Exo-Assisted walking trials (Fig. 6A). Compared to the shod trial, their metabolic cost of transport was 3% lower while walking with their passive AFOs, yet 17% lower while walking with the powered exoskeleton. Three participants completed Exo-Assisted walking trials with three different magnitudes of plantar-flexor assistance (Fig. 6B-D). Two participants (P3 and P5) exhibited reduced improvement in metabolic cost of transport during walking with assistance greater than 0.25 and 0.26 Nm·kg−1 of peak plantar-flexor assistance, respectively, while the greatest improvement in metabolic cost of transport for participant P1 occurred with their largest torque setpoint (0.29 Nm·kg−1).
Fig. 6. Single subject experiments.
(A) The effects of walking condition on metabolic cost of transport for the participant (P2) who completed shod, AFO, Zero-Torque-Ctrl, and Exo-Assisted walking trials on their final visit. (B-D) The effects of different magnitudes of untethered plantar-flexion assistance on metabolic cost of transport for three participants (P1, P3, and P5) who completed multiple Exo-Assisted walking trials on their final visits. a See note in supplemental material regarding recording of the Zero-Torque-Ctrl trial on a separate visit for P3.
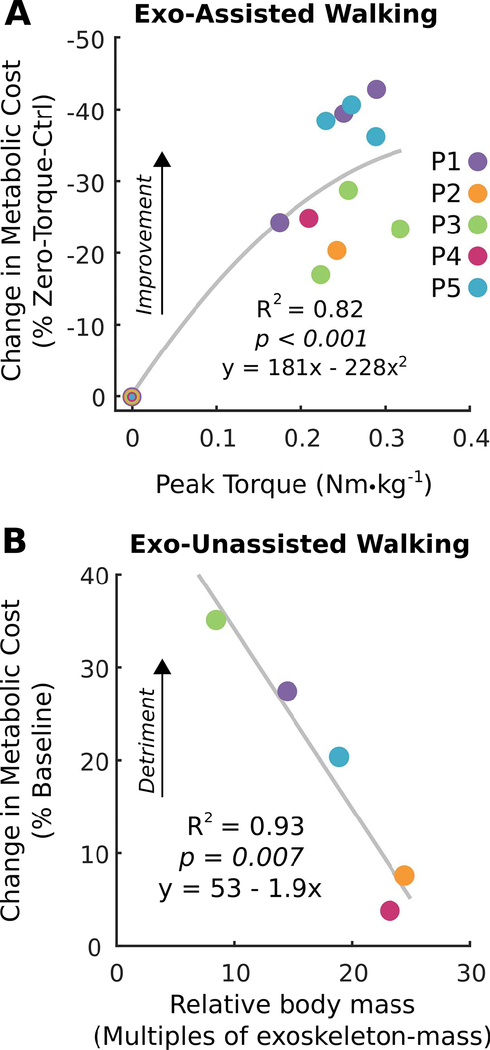
Change in metabolic cost was highly related to the amount of plantar-flexion assistance provided from the exoskeleton (R2 = 0.82, p < 0.001; Fig. 7A). A reduction in metabolic cost increased approximately linearly as assistance increased before starting to level off around 0.25 Nm·kg−1. To evaluate the metabolic cost associated with wearing the device, we also collected respiratory data during walking under Zero-Torque-Ctrl. We found that the participant’s body mass relative to exoskeleton mass was highly related to the change in walking economy during the Zero-Torque-Ctrl trials (R2 = 0.93, p = 0.007; Fig. 7B). Within the range of body masses tested, the metabolic cost of transport associated with wearing the device decreased by 19% for every 10 times that body mass was greater than exoskeleton mass.
Fig. 7. Relationships between assisted and unassisted exoskeleton walking on metabolic cost.
(A) The relationship between peak torque during walking with untethered exoskeleton assistance and the change in metabolic cost relative to walking unassisted with the exoskeleton (2nd order polynomial regression; n = 16, p < 0.001). (B) The relationship between body mass and the change in metabolic cost during walking unassisted with the untethered exoskeleton relative to baseline walking (linear regression; n = 5, p = 0.007).
All participants perceived higher exertion during walking with exoskeleton assistance compared to baseline (Supplemental Table III). Three participants preferred walking with exoskeleton assistance, one participant preferred their baseline condition, and one participant was undecided (Supplemental Table III). Three participants articulated how exoskeleton assistance helped them walk (Supplemental Table IV).
IV. DISCUSSION
The first goal of this study was to create a wearable assistive device capable of reducing the metabolic cost of walking in young individuals with compromised balance, strength, and selective motor control. We accomplished this goal through the design of a lightweight, low-profile, and battery-powered ankle exoskeleton that can be easily customized to fit a wide range of statures, including young children, adolescents, and adults. The timing of exoskeleton assistance was controlled using a simple and intuitive real-time control strategy and embedded sensors that was robust to variable walking patterns. The untethered design was used for all experiments in this study to provide insight for future investigations on the use of wearable assistance to improve mobility at home and in the community.
The clinical objective of this study was to evaluate if the untethered robotic ankle exoskeleton could improve walking economy in an initial group of children and young adults with gait deficits from CP. Collectively, participants completed over 10.8 hours of exoskeleton walking during 37 study visits lasting a total of 62 hours. This study indicated that the novel assistive device significantly improved the metabolic cost of transport in a diverse cohort with varying degrees of impairment and large differences in age, body mass, and walking ability. Our participants had a baseline metabolic cost of transport that was 80% above that of unimpaired children and young adults [32]. The average metabolic improvement during walking with exoskeleton assistance resulted in an average energy cost of walking that was 30% closer to normal levels. This improvement was roughly twice that reported for walking with passive orthotics [33]. One participant (P2) in our study completed shod, AFO, and Exo-Assisted trials. We found that, compared to the shod trial, walking with the AFO improved walking economy by 3%, whereas walking with the exoskeleton improved walking economy by 14%.
We found that metabolic cost of transport was highly sensitive, and linearly related, to the magnitude of plantar-flexor assistance across the range of magnitudes tested. For participants in this study, an average of 0.26 Nm·kg−1 of plantarflexor assistance proved most beneficial for reducing metabolic cost of transport. Single subject experiments revealed that increasing assistance above this amount was beneficial for one participant, but detrimental for two others. We theorize that too much assistance may become destabilizing. This finding demonstrates the need for highly customizable torque output in this patient population. Human in the loop optimization, like the technique reported by Zhang et al. [16], may improve outcomes.
A primary challenge in the design of an untethered exoskeleton intended to reduce the metabolic cost of walking is that simply adding mass to the body, particularly distal portions of the leg, increases the energy required to move the body. We found that the user’s body mass relative to the mass of the exoskeleton was significantly related to the increase in metabolic cost associated with simply wearing the exoskeleton. For example, the exoskeleton for our youngest and lightest participant (15.7 kg) was 11.8% of their body mass. The metabolic burden of wearing the device, as assessed during the Zero-Torque-Ctrl trial, was very large (a 35% increase). Therefore, while exoskeleton assistance improved walking economy by 39% relative to walking with ZeroTorque-Ctrl, the net reduction below baseline was only 4%. Our results suggest a threshold whereby powered assistance relative to the added mass of the exoskeleton no longer benefits the user. This captures a fundamental challenge for powered assistive devices designed for young children.
An individual’s perception of benefit for a mobility aid is an important consideration in the development of assistive technologies. Surprisingly, and despite the measured reduction in metabolic cost of transport, none of the participants perceived reduced exertion during walking with exoskeleton assistance. In fact, participants perceived the opposite. This was likely due the novelty of walking with powered assistance, increased focus, and the added sensation wearing the device. Tellingly, the majority of the participants preferred walking with exoskeleton assistance compared to baseline, while only one participant preferred the opposite.
The long-term goal of this research is to provide a more effective mobility aid for individuals with gait pathology, used in selective intervals to improve quality of life. It is important that users of the device do not become dependent on powered assistance. Therefore, the present work may complement important research focused on encouraging improved motor control and strength for individuals with CP [34]–[36]. Due to the ability of our ankle exoskeleton to provide equal amounts of plantar-flexor and dorsi-flexor torque, there is the potential for the device to be used in a rehabilitation context, providing plantar-flexor resistance with the goal of strengthening the ankle plantar-flexor muscles during therapy.
This pilot study had several limitations that should be considered when interpreting these findings. First, the sample size was very small. Nevertheless, it was comparable to the number of participants in similar prominent multi-visit repeated-measures studies (e.g. n = 6 [36]), and was sufficient to demonstrate device efficacy through statistically and clinically significant improvements [37]. Due to the pioneering nature of this work and the significant time commitment and resources required to conduct a multi-visit study in individuals with neuromuscular deficits, the present sample size was selected to confirm the efficacy of the device and provide insight for the design of future larger-scale trials. Another limitation to note is that while this study shows that improved energy cost during walking with assistance is achievable for individuals with CP, as assessed via rigorous within visit testing, follow-up studies should investigate day-to-day variability. Furthermore, while participants took seated breaks between trials, and trial order was randomized across participants, fatigue or treadmill acclimation was not assessed. Therefore, our results may be influenced by the order in which participants completed the walking conditions. Completing a baseline trial at the end of the visit may exaggerate the effect of the device. Walking trials during experimental measurement were five minutes long, a length that was selected as an achievable duration across the wide range of participants, and which balanced the requirement for metabolic assessment at steady-state while also minimizing the potential effects of fatigue. Future studies should investigate how walking duration and assessment order affects outcomes. Non-zero torque applied to the ankle during the Zero-Torque-Ctrl condition, while minimal, may influence our findings from that condition. Lastly, the same battery was used for all participants. Ideally, different batteries would have been selected to achieve the same runtime across participants; a lower capacity battery for the lighter participants may have slightly improved metabolic outcomes.
In conclusion, this pilot study provides evidence that a lightweight untethered ankle exoskeleton can result in clinically significant improvements in walking economy for children and young adults with CP. Improvements were observed across a wide range of participant characteristics. This study provides initial evidence for the further investigation of powered plantar-flexor assistance for augmenting mobility in children and young adults with neurological disorders. Findings from this study, including exoskeleton training times, and the relationships between metabolic cost of transport, the magnitude of assistance and relative exoskeleton mass, will likely prove beneficial for the design of future clinical trials.
Supplementary Material
Acknowledgments
This study was supported by NIH grant number 1R03HD094583–01 and Arizona Department of Health Serves grant number ADHS18–198864.
REFERENCES
- [1].Aisen ML et al. , “Cerebral palsy: clinical care and neurological rehabilitation,” Lancet Neurol, vol. 10, no. 9, pp. 844–852, 2011. [DOI] [PubMed] [Google Scholar]
- [2].Rose J, Gamble JG, Burgos A, Medeiros J, and Haskell WL, “Energy expenditure index of walking for normal children and for children with cerebral palsy,” Dev Med Child Neurol, vol. 32, no. 4, pp. 333–340, 1990. [DOI] [PubMed] [Google Scholar]
- [3].Maltais DB, Pierrynowski MR, Galea VA, and Bar-Or O, “Physical activity level is associated with the O2 cost of walking in cerebral palsy,” Med Sci Sport. Exerc, vol. 37, no. 3, pp. 347–353, 2005. [DOI] [PubMed] [Google Scholar]
- [4].Damiano DL, “Activity, activity, activity: Rethinking our physical therapy approach to cerebral palsy,” Phys. Ther, vol. 86, no. 11, pp. 1534–1540, 2006. [DOI] [PubMed] [Google Scholar]
- [5].Bottos M and Gericke C, “Ambulatory capacity in cerebral palsy: Prognostic criteria and consequences for intervention,” Developmental Medicine and Child Neurology, vol. 45, no. 11 pp. 786–790, 2003. [DOI] [PubMed] [Google Scholar]
- [6].Winter DA, Biomechanics and motor control of human gait: normal, elderly and pathological. 1991. [Google Scholar]
- [7].Olney SJ, MacPhail HE, Hedden DM, and Boyce WF, “Work and power in hemiplegic cerebral palsy gait,” Phys. Ther, vol. 70, no. 7, pp. 431–438, 1990. [DOI] [PubMed] [Google Scholar]
- [8].Winters TF Jr, Gage JR, and Hicks R, “Gait patterns in spastic hemiplegia in children and young adults,” J. Bone Jt. Surg. - Ser. A, vol. 69, no. 3, pp. 437–441, 1987. [PubMed] [Google Scholar]
- [9].McNee AE, Shortland AP, Eve LC, Robinson RO, and Gough M, “Lower limb extensor moments in children with spastic diplegic cerebral palsy,” Gait Posture, vol. 20, no. 2, pp. 171–176, 2004. [DOI] [PubMed] [Google Scholar]
- [10].Bjornson KF, Belza B, Kartin D, Logsdon R, and McLaughlin JF, “Ambulatory physical activity performance in youth with cerebral palsy and youth who are developing typically,” Phys Ther, vol. 87, no. 3, pp. 248–257, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ries AJ, Novacheck TF, and Schwartz MH, “The efficacy of anklefoot orthoses on improving the gait of children with diplegic cerebral palsy: a multiple outcome analysis,” PM&R, vol. 7, no. 9, pp. 922–929, 2015. [DOI] [PubMed] [Google Scholar]
- [12].Kerkum YL, Buizer AI, van den Noort JC, Becher JG, Harlaar J, and Brehm M-A, “The effects of varying ankle foot orthosis stiffness on gait in children with spastic cerebral palsy who walk with excessive knee flexion,” PLoS One, vol. 10, no. 11, p. e0142878, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dreher T et al. , “Long-term results after gastrocnemius-soleus intramuscular aponeurotic recession as a part of multilevel surgery in spastic diplegic cerebral palsy,” J Bone Jt. Surg Am, vol. 94, no. 7, pp. 627–637, 2012. [DOI] [PubMed] [Google Scholar]
- [14].Sutherland DH, Kaufman KR, Wyatt MP, Chambers HG, and Mubarak SJ, “Double-blind study of botulinum A toxin injections into the gastrocnemius muscle in patients with cerebral palsy,” Gait Posture, vol. 10, no. 1, pp. 1–9, 1999. [DOI] [PubMed] [Google Scholar]
- [15].Damiano DL, Alter KE, and Chambers H, “New clinical and research trends in lower extremity management for ambulatory children with cerebral palsy,” Phys Med Rehabil Clin N Am, vol. 20, no. 3, pp. 469–491, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang J et al. , “Human-in-the-loop optimization of exoskeleton assistance during walking,” Science (80-. )., vol. 356, no. 6344, pp. 1280–1283, 2017. [DOI] [PubMed] [Google Scholar]
- [17].Sawicki GS and Ferris DP, “Mechanics and energetics of level walking with powered ankle exoskeletons,” J Exp Biol, vol. 211, no. Pt 9, pp. 1402–1413, 2008. [DOI] [PubMed] [Google Scholar]
- [18].Collins SH, Wiggin MB, and Sawicki GS, “Reducing the energy cost of human walking using an unpowered exoskeleton,” Nature, vol. 522, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mooney LM, Rouse EJ, and Herr HM, “Autonomous exoskeleton reduces metabolic cost of walking,” 2014 36th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBC 2014, vol. 11, no. 1, pp. 3065–3068, 2014. [DOI] [PubMed] [Google Scholar]
- [20].Quinlivan BT et al. , “Assistance magnitude versus metabolic cost reductions for a tethered multiarticular soft exosuit,” Sci. Robot, vol. 2, no. 2, p. eaah4416, 2017. [DOI] [PubMed] [Google Scholar]
- [21].Panizzolo FA et al. , “A biologically-inspired multi-joint soft exosuit that can reduce the energy cost of loaded walking,” J. Neuroeng. Rehabil, vol. 13, no. 1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Awad LN et al. , “A soft robotic exosuit improves walking in patients after stroke,” Sci. Transl. Med, vol. 9, no. 400, 2017. [DOI] [PubMed] [Google Scholar]
- [23].Van Dijk W, Meijneke C, and Van Der Kooij H, “Evaluation of the achilles ankle exoskeleton,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 25, no. 2, pp. 151–160, 2017. [DOI] [PubMed] [Google Scholar]
- [24].Witte KA, Zhang J, Jackson RW, and Collins SH, “Design of two lightweight, high-bandwidth torque-controlled ankle exoskeletons,” in Proceedings - IEEE International Conference on Robotics and Automation, 2015, vol. 2015–June, no. June, pp. 1223–1228. [Google Scholar]
- [25].Browning RC, Modica J, Kram R, and Goswami A, “The Effects of Adding Mass to the Legs on the Energetics and Biomechanics of Walking,” Med Sci Sport. Exerc, vol. 39, no. 3, pp. 515–525, 2007. [DOI] [PubMed] [Google Scholar]
- [26].Francis CA, Lenz AL, Lenhart RL, and Thelen DG, “The modulation of forward propulsion, vertical support, and center of pressure by the plantarflexors during human walking,” Gait Posture, vol. 38, no. 4, pp. 993–997, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hansen AH, Childress DS, Miff SC, Gard SA, and Mesplay KP, “The human ankle during walking: Implications for design of biomimetic ankle prostheses,” J. Biomech, vol. 37, no. 10, pp. 1467–1474, 2004. [DOI] [PubMed] [Google Scholar]
- [28].Gasparri GM, Bair MO, Libby R, and Lerner ZF, “Verification of a Robotic Ankle Exoskeleton Control Scheme for Gait Assistance in Individuals with Cerebral Palsy,” in IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), 2018, p. Accepted. [Google Scholar]
- [29].Yelling M, Lamb KL, and Swaine IL, “Validity of a Pictorial Perceived Exertion Scale for Effort Estimation and Effort Production During Stepping Exercise in Adolescent Children,” Eur. Phys. Educ. Rev, vol. 8, no. 2, pp. 157–175, Jun. 2002. [Google Scholar]
- [30].Brockway JM, “Derivation of forulae used to calculate energy expenditure in man,” Hum. Nutr. Clin. Nutr, vol. 41C, pp. 463–471, 1987. [PubMed] [Google Scholar]
- [31].Maxwell Donelan J, Kram R, and Arthur D K., “Mechanical and metabolic determinants of the preferred step width in human walking,” Proc. R. Soc. B Biol. Sci, vol. 268, no. 1480, pp. 1985–1992, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weyand PG, Smith BR, Puyau MR, and Butte NF, “The massspecific energy cost of human walking is set by stature,” J. Exp. Biol, vol. 213, no. 23, pp. 3972–3979, 2010. [DOI] [PubMed] [Google Scholar]
- [33].Brehm MA, Harlaar J, and Schwartz M, “Effect of ankle-foot orthoses on walking efficiency and gait in children with cerebral palsy,” J. Rehabil. Med, vol. 40, no. 7, pp. 529–534, 2008. [DOI] [PubMed] [Google Scholar]
- [34].Burdea GC, Cioi D, Kale A, Janes WE, Ross SA, and Engsberg JR, “Robotics and gaming to improve ankle strength, motor control, and function in children with cerebral palsy–a case study series.,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 21, no. 2, pp. 165–73, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dodd KJ, Taylor NF, and Damiano DL, “A systematic review of the effectiveness of strength-training programs for people with cerebral palsy,” Archives of Physical Medicine and Rehabilitation, vol. 83, no. 8 pp. 1157–1164, 2002. [DOI] [PubMed] [Google Scholar]
- [36].Kang J, Martelli D, Vashista V, Martinez-Hernandez I, Kim H, and Agrawal SK, “Robot-driven downward pelvic pull to improve crouch gait in children with cerebral palsy,” Sci. Robot, vol. 2, no. 8, p. eaan2634, 2017. [DOI] [PubMed] [Google Scholar]
- [37].Maltais D, Bar-Or O, Galea V, and Pierrynowski M, “Use of orthoses lowers the O(2) cost of walking in children with spastic cerebral palsy.,” Med. Sci. Sports Exerc, vol. 33, no. 2, pp. 320–325, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.