Abstract
Ultraviolet light causes skin cancer. Salicylic acid and other molecular filters absorb damaging radiation but are washed away readily. Conjugation to a collagen mimetic peptide is shown to retain salicylic acid on collagen-containing skin surrogates after repeated washing. This strategy, which is highly modular, could enhance the water-resistance of sunscreens.
Graphical Abstract
A small-molecule UV-filter with a pendant collagen mimetic peptide anneals to a collagen-laden surface and protects against UV radiation.
Skin is our largest organ.1 Our skin not only protects underlying muscles, bones, ligaments, and internal organs, but also regulates body temperature and is the conduit for the sensations of touch, heat, and cold. Ultraviolet (UV) radiation from the sun damages skin, leading to burns, premature aging, immunosuppression, and cancer.2–13 In the US, skin cancer is more prevalent than all other types of cancer combined.12,14 Accordingly, UV radiation is a major public health threat. The risk can, however, be reduced by the proper use of sunscreens.2,5–9,15
UV radiation is divided into three types, based on wavelength: A, B, and C. Type C is blocked by atmospheric ozone, but UVA (320–400 nm) and UVB (290–320 nm) can penetrate human skin and damage DNA and other biomolecules.3,4,11,12 Hence, sunscreens contain molecular “filters” to absorb UVA and UVB radiation.16,17 Typical filters are small aromatic compounds, such as salicylates, cinnamates, benzophenones, or derivatives of p-aminobenzoic acid.16,18
Despite the widespread availability of sunscreens, public compliance with their use is a problem.19,20 Moreover, the skin of patients with autoimmune diseases (e.g., psoriasis, eczema, vitiligo, or lupus) or who take immunosuppressant drugs is highly photosensitive, and these patients have an especially high risk of skin cancer.5–9,21 Organic chemists have addressed this issue by attaching lipophilic moieties to filters. The ensuing hydrophobic interactions deter water and sweat from washing away an applied sunscreen. Unfortunately, these hydrophobic interactions are not only weak and short-lived, but also lead to undesirable greasiness that diminishes compliance.22
Collagen is the most abundant protein in the human body and the primary component of skin.23 Collagen strands form triple helices that assemble into higher-order structures. Natural collagen contains loops or other interruptions in its triple helix,24–26 and these regions provide binding sites for collagen mimetic peptides (CMPs27–29).30–33 Damaged skin, which is more vulnerable than healthy skin to UV radiation,5–9,21 is likely to contain additional binding sites. In previous work, we used CMPs to anneal pendant dyes and a cytoactive factor to collagen.32,34 We reasoned that that a CMP could likewise and beneficially anchor a pendant UV-filter. Herein, we test that hypothesis and its manifestations.
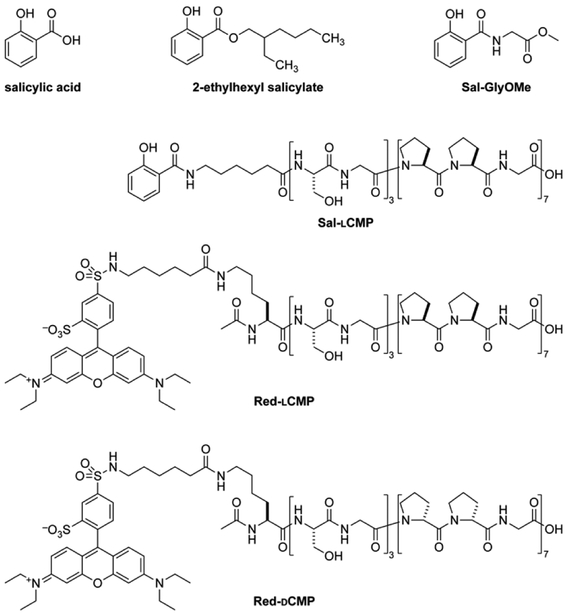
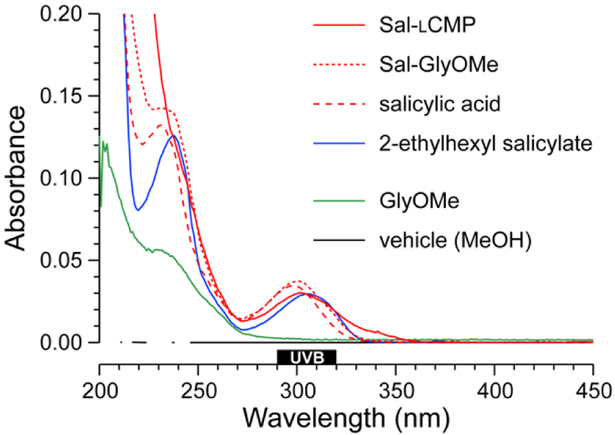
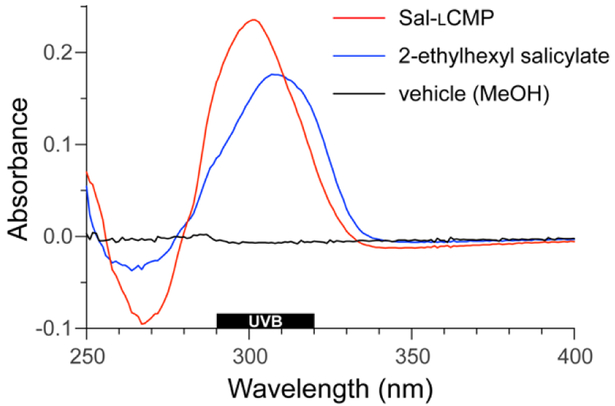
For a proof-of-concept, we selected salicylic acid (Sal) because its 2-ethylhexyl ester is a common ingredient in commercial sunscreens (Fig. 1). We reasoned that the carboxyl group of salicylic acid could be tethered to a CMP via an amide bond. We were aware, however, that the absorbance of a UV-active molecule is sensitive to its substituents. Accordingly, an amide of salicylic acid could have a different absorbance profile than the free acid or 2-ethylhexyl ester. To search for such a perturbation, we synthesized Sal-GlyOMe, which is the glycine methyl ester of salicylic acid. We found that Sal-GlyOMe maintains absorbance of UVB radiation comparable to that of salicylic acid and its 2-ethylhexyl ester (Fig.2).
Fig. 1.
Structures of salicylic acid, its CMP conjugate (Sal-lCMP), and related molecules used in this work.
Fig. 2.
UV spectra of solutions of Sal-lCMP and related analytes (10 µM) in methanol.
Confident that an amide bond does not compromise the UVB absorption of salicylic acid, we synthesized a salicylic acid–CMP conjugate (Sal-lCMP) by segment condensation35 on a solid support. As the collagen segment, we chose (lPro-lPro-Gly)7, which contains only l-proline and glycine residues. This peptide does not form a stable triple helix with itself but does form stable triple helices with natural collagen strands and is not toxic to dermal fibroblast cells.32,34 In the conjugate, the collagen segment is separated from the N-terminal salicylic acid by a 6-aminohexanoic acid spacer (Fig. 1). As with Sal-GlyOMe, we found that the collagen sequence and spacer does not affect the absorbance of UVB radiation (Fig. 2). To create a sensitive probe for collagen binding, we also synthesized conjugates that simply link lCMP or its diastereomer, dCMP, to Rhodamine Red™-X, which is a fluorescent dye (Fig. 1). dCMP contains d-proline residues and cannot form a triple helix with natural collagen strands. (For experimental details on chemical and peptide synthesis, see: Supplementary Information, Section III.)
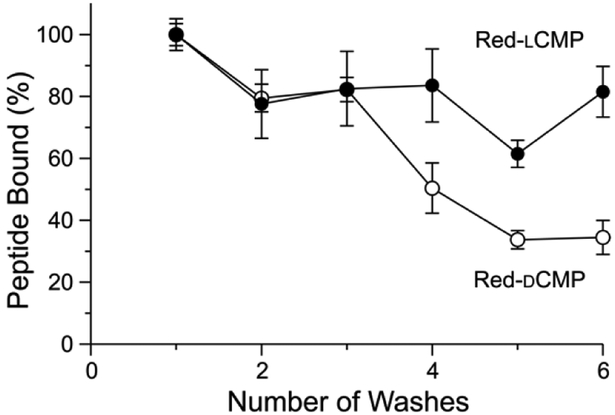
Next, we evaluated the adherence of CMP conjugates to a collagen-laden surface. A solution of Red-lCMP and Red-dCMP were added to collagen-coated wells in a plate. The solution was removed, and adherent Red-lCMP and Red-dCMP were quantified with a plate reader. We found that, as expected, Red-lCMP was retained preferentially compared to Red-dCMP after multiple washes (Fig. 3).
Fig. 3.
Graph showing the adherence of Red-lCMP and Red-dCMP to collagen-coated wells after a series of washes. Fluorescence was measured with ex: 560 nm and em: 580 nm, and values are the mean ± SD from triplicate measurements. (For experimental details, see: Supplementary Information, Section IV.)
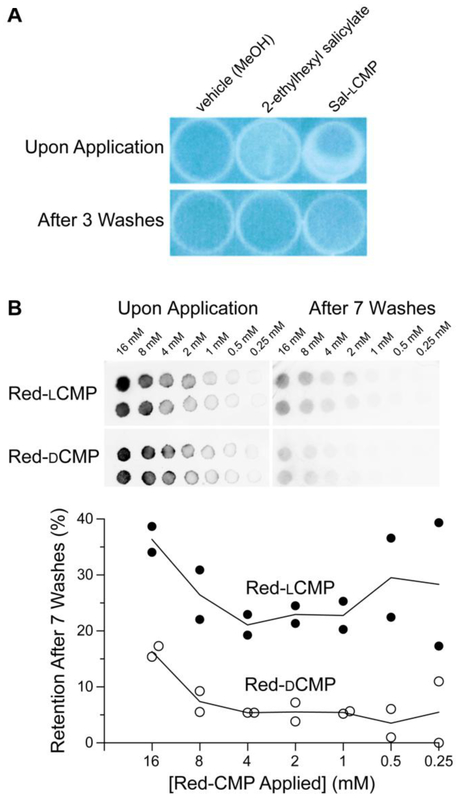
Having demonstrated that an lCMP strand will adhere to a collagen surface, we used cyanotyping to provide a visual readout of UV absorbance by Sal-lCMP. Cyanotyping was invented in 1842 by Sir John Herschel36 and became well-known for its use in photography and in the generation of architectural blueprints. Cyanotyping relies on paper coated with ferric ammonium citrate ((NH4)5Fe(C6H4O7)2) and potassium ferricyanide (K3[Fe(CN)6]). Both of these salts are soluble in water. Upon irradiation with UV light, Fe(III) from the ferric ammonium citrate is reduced to Fe(II). Fe(II) forms an insoluble salt: potassium ferric hexacyanoferrate, which is known as “Prussian blue”.38 The image develops in water, which removes soluble salts. In our experiment, the collagen-coated wells were UV-transparent. Accordingly, the wells were placed between cyanotype paper and a UV light source. Sal-lCMP or 2-ethylhexyl salicylate was added to the wells, which were then exposed to UV light. As the paper under wells containing either Sal-lCMP or 2-ethylhexyl salicylate remained white (Fig. 4A, top), we concluded that these two compounds protected against UV irradiation. After repeated washing, the containing 2-ethylhexyl salicylate appeared to be identical to an untreated well whereas the well containing Sal-lCMP retained protection against UV irradiation (Fig. 4A, bottom).
Fig. 4.
Images showing the adherence of CMP conjugates and related analytes to collagen surfaces, before and after washing. (A) Photographs of cyanotype paper after being covered by collagen-coated wells that had been treated with Sal-lCMP or a related analyte. (B) Top, Inverse-fluorescence images of Vitro-skin® spotted with 5 µL of an aqueous solution of Red-lCMP or Red-dCMP (16→0.25 mM). Bottom, Graph of the data as quantified with ImageJ software.37 For experimental details, see: Supplementary Information, Sections IV–VI.
Next, we tested our strategy with an in vitro skin model. Vitro-skin®, which is formulated with collagen and has been used previously to assess sunscreens.39,40 As with the collagen wells, we applied Red-lCMP and Red-dCMP (here, 80→1.25 nmol) to Vitro-skin® surface to test for adherence. After multiple washes, the lCMP conjugate was retained to a greater extent than was the dCMP conjugate, regardless of the amount applied initially (Fig. 4B).
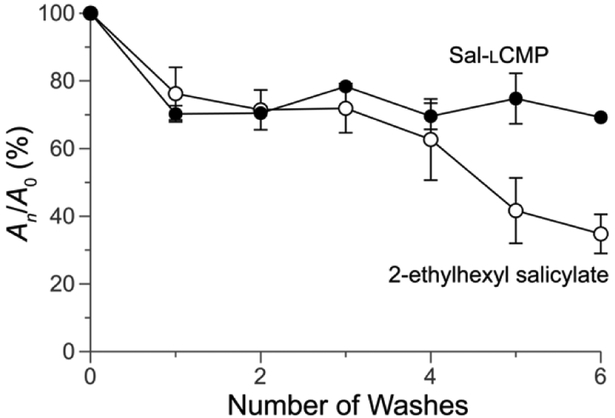
Finally, we sought a direct comparison of the efficacy of Sal-lCMP and the commercial sunscreen, 2-ethylhexyl salicylate. Towards that end, we spread Sal-lCMP and 2-ethylhexyl salicylate on the surface of Vitro-skin®. Then, we obtained the absorption spectra of the surfaces with a solid-state UV-vis spectrometer. Again, we observed comparable UV-vis spectra, showing greatest absorbance in the UVB range (Fig. 5). Next, we tested the longevity of the two sunscreens through a series of washes. The monitoring at 300 nm shows that Sal-lCMP and 2-ethylhexyl salicylate diminishes by 30% after the first wash (Fig. 6). With further washes, Sal-lCMP maintains UV absorption whereas that of 2-ethylhexyl salicylate continues to diminish. This result also indicates that the Sal-lCMP conjugate not only protects against UVB radiation, but also enhances the longevity of that protection compared to 2-ethylhexyl salicylate.
Fig. 5.
Solid-state UV spectra of Vitro-skin® treated with a methanolic solution of Sal-lCMP and 2-ethylhexyl salicylate at 0.14 µmol/cm2.
Fig. 6.
Graph showing the adherence of Sal-lCMP and 2-ethylhexyl salicylate to Vitro-skin®, before and after washing. Absorbance was measured at 300 nm initially (A0) and after a wash (An), and values are the mean ± SD from triplicate measurements. For experimental details, see: Supplementary Information, Section VII.
Conclusions
We conclude that CMPs can anchor a pendant UV-filter on a collagen surface through multiple washes. There, the filter is able to absorb UV light. Hence, the use of a CMP tether merits consideration as a means to endow sunscreens with water resistance. This approach could be especially beneficial to patients with photosensitive skin. In addition, anchoring to collagen could diminish any systemic cytotoxicity of a sunscreen, expediting approval from regulatory agencies. Future applications could benefit from the multimeric display of UV-filters on a single CMP as well as from the use of state-of-the-art UV-filters18,41 and CMPs containing l-fluoroproline residues, which can anneal extremely strongly to natural collagen.32
Supplementary Material
Acknowledgements
We are grateful to Dr. M. M. Vestling for advice and assistance. A.J.E. was supported by Chemistry–Biology Interface Training Grant T32 GM008505 (NIH). This work was supported by Grant R01 AR044276 (NIH).
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic Supplementary Information (ESI) available: Synthetic and analytical procedures. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Jablonski NG, Skin: A Natural History, University of California Press, Berkeley, CA, 2013. [Google Scholar]
- 2.Green A, Williams G, Nèale R, Hart V, Leslie D, Parsons P, Marks GC, Gaffney P, Battistutta D, Frost C, Lang C and Russell A, Lancet, 1999, 354, 723–729. [DOI] [PubMed] [Google Scholar]
- 3.Gasparro FP, Environ. Health Perspect, 2000, 108, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong BK and Kricker A, J. Photochem. Photobiol. B, 2001, 63, 8–18. [DOI] [PubMed] [Google Scholar]
- 5.van der Pols JC, Williams GM, Pandeya N, Logan V and Green AC, Cancer Epidemiol. Biomarkers Prev, 2006, 15, 2546–2548. [DOI] [PubMed] [Google Scholar]
- 6.Moyal DD and Fourtanier AM, J. Am. Acad. Dermatol, 2008, 58, S149–S154. [DOI] [PubMed] [Google Scholar]
- 7.Green AC, Williams GM, Logan V and Strutton GM, J. Clin. Oncol, 2011, 29, 257–263. [DOI] [PubMed] [Google Scholar]
- 8.Hughes MC, Williams GM, Baker P and Green AC, Ann. Intern Med, 2013, 158, 781–790. [DOI] [PubMed] [Google Scholar]
- 9.Darvin ME, Richter H, Ahlberg S, Haag SF, Meinke MC, Le Quintrec D, Doucet O and Lademann J, J. Biophotonics, 2014, 7, 735–743. [DOI] [PubMed] [Google Scholar]
- 10.Dawes JM, Antunes-Martins A, Perkins JR, Paterson KJ, Sisignano M, Schmid R, Rust W, Hildebrandt T, Geisslinger G, Orengo C, Bennett DL and McMahon SB, PLoS One, 2014, 9, e93338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shafie Pour N, Saeedi M, Morteza Semnani K and Akbari J, Pediatr. Rev, 2015, 3, 1–7. [Google Scholar]
- 12.Rogers HW, Weinstock MA, Feldman SR and Coldiron BM, JAMA Dermatol, 2015, 151, 1081–1086. [DOI] [PubMed] [Google Scholar]
- 13.Quist SR, Wiswedel I, Quist J and Gollnick HP, Acta Derm. Venereol, 2016, 96, 910–916. [DOI] [PubMed] [Google Scholar]
- 14.Society AC, Cancer Facts & Figures 2017, American Cancer Society, Atlanta, GA, 2017. [Google Scholar]
- 15.Seite S and Fourtanier AM, J. Am. Acad. Dermatol, 2008, 58, S160–S166. [DOI] [PubMed] [Google Scholar]
- 16.Forestier S, J. Am. Acad. Dermatol, 2008, 58, S133–S138. [DOI] [PubMed] [Google Scholar]
- 17.Jansen R, Osterwalder U, Wang SQ, Burnett M and Lim HW, J. Am. Acad. Dermatol, 2013, 69, 867.e861–867.e814. [DOI] [PubMed] [Google Scholar]
- 18.Mancuso JB, Maruthi R, Wang SQ and Lim HW, Am. J. Clin. Dermatol, 2017, 18, 643–650. [DOI] [PubMed] [Google Scholar]
- 19.Diffey B, Photodermatol. Photoimmunol. Photomed, 2009, 25, 233–236. [DOI] [PubMed] [Google Scholar]
- 20.Diffey B and Osterwalder U, Photochem. Photobiol. Sci, 2017, 16, 1519–1523. [DOI] [PubMed] [Google Scholar]
- 21.Green A, Williams G, Nèale R, Hart V, Leslie D, Parsons P, Marks GC, Gaffney P, Battistutta D, Frost C, Lang C and Russell A, Lancet, 1999, 354, 723–729. [DOI] [PubMed] [Google Scholar]
- 22.Solky BA, Phillips PK, Christenson LJ, Weaver AL, Roenigk RK and Otley CC, J. Am. Acad. Dermatol, 2007, 57, 67–72. [DOI] [PubMed] [Google Scholar]
- 23.Shoulders MD and Raines RT, Annu. Rev. Biochem, 2009, 78, 929–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paterlini MG, Nemethy G and Scheraga HA, Biopolymers, 1995, 35, 607–619. [DOI] [PubMed] [Google Scholar]
- 25.Long CG, Thomas M and Brodsky B, Biopolymers, 1995, 35, 621–628. [DOI] [PubMed] [Google Scholar]
- 26.Leikina E, Mertts MV, Kuznetsova N and Leikin S, Proc. Natl. Acad. Sci. USA, 2002, 99, 1314–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beriso R, De Simone A, Ruggiero A, Improta R and Vitagliano L, J. Pept. Sci, 2008, 15, 131–140. [DOI] [PubMed] [Google Scholar]
- 28.Fields GB, Org. Biomol. Chem, 2010, 8, 1237–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siebler C, Erdmann RS and Wennemers H, Chimia, 2013, 67, 891–895. [DOI] [PubMed] [Google Scholar]
- 30.Miles CA and Bailey AJ, Micron, 2001, 32, 325–332. [DOI] [PubMed] [Google Scholar]
- 31.Mo X, An Y, Yun CS and Yu SM, Angew. Chem. Int. Ed, 2006, 45, 2267–2270. [DOI] [PubMed] [Google Scholar]
- 32.Chattopadhyay S, Murphy CJ, McAnulty JF and Raines RT, Org. Biomol. Chem, 2012, 10, 5892–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chattopadhyay S and Raines RT, Biopolymers, 2014, 101, 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chattopadhyay S, Guthrie KM, Teixeira L, Murphy CJ, Dubielzig RR, McAnulty JF and Raines RT, J. Tissue Eng. Regen. Med, 2014, 10, 1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellison AJ, VanVeller B and Raines RT, Peptide Sci, 2015, 104, 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ware M, Hist. Photogr, 1998, 22, 371–379. [Google Scholar]
- 37.Schneider CA, Rasband WS and Eliceiri KW, Nat. Methods, 2012, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawrence GD and Fishelson S, J. Chem. Educ, 1999, 76, 1199–1200. [Google Scholar]
- 39.Diffey BL, Tanner PR, Matts PJ and Nash JF, J. Am. Acad. Dermatol, 2000, 43, 1024–1035. [DOI] [PubMed] [Google Scholar]
- 40.Stanfield J, Osterwalder U and Herzog B, Photochem. Photobiol. Sci, 2010, 9, 489–494. [DOI] [PubMed] [Google Scholar]
- 41.Bruge F, Tiano L, Astolfi P, Emanuelli M and Damiani E, PLoS One, 2014, 9, e83401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.