Abstract
[(L)CuII(O2•−)]+ (i.e., cupric-superoxo) complexes, as the first and/or key reactive intermediates in (bio)chemical Cu-oxidative processes, including in the monooxygenases PHM and DβM, have been systematically stabilized by intramolecular hydrogen bonding within a TMPA ligand-based framework. Also, gradual strengthening of ligand-derived H-bonding dramatically enhances the [(L)CuII(O2•−)]+ reactivity toward hydrogen-atom abstraction (HAA) of phenolic O−H bonds. Spectroscopic properties of the superoxo complexes and their azido analogues, [(L)CuII(N3−)]+, also systematically change as a function of ligand H-bonding capability.
The activation of molecular oxygen with copper is of considerable interest in nature, as well as in oxidative synthetic procedures and in addressing energy concerns (e.g., fuel-cell O2 reduction catalysts).1 Reduction of O2 with CuI leads to complexes with singly or doubly reduced dioxygen (superoxo or (hydro)peroxo, respectively); further important downstream intermediates (viz. CuII−O•) form following reductive O−O cleavage.2 Various CuI−O2 derived species are suggested to initiate substrate attack leading to O atom incorporation i.e., net hydroxylation in copper monooxygenases such as peptidylglycine-α-hydroxylating monooxygenase (PHM) and dopamine-βmonooxygenase (DβM).3a-e For these cases, along with the dehydrogenation of RCH2OH in galactose oxidase (GO), copper amine oxidase iminosemiquinone H atom abstraction (HAA), and GO cofactor biogenesis (HAA from cysteine S− H),3f a mononuclear cupric superoxide (CuII−O2•−) intermediate is implicated in effecting key H atom abstraction (HAA) chemistry.3b,c,4
Thus, there have been considerable efforts in the design of appropriate synthetic ligands to probe the factors affecting the formation, stabilization, and reactivity of these metastable [LCuII(O2•−)]+ species.2b,c,5 For example, increasing electron donation to Cu,5 providing steric interactions,6 controlling coordination geometry,7 and employing cryogenic conditions and suitable solvents5–8 have each been shown to stabilize the superoxide species and prevent its subsequent reaction with a second equiv of the CuI precursor to yield thermodynamically favored binuclear peroxo-bridged dicopper(II)complexes(Eqs1 and 2).9
| (1) |
| (2) |
H-bonding in the secondary coordination sphere is known to stabilize binuclear [{(L)CuII}2(O22−)]2+ and mononuclear [(L)CuII(OOH)]+ complexes with N3O and N4 ligand architecture.11 Considering extensive recent efforts in studying H-bonding influences on model complexes12 and in biological systems (for example, this plays a crucial role in stabilizing and activating O2-intermediates,13 effecting nitrite reduction,14 and facilitating neural NOS activity15), the importance of H-bonding on [(L)CuII(O2•−)]+ chemistries still remains a fundamental and critical area for study.
In this report, we demonstrate that the imposition of progressively enhancing H-bonding moieties in a series of TMPA-based L−Cu complexes {Figure 1; pKas of related relevant organic groups: pentafluorobenzylamine (~7) < benzylamine (9.3),16 and note that pivalamide and other amides are more acidic than 2° amines} (i) inhibits the formation of corresponding binuclear trans-μ−1,2-peroxo [{(L)CuII}2(O22−)]2+ complex, in favor of monocopper [(L)-CuII(O2•−)]+ species, while (ii) also significantly enhances the HAA reactivity of the superoxo species toward phenolic substrates.
Figure 1.
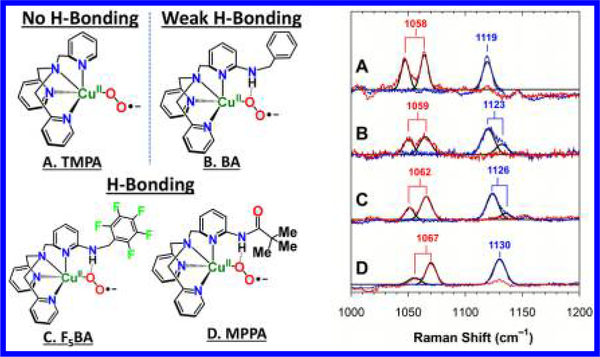
[(L)CuII(O2•−)]+ complexes without (A, L = TMPA; tris(2pyridyl-methyl)amine) and with internal H-bonding substituents (B−D, L = BA, F5BA, and MPPA), and corresponding resonance Raman spectra (λex = 413.1 nm) of complexes A−D in frozen 2methyltetrahydrofuran (MeTHF) solutions prepared with 16O2 (blue) and 18O2 (red). Labels indicate the center of the Fermi doublets. See Supporting Information for tabulation of ν(Cu−O) values.10
When a 0.4 mM solution of [(TMPA)CuI(MeCN)]B(C6F5)4 in MeTHF was subjected to dioxygen bubbling at −135 °C and allowed to equilibrate for 30 min, the resulting mixture consisted oftheknown9 end-on superoxide(i.e.,CuII−O−O•−)complex A {[(TMPA)CuII(O2•−)]+} (63% based on Cu (see Section 4.3(a) in the SI); λmax = 423 nm, ε = 5600 M−1cm−1; 752 nm)10 and the known trans-peroxide dicopper(II) complex [{(TMPA)CuII}2(O22−)]2+ (37%; λmax = 525, 610 nm) (Figure 2).10 With its ligand derived weak H-bonding (vide infra),17 O2-bubbling of [(BA)CuI)]+ significantly increased relative amounts of B {[(BA)CuII(O2•−)]+} (83% (see Section 4.3(b) in the SI); λmax = 418 nm, ε = 4100 M−1 cm−1; 750 nm), compared to [{(BA)CuII}2(O22−)]2+ (17%; λmax = 500, 605 nm).10 However, dramatic stabilization is observed with utilization of F5BA, a new ligand capable of stronger H-bonding than in BA, cupricsuperoxide [(F5BA)CuII(O2•−)]+ (414 nm, ε = 4000 M−1 cm−1; 748 nm), is generated exclusively.10 Employing MPPA,11c,18 possessing the strongest H-bonding moiety, complex D {[(MPPA)CuII(O2•−)]+} is formed. These superoxo species, C and D, are very stable in solution (−135 °C); they are fully formed, and there appears to be no hint of any peroxide analog present.10,19
Figure 2.
UV−vis spectra of oxygenated mixtures of ~0.4 mM of [(L)CuI]B(C6F5)4 (L = TMPA, BA, F5BA, and MPPA) at −135 °C in MeTHF after 30 min. H-bonding stabilizes superoxide species and prevents trans-peroxide formation (see Figures S1−S4).10
This systematic H-bonding enhancement of [(L)-CuII(O2•−)]+ stabilization is further supported by corresponding UV−vis data obtained at [(L)CuI]+ initial concentrations of 0.1 mM (see Figures S5−S8).10 Analogous to synthetic and protein derived oxy-heme adducts (formally FeIII−O2•− species), the primary effect of the H-bonding (e.g., for the distal His residue in hemo- or myoglobins) is to dramatically decrease the rate of O2 dissociation, hence lowering k−1 thereby increasing K1 (Eq 1). With stronger H-bonding, [(L)CuII(O2•−)]+ species becomes systematically more stable across B−D. Without the H-bond in [(TMPA)CuII(O2•−)]+(A), k−1 is significant, more Cu(I) becomes available, and [(L)CuII(O2•−)]+ readily reacts to yield the thermodynamic [{(L)CuII}2(O22−)]2+ product (Eq 2).
Resonance Raman (rR) spectroscopy was used to characterize the effect of H-bonding in the series of superoxide complexes A−D in frozen MeTHF solution. Laser excitation (413.1 nm) of each superoxide complex (~1 mM) revealed two sets of 16/18O isotope-sensitive enhanced modes at around 460 and 1120 cm−1 (Figure 1), which are characteristic of ν(Cu−O) and ν(O−O), respectively (the latter appears as a Fermi doublet (Figure 1), consistent with previous observations21), of end-on cupric superoxides (see Section 5 in the SI for details).5a,c,6a,7,18,21b,c
Interestingly, a systematic trend is observed for the A−D complexes where the ν(O−O)values18 increasedwithenhancing H-bonding ability of the systematically stronger N−H dipoles present across B−D. Analogous to previously reported cases,18,21c we presume the observed incremental trend in ν(O−O) across B−D is caused by enhanced structural vibrational coupling and alignment of increased O−O dipole opposite to the systematically increasing N−H dipole. [(TMPA)CuII(O2•−)]+ (A), without any H-bonding moiety, exhibited the lowest (weakest) O−O stretch as expected.
In parallel, the doubly occupied superoxide πσ* (LMCT donor MO) undergoes more electrostatic stabilization compared to the CuII-dz2 (LMCT acceptor MO) with increasing H-bonding across the A−D [(L)CuII(O2•−)]+ series, which increases the energy of the LMCT (πσ* → CuII-dz2) transition;10 hence, a systematic blue shift is observed in the higher energy LMCT absorption, ranging from 423 to 410 nm across A−D (Figure 2 and Table 1).
Table 1.
Summary of Spectroscopic Features (UV-vis, IR, and rRaman) for [(L)CuII(O2•−)]+ and [(L)CuII(N3−)]+ Complexes
| TMPA | BA | F5BA | MPPA | |
|---|---|---|---|---|
| rR O−O stretch {Δ18O2} (cm−1) | 1119 {−61} | 1123 {−64} | 1126 {−64} | 1130 {−63} |
| λmax CuII−O2•− (nm) | 423 | 418 | 414 | 410 |
| λmax CuII−N3− (nm) | 413 | 402 | 399 | 395 |
| IR N≡N stretch (cm−1) | 2046 | 2049 | 2052 | 2063 |
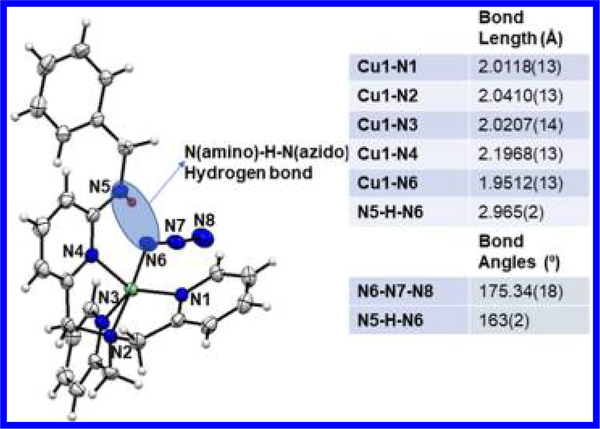
To obtain better insight into the H-bonding in these [(L)CuII(O2•−)]+ complexes, we examined the structures of the azido analogs, [(BA)CuII(N3−)]ClO4, [(F5BA)CuII(N3−)]ClO4, and [F5BA)CuII(OClO3)(acetone)]ClO4 (for syntheses, see the SI).10 Azide ion bound to CuII complexes have been previously used to model both electronic and connectivity structural aspects for coordination of a peroxide dianion.22 With respect to a cupric-superoxide complex, a CuII−N3 moiety would be expected to have a very similar CuII−N−N−N binding mode (e.g., bond distances and the <CuII−N−N angle). The crystal structure of [(BA)CuII(N3−)]+ (Figure 3) experimentally determined via single-crystal X-ray crystallography revealed a weak but clearly observable intramolecular H-bond found between the benzyl amino N−H hydrogen atom and the azido N atom (2.17(2) Å) proximal to the Cu(II) ion. The Namino−H…Nazido distance is 2.965(2) Å with <Namino−H···Nazido = 163(2)°. Similar H-bonding is observed in the F5BA analog mentioned above.10 This interaction, also observed in other CuII(X) (X = N3−, −OOH, or peroxo-Cu) instances with TMPA based ligands possessing internal H-bonding groups,11,23 supports the notion that the H-bonding in complexes B−D occurs to the proximal O atom, as depicted in Figure 1.
Figure 3.
Displacement ellipsoid plot (50% probability level) of the cationic [(BA)CuII(N3−)]+ at 110(2) K.Alist of relevant bond distances and angles is also provided. The H-bonding between the benzylamineand the proximal azide nitrogen is highlighted
To further support our suppositions concerning the Hbonding in these [(L)CuII(O2•−)]+ complexes, we examined the antisymmetric N3− IR stretch (see Section 7 in the SI)10 for thefull series of complexes [(L)CuII(N3−)]+. The characteristic νN−N values (Table 1) increased progressively with H-bonding strength across A−D, presumably due to an increase in the triplebond character of the azido ligand, resulting from removal of electron density from the copper-bound Nazido atom, mirroring the increase in rRaman O−O stretches in A−D. Additionally, the azido → CuII LMCT band shifts progressively to higher energy with increased ligand H-bonding (Table 1, also see Section 7 in the SI),10 consistent with the increasing trend in O2•−→ CuII LMCT energy across A−D.
Elucidation of the oxidative capability and scope of reactivity for [(L)CuII(O2•−)]+ complexes is a major objective. We expected that systematic increased H-bonding to the O2•− should stabilize its π* orbital and also increase its electrophilicity. Indeed, we have found a striking difference in the reactivity toward phenols (electrophilic HAA) with varying O−H bond strengths, across the A−D series.
Reactions with 2,6-di-tert-butyl-4-methoxyphenol (DTBP) withaweak O−Hbond(BDE= 82.0kcal/molinDMSO24) were first investigated. [(TMPA)CuII(O2•−)]+ (A) reacts with a second-order rate constant of 3.11 × 10−1 M−1 s−1 (−135 °C, MeTHF; Figure 4) as determined by monitoring absorbance decrease at 752 nm due to A. [(TMPA)CuIIOOH]+ (~80% yield, see Figure S42) and the corresponding phenoxyl radical were observed, demonstrating a net H atom transfer from phenol to superoxo.5b,10 Importantly the corresponding peroxodicopper(II) complex [{(TMPA)CuII}2(O22−)]2+ is observed to be completely unreactive toward phenols (see Figure S27).10
Figure 4.
(a) UV−vis monitoring of the reaction of A and 2,6-di-tertbutyl-4-methoxyphenol; (b) contrasting rates of oxidation by A−D (k2 values are given in the text); (c) UV−vis spectra of reaction of A with 4methoxyphenol, showing no reaction; (d) reaction of D with 4methoxyphenol and (inset) kinetic trace of the decay of 741 nm band of D. See Section 6 in the SI for details.
The cupric superoxo complexes possessing intramolecular Hbonds (B−D) react more efficiently with DTBP under the same conditions (Figure 4b), with k2 = 5.73 × 10−1 M−1 s−1 (B), 11.47 × 10−1 M−1 s−1 (C), and 9.88 × 10−1 M−1 s−1 (D). In each case, the phenoxy radical was directly observed by both EPR (g ≈ 2.0) and UV−vis (407 nm) spectroscopies (see Section 9.2 in the SI).10 Formation of the hydroperoxo product (86% yield) by C via net HAA from DTBP is also verified by spectrophotometric quantitative analysis of the amount of H2O2 released upon acidification; see Section 9.3 in the SI. Although the spectroscopic data imply that D is the most activated in the series, it is less reactive toward DTBP than C. We presume that this apparent anomaly is due to the larger steric interaction of the tert-butyl group in D, inhibiting bulky substrate approach and decreasing the advantage D should have, with its strongest Hbonding pivalamido group. Notably, C could also exert π−π stacking interactions with DTBP, facilitating a much favorablesubstrate approach compared to D, hence resulting in its slightly enhanced reaction rate.
Further, we tested our [(L)CuII(O2•−)]+ complexes A−D toward 4-methoxyphenol (MP), a substrate with a stronger O− H bond (BDE = 87.6 kcal/mol in DMSO24). [(TMPA)-CuII(O2•−)]+ was completely unreactive toward 250 equiv of MP at −135 °C (Figure 4c). [(BA)CuII(O2•−)]+ (B) exhibited exceedingly slow reactivity (completion took >6 h), and [(F5BA)CuII(O2•−)]+ (C) reacted considerably faster.10 However, the kinetic behavior was complicated in these cases, and the final copper complex product could not be identified, similar to the observation by Tolman and co-workers25 in MP HAA by a CuIII-hydroxide complex. The MP reaction with [(MPPA)CuII(O2•−)]+ (D) was much cleaner and followed clear secondorder decay (k2 = 2.33 × 10−2 M−1 s−1). The corresponding hydroperoxo-Cu(II) product was also observed in UV−vis spectroscopy (Figure 4d). This is the first example of a much stronger phenol being oxidized by a mononuclear cupric superoxide compared to the previously reported cases.
In conclusion, we report for the first time how H-bonding strength in the ligand secondary coordination sphere can be tuned to systematically stabilize cupric superoxide ([LCuII(O2•−)]+) species, control the superoxide−peroxide equilibrium, and concurrently activate the superoxide toward enhanced exogenous O−H substrate oxidation. This was achieved by systematically increasing the electrophilicity of [LCuII(O2•−)]+ via implementation of differing H-bonding moieties. The structures of the [(L)CuII(N3−)]+ analogs suggest that H-bonding occurs to the proximal O-atom in ([LCuII(O2•−)]+) complexes. Further use of H-bonding or other ligand modifications in order to generate cupric−superoxide complexes with greater oxidative capabilities is being explored.
Supplementary Material
∎ ACKNOWLEDGMENTS
The research support of the NIH (DK031450 to E.I.S.; GM28962 to K.D.K.) is gratefully acknowledged.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b04671.
Synthetic and analytical details (methodologies and UV− vis, EPR, NMR, and FT-IR spectra) (PDF)
Crystallographic data for [(BA)CuII(N3)](ClO4) (CIF), [(F5BA)CuI]2 (CIF), [(F5BA)CuII(N3)](OTf) (CIF), and [(F5BA)CuII(CH3COCH3)(OClO3)](ClO4) (CIF)
The authors declare no competing financial interest.
∎ REFERENCES
- (1).McCann SD; Stahl SS Acc. Chem. Res. 2015, 48, 1756. [DOI] [PubMed] [Google Scholar]
- (2).(a) Lee JY; Karlin KD Curr. Opin. Chem. Biol. 2015, 25, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Elwell CE; Gagnon NL; Neisen BD; Dhar D; Spaeth AD; Yee GM; Tolman WB Chem. Rev. 2017, 117, 2059. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Itoh S Acc. Chem. Res. 2015, 48, 2066. [DOI] [PubMed] [Google Scholar]
- (3).(aKlinman JP Chem. Rev. 1996, 96, 2541. [DOI] [PubMed] [Google Scholar]; (b) Klinman JP J. Biol. Chem. 2006, 281, 3013. [DOI] [PubMed] [Google Scholar]; (c) Solomon EI; Heppner DE; Johnston EM; Ginsbach JW; Cirera J; Qayyum M; KieberEmmons MT; Kjaergaard CH; Hadt RG; Tian L Chem. Rev. 2014, 114, 3659. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Whittaker JW Chem. Rev. 2003, 103, 2347. [DOI] [PubMed] [Google Scholar]; (e) Cowley RE; Tian L; Solomon EI Proc. Natl. Acad. Sci. U. S. A. 2016, 113, 12035. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Cowley RE; Cirera J; Qayyum MF; Rokhsana D; Dooley DM;Hedman B; Hodgson KO;Solomon EI J. Am. Chem. Soc. 2016, 138, 13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Prigge ST; Eipper BA; Mains RE; Amzel LM Science 2004, 304, 864. [DOI] [PubMed] [Google Scholar]
- (5).(a) Maiti D; Fry HC; Woertink JS; Vance MA; Solomon EI; Karlin KD J. Am. Chem. Soc. 2006, 129, 264. [DOI] [PubMed] [Google Scholar]; (b) Lee JY; Peterson RL; Ohkubo K; Garcia-Bosch I; Himes RA; Woertink J; Moore CD; Solomon EI; Fukuzumi S; Karlin KD J. Am. Chem. Soc. 2014, 136, 9925. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kim S; Lee JY; Cowley RE; Ginsbach JW; Siegler MA; Solomon EI; Karlin KD J. Am. Chem. Soc. 2015, 137, 2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) Würtele C; Gaoutchenova E; Harms K; Holthausen MC; Sundermeyer J; Schindler S Angew. Chem., Int. Ed. 2006, 45, 3867. [DOI] [PubMed] [Google Scholar]; (b) Fujisawa K; Tanaka M; Morro-oka Y; Kitajima NJ Am. Chem. Soc. 1994, 116, 12079. [Google Scholar]
- (7).Kunishita A; Kubo M; Sugimoto H; Ogura T; Sato K; Takui T; Itoh SJ Am. Chem. Soc. 2009, 131, 2788. [DOI] [PubMed] [Google Scholar]
- (8).Zhang CX; Kaderli S; Costas M; Kim E; Neuhold YM; Karlin KD; Zuberbuhler AD Inorg. Chem. 2003, 42, 1807. [DOI] [PubMed] [Google Scholar]
- (9).(a) Karlin KD; Kaderli S; Zuberbühler AD Acc. Chem. Res 1997, 30, 139. [Google Scholar]; (b) Karlin KD; Wei N; Jung B; Kaderli S; Zuberbuhler AD J. Am. Chem. Soc. 1991, 113, 5868. [Google Scholar]
- (10). See Supporting Information.
- (11).(a) Wada A; Harata M; Hasegawa K; Jitsukawa K; Masuda H; Mukai M; Kitagawa T; Einaga H Angew. Chem., Int. Ed. 1998, 37, 798. [DOI] [PubMed] [Google Scholar]; (b) Yamaguchi S; Masuda H Sci. Technol. Adv. Mater. 2005, 6, 34. [Google Scholar]; (c) Yamaguchi S; Wada A; Funahashi Y; Nagatomo S; Kitagawa T; Jitsukawa K; Masuda H Eur. J. Inorg. Chem. 2003, 42, 4378. [DOI] [PubMed] [Google Scholar]; (d) Yamaguchi S; Kumagai A; Nagatomo S; Kitagawa T; Funahashi Y; Ozawa T; Jitsukawa K; Masuda H Bull. Chem. Soc. Jpn. 2005, 78, 116. [Google Scholar]; (e) Mann SI; Heinisch T; Ward TR; Borovik AS J. Am. Chem. Soc. 2017, 139, 17289. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Dahl EW; Dong HT; Szymczak NK Chem. Commun. 2018, 54, 892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Nagaraju P; Ohta T; Liu J-G; Ogura T; Naruta Y Chem. Commun. 2016, 52,7213. [DOI] [PubMed] [Google Scholar]; (b) Matson EM;Park YJ;Fout AR J. Am. Chem. Soc. 2014, 136, 17398. [DOI] [PubMed] [Google Scholar]; (c) Macbeth CE; Gupta R; MitchellKoch KR; Young VG Jr ; Lushington GH; Thompson WH; Hendrich MP; Borovik AS J. Am. Chem. Soc. 2004, 126, 2556. [DOI] [PubMed] [Google Scholar]; (d) Borovik AS Acc. Chem. Res. 2005, 38, 54. [DOI] [PubMed] [Google Scholar]
- (13).(a) Yang J; Kloek AP; Goldberg DE; Mathews FS Proc. Natl. Acad. Sci. U. S. A. 1995, 92, 4224. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lukin JA; Simplaceanu V; Zou M; Ho NT; Ho C Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 10354. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Arroyo Mañez P; Lu C; Boechi L; Martí MA; Shepherd M; Wilson JL; Poole RK; Luque FJ; Yeh SR; Estrin DA Biochemistry 2011, 50, 3946. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Sheng Y; Abreu IA; Cabelli DE; Maroney MJ; Miller AF; Teixeira M; Valentine JS Chem. Rev. 2014, 114, 3854. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Huang X; Groves JT Chem. Rev. 2018, 118, 2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Merkle AC; Lehnert N Dalt. Trans 2012, 41, 3355. [DOI] [PubMed] [Google Scholar]
- (15).Poulos TL; Li H Acc. Chem. Res. 2013, 46, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).(a) Advanced Chemistry Development (ACD/Labs) Software, V11.02; ACD/Labs, 2018. [Google Scholar]; (b) Hall HK J. Am. Chem. Soc. 1957, 79, 5441. [Google Scholar]; (c) Mareque Rivas JC; Hinchley SL; Metteau L; Parsons S DaltonTrans. 2006, 2316. [DOI] [PubMed] [Google Scholar]
- (17).Kim S; Saracini C; Siegler MA; Drichko N; Karlin KD Inorg.Chem. 2012, 51, 12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Peterson RL; Himes RA; Kotani H; Suenobu T; Tian L; Siegler MA; Solomon EI; Fukuzumi S; Karlin KD J. Am. Chem. Soc. 2011, 133, 1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19). A similar correlation between the H-bonding strength of the ligand and the stability of the superoxo complex is also observed at temperatures above −135 °C, where cupric superoxide adducts with weak or no H-bonding readily transform into the corresponding transperoxide species when compared with strongly H-bonded systems.)
- (20).(a) Momenteau M; Reed CA Chem. Rev. 1994, 94, 659. [Google Scholar]; (b) Birukou I; Schweers RL; Olson JS L. Biol. Chem. 2010, 12, 8840. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Momenteau M; Lavalette DJ Chem. Soc., Chem. Commun 1982, 341. [Google Scholar]
- (21).The O−O stretch was observed as a Fermi resonance in at least one isotopologue of each complex, where ν(O−O) is split by an unenhanced mode with the same symmetry at similar energy. This assignment is supported by the different relative intensities of the doublet between 16Oand 18Oisotopologues, which is expected for Fermi resonance between isotope-sensitive and isotope-insensitive modes. The presence of multiple species is less likely since the ratio in a mixture should be independent of the isotopologue. Fermi resonances have been observed in other cupric superoxides; Ref 18.Woertink JS; Tian L; Maiti D; Lucas HR; Himes RA; Karlin KD; Neese F; Würtele C; Holthausen MC; Bill E; Sundermeyer J; Schindler S; Solomon EI Inorg. Chem. 2010, 49, 9450.Peterson RL; Ginsbach JW; Cowley RE; Qayyum MF; Himes RA; Siegler MA; Moore CD; Hedman B; Hodgson KO; Fukuzumi S; Solomon EI; Karlin KD J. Am. Chem. Soc. 2013, 135, 16454.Cao R; Saracini C; Ginsbach JW; Kieber-Emmons MT; Siegler MA; Solomon EI; Fukuzumi S; Karlin KDJ Am. Chem. Soc. 2016, 138, 7055 The pre-interaction values of ν(O−O) for A−D given in Figure 1 were determined from the intensity-weighted average of the two observed vibrations.
- (22).Pate JE; Ross PK; Thamann TJ; Reed CA; Karlin KD; Sorrell TN; Solomon EI J. Am. Chem. Soc. 1989, 111, 5198. [Google Scholar]
- (23).Wada A; Honda Y; Yamaguchi S; Nagatomo S; Kitagawa T; Jitsukawa K; Masuda H Inorg. Chem. 2004, 43, 5725. [DOI] [PubMed] [Google Scholar]
- (24).Warren JJ; Tronic TA; Mayer JM Chem. Rev. 2010, 110, 6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Dhar D; Yee GM; Markle TF; Mayer JM; Tolman WB Chem. Sci. 2017, 8, 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.