Abstract
Early mobilization of critically ill patients in the Intensive Care Unit (ICU) can prevent adverse outcomes such as delirium and post-discharge physical impairment. To date, no studies have characterized activity of sepsis patients in the ICU using granular actigraphy data. This study characterizes the activity of sepsis patients in the ICU to aid in future mobility interventions. We have compared the actigraphy features of 24 patients in four groups: Chronic Critical Illness (CCI) sepsis patients in the ICU, Rapid Recovery (RR) sepsis patients in the ICU, non-sepsis ICU patients (control-ICU), and healthy subjects. We used a total of 15 statistical and circadian rhythm features extracted from the patients’ actigraphy data collected over a five-day period. Our results show that the four groups are significantly different in terms of activity features. In addition, we observed that the CCI and control-ICU patients show less regularity in their circadian rhythm compared to the RR patients. These results show the potential of using actigraphy data for guiding mobilization practices, classifying sepsis recovery subtype, as well as for tracking patients’ recovery.
I. INTRODUCTION
Sepsis is defined as an inflammatory body response to infection, with severe sepsis and septic shock being its more severe forms (1). Sepsis has a high prevalence rate of up to 30% in the Intensive Care Unit (ICU) (2). Sepsis prevalence has been increasing, possibly due to progressive aging of population, and the existence of more comorbidities (3–5). Sepsis can negatively affect health outcomes in ICU patients, including higher chance of mortality, longer length of stay in the ICU, higher chance of need for specialized care after discharge, and long-term decline in cognitive and functional abilities after discharge (3, 6–10). With higher rates of sepsis in the ICU, there is an increasing population of sepsis survivors that will be dealing with its consequences.
Sepsis patients, who do not die early, can be classified into two recovery subtypes: Chronic Critical Illness (CCI) and Rapid Recovery (RR). CCI type is defined as an ICU length of stay greater than or equal to 14 days with the evidence of persistent organ dysfunction. Patients with ICU length of stay of less than 14 days also qualify for CCI if they are discharged to another hospital, a long-term acute care facility, or to hospice, and demonstrate evidence of organ dysfunction at the time of discharge. Patients who do not meet the criteria for CCI or early death, are classified as RR (11, 12). CCI patients have a greater incidence of secondary infections (11).
Early detection of sepsis can significantly affect patient recovery rate (13–16). Current methods of diagnosis rely on physiological signals such as heart rate and core body temperature or laboratory tests such as procalcitonin (17). Despite these efforts, sepsis is still unrecognized and underreported (18). Besides diagnosing sepsis, identifying sepsis recovery subtypes can also allow for timely interventions that can reduce sepsis duration and severity.
While electronic health records data and several physiological signals have been used for sepsis detection and its risk prediction (19, 20), other information such as functional status and activity level have rarely been examined. Typically, questionnaire-based assessment tools are used for assessing patient activity and functional status in the ICU or after discharge (21, 22). However, these tools often introduce uncertainties such as recall bias and subjectivity. In recent years, actigraphy methods have been used in various studies for continuous, noninvasive, and objective assessment of activity over long periods (23, 24). Continuous and accurate activity measurement in the ICU can also guide mobilization interventions, and lead to improved patient outcomes (25, 26).
In this study, we used actigraphy to characterize activity of sepsis patients in the ICU. We have examined activity patterns of CCI and RR sepsis patients in comparison with both non-sepsis ICU patients and healthy subjects. To our knowledge, this is the first study to use actigraphy data to characterize sepsis recovery subtypes. In addition, we compared the circadian rhythm of CCI and RR sepsis patients with both non-sepsis ICU patients and healthy subjects. Circadian rhythm is important for maintaining health in humans, and can be affected by both sepsis and length of ICU stay. To our knowledge, the effect of sepsis on circadian rhythm of physical activity has not been studied before, since it requires continuous activity measurement. Therefore, we examined patients’ circadian rhythm to understand how sepsis affects the diurnal rhythmicity of physical activity for each sepsis recovery subtype, compared to non-sepsis ICU patients and healthy subjects.
The rest of the paper is as follows: we first explain the dataset description and analysis methods in section II, we will describe the results in section III, and finally we will discuss the results in section IV.
II. Methods
A. Data Collection
We collected actigraphy data from 14 patients admitted to UF Shands hospital ICU between 04/2016–06/2017, and from 10 community-dwelling subjects. All subjects were consented to participate in the study prior to enrollment, and all procedures were approved by the University of Florida Institutional Review Board (IRB). Participants included sepsis ICU patients from both CCI and RR groups, non-sepsis ICU patients, and healthy subjects. The participants wore an ActiGraph GT3X on their dominant wrist. ActiGraph GT3X is an accelerometer unit used for continuous and noninvasive measurement of human physical movement. In this study, we used activity expressed in counts per minute. ICU patients wore the device for the duration of their stay in the ICU, and healthy subjects wore the device for two weeks. Five days of actigraphy data was used for analysis for all participants.
B. Analysis
First, we removed days with non-weartime longer than one hour at a time. We used the vector magnitude activity counts calculated as in Equation (1) for features extraction. We calculated the average and confidence intervals for all patients in each group for visualizing the difference in activity patterns among different groups. We compared CCI and RR activity to both healthy subjects and control-ICU patients to examine the effect of ICU admission on patients’ activity and circadian rhythm.
| (1) |
In Equation (1), x, y, and z are the activity counts in the three Cartesian basis vectors.
We extracted 10 statistical features as well as circadian rhythm features to summarize the 5-day activity data. We used these features to compare the distribution of features among the four participant groups. For features that did not have a normal distribution, we used nonparametric tests to describe the feature distributions, and to assess the differences among groups. All analysis were done using R (version 3.1.3) (27).
To extract the circadian rhythm features, we used a non-linear transformation of the traditional cosine curve, using the anti-logistic function in the sigmoidal family, as in Equation (2), (28). We extracted the following features on the activity’s circadian rhythm from the fitted nonlinear model for each patient: min, alpha, beta, phase, amplitude, and mesor. Min is the minimum value of the fitted model. Phase is the time of day the fitted model’s peak occurs. Mesor is the adjusted mean value. Amplitude is the difference between the minimum and maximum of the fitted model. Alpha determines whether the peaks of the model’s fitted values are wider than their troughs, and beta determines whether the model rises and falls more steeply than a cosine curve. We performed the parameter estimation in two stages. In the first stage, the parameters of the traditional cosine curve were estimated by linear least squares projection of the data onto sine and cosine curves of 24h period. The linear model coefficients were then transformed in a non-linear manner into mesor (estimated by the mean in this case), amplitude and phase. Next, parameters of the extended cosine model were estimated using non-linear least squares, with the starting values of the parameters computed from mesor, amplitude, and phase of the best-fitting cosine curve (28).
| (2) |
III. Results
Table I shows the demographic characteristics of participants in each group. Inclusion criteria included age of 18 years or older. Participants’ age and gender do not differ significantly between groups, except for RR group’s age that was different from healthy subjects. Fig. 1 shows the average and confidence interval of the patients’ activity in all four groups over five days. Healthy subjects’ activity is significantly higher than the other three groups, as expected. Also, the RR group’s activity is higher than both CCI and control-ICU groups. However, CCI group and control-ICU group have similar activity over five days. Another difference between the groups is that healthy subjects have a clear circadian rhythm, while RR subjects have a less distinct circadian rhythm. The CCI and control-ICU subjects do not show any rhythmicity and difference between the daytime and nighttime activity.
TABLE I.
Characteristics of study cohort. Control-ICU: non-sepsis ICU patients, CCI: chronic critical illness, RR: rapid recovery, control-healthy: healthy subjects. N: number of patients. Un: Unavailable. a: Kruskal-Wallis analysis of variance by ranks.
| Variable | Control-ICU, N=3 | CCI, N=5 | RR, N=6 | Control-healthy, N=10 | Pa |
|---|---|---|---|---|---|
| Age, median (25%, 75%) |
68 (59,79) |
63 (51, 69) |
58 (52.3, 64.8) |
67 (66,67.8) |
0.152 |
| Gender, number of female (%) |
2 (0.66) |
2 (0.4) |
4 (0.66) |
4 (0.4) |
0.568 |
| Race, number of white (%) |
3 (100) |
5 (100) |
6 (100) |
Un |
1 |
| Primary Diagnosis Group, number (%) | 0.730 | ||||
| - → Certain infectious and parasitic diseases | 0 (0) | 2 (40) | 2 (33.3) | Un | |
| - → Diseases of the digestive system | 3 (100) | 2 (40) | 2 (33.3) | Un | |
| - → Malignant neoplasms of female genital organs | 0 (0) | 0 (0) | 1 (16.7) | Un | |
| - → Injury, poisoning and certain other consequences of external causes | 0 (0) 0 (0) |
0 (0) 1 (20) |
1 (16.7) 0 (0) |
Un Un |
|
| - → Diseases of the nervous system |
Figure 1.
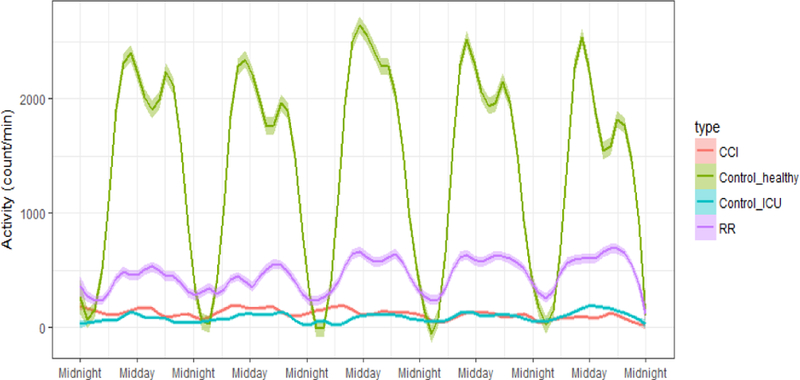
Average activity of four groups of patients –CCI: chronic critical illness patients, RR: Rapid Recovery patients, control-ICU: non-sepsis ICU patients, control-healthy: healthy subjects. For all groups, confidence interval is shown by a transparent band around the average values.
Table II shows the distribution of statistical features for the four groups. All features were statistically different among the four groups (except for the start of 10-hour maximum activity window). The extracted features are different between healthy subjects and all the other groups (Fig.1). Half of the features are statistically different between the RR group and all other groups, with a significant gap between RR group and other groups, as seen in Fig.1. None of the features were statistically different between the CCI and non-sepsis ICU patients (Fig.1).
TABLE II.
Distribution of statistical features extracted from activity data of all groups.
| Variable | Control-ICU, N=3 | CCI, N=5 | RR, N=6 | Control-healthy, N=10 | pa |
|---|---|---|---|---|---|
| Mean of activity of the whole day |
31.1 (22.8, 128)b,d | 140 (83.6, 168)b,d | 428 (313, 551)b,c,e | 1328 (1062, 1742)c,d,e | <0.001 |
| Standard deviation of activity of the whole day |
137 (113, 308)b,d | 346 (249, 386)b,d | 701 (586, 990)b,c,e | 2049 (1663, 2107)c,d,e | <0.001 |
| M10 | 25513 (18571, 113443)b,d | 126226 (67371, 151684)b,d | 317549 (252610, 492412)b,c,e | 1398892 (1083188, 1685662)c,d,e | <0.001 |
| Time of M10 | 437 (369, 572) | 491 (405, 568) | 602 (517, 616) | 513 (418, 580) | 0.585 |
| L5 | 984 (709, 11603)d | 12014 (10729, 17500)b,d | 59283 (43613, 73749)c,e | 23062 (21363, 26593)c | 0.002 |
| Time of L5 | 389 (257, 515)b | 696 (571, 792)b | 298 (166 ,436)b | 49.9 (26.7, 78.2)c,d,e | 0.002 |
| RA | 0.84 (0.82, 0.90) | 0.78 (0.78, 0.86)b | 0.74 (0.68, 0.81)b | 0.96 (0.95, 0.97)c,d | 0.001 |
| RMSSD | 143 (123, 327)b,d | 364 (253, 369)b,d | 760 (593, 912)b,c,e | 1297 (1149, 1447)c,d,e | <0.001 |
| RMSSD/SD | 1.11 (1.09, 1.13)b | 1.05 (1.05, 1.07)b | 0.97 (0.91, 1.06)b | 0.66 (0.62, 0.69)c,d,e | <0.001 |
| Number of immobile minutes | 1239 (1040, 1287)b,d | 1046 (1029, 1102)b,d | 651 (621, 682)b,c,e | 564 (517, 590)c,d,e | <0.001 |
Control-ICU: non-sepsis ICU patients, CCI: chronic critical illness, RR: rapid recovery, control-healthy: healthy subjects. Data are median and interquartile range (25%, 75%) values.
Kruskal-Wallis analysis of variance by ranks
significantly different from control-healthy patients (p<0.01)
significantly different from CCI patients (p<0.05)
significantly different from RR patients (p<0.05)
significantly different from control-ICU patients (p<0.05). N: number of patients. M10: activity over the 10-hour window with maximum activity. L5: activity over the 5-hour window with minimum activity. RA: relative amplitude (defined as). RMSSD: root mean square of sequential of sequential differences. SD; standard deviation.
We also visualized the differences between the circadian rhythm of the four groups’ activity data. Fig. 2 shows the fitted non-linear model for one example patient per each group (other patients are not depicted due to space constraints). As in Fig. 1, the healthy subject and RR patient show a clearer rhythmicity in their activity, compared with the control-ICU and CCI patients. Table III shows the distribution of circadian features for all patients, extracted using a sigmoidally transformed cosine function. Here, only the amplitude feature was significantly different between the four groups (p<0.001).
Table III.
Distribution of statistical features extracted for activity data for all groups.
| Variable | Control-ICU, N=3 | CCI, N=5 | RR, N=6 | Control-healthy, N=10 | pa |
|---|---|---|---|---|---|
| Min | 0.55 (0.39, 1.02) | 0.99 (0.67, 1.06) | 1.94 (0.84, 2.61) | 0.79 (0.49, 1.13) | 0.475 |
| Amplitude | 0.72 (0.44, 1.07)b | 0.77 (0.60, 1.08)b | 0.81 (0.61, 2.43)b | 5.64 (4.97, 6.04)c,d,e | <0.001 |
| Phase | 6.21 (4.71, 10.66) | 13.09 (12.81, 15.71) | 15.41 (13.67, 16.14) | 14.75 (13.78, 15.23) | 0.281 |
| Alpha | −0.13 (−0.15, 0.43)b | 0.20 (−0.65, 0.27) | −0.50 (−0.74, −0.04) | −0.44 (−0.47, −0.35)e | 0.249 |
| Beta | 27.34 (16.02, 291.46) | 51.77 (40.81, 56.69) | 48.13 (18.91, 78.86) | 14.47 (8.16, 33.93) | 0.398 |
Control-ICU: non-sepsis ICU patients, CCI: chronic critical illness, RR: rapid recovery, control-healthy: healthy subjects. Data are median and interquartile range (25%, 75%) values.
Kruskal-Wallis analysis of variance by ranks
significantly different from control-healthy patients (p<0.01)
significantly different from CCI patients (p<0.05)
significantly different from RR patients (p<0.05)
significantly different from control-ICU patients (p<0.05). N: number of patients.
IV. Discussion
We showed that there are significant differences in the activity profiles of CCI and RR sepsis patients during their stay in the ICU. As expected, actigraphy features of CCI, RR, and non-sepsis ICU patients were significantly different from healthy subjects. This concurs with previous non-actigraphy studies of functional status in sepsis and ICU patients. Among the circadian rhythm features, amplitude was the only feature that was significantly different among the four groups. However, a larger population might show a larger difference in the distribution of other circadian rhythm features as well. Differences in physical activity of the patients may become more evident over longer stay in the ICU, and also due to the muscle deterioration caused by sepsis over time. This is the first study that uses actigraphy methods to objectively and continuously measure sepsis patients’ activity during their ICU stay, and to compare it to non-sepsis ICU patients and healthy subjects. The significant differences in actigraphy features show that they can potentially be used for automatic detection of sepsis severity and recovery subtype.
One main limitation of the study was the small number of participants in each group, which might have contributed to inability to capture potential differences in features among the four groups. The small sample size also resulted in a limited age range, with the sample generally consisting of older adult patients. Another limiting factor was that the participants were not matched according to their comorbidity and primary diagnoses. This may contribute to intragroup diversity when unaccounted for. In the future, we will investigate the discriminating power of more diverse actigraphy features within a larger and more diverse population.
Figure 1.
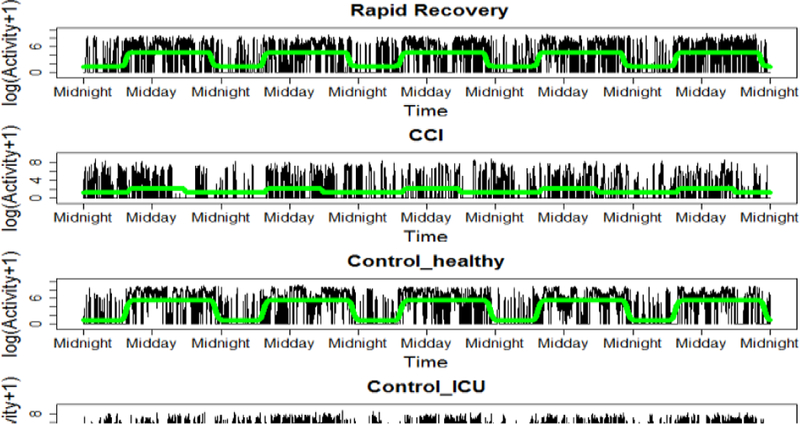
Nonlinear fitted model (green) used for circadian rhythm features extraction, for one patient per group. CCI: chronic critical illness patients, control-ICU: non-sepsis ICU patients, control-healthy: healthy subjects.
Acknowledgment
This work is supported by the following grants: NIH/NIGMS P50 GM111152 (SCB, AB, TOB), NIH/NIA P03 AG056444 (SCB), NIH/NIA P30 AG028740 (PR), and NIH/NIGMS RO1 GM-110240 (PR, AB).
Contributor Information
Anis Davoudi, Biomedical Engineering Department, University of Florida, Gainesville, FL 32611 USA.
Duane B. Corbett, Department of Aging and Geriatric Research, University of Florida, Gainesville, FL 32610, USA
Tezcan Ozrazgat-Baslanti, Department of Medicine, University of Florida, Gainesville, FL 32610 USA.
Azra Bihorac, Department of Medicine, University of Florida, Gainesville, FL 32610 USA.
Scott C. Brakenridge, Department of Surgery, University of Florida, Gainesville, FL 32610 USA
Todd M. Manini, Department of Aging and Geriatric Research, University of Florida, Gainesville, FL 32610, USA
Parisa Rashidi, Biomedical Engineering Department, University of Florida, Gainesville, FL 32611 USA.
References
- 1.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31(4):1250–6. Epub 2003/04/12. doi: 10.1097/01.ccm.0000050454.01978.3b. PubMed PMID: 12682500. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006;34(2):344–53. Epub 2006/01/21. PubMed PMID: 16424713. [DOI] [PubMed] [Google Scholar]
- 3.Nasa P, Juneja D, Singh O. Severe sepsis and septic shock in the elderly: An overview. World Journal of Critical Care Medicine 2012;1(1):23–30. doi: 10.5492/wjccm.v1.i1.23. PubMed PMID: PMC3956061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suarez De La Rica A, Gilsanz F, Maseda E. Epidemiologic trends of sepsis in western countries. Annals of Translational Medicine 2016;4(17):325. doi: 10.21037/atm.2016.08.59. PubMed PMID: PMC5050194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert review of anti-infective therapy 2012;10(6):701–6. doi: 10.1586/eri.12.50. PubMed PMID: PMC3488423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pittet D, Rangel-Frausto S, Li N, Tarara D, Costigan M, Rempe L, et al. Systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock: incidence, morbidities and outcomes in surgical ICU patients. Intensive Care Med 1995;21(4):302–9. Epub 1995/04/01. PubMed PMID: 7650252. [DOI] [PubMed] [Google Scholar]
- 7.Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. Jama 1997;277(13):1058–63. Epub 1997/04/02. PubMed PMID: 9091694. [PubMed] [Google Scholar]
- 8.Rowe T, Araujo KLB, Van Ness PH, Pisani MA, Juthani-Mehta M. Outcomes of Older Adults With Sepsis at Admission to an Intensive Care Unit. Open Forum Infectious Diseases 2016;3(1):ofw010. doi: 10.1093/ofid/ofw010. PubMed PMID: PMC4766385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leibovici L Long-term consequences of severe infections. Clinical Microbiology and Infection 2013;19(6):510–2. doi: 10.1111/1469-0691.12160. [DOI] [PubMed] [Google Scholar]
- 10.Götz T, Günther A, Witte OW, Brunkhorst FM, Seidel G, Hamzei F. Long-term sequelae of severe sepsis: cognitive impairment and structural brain alterations – an MRI study (LossCog MRI). BMC Neurology 2014;14:145-. doi: 10.1186/1471-2377-14-145. PubMed PMID: PMC4105508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stortz JA, Murphy TJ, Raymond SL, Mira JC, Ungaro R, Dirain ML, et al. Evidence for Persistent Immune Suppression in Patients WHO Develop Chronic Critical Illness After Sepsis. Shock 2017. Epub 2017/09/09. doi: 10.1097/shk.0000000000000981. PubMed PMID: 28885387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loftus TJ, Mira JC, Ozrazgat-Baslanti T, Ghita GL, Wang Z, Stortz JA, et al. Sepsis and Critical Illness Research Center investigators: protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open 2017;7(7):e015136 Epub 2017/08/03. doi: 10.1136/bmjopen-2016-015136. PubMed PMID: 28765125; PubMed Central PMCID: PMCPMC5642775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yealy DM, Huang DT, Delaney A, Knight M, Randolph AG, Daniels R, et al. Recognizing and managing sepsis: what needs to be done? BMC Medicine 2015;13:98. doi: 10.1186/s12916-015-0335-2. PubMed PMID: PMC4410741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mok K, Christian MD, Nelson S, Burry L. Time to Administration of Antibiotics among Inpatients with Severe Sepsis or Septic Shock. The Canadian Journal of Hospital Pharmacy 2014;67(3):213–9. PubMed PMID: PMC4071083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joo YM, Chae MK, Hwang SY, Jin S- C, Lee TR, Cha WC, et al. Impact of timely antibiotic administration on outcomes in patients with severe sepsis and septic shock in the emergency department. Clinical and Experimental Emergency Medicine 2014;1(1):35–40. doi: 10.15441/ceem.14.012. PubMed PMID: PMC5052817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coelho FR, Martins JO. Diagnostic methods in sepsis: the need of speed. Revista da Associação Médica Brasileira (English Edition) 2012;58(4):498–504. doi: 10.1016/S2255-4823(12)70236-9. [DOI] [PubMed] [Google Scholar]
- 17.Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med 2006;34(7):1996–2003. Epub 2006/05/23. doi: 10.1097/01.ccm.0000226413.54364.36. PubMed PMID: 16715031. [DOI] [PubMed] [Google Scholar]
- 18.Ellis AL, John J, Kinasewitz GT. Sepsis Remains an Underrecognized and Underreported Condition. CHEST 138(4):393A. doi: 10.1378/chest.10585. [DOI] [Google Scholar]
- 19.Nguyen SQ, Mwakalindile E, Booth JS, Hogan V, Morgan J, Prickett CT, et al. Automated electronic medical record sepsis detection in the emergency department. PeerJ 2014;2:e343. doi: 10.7717/peerj.343. PubMed PMID: PMC3994640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desautels T, Calvert J, Hoffman J, Jay M, Kerem Y, Shieh L, et al. Prediction of Sepsis in the Intensive Care Unit With Minimal Electronic Health Record Data: A Machine Learning Approach. JMIR Medical Informatics 2016;4(3):e28. doi: 10.2196/medinform.5909. PubMed PMID: PMC5065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Khalaf MS, Al Ehnidi FH, Al-Dorzi HM, Tamim HM, Abd-Aziz N, Tangiisuran B, et al. Determinants of functional status among survivors of severe sepsis and septic shock: One-year follow-up. Ann Thorac Med 2015;10(2):132–6. Epub 2015/04/02. doi: 10.4103/1817-1737.150731. PubMed PMID: 25829965; PubMed Central PMCID: PMCPMC4375742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christakou A, Papadopoulos E, Patsaki E, Sidiras G, Nanas S. Functional Assessment Scales in a General Intensive Care Unit. 2013 2013;8(4):7 Epub 2013–09-28. doi: 10.2015/hc.v8i4.552. [DOI] [Google Scholar]
- 23.Kamdar BB, Kadden DJ, Vangala S, Elashoff DA, Ong MK, Martin JL, et al. Feasibility of Continuous Actigraphy in Patients in a Medical Intensive Care Unit. Am J Crit Care 2017;26(4):329–35. Epub 2017/07/03. doi: 10.4037/ajcc2017660. PubMed PMID: 28668919; PubMed Central PMCID: PMCPMC5629184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ, et al. The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behavioral Sleep Medicine 2015;13(sup1):S4–S38. doi: 10.1080/15402002.2015.1046356. [DOI] [PubMed] [Google Scholar]
- 25.Hodgson CL, Berney S, Harrold M, Saxena M, Bellomo R. Clinical review: Early patient mobilization in the ICU. Critical Care 2013;17(1):207-. doi: 10.1186/cc11820. PubMed PMID: PMC4057255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green M, Marzano V, Leditschke IA, Mitchell I, Bissett B. Mobilization of intensive care patients: a multidisciplinary practical guide for clinicians. Journal of Multidisciplinary Healthcare 2016;9:247–56. doi: 10.2147/JMDH.S99811. PubMed PMID: PMC4889100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Development Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for statistical Computing; 2010. [Google Scholar]
- 28.Marler MR, Gehrman P, Martin JL, Ancoli-Israel S. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med 2006;25(22):3893–904. Epub 2005/12/29. doi: 10.1002/sim.2466. PubMed PMID: 16381069. [DOI] [PubMed] [Google Scholar]


