Abstract
Type 2 diabetes is one of the leading pathologies that increases the risk of improper wound healing. Obesity has become a major risk factor for this disease that is now considered to be the 4th highest cause of preventable blindness according to the World Health Organization. The cornea is the most densely innervated structure in the human body and senses even the slightest injury. In diabetes, decreased corneal sensitivity secondary to diabetic peripheral neuropathy can lead to increased corneal abrasion, ulceration, and even blindness. In this study, a diet induced obesity (DIO) mouse model of pre-Type 2 diabetes was used to characterize changes in sensory nerves and P2X7, a purinoreceptor, a pain receptor, and an ion channel that is expressed in a number of tissues. Since our previous studies demonstrated that P2X7 mRNA was significantly elevated in diabetic human corneas, we examined P2X7 expression and localization in the DIO murine model at various times after being fed a high fat diet. Fifteen weeks after onset of diet, we found that there was a significant decrease in the density of sub-basal nerves in the DIO mice that was associated with an increase in tortuosity and a decrease in diameter. In addition, P2X7 mRNA expression was significantly greater in the corneal epithelium of DIO mice, and the increase in transcript was enhanced in the central migrating and peripheral regions after injury. Interestingly, confocal microscopy and thresholding analysis revealed that there was a significant increase in P2X7 distal to the injury, which contrasted with a decrease in P2X7-expressing stromal sensory nerves. Therefore, we hypothesize that the P2X7 receptor acts to sense changes at the leading edge following an epithelial abrasion, and this fine-tuned regulation is lost during the onset of diabetes. Further understanding of the corneal changes that occur in diabetes can help us better monitor progression of diabetic complications, as well as develop new therapeutics for the treatment of diabetic corneal dysfunction.
Keywords: Wound healing, Purinoreceptor P2X7, Corneal sensory nerves, Pre-type 2 diabetes
1. Introduction
Type 2 diabetes is one of the leading pathologies that increase the risk of improper wound repair in many tissues, and obesity, which is a major risk factor for diabetes, accounts for 95% of all new cases each year. The cornea is an excellent model for the study of diabetic wound repair for it is a relatively simple highly innervated avascular tissue, which is oxygenated via diffusion and requires strict homeostatic control to maintain transparency. Repair of a wound involves a number of processes, including cell adhesion, migration, and proliferation, as well as matrix deposition and tissue remodeling (Chi and Trinkaus-Randall, 2013; Stepp et al., 2014). Complications, such as impaired wound healing and decreased corneal sensitivity that is secondary to diabetic peripheral neuropathy, have been detected in the diabetic cornea (Cai et al., 2014). Together, these can lead to increased recurrent corneal abrasions, epithelial fragility, ulceration, and even blindness (Ljubimov, 2017).
The risk of corneal pathologies associated with diabetes has been correlated with the density of sensory nerves, which have a major role in neuropathic pain (Belmonte et al., 2004; Tervo and Moilanen, 2003). The sub-basal nerves form a dense network of which a subset penetrates into the apical cells (Marfurt et al., 2010; Müller et al., 2003; Stepp et al., 2017). In vitro co-culture studies demonstrated that neuronally-released factors facilitate epithelial cell-cell communication; however, this communication was altered when nerves were placed under compromised environments, such as hypoxia (Lee et al., 2014; Oswald et al., 2012). Most recently, there has been strong evidence that corneal epithelial cells provide support for the sensory nerves in a similar way that glial cells do for cranial nerves (Stepp et al., 2017).
The earliest documented response to injury in the corneal epithelium was the release of nucleotides from damaged cells and the subsequent Ca2+ mobilization from and along the wound site (Klepeis et al., 2001). This release of ATP causes activation of specific trans-membrane purinoreceptors (Klepeis et al., 2001; Weinger et al., 2005). The availability and degradation of these active metabolites are subject to control by ectonucleotidases, which play a role in ligand activity, regulation, and receptor activation (Abbracchio et al., 2009). The purinoreceptors, P2X7 and P2Y2, have been identified as major players in the corneal injury response. The G-protein-coupled P2Y receptor causes an increase in intracellular Ca2+ via inositol 1,4,5-trisphosphate receptor-mediated signaling, while the P2X receptors gate ions and ATP from the extracellular environment (Ralevic and Burnstock, 1998). There is a coordination in the regulation of the P2Y2 and P2X7 receptors, with P2Y2-mRNA expression increasing in response to injury and P2X7-mRNA expression decreasing (Minns and Trinkaus-Randall, 2016). Furthermore, the P2X7 receptor undergoes an injury dependent change in localization, which resolves after repair and stratification (Minns et al., 2016).
Unwounded diabetic human corneas express P2X7 mRNA at a significantly higher level than age-matched controls (Mankus et al., 2011). We hypothesize that the P2X7 receptor normally acts by sensing changes at the leading edge of the wound; however, this regulation is altered in the diabetic cornea. Therefore, we placed C57BL/6J mice on a high fat chow diet to induce obesity (DIO), thus creating a pre-Type 2 diabetic murine model. These DIO mice displayed a loss of the prototypical corneal epithelial nerve whorl and expressed significantly higher levels of P2X7 mRNA than controls. In addition, there was a change in P2X7 localization in the epithelium and stroma of wounded corneas. Diffuse localization of P2X7 was detected at the wound edge and nerve integrity changed with time on the enhanced fat diet. Finally, GM130, a marker of the cis-golgi, became diffuse; indicating that trafficking of proteins may be disrupted in the DIO tissue. Further understanding of the corneal changes that occur with diabetes can help us better monitor the progression of diabetic complications, as well as develop new therapeutics for the treatment of diabetic corneal dysfunction.
2. Materials and methods
2.1. Tissue preparation
The research protocol conformed to the standards of the Association for Research in Vision and Ophthalmology for the Use of Animals in Ophthalmic Care and Vision Research and the Boston University IACUC. C57BL/6J mice were obtained at 7.5, 15, and 22 wks of age from Jackson Laboratory (The Jackson Laboratory; Bar Harbor, ME). Control mice (Ctrl) were maintained on the Control Diet D12450B (10 kcal% fat, 3.8 kcal/g), while the type 2 pre-diabetic diet induced obesity (DIO) mice were fed a High Fat Diet (HFD) D12492 (60 kcal% fat, 5.2 kcal/g). Prior to delivery, body weight, blood glucose, and glucose tolerance tests were performed and measurements were obtained from Jackson laboratory (Table 1). The physiological data summary confirms that the DIO mice developed obesity, mild hyperglycemia, dyslipidemia, and impaired glucose tolerance. DIO blood glucose remained elevated at 120 min (mins) at both 8 and 16 weeks. In addition, there was no difference in bone mineral density in DIO mice compared with Control.
Table 1.
Physiological data summary.
| Analysis | 8 Weeks | 16 Weeks | 26 Weeks | |||
|---|---|---|---|---|---|---|
| DIO | Ctrl | DIO | Ctrl | DIO | Ctrl | |
| Mean Weight (grams) | 24 | 22.9 | 40.8 | 32.4 | 49.5 | 36.7 |
| Mean Fat (grams) | 8.4 | 5.3 | 19.5 | 8.8 | 27.8 | 15.2 |
| Triglycerides (mg/dL) | 138 | 119 | 155 | 106 | 152 | 96 |
| HbAlc (NGSP%) | 5.7 | 5.7 | 6.4 | 5.8 | 6.7 | 6.1 |
| 8 weeks | 16 weeks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mins/Mice | 0 | 30 | 60 | 90 | 120 | 0 | 30 | 60 | 90 | 120 | |
| Blood Glucose (mg/dL) [After glucose tolerance test] | DIO | 125 | 500 | 450 | 400 | 400 | 150 | 550 | 525 | 500 | 500 |
| Ctrl | 75 | 375 | 325 | 300 | 275 | 75 | 400 | 275 | 250 | 230 | |
Upon delivery, mice were acclimated for 24 h before the experiment began. This acclimation time was needed, since in preliminary experiments, there was a variability detected in the results that was due to the stress from traveling. At the appropriate time, the mice were euthanized and a minimum of 3 corneas per time point and condition were analyzed.
2.2. Corneal abrasions
To examine localization of P2X7 in response to wound healing, debridement wounds were performed, corneas were prepared for organ culture, and wound healing was assessed after 6 and 12 h, as described (Lee et al., 2014; Minns et al., 2016). Organ culture was used since it maintains the tissue in its proper 3D environment. Briefly, a 1.5-mm epithelial debridement wound was made, eyes were enucleated, and corneas were dissected leaving an intact scleral rim. Corneas were inverted, and the endothelial cavity was filled with Dulbecco’s Modified Eagles medium (DMEM) supplemented with 0.75% low agar. Then, the corneas were placed endothelial-side down into 35 mm2 6-well plates, DMEM media (containing 100μ/ml penicillin and 100 μg/ml streptomycin) was added to reach the sclera, and the corneas were incubated at 37 °C and 5% CO2. To determine extent of wound healing over time, corneas were stained with 1% methylene blue in PBS (pH 7.4) at indicated times after injury (Minns et al., 2016).
2.3. Quantitative real-time polymerase chain reaction (qRT-PCR)
Fifteen-wk old DIO and Control mouse epithelial and stromal mRNA was isolated, as described (Lee et al., 2014; Minns et al., 2016). In brief, the tissue was lysed in RNA lysis buffer, cells were homogenized (Polytron homogenizer) to ensure complete lysis, mRNA was isolated (RNeasy spin column kit: Fisher Scientific, Pittsburgh, PA), and normalized concentrations were reverse transcribed (Lee et al., 2014; Minns et al., 2016). qRT-PCR was carried out using verified Taqman probes (Life Technologies; Grand Island, NY) for target or 18S genes on an ABI 7300 thermal cycler (ThermoFisher; Waltham, MA). Relative expression was determined using the ΔΔCt method (Livak and Schmittgen, 2001) and 18S was used as a housekeeping gene.
2.4. Immunofluorescence
Indirect-immunofluorescent (IF) staining for purinergic receptor P2X7 was performed on mouse corneas (Lee et al., 2014; Minns et al., 2016). Briefly, corneas were fixed in 4% paraformaldehyde, incubated overnight at 4 °C in either anti-P2X7 E1E8T Rabbit mAb (1:200: Cell Signaling Technology; Danvers, MA) or anti-CD45 [#550539 (1:5: B.D. Pharmigen; San Jose CA], washed in phosphate buffered saline (PBS) containing 0.02% Tween solution for 4 h, and then incubated with the appropriate Alexa Fluor secondary antibody (Minns et al., 2016). RAW264.7 cells, a mouse monocytic leukemic cell line (macrophages: ATCC; Manassas, VA), were used as a positive control for CD45.
To stain the corneas for sensory nerves, they were fixed in 4% paraformaldehyde and then further fixed using a methanol fixation protocol (Pal-Ghosh et al., 2016). After methanol fixation, corneas were subjected to a rehydration series with increasing concentrations of Triton X-100 in PBS. After which, they were washed with PBS, blocked with 4% bovine serum albumin (BSA) solution, incubated with anti-Tuj1 (1:200: Cell Signaling Technology) overnight at 4 °C, washed in PBS containing 0.02% Tween solution, blocked again, and incubated with secondary antibody (1:200: Invitrogen; Waltham, MA) at room temperature.
Staining for Cis-Golgi was performed using GM130 (1:100: Abcam; Cambridge,MA) and the FMK-2201 mouse on mouse kit (Cell Signaling, Vector Laboratories, Burlingame, CA). Corneas were washed with PBS and Triton-X (0.1%) prior to blocking using the protocol recommended by the manufacturer. After staining, corneas were imaged for specific proteins compared to the secondary control using confocal microscopy.
2.5. Confocal microscopy
To examine the sensory innervation, butterfly slits were made in whole corneas to facilitate flattening of the tissue. To examine localization of P2X7, CD45, and GM130, corneas were sectioned into radial wedges so that each wedge contained the injury site and the region distal from the wound toward the sclera. All tissues were sandwiched between two glass coverslips and imaged en face from the apical epithelium toward the stroma with either a 20× objective (whole cornea) or 40× oil objective at a 0.5× zoom (radial wedge). Fluorescent gain levels were set using secondary control samples and were not changed, and the pinhole size was kept at 1 Airy Unit across all images. For the whole corneas, nine tiles (3 × 3) were taken of the cornea and the Z-stack ranges of the tiles were set to include the fine apical nerves, which descended posteriorly through the large stromal nerve bundles. Each Z-stack consisted of 115 averaged images/stack or tile. Optimal optical sections were determined at 0.94 nm using the automated function within the Zen-Black software, and this distance was maintained as a constant throughout all images. These images were taken on the Zeiss LSM 700 inverted confocal microscope (Carl Zeiss Microscopy, LLC; Thornwood, NY). The nine individual z-stacks were stitched together with a FIJI/ImageJ plug-in (NIH, Bethesda, MD; http://imagej.nih.gov/ij/), which allowed for the visualization of the majority of the cornea within a single maximum projection image. For presentation, the stitched together maximum projection images were divided into three regions: stromal, sub-basal, and apical.
For the radial wedges, each maximum projection image was taken as a 5-layer Z-stack with each layer being 0.360 μm apart, with a total Z-stack range of 1.44 μm. Within each experiment, image settings were reused to eliminate any changes in data by varying confocal settings. Thus, any differences could be attributed to innate physiological changes caused by the administration of the HFD. These images were taken on the Zeiss LSM 700 inverted confocal microscope (Carl Zeiss) and image analyses was performed using FIJI/ImageJ.
Maximum projection images of the cis-golgi apparatus were acquired with a Zeiss 880 confocal microscope (Carl Zeiss) using the AIRyScan Super-Resolution function. Fluorescent gain levels were set using secondary control samples, images were acquired with a 63× oil objective at a 2× zoom, and the pinhole size was kept at 1 Airy Unit. Images were obtained using ZEN-Black and analyzed using proprietary ZEN software (Carl Zeiss).
2.6. Image analysis
Localization of P2X7 was quantified using the FIJI/ImageJ software program using a common threshold to minimize background noise. Using the polygon selection tool, regions of interest were outlined in 40 μm intervals from 0 to 200 μm starting from the leading edge of the wound. The Area fraction of pixels above the set threshold was measured for each 40 μm region among each layer within the z-stack to acquire values above background. Area fraction values were used to account for the variance in epithelial thickness between samples, thus providing an assessment of P2X7 within each region of interest. These percentages were averaged across the layers in the Z-stack for each 40 μm interval and were analyzed using GraphPad Prism 5 software for statistical analysis (GraphPad Software; San Diego, CA). Qualitative visual representations of Golgi localization were created using ZEN 2 lite software’s 2.5D image display feature.
To measure corneal epithelial and stromal thicknesses, cross-section images perpendicular to the corneal surface were analyzed using ImageJ. The thickness of the epithelial layer (from the apical surface of the epithelial cells to the basal lamina) and the stromal layer (from the basal lamina to Descemet’s membrane) was measured by drawing a region of interest perpendicular to the tangent of the curve of the corneal surface at a given point (ImageJ). Three measurements of epithelial and stromal thickness were taken at different points along each cross-sectional image. This was repeated in 3 different Control and DIO corneas, and the average epithelial and stromal thicknesses were calculated.
Nerve density and morphology were calculated for both Control and DIO corneas using the FIJI/ImageJ software program. Nerve density was measured using the threshold feature with the threshold set to 50–255 to determine values above background. The threshold was maintained throughout all optical sections and across all images. The nine tiles for each cornea were stitched into a single Z-stack compiled. It was then sub-divided morphologically into apical, sub-basal, and stromal regions. Areas of overlap between these layers were excised prior to measuring nerve density (e.g. intervening stromal nerves were removed from Z-stacks of the sub-basal region). A total area of the threshold nerves was measured for each region, which was then divided by the difference of the total area of the Z-stack and the excised sections. These values were compared to the Sholl program in Fiji and found to more accurately represent the data.
To determine nerve fiber diameter in ImageJ, a straight line was drawn perpendicular to a sub-set of nerves within the Z-stack of the sub-basal nerve plexus. The Plot Profile feature was used to graph the pixel intensity along this line, and each peak on the graph represented a nerve that crossed the line. The width of the base of each peak was determined, and all measurements were automatically scaled to μm and then averaged for Control and DIO. This process was performed for 80 Control and 68 DIO nerves in 4 and 3 corneas, respectively.
To evaluate tortuosity of the nerves in the sub-basal region, we defined tortuosity of a nerve as the ratio of the length of the nerve to the straight-line distance from one end of the nerve to the other, otherwise known as Euclidean distance. Using the freehand-line tool in ImageJ, the longest continuous segment of each nerve was manually traced and measured. Then, the straight-line tool was used to measure the Euclidean distance of the traced segment. Finally, the tortuosity of each nerve fiber was calculated and averaged for Control and DIO. This process was performed on over 20 Control and DIO nerve fibers in a minimum of 3 corneas (15 wks).
2.7. Statistical analysis
Values were presented as the mean ± standard error of the mean (SEM) of at least 3 independent experiments. Statistical significance was determined by unpaired, one-tailed Student’s t-test or two-way ANOVA with Sidak’s post hoc test using GraphPad Prism 5. Data was considered statistically significant when p < 0.05.
3. Results
3.1. Characterization of model
As Type 2 diabetes is a serious health risk in the human population, we used the pre-type 2 diabetic model to examine changes in the cornea. By 15 wks, the mice that were fed HFD exhibited oily skin, greatly increased body weight, and greater lethargy than control mice. Glucose tolerance test data revealed that after 8 wks of HFD, the mice had elevated blood glucose levels, and by 22 wks, there was a decrease in the capacity to metabolize glucose in the DIO mice (data sent to us from Jackson Laboratory). These data demonstrate that the DIO model simulates a pre-type 2 diabetic condition, where glucose digestion was impaired (Brownlee, 2005).
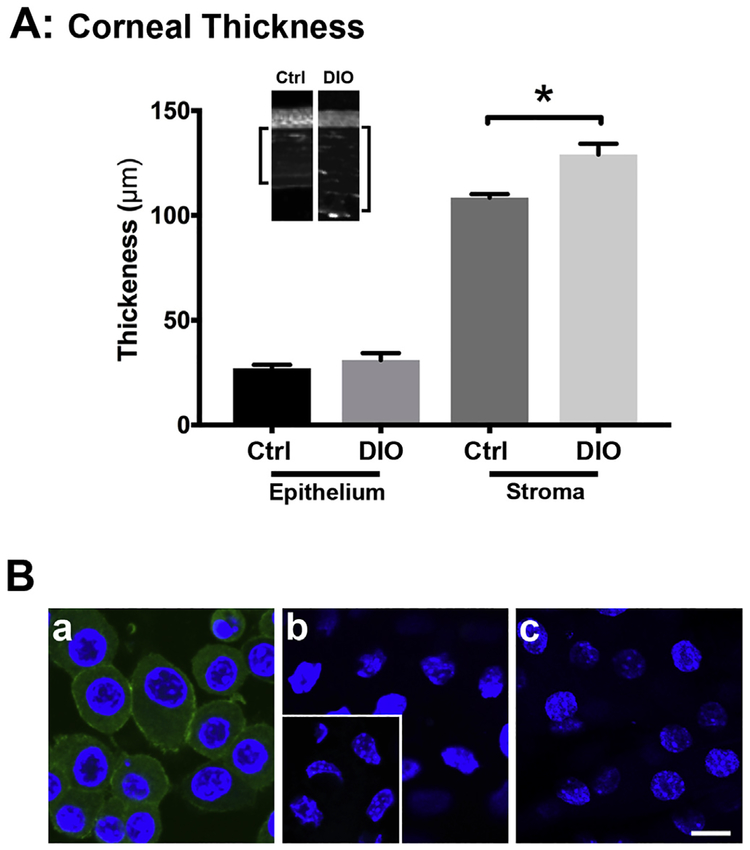
At 15 wks after being placed on HFD, the thickness of DIO and control corneas was compared. As seen in Fig. 1A, there was no significant difference in DIO and control (Ctrl) epithelial thickness; however, the DIO stromas were significantly thicker than control by more than 10% (*p < 0.05). Representative cross-sections of DIO and control corneas were presented (Fig. 1A, inset). To determine if the increased stromal thickness could be attributed to inflammation, corneas were stained for CD45 and compared to macrophages, CD45’s positive control. As seen in Fig. 1B, CD45 was detected in macrophages (Fig. 1B.a), but not detected in either Control or DIO stromal cells (Fig. 1B.b and c, respectively), which were similar to negative control (Fig. 1B.b inset).
Fig. 1. Structural changes in Control and DIO mice analyzed at 15 wks.
A) Graph of corneal thickness of Ctrl (C57BL/6 control) and DIO (Type 2 diabetic) mice. The epithelium and stroma were measured in at least 3 randomly selected corneas. There was no difference in epithelial thickness; however, the stroma of the DIO mice was significantly thicker than Ctrl (*p < 0.05; T-test). B) Representative CD45 indirect immunofluorescence images of a) macrophages (positive control), b) Ctrl mouse stromal cells, and c) DIO mouse stromal cells. CD45-positive staining (green) was present in the macrophages (a); however, it was not observed in the Ctrl or DIO mouse samples (b and c, respectively). No staining was observed in the negative secondary antibody control (b, inset). Bar = 10 μm for all images, including the inset. Blue = DAPI, a nuclear counterstain. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Regional changes in innervation
Nine tiles of optical (3 × 3) were taken en face, and the Z-stacks were stitched together to form one large Z-stack of the entire area of interest. Images were then examined from the apical epithelial nerve endings through the stroma for both DIO and control mice (see Methods). For each eye, corneal nerves in the apical, sub-basal, and stromal regions were examined and maximum projection images were produced for each region (Fig. 2A.a–f). The 3 regions were designated by DAPI staining throughout the cornea (data not shown). In all control mice examined (Fig. 2A.a,c,e), the exquisite organization of the central whorl was present (He and Bazan, 2016; Müller et al., 2003). Variations in the distinct whorl pattern were detected by 7.5 wks in the DIO mice (data not shown); however, in the 15-wk DIO mouse corneas, the integrity of the whorl was lost (Fig. 2A.c,d). By 22 wks, there was no further difference (data not shown). Therefore, a thorough analysis of regional changes in the sensory nerves was performed at the 15-wk time point (Fig. 2A.a–f).
Fig. 2. Regional innervation in Control and DIO mice analyzed at 15 weeks.
Control and DIO eyes were stained with Tuj1 (green) and imaged en face from the apical surface of the cornea through the stroma. A.a-f) En face images represent 9 separate z-stack images stitched together. The 3 regions are subsets of continuous scans and are presented as maximum projection. A.a-b) Apical region, A.c-d) Sub-basal corneal nerve plexus of intraepithelial nerves, and A.e-f) Stromal nerves. A.a-d) Represent a compression of 25 optical sections, and A.e-f) Represent a compression of 50 optical sections. In A.c-d) The corneal nerves form a dense basal plexus in Control (A.c), which is lost in the DIO mice (A.d). Scale bar = 250 μm. B) Graphs of the nerve density in the three regions shown in (A): B.a) Apical, B.b) Sub-basal, and B.c) Stromal. A significant difference in nerve density in the sub-basal intraepithelial nerves was observed, (*p = 0.01; unpaired T-test). N = minimum of 3 independent experiments. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The nerve fibers arise from stromal branches that enter the cornea and then branch in the limbal scleral interface. The density of nerves in each region was analyzed using a number of methods due to fragmentation of the DIO corneal epithelial nerves. The stromal nerves from both the DIO and control mice are thicker than the nerves in the sub-basal and apical regions with each stromal nerve composed of hundreds of axons, while each sub-basal nerve has 15–20 axons. At 7.5 wks in DIO mice, the nerves that would normally form a part of the whorl appeared to be shorter and fragmented in the regional images of the corneas, the nerve density was 30% less dense than control, and there was a high degree of variability (data not shown). By 15 wks in DIO mice (Fig. 2B.a–c), the nerve density in the sub-basal region (Fig. 2B.b) was ~3 times less dense than that of control (*p = 0.01). The apical nerve endings in the control corneas appeared as numerous split endings, as previously described (Müller et al., 2003; Rózsa and Beuerman, 1982). While there is a major change in the maximum projection images of the sub-basal region (Fig. 2A.c–d) that difference was not detected in the apical region (Fig. 2A.a–b) or stromal (Fig. 2A.e–f) regions. Together these data indicate that the fragmentation of sensory nerves occurs when animals are fed the HFD.
We compared the nerves 30 min after an epithelial abrasion was performed in vivo in order to examine for tortuosity and density in the DIO and control corneas. As seen in Fig. 3A and B, maximum projection en face images were obtained of the nerves that extended to the wound edge of the abrasion (Fig. 3A and B; dotted lines indicate wound edge). Tortuosity of nerves (Fig. 3C) and nerve diameter (Fig. 3D) were examined. Nerves from the DIO mice were found to be 6% more tortuous (***p < 0.001) and 20% narrower (***p < 0.001) than those in the control group (Fig. 3C and D).
Fig. 3. Corneal nerves at wound edge of Control and DIO mice analyzed at 15 weeks.
Corneas of Control (A) and DIO (B) mice were wounded and stained with Tuj1 (green) 30 min after wounding. DAPI (blue) was used as a nuclear counterstain. The red-dotted line indicates the wound edge. Corneas were imaged en face, and images are representative maximum projections. Scale bar = 250 μm. C) Graph of Tortuosity, and D) Graph of nerve diameter. Significance was determined using a parametric unpaired t-test assuming equal variance between the two groups (***p < 0.001; T-test). N = a minimum of 3 independent experiments. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Expression and localization of P2X7 in vivo
Investigators have demonstrated that P2X7 is a pain receptor, and we hypothesize that it contributes to the avoidance of noxious stimuli, thereby protecting the eye. Previously, we demonstrated that P2X7 mRNA was expressed in rat trigeminal ganglion extracts, cultured trigeminal nerves and glial cells (Oswald et al., 2012), and human corneal limbal epithelial cells. In addition, we previously demonstrated that there was a 7-fold increase in P2X7 expression in diabetics compared to their age-matched controls (Mankus et al., 2011). The data from our current study agrees with these findings. As seen in Fig. 4, P2X7 mRNA was found to be 7–10-fold greater in DIO mouse corneal epithelium than their age-matched control counterparts after 15 wks of HFD (**p < 0.01).
Fig. 4. P2X7-mRNA expression increased in DIO corneal epithelium.
Graph of P2X7 mRNA from Control and DIO mouse corneal epithelium shows that mice subjected to HFD (DIO) had a greater than 10-fold increase in P2X7-mRNA expression than their age-matched control counterparts. (**p < 0.01; 2-way ANOVA). N = a minimum of 3 independent experiments.
After examining the change in corneal nerves and demonstrating that there was a change in P2X7 mRNA, we asked if there was a change in localization of P2X7 in stromal nerves in vivo. As expected, no significant difference was observed in P2X7-positive stromal nerves between 7.5- and 15-wk controls (Fig. 5B). Neither was there a difference between the 7.5-wk control and DIO stromal nerves (Fig. 5B). However, at 15 wks, there was a significant decrease in P2X7-positive stromal nerves in DIO and control mice (Fig. 5A and B; ***p < 0.001). Together these results indicate that length of time on HFD altered the regulation of P2X7 and could potentially regulate wound repair and cell-cell communication.
Fig. 5. P2X7 stromal nerves decreased with increasing time on a high fat diet.
A) Representative images of Control (A.a) and DIO (A.b) stromal nerves stained with P2X7 at 15 wks. Bar = 250 μm. B) Graph of the P2X7-positive stromal nerve density. By 15 wks on the HFD (DIO), the density of P2X7-positive stromal nerves decreased significantly, as compared with Controls (Ctrl: ***p < 0.001; 2-way ANOVA). N = a minimum of 3 independent experiments.
3.4. Expression and localization of P2X7 ex vivo
To examine the role of the epithelium in the regulation of P2X7, mRNA expression was compared after an epithelial abrasion of 15-wk DIO and control corneas. Corneal organ cultures were incubated for 12 h after injury, and tissue from the wounded and peripheral regions were evaluated for expression, as described (Minns et al., 2016). Previously, we demonstrated that placing corneas in organ culture severed the stromal nerves and the sensory nerves degenerated within 6 h (Stepp et al., 2014). Thus, changes in P2X7 that occur in unwounded compared to wounded corneas reflect differences in corneal epithelial cell P2X7 expression independent of the confounding influence of the sensory nerves, which are no longer present (Fig. 6). For each mouse type (Control and DIO), the unwounded corneas were normalized to 1 and the mRNA expression was compared. As seen in Fig. 6A, there was a significantly higher expression of P2X7 mRNA found in the DIO wounded area, where the cells had migrated post injury, and the peripheral region, which is the area surrounding the wounded area (***p < 0.001). As seen in Fig. 6B, the rate of wound healing was delayed in the DIO corneas. Control corneas healed consistently by 18 h (Fig. 6B.a, arrow indicates wound area), while in a number of experiments, the DIO corneas had not fully healed (76 ± 10% wound closure; Fig. 6B.b, arrow indicates leading edge). Together these data indicate that P2X7 misregulation may be associated with the lack of repair in the DIO model.
Fig. 6. P2X7-mRNA expression was elevated in DIO corneas maintained in organ culture for 12 h.
A) Expression of P2X7 mRNA was determined for epithelial cells that had migrated into the wound area (Wound area) and for the surrounding peripheral area (Periphery) compared to unwounded for Control and DIO (***p < 0.001; 2-way ANOVA). B) 18 h after abrasion, both Control and DIO corneas were stained for P2X7. Cross-section of corneas showed that wound repair was attenuated in the DIO mice (B.b) as compared to Control (B.a). Arrows indicate the healing wound area (B.a) or leading edge (B.b). N = a minimum of 3 independent experiments. Bar = 50 μm.
Immunohistochemical analysis of P2X7 in the intact and wounded corneal epithelium of Control and DIO mice was performed at 7.5, 15, and 22 wks P2X7 localization was consistently more diffuse in the DIO corneas. After 7.5 wks in the unwounded control corneas, P2X7 was present on the apical side of the basal cell nuclei (Fig. 7A), while it was more diffuse in the DIO corneas (Fig. 7B). Surprisingly, there was enhanced staining in the endothelium of the DIO mice. Six hrs after abrasion, P2X7 localization was present in the apical epithelium leading down to the wound edge in Control mice (Fig. 7C; arrow indicates the tip of the leading edge), while it was present throughout the epithelium in the DIO (Fig. 7D; arrow indicates the tip of the leading edge). At 15 and 22 wks, P2X7 was examined at both 6 and 12 h after injury (Fig. 8). P2X7 localization in 15-wk corneas was elevated distal to the leading edge (asterisk) in the DIO corneal epithelium with staining more intense along the apical surface (Fig. 8B,D), as compared with control corneas (Fig. 8A,C). By 22 wks, P2X7 localization remained elevated in the DIO epithelium at 6 h (Fig. 8F), and at 12 h, P2X7 was localized at the leading edge in the DIO mice (Fig. 8H). The increase in overall localization appeared to positively correlate with age-of-mouse and length-of-time spent on HFD.
Fig. 7. P2X7 localization in 7.5-wk-old Control and DIO mice.
Representative P2X7 immunolocalization images of A, B) Unwounded and C,D) Wounded corneas: 6-hrs post-epithelial abrasion wound on Control (A, C) and DIO (B, D) mouse corneas. Arrows indicate the leading edge of the migrating epithelium. N = a minimum of 3 independent experiments. Bar = 50 μm.
Fig. 8. P2X7 localization in 15- and 22-wk-old Control and DIO mice.
Representative immunofluorescent images of corneas stained with P2X7 (A, B, E, and F) 6 h and (C, D, G, and H) 12 h after epithelial abrasion on mice fed a control chow diet (Control) compared with HFD (DIO) for 15 (A–D) or 22 (E–H) wks. Asterisks indicate the leading edge of migrating epithelium. N = a minimum of 3 independent experiments. Bar = 34.68 μm.
To determine if there was a change in P2X7 localization after injury, we examined the corneal epithelium and stroma adjacent and distal to the wound edge. To examine changes in the epithelium, 15- and 22-wk Control and DIO corneal epithelium either 6 or 12 h after wounding were examined by dividing the cross-sectional image of the epithelium into 5 × 40 μm (length) regions of interest (ROI), starting from the tip of the leading edge and moving distally toward the sclera. An example of this measurement is seen in Fig. 9A. The fluorescence area fraction ratio of each ROI was measured and graphed (Fig. 9B). The rapid decrease in fluorescence that was detected in the control was similar to earlier studies (Minns et al., 2016) and was absent in the DIO (Fig. 9B). After 15 wks, there was no significant difference 6 h after abrasion (Fig. 9B.a). At 12 h (Fig. 9B.b), while the fluorescence remained elevated distal from the wound, the values were only significant in the most distal region (*p < 0.05). When the 22-wk-old Control and DIO corneas were abraded and allowed to heal for 6 h (Fig. 9B.c) or 12 h (Fig. 9B.d), the ROIs proximal to the leading edge 12 h after abrasion (Fig. 9B.d) showed significant statistical differences between Control and DIO mice (*p < 0.05, **p < 0.01); however, at both time points (6 and 12 h) for the 22-wk-old samples (Fig. 9B.c,d), the ROIs distal to the leading edge demonstrated elevated P2X7, with the fourth distal region (120–160 μm) at 12 h after abrasion trended toward significance (Fig. 9B.d; p = 0.1). Linear regression analyses were also performed and the correlation with distance was less in the DIO, as predicted. These results suggest that there may be a delayed response in the diabetic model to increase trafficking towards the leading edge. We hypothesize that age and length of time on the HFD do play a role in the demonstrated change in P2X7 localization and trafficking, along with a decreased rate of wound healing.
Fig. 9. P2X7 localization analyzed in the healing epithelium of 15- and 22-wk-old Control and DIO mice.
A) Representative immunofluorescent images of the leading edge of corneal epithelium stained with P2X7 before and after thresholding was applied. The second image demonstrates the 40 μm regions of interest (ROI) used for analysis. B) Epithelium: Graphs of 40 μm ROI analysis in 15- and 22-wk-old Control and DIO mice at 6 and 12 h post-debridement. C) Stroma: Graphs of ROI analysis in 15- and 22-wk-old Control and DIO mice at 12 h post-debridement. Graphs represent calculated average % area above threshold ± SEM. N = a minimum of 3 independent experiments. Asterisks denote statistically significant difference between Control and DIO values within the ROI region (*p < 0.05, **p < 0.01).
After analyzing the significant differences in displayed fluorescence correlated to P2X7 trafficking within the epithelium between Control and DIO mice, we applied similar analyses to test if these results were consistent within the underlying stroma. Utilizing the same thresholding method described above, we compared corneas from both 15-and 22-wk models 12 h after injury at 3 regions behind the leading edge (LE: 0–40 μm, Mid: 80–120 μm, Distal: 160–200 μm). No significant differences between Control and DIO models were detected (Fig. 9C).
3.5. Apical-basal polarity of cis-golgi in DIO mice
To understand if the changes in localization of P2X7 were due to altered protein trafficking, we examined the localization of the cis-golgi in Control and DIO corneas using the antibody GM-130. GM-130 localizes to the cis-golgi, which has been shown to play critical roles in GTPase-signal transduction pathways at the leading edge of migrating cells. A 2.5D qualitative analysis of the images was performed using a LUT intensity (Fig. 10A–C) with the angle of analysis shown in Fig. 10C. The GM-130 demonstrated a classical arching localization over the nucleus in the control (Fig. 10A, arrow). The overall fluorescence in the DIO samples displayed more intense colors, and intense staining was detected at the leading edge on the distal side of the nucleus suggesting altered trafficking (Fig. 10B, arrow indicates leading edge).
Fig. 10. GM130 is diffusely localized in 15-wk-old DIO mice 12 h after corneal abrasion.
Representative images of A) Control (Ctrl) and B) DIO corneas stained with GM130 (green) obtained with a 40× objective from a Zeiss 880 confocal microscope with AIRyScan, presented as 2.5D using a LUT intensity wedge to demonstrate the difference in GM130 localization and intensity. C) Secondary control demonstrating the angle that the tissues were imaged. N = a minimum of 3 independent experiments. DAPI (blue) was used as a nuclear counterstain. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
The diabetic cornea is of high interest since patients exhibit decreased corneal sensitivity, which renders the cornea more vulnerable to trauma. Furthermore, diabetic blindness has become a national public health issue with the enhanced prevalence of Type 2 diabetes in the human population (W.H.O). In addition, these patients present with bilateral changes that correlate with progression of corneal sensory loss and somatic neuropathy (Davidson et al., 2012; Kalteniece et al., 2018; Malik et al., 2005; Rosenberg et al., 2000). Interestingly, these changes are even seen in patients with impaired glucose tolerance not meeting the criteria for Type 2 diabetes mellitus.
Previously, wound healing in diabetics was thought to be compromised in part by an altered composition of the basal lamina (Ljubimov, 2017). More recently, investigators have shown that it is more complicated and that the corneal nerve fibers and the surrounding epithelium are required to maintain a healthy cornea that promotes wound healing (Müller et al., 2003). To examine changes in a controlled manner, a number of models have been developed to study Type 1 and 2 diabetes that range from chemical induction by streptozotocin to a high fat diet (HFD), which is a natural inducer of obesity and causes a decrease in glucose tolerance (Davidson et al., 2014; Yorek et al., 2015). The latter is the model for pre-Type 2 diabetes.
In the present study, we reported changes in the cornea that occurred over time in C57BL/6J mice after being exposed to HFD causing diet induced obesity (DIO) and pre-type 2 diabetes. The DIO mice exhibited significantly thicker stromas than control mice, and negligible CD45 fluorescence in the stromas compared to positive control macrophages, suggesting that the difference was due to changes in hydration, not cellular infiltration. While the DIO mice were leptin responsive, we did not detect changes in the epithelium. However, our preliminary studies demonstrated that there was less protein obtained from DIO corneal epithelium.
In contrast, the ob/ob mice, which are deficient in leptin, have both a thicker epithelium and abnormal intracellular spaces (Ottaway et al., 2015; Ueno et al., 2014). While most previous studies did not differentiate between epithelial and stromal thickness, a number of them reported an increase in corneal thickness. These studies postulated that it was correlated with the duration of diabetes and hemoglobin A1c levels (El-Agamy and Alsubaie, 2017; Su et al., 2008).
The confocal imaging of whole corneas revealed that corneal nerves of 15-wk-old mice fed HFD had decreased nerve density in the region of the sub-basal nerve plexus resulting in a deterioration of the central whorl seen in corneas from non-diabetic mice. However, there was no significant difference in apical or stromal nerve density. However, we did consistently detect increased background Tuj1 stain speckling in the diabetic stroma, which may be an indicator of damaged or dying nerves in diabetic corneas. In addition, there was a significant increase in nerve tortuosity and decreased nerve diameter in the diabetic sub-basal nerve plexus.
These analyses complement the in vivo corneal microscopy (IVCM) performed on humans for assessment of corneal nerve status (Tervo et al., 2016). Their research demonstrates that with IVCM the resolution of the corneal nerves is more limited and a significant proportion of the sub-basal and anterior stromal corneal nerve fibers may be missed, indicating the importance of histochemical studies. Therefore, assessing the status of epithelial nerve terminals, whose morphology is associated with the sensory modality of their parent axons, is difficult with IVCM. Other investigators have added to the knowledge of sensory nerves by comparing the central and inferior regions by IVCM (Kalteniece et al., 2018).
Recent studies on human corneal nerve thickness are contradictory and include reports of both decreased (Brines et al., 2018; Ziegler et al., 2014) and increased (Mocan et al., 2006) nerve diameter. Our results of decreased corneal nerve diameter in the murine model correspond with those of Ziegler and colleagues (Ziegler et al., 2014) and with those demonstrating decreased nerve diameter of unmyelinated sural nerves in diabetes (Malik et al., 2005). In addition, Brines reported a decrease in the number and complexity of nerve fiber bundles in severe diabetic neuropathy (Brines et al., 2018). Since corneal nerves in the sub-basal nerve plexus are unmyelinated, the results on sural nerves may suggest a common pathological cause of decreased nerve integrity in diabetes.
We examined tortuosity of the nerves in this region using methods suggested by investigators (Grisan et al., 2008; Lagali et al., 2015). These take into account the number of turns, the amplitude of each turn and the overall curve of the nerve. However, as it is unknown how short-range and long-range tortuosity [as defined by Lagali (Lagali et al., 2015)] each relate to nerve pathology, we used the arc/cord measurement of tortuosity (Grisan et al., 2008) that takes into consideration both subtypes of tortuosity. Any inaccuracy of measurement in our study should then be mitigated by the inclusion of nerves with an apparent un-curved path. Interestingly, the early studies of nerve crush injury (Kawabuchi et al., 1998) suggest that increased nerve tortuosity may reflect nerve regeneration. The underlying hypotheses from their work suggest that tortuosity is indicative of nerve regeneration along with ongoing nerve damage. This hypothesis is worth further investigation as the corneal nerves are continually regenerating, and we believe there may be a potential mis-regulation in the balance of regeneration and degeneration.
While a decrease in apical nerve density has been reported to accompany and even precede sub-basal nerve plexus decrease in diabetes (Davidson et al., 2012), we found no statistical difference in apical nerve terminal density between the two groups at 15 or 22 wks. This incongruence seems counterintuitive, as one would predict a decrease in the sub-basal plexus to result in decreased density of apical endings. Cai (Cai et al., 2014) used three-dimensional imaging and found that the nerves initially lost in the diabetic sub-basal region were non-branching fibers, and these observations support our results in the murine model, indicating that a substantial sub-basal nerve plexus loss would be required before a significant apical loss would be detected. Recently, IVCM studies indicated changes in specific regions of the cornea were correlated with regional neuropathy (Kalteniece et al., 2018), suggesting that the loss in corneal nerves in diabetic patients occurred at the inferior whorl compared to the central cornea. Studies from our model did not detect a similar difference in pattern, but the enhanced detail of the nerves detected with histochemical and confocal analyses may explain this difference (Tervo et al., 2016). Earlier studies on the sub-basal nerve plexus indicated that a density loss was not associated with sensory loss (Rosenberg et al., 2000). Corneal sensation is initially transmitted by apical fibers (Ivanusic et al., 2013) and, as we have shown, these fibers remained unchanged. As our study did not measure changes in sensitivity over time, we are unable to determine whether our results represent changes prior to the onset of symptomatic neuropathy. Moreover, the study of pain is complicated as it relates to centralization of the sensory signals, which are transmitted to the brain and remain “on” whether the nerves are there or not. It is speculated that time is required to stop these brain derived pain signals.
Previously, in vitro studies suggested that the intact sensory nerves provide trophic support to corneal epithelial cells through the release of neurotransmitters and neuropeptides. In fact, co-culture studies demonstrated that calcium mobilization of corneal epithelial cells was significantly different when the epithelium was exposed to nerve culture medium and the nerves were exposed to hypoxic conditions (Lee et al., 2014). These findings are supported by early studies suggesting that impaired corneal innervation, demonstrated in diabetes, was due to diminished trophic support (García-Hernández et al., 2011). Additional studies by Xu and colleagues demonstrated that corneas incubated in the presence of high glucose exhibited diminished signaling through the epidermal growth factor pathway (Xu et al., 2009). As the latter studies were performed in organ culture, nerves were absent and their studies support the recent hypothesis proposed by Stepp and colleagues that the corneal epithelium has a supportive role for nerves and act as glia (Stepp et al., 2017).
One major change that we detected previously was a significant upregulation in P2X7 in corneal epithelial samples from diabetics compared to age-matched controls (Mankus et al., 2011). Preliminary studies from corneal epithelium of streptozotocin mice also showed a similar increase. P2X7 is both a pain receptor and a channel protein that is expressed by corneal epithelium and nerves and is activated by ATP (Abbracchio et al., 2009; Mankus et al., 2011; Oswald et al., 2012). Elevation of P2X7 has been detected in human fibroblasts (Solini et al., 2000), and patients with Type 2 diabetes exhibit an increase in P2X7 on peripheral blood mononuclear cells compared to controls (García-Hernández et al., 2011). Together these data led us to examine changes over time in the corneal epithelium of DIO mice compared to control. In control tissue, the distinct localization of P2X7 at the leading edge after injury was thought to be due to the role of purinergic receptors in promoting rapid cytoskeletal rearrangements at the leading edge (Minns et al., 2016). One hypothesis for the change in localization of the pore protein to the leading edge after injury was that it could act as a sensor. For example, we have preliminary data in the DIO mice indicating that the localization of Pannexin1, which serves as a passageway for ATP to the exterior, was markedly decreased. As these 2 proteins have been shown to associate in vitro, the misregulation of both may alter localization and repair.
Given that the localization of P2X7 was less distinct and more widespread throughout the epithelial tissue behind the wound in DIO models that are subject to HFD, we hypothesized that trafficking of the purinergic receptor may be less efficient in diabetics. The length of time spent on the HFD appeared to correlate with a more profound difference in the localization of P2X7 behind the leading edge of the wound. The changes in the pore proteins are associated with striking changes in the localization of GM-130, which localizes to the cis-Golgi. However, an alternative hypothesis that there is a change in the glycosylation of P2X7 has not been addressed. Further studies are warranted as altered glycosylation has been detected in diabetic corneas (McDermott et al., 2003). The changes found in DIO mice may suggest that the epithelial cells are attempting to compensate for the loss of innervation by enhancing the overall expression and localization of the receptor.
In summary, the elevation of P2X7 in diabetic patients (Mankus et al., 2011) is also present in a natural pre-diabetic murine model. While we still don’t understand how its misregulation retards migration, the elevated protein may provide a way of targeting tissue at early stages of disease. The ideal experiments of the future could also employ imaging technologies to modulate cell communication at different points of nerve fragmentation in diabetics to study how the neuropathy affects wound healing.
Acknowledgements
The authors would like to thank Drs. Andrew Taylor of Boston University School of Medicine and James Zieske of Schepens Eye Research for helpful discussions, antibody to CD45 and critical review of the manuscript. The authors would also like to thank the critical review and editorial work of Audrey Hutcheon, BS.
Funding
This work was supported by the National Institutes of Health [EY06000 (V.T-R)], gs3:Massachusetts Lions Eye Research Fund and the New England Corneal Transplant Fund to Boston University.
Abbreviations:
- P2X7
purinoreceptor P2X7
- P2Y2
purinoeceptor P2Y2
- ROI
Region of interest
- Ca2+
calcium
- DIO
diet induced obesity
- HFD
high fat diet
- PBS
phosphate buffered saline
- ATP
Adenosine triphosphate
- BSA
bovine serum albumin
- DMEM
Dulbecco’s modified eagles medium
- wk
week
- hr
hour
- Tuj1
tubulin beta III
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.exer.2018.06.001.
Conflicts of interest
The authors declare no competing or financial interests.
References
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H, 2009. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 32, 19–29. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Acosta MC, Gallar J, 2004. Neural basis of sensation in intact and injured corneas. Exp. Eye Res. 78, 513–525. [DOI] [PubMed] [Google Scholar]
- Brines M, Culver DA, Ferdousi M, Tannemaat MR, van Velzen M, Dahan A, Malik RA, 2018. Corneal nerve fiber size adds utility to the diagnosis and assessment of therapeutic response in patients with small fiber neuropathy. Sci. Rep 8, 4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M, 2005. The pathobiology of diabetic complications: a unifying mechanism.Diabetes 54, 1615–1625. [DOI] [PubMed] [Google Scholar]
- Cai D, Zhu M, Petroll WM, Koppaka V, Robertson DM, 2014. The impact of type 1 diabetes mellitus on corneal epithelial nerve morphology and the corneal epithelium. Am. J. Pathol 184, 2662–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi C, Trinkaus-Randall V, 2013. New insights in wound response and repair of epithelium. J. Cell. Physiol 228, 925–929. [DOI] [PubMed] [Google Scholar]
- Davidson EP, Coppey LJ, Kardon RH, Yorek MA, 2014. Differences and similarities in development of corneal nerve damage and peripheral neuropathy and in diet-induced obesity and type 2 diabetic rats. Invest. Ophthalmol. Vis. Sci 55, 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EP, Coppey LJ, Yorek MA, 2012. Early loss of innervation of cornea epithelium in streptozotocin-induced type 1 diabetic rats: improvement with Ilepatril treatment. Invest. Ophthalmol. Vis. Sci 53, 8067–8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agamy A, Alsubaie S, 2017. Corneal endothelium and central corneal thickness changes in type 2 diabetes mellitus. Clin. Ophthalmol 11, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Hernández MH, Portales-Cervantes L, Cortez-Espinosa N, Vargas-Morales JM, Fritche Salazar JF, Rivera-López E, Rodríguez-Rivera JG, Quezada-Calvillo R, Portales-Pérez DP, 2011. Expression and function of P2X(7) receptor and CD39/Entpd1 in patients with type 2 diabetes and their association with biochemical parameters. Cell. Immunol 269, 135–143. [DOI] [PubMed] [Google Scholar]
- Grisan E, Foracchia M, Ruggeri A, 2008. A novel method for the automatic grading of retinal vessel tortuosity. IEEE Trans. Med. Imaging 27, 310–319. [DOI] [PubMed] [Google Scholar]
- He J, Bazan HEP, 2016. Neuroanatomy and neurochemistry of mouse cornea. Invest. Ophthalmol. Vis. Sci 57, 664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanusic JJ, Wood RJ, Brock JA, 2013. Sensory and sympathetic innervation of the mouse and Guinea pig corneal epithelium. J. Comp. Neurol 521, 877–893. [DOI] [PubMed] [Google Scholar]
- Kalteniece A, Ferdousi M, Petropoulos I, Azmi S, Adam S, Fadavi H, Marshall A, Boulton AJM, Efron N, Faber CG, Lauria G, Soran H, Malik RA, 2018. Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Sci. Rep 8, 3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabuchi M, Chongjian Z, Islam AT, Hirata K, Nada O, 1998. The effect of aging on the morphological nerve changes during muscle reinnervation after nerve crush. Restor. Neurol. Neurosci 13, 117–127. [PubMed] [Google Scholar]
- Klepeis VE, Cornell-Bell A, Trinkaus-Randall V, 2001. Growth factors but not gap junctions play a role in injury-induced Ca2+ waves in epithelial cells. J. Cell Sci 114, 4185–4195. [DOI] [PubMed] [Google Scholar]
- Lagali N, Poletti E, Patel DV, McGhee CNJ, Hamrah P, Kheirkhah A, Tavakoli M, Petropoulos IN, Malik RA, Utheim TP, Zhivov A, Stachs O, Falke K, Peschel S, Guthoff R, Chao C, Golebiowski B, Stapleton F, Ruggeri A, 2015. Focused tortuosity definitions based on expert clinical assessment of corneal subbasal nerves. Invest. Ophthalmol. Vis. Sci 56, 5102–5109. [DOI] [PubMed] [Google Scholar]
- Lee A, Derricks K, Minns M, Ji S, Chi C, Nugent MA, Trinkaus-Randall V, 2014. Hypoxia-induced changes in Ca(2+) mobilization and protein phosphorylation implicated in impaired wound healing. Am. J. Physiol. Cell Physiol 306, C972–C985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, 2017. Diabetic complications in the cornea. Vis. Res 139, 138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik RA, Tesfaye S, Newrick PG, Walker D, Rajbhandari SM, Siddique I,Sharma AK, Boulton AJM, King RHM, Thomas PK, Ward JD, 2005. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia 48, 578–585. [DOI] [PubMed] [Google Scholar]
- Mankus C, Rich C, Minns M, Trinkaus-Randall V, 2011. Corneal epithelium expresses a variant of P2X(7) receptor in health and disease. PLoS One 6, e28541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF, Cox J, Deek S, Dvorscak L, 2010. Anatomy of the human corneal innervation. Exp. Eye Res 90, 478–492. [DOI] [PubMed] [Google Scholar]
- McDermott AM, Xiao TL, Kern TS, Murphy CJ, 2003. Non-enzymatic glycation in corneas from normal and diabetic donors and its effects on epithelial cell attachment in vitro. Optometry 74, 443–452. [PubMed] [Google Scholar]
- Minns MS, Teicher G, Rich CB, Trinkaus-Randall V, 2016. Purinoreceptor P2X7 regulation of Ca(2+) mobilization and cytoskeletal rearrangement is required for corneal reepithelialization after injury. Am. J. Pathol 186, 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minns MS, Trinkaus-Randall V, 2016. Purinergic signaling in corneal wound healing: a tale of 2 receptors. J. Ocul. Pharmacol. Ther 32, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocan MC, Durukan I, Irkec M, Orhan M, 2006. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea 25, 769–773. [DOI] [PubMed] [Google Scholar]
- Müller LJ, Marfurt CF, Kruse F, Tervo TMT, 2003. Corneal nerves: structure, contents and function. Exp. Eye Res 76, 521–542. [DOI] [PubMed] [Google Scholar]
- Oswald DJ, Lee A, Trinidad M, Chi C, Ren R, Rich CB, Trinkaus-Randall V, 2012. Communication between corneal epithelial cells and trigeminal neurons is facilitated by purinergic (P2) and glutamatergic receptors. PLoS One 7, e44574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaway N, Mahbod P, Rivero B, Norman LA, Gertler A, D’Alessio DA, Perez-Tilve D, 2015. Diet-induced obese mice retain endogenous leptin action. Cell Metab 21, 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Ghosh S, Pajoohesh-Ganji A, Tadvalkar G, Kyne BM, Guo X, Zieske JD, Stepp MA, 2016. Topical Mitomycin-C enhances subbasal nerve regeneration and reduces erosion frequency in the debridement wounded mouse cornea. Exp. Eye Res 146, 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G, 1998. Receptors for purines and pyrimidines. Pharmacol. Rev 50, 413–492. [PubMed] [Google Scholar]
- Rosenberg ME, Tervo TM, Immonen IJ, Müller LJ, Grönhagen-Riska C,Vesaluoma MH, 2000. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest. Ophthalmol. Vis. Sci 41, 2915–2921. [PubMed] [Google Scholar]
- Rózsa AJ, Beuerman RW, 1982. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain 14, 105–120. [DOI] [PubMed] [Google Scholar]
- Solini A, Chiozzi P, Falzoni S, Morelli A, Fellin R, Di Virgilio F, 2000. High glucose modulates P2X7 receptor-mediated function in human primary fibroblasts. Diabetologia 43, 1248–1256. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Tadvalkar G, Hakh R, Pal-Ghosh S, 2017. Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves. Glia 65, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA, Zieske JD, Trinkaus-Randall V, Kyne BM, Pal-Ghosh S, Tadvalkar G, Pajoohesh-Ganji A, 2014. Wounding the cornea to learn how it heals. Exp. Eye Res 121, 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su DHW, Wong TY, Wong W-L, Saw S-M, Tan DTH, Shen SY, Loon S-C, Foster PJ, Aung T, Singapore Malay Eye Study, G, 2008. Diabetes, hyperglycemia, and central corneal thickness: the Singapore Malay Eye Study. Ophthalmology 115, 964–968 e961. [DOI] [PubMed] [Google Scholar]
- Tervo T, Holopainen J, Belmonte C, 2016. Confocal microscopy of corneal nerves-a limited but still useful technique to evaluate peripheral neuropathies. JAMA Ophthalmol 134, 990–991. [DOI] [PubMed] [Google Scholar]
- Tervo T, Moilanen J, 2003. In vivo confocal microscopy for evaluation of wound healing following corneal refractive surgery. Prog. Retin. Eye Res 22, 339–358. [DOI] [PubMed] [Google Scholar]
- Ueno H, Hattori T, Kumagai Y, Suzuki N, Ueno S, Takagi H, 2014. Alterations in the corneal nerve and stem/progenitor cells in diabetes: preventive effects of insulin-like growth factor-1 treatment. Int. J. Endocrinol 2014, 312401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger I, Klepeis VE, Trinkaus-Randall V, 2005. Tri-nucleotide receptors play a critical role in epithelial cell wound repair. Purinergic Signal 1, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K-P, Li Y, Ljubimov AV, Yu F-SX, 2009. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes 58, 1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorek MS, Obrosov A, Shevalye H, Holmes A, Harper MM, Kardon RH, Yorek MA, 2015. Effect of diet-induced obesity or type 1 or type 2 diabetes on corneal nerves and peripheral neuropathy in C57Bl/6J mice. J. Peripher. Nerv. Syst.: JPNS 20, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I, Brüggemann J, Strom A, Peschel S, Köhler B, Stachs O, Guthoff RF, Roden M, German Diabetes Study G, 2014. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes 63, 2454–2463. [DOI] [PubMed] [Google Scholar]