Abstract
Purpose
While metastasis to the thyroid from a primary cancer remote to the thyroid is uncommon, current imaging techniques have improved detection of these intrathyroid metastases. The purpose of this study was to evaluate the 18F-PET/CT appearance of intrathyroid metastases and assess the impact of detection on patient management.
Methods
The 18F-PET/CT appearance of intrathyroid metastasis, including standardized uptake value (SUV), disease extent, and the effect on patient management following diagnosis were retrospectively reviewed. Inclusion criteria included 18F-PET/CT imaging and diagnosis of the intrathyroid metastasis matching the remote primary tumor.
Results
Intrathyroid metastasis were detected in 24 patients. The intrathyroid metastases presented on 18F-PET/CT as focal nodular uptake (n = 21), multiple nodular uptake (n = 2), or diffuse uptake/infiltration of the thyroid gland (n = 1). The SUV ranged between 3.9 and 42 (median 12.5 ± 7.5); in 2 patients, the FDG-avidity was minimal. On 18F-PET/CT, distant metastases were present outside the neck (n = 18), or limited to the neck (n = 6). In 2 of these 6 patients, the thyroid was the only site of metastatic disease. Due to the metastatic disease, the therapy was changed in 23 of 24 patients; 1 patient was lost to follow-up.
Conclusion
In any patient with a previous or current history of an extrathyroid malignancy, an 18FDG-avid thyroid mass or diffuse infiltration of the thyroid on 18F-PET/CT should be considered a potential intrathyoid metastasis until proven otherwise. Knowledge of an intrathyroid metastasis may impact patient management, especially if the thyroid or neck are the only sites of metastatic disease.
Keywords: PET/CT, Thyroid, Metastasis, Standardized uptake value (SUV)
1. Introduction
Intrathyroid metastasis from an extrathyroid primary cancer is uncommon clinically. In the past, metastases to the thyroid have been most commonly detected at autopsy with a reported incidence ranging from 1.25% to 24%.[1–14] While clinical findings may be subtle, detection of an intrathyroid metastasis from an extrathyroid primary tumor has improved with current imaging techniques, including 18F-PET/CT. To the best of our knowledge, previous descriptions of the 18F-PET/CT of intrathyroid metastases are limited to case reports[15–21] and there are no reports about how detection of an intrathyroid metastasis effects management. As knowledge of metastasis specific to the thyroid gland could potentially change patient management, the purpose of this study is to report on our experience with 18F-PET/CT on intrathyroid metastases.
2. Materials and Methods
The Institutional Review Board approved this study and waived the requirement for informed consent. Data acquisition was performed in compliance with all applicable Health Insurance Portability and Accountability Act regulations. Fifty-five patients have been diagnosed with a cytologically-proven intrathyroid metastases matching an extrathyroid primary tumor at our institution between 2002 and 2016. From this group, those patients in whom an 18F-PET/CT study was obtained were included in this study. A retrospective review of the patient demographics and 18F-PET/CT appearance of intrathyroid metastases from a remote primary tumor was performed.
18F-PET/CT scans were performed on a dedicated PET/CT system (Discovery ST, STe, or RX, General Electric Medical Systems, Milwaukee, WI). Scans were acquired to include a region from the orbits through the mid thighs. Scans were acquired 60 to 90 minutes after intravenous administration of 18F-PET/CT. PET studies were acquired in either 2-dimensional or 3-dimensional acquisition mode at 3–5 minutes per bed position (depending on the patient body mass index).
3. Results
3.1 Demographics
Intrathyroid metastases were detected on 18F-PET/CT scans in 24 patients, 10 men and 14 women, age range 44–77 years (median 58.5 ± 9.8 years). Sites of primary tumor were as follows: lung (n = 9), breast (n = 5), head and neck (n = 5), melanoma (n = 2), colon (n = 1), neuroendocrine tumor of the adrenal gland (n = 1), and synovial sarcoma of the C1 level (n = 1). The patient demographics, indication for PET/CT imaging and staging, when available, is provided in Table 1.
Table 1.
Patients’ demographics
| Patient # | Age/Sex | Primary cancer | PET/CT indication | Staging |
|---|---|---|---|---|
| 1 | 54/F | Breast | Staging | T1N0M0 |
| 2 | 64/M | Lung | Staging | T2N2M1 |
| 3 | 63/M | Melanoma | Staging | Clark’s level II |
| 4 | 59/F | Melanoma | Staging | Stage III |
| 5 | 40/F | Neuroendocrine adrenal | Staging | NA |
| 6 | 79/M | Lung | Staging | T2N2M0 |
| 7 | 45/M | SCC-RMT | Staging | T1N0MO |
| 8 | 66/M | Lung | Staging | T1N3M0 |
| 9 | 77/F | Colon | Thyroid mass | T1N3M0 |
| 10 | 60/F | SCC-tonsil | Prior US FNA | T1N2CMO |
| 11 | 52/F | Lung | Prior US FNA | NA |
| 12 | 44/F | Lung | Staging | T1N0M0 |
| 13 | 48/F | Breast | Staging | NA |
| 14 | 67/M | Synovial sarcoma C1 ring | Staging | NA |
| 15 | 49/M | SCC-tonsil | Staging | T2N2bMO |
| 16 | 50/F | Breast | Staging | T2 N1MX |
| 17 | 59/F | Lung | Prior US FNA | T3N3M0 |
| 18 | 52/F | Breast | Staging | T1N0M0 |
| 19 | 57/M | Lung | Staging | T2N3M0 |
| 20 | 58/F | Lung | Staging | T4N3M1 |
| 21 | 61/M | SCC-BOT | Prior US FNA | T2N2CMO |
| 22 | 65/F | Breast | Prior US FNA | T4N0M0 |
| 23 | 51/M | Lung | Staging | Stage IV |
| 24 | 67/F | SCC nasal cavity | Staging | T2N2M0 |
Note. SCC: squamous cell carcinoma; RMT: retromolar trigone; BOT: base of tongue; FNA: fine needle aspiration.
The time from primary tumor diagnosis to 18F-PET/CT imaging demonstrating the intrathyroid metastasis ranged from 2–141 months (median 16 months). Clinically, the patients were asymptomatic (n = 16), presented with a palpable neck/thyroid mass (n = 7), or neck pain (n = 1). The time from 18F-PET/CT to diagnosis of the intrathyroid metastasis ranged between 121 days before the 18F-PET/CT to 53 days after (median 4 ± 29 days after the 18F-PET/CT).
3.2 Imaging appearance
The intrathyroid metastases presented on 18F-PET/CT as a focal, solitary nodular uptake (n = 21) (see Figure 1), multiple discrete nodular uptake (n = 2) (see Figure 2), or diffuse uptake/infiltration of the thyroid gland (n = 1) (see Figure 3). The SUV ranged between 3.9 and 42 (median 12.3 ± 7.5); in 2 patients, the FDG-avidity was minimal (see Figure 4). On 18F-PET/CT, sites of extrathyroid metastasis included neck nodes (n = 13) and distant metastases outside the neck (n = 18). Metastatic disease was limited to the neck in 6 patients. In 2 of these 6 patients (patients #11 and #22), the thyroid was the only site of metastatic disease (see Table 2).
Figure 1.
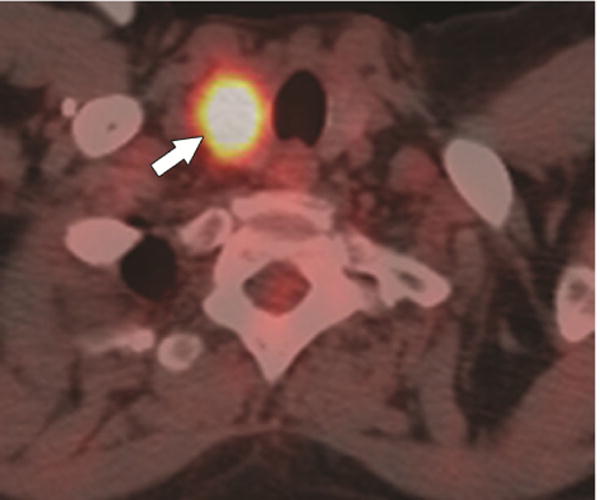
A 54-year-old female with breast cancer (patient #1). 18F-PET/CT, axial plane, shows a solitary nodular focus of uptake in the right thyroid gland (SUV = 15.4) (arrow).
Figure 2.
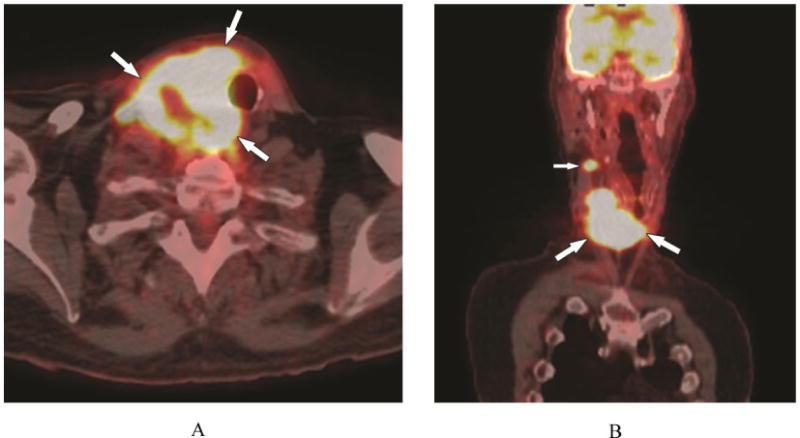
A 67-year-old male with synovial sarcoma of the C1 ring (patient #14). A) 18F-PET/CT, axial plane, shows multiple nodular foci of uptake (SUV = 21.8) in the thyroid and isthmus (arrows). B) 18F-PET/CT, coronal plane, multiple nodular foci of uptake in the right thyroid (large arrows) and right mid neck node (small arrow). Note lack of FDG activity in the left lobe.
Figure 3.
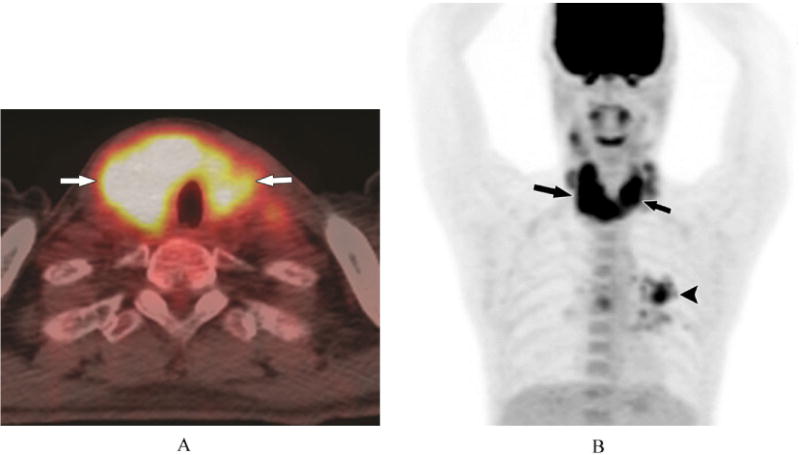
A 51-year-old male with history of lung cancer (patient #23). A) 18F-PET/CT, axial plane, show diffuse uptake throughout the thyroid gland (SUV = 9.4) (arrows). B) 18F-PET/CT, 3D image, diffuse uptake in the thyroid gland (large arrows) and a left lung metastasis (small arrow).
Figure 4.

A 44-year-old female with lung cancer (patient #12). A) 18F-PET/CT, axial plane, demonstrates minimal FDG avidity in the left lobe of the thyroid gland (arrow). B) 18F-PET/CT, axial plane, shows bilateral lower neck FDG avid nodes (arrows).
Table 2.
PET/CT findings
| Patient # | PET/CT Findings | SUV | Neck nodes | Distant metastasis |
|---|---|---|---|---|
| 1 | Solitary uptake | 15.4 | None | Lung, axilla |
| 2 | Solitary uptake | 42 | None | Subcarinal, chest wall |
| 3 | Solitary uptake | 12.7 | None | Brain |
| 4 | Solitary uptake | 9 | None | Brain |
| 5 | Solitary uptake | 3.9 | None | Thyroid, adrenal |
| 6 | Solitary uptake | 9.2 | None | Larynx, mediastinum |
| 7 | Solitary uptake | 8.6 | None | Tongue base, lung, adrenal |
| 8 | Solitary uptake | 12.8 | None | Adrenal |
| 9 | Solitary uptake | 10.4 | Right | Adrenal |
| 10 | Solitary uptake | 13.3 | Bilateral | Hilum |
| 11 | Multiple nodular | 15.8 | None | None |
| 12 | Solitary uptake | Minimal | Bilateral | Lung, pelvis |
| 13 | Solitary uptake | Minimal | Left | Lung, mediastinum |
| 14 | Solitary uptake | 21.8 | Right | Lung |
| 15 | Solitary uptake | 12.4 | Right | Retropharyngeal nodes |
| 16 | Multiple nodular | 6.6 | Right | Live, bone |
| 17 | Solitary uptake | 12.1 | None | Brain |
| 18 | Solitary uptake | 16.5 | Right | None |
| 19 | Solitary uptake | 13.4 | Right | Mediastinum |
| 20 | Solitary uptake | 15.3 | Bilateral | Breast, mediastinum |
| 21 | Solitary uptake | 12.2 | Midline | None |
| 22 | Solitary uptake | 7.9 | None | None |
| 23 | Diffuse | 9.4 | Bilateral | Lung, subcarinal |
| 24 | Solitary uptake | 9 | Right | None |
3.3 Patient management and survival
Due to the metastatic disease, the patient’s therapy was changed in 23 of 24 patients; 1 patient was lost to follow-up. The treatment in these 23 patients included the addition of chemotherapy (n = 20), chemotherapy with neck radiation (n = 1), and total thyroidectomy and neck dissection (n = 1) and hospice referral (n = 1). Four of the 23 patients were undergoing chemotherapy at the time that the intrathyroid metastasis was discovered and was subsequently changed (see Table 3). As of this writing, 19 of the 23 (83%) patients are deceased. These patients lived from 13 days to 8 years 5 months (median 1 year 2 months) after the diagnosis of the intrathyroid metastases. Four patients (17%) are still alive at the time of this report.
Table 3.
Initial management and change in management
| Patient # | Inintial manangement | Change in management |
|---|---|---|
| 1 | None | Doxil, Cytoxan |
| 2 | None | Paclitaxel, Carboplatin, Tarceva |
| 3 | Temodar, Thalidomide | Docetaxel |
| 4 | None | Temodar |
| 5 | None | Chemotherapy |
| 6 | None | Chemotherapy |
| 7 | None | Docetaxil, Caroplatin |
| 8 | Pemetrexed, Carboplatin | Erlotinib |
| 9 | None | Folfiri, Avastin |
| 10 | None | Thyroidectomy, bilateral neck dissection |
| 11 | None | Emetrexed, Carboplatin |
| 12 | None | Arboplatin, Paclitaxel |
| 13 | None | Xeloda |
| 14 | None | Hospice |
| 15 | None | Carboplatin, Certuximab |
| 16 | None | Capecitabine, Ixabepilone |
| 17 | None | Carboplatin, Paclitaxel |
| 18 | Trastuzumb, Arimidex | Trastuzumab, radiation |
| 19 | None | Erbitux with Gemcitabine |
| 20 | None | Lost to follow-up |
| 21 | None | Taxotere, Cisplatin, Tarceva |
| 22 | Aarimidex | Ixabepilone, Bevacizumab |
| 23 | None | Pemetrexed, Carboplatin,Bevacizumab |
| 24 | None | Doxetaxel, Cisplatin |
4. Discussion
While intrathyroid metastases are rare, we have noticed an increased number of cases at our institution. This is likely due to increased awareness and improved detection with advancing technology. Our results demonstrate that intrathyroid metastases present on 18F-PET/CT predominately as solitary nodules, but can also occur as multiple nodules or as diffuse uptake/infiltration throughout the gland with a median SUV of 12.3. This is in contradistinction to the normal thyroid gland that usually shows low or absent 18F-FDG uptake.[22,23]
Autopsy series show that the breast and lungs are the most common tumors that metastasize to the thyroid gland.[1,24–26] In clinical series however, renal cell carcinoma was the most frequent source of the metastasis.[4,9,27,28] In our series, the lung was the most common primary site of an intrathyroid metastasis (see Table 1). As we are a referral center for cancer, with subspecialization for certain cancer types, an estimation of the frequency of metastasis would be biased. No relation was noted in our series between the type of intrathyroid metastases or SUV, the site of the primary lesion, or the stage of the primary tumor on initial diagnosis.
In our study, the majority of intrathyroid metastases presented on 18F-PET/CT as focal solitary nodular uptake, similar to other benign or malignant primary thyroid lesions.[10,14] While analysis of the neck nodes was not the focus of this study, abnormal neck lymph nodes were present in 13 of 24 (54%) patients. In contradistinction, metastasis to regional neck lymph nodes has been reported to occur in only 19.4% of primary thyroid malignancies.[29] In addition, metastases to locations outside the neck were present in 18 of 24 (75%) patients. The presence of FDG avid nodules in the thyroid in patients with an extrathyroid malignancy, abnormal neck nodes, and/or lesions outside the neck, should further increase suspicion for intrathyroid metastasis. Both patients in our series that presented with thyroid nodules demonstrating minimal 18FDG-avidity had cervical adenopathy and distant metastasis.
Intrathyroid metastases can also present as multiple discrete nodular uptake or diffuse thyroid uptake/infiltration mimicking thyroiditis. In the absence of metastatic adenopathy, diffuse metastatic infiltration of the thyroid from an extrathyroid primary disease cannot be distinguished from thyroiditis.[30–33]
The SUV of a thyroid nodule is not predictive of malignancy. On PET/CT, imaging obtained for non-thyroid disorders, incidental thyroid uptake is either focal or diffuse, often seen with primary thyroid carcinoma or thyroiditis with an SUV of 10.7 ± 7.8 for focal lesion and mean of 7.7 (4.3–13.4) for diffuse lesions.[10,14] This is similar to our series, were the SUV of intrathyroid metastases were median 12.3 ± 7.5 and 9.4, respectively. The optimal SUV max cutoff value to differentiate benign from malignant lesions however, has not been fully defined, and could be the subject of further study.
Radiologists interpreting 18F-PET/CT should be aware of these different uptake patterns at presentation. This is especially true for metastases limited to the thyroid and neck which may be confused for primary thyroid cancer. Alternate imaging such as ultrasound with fine needle aspiration may be used as the next step to differentiate between these findings.
Limitations of the study include the retrospective nature of the review and the relatively small number of cases. Future directions could include attempts to differentiate metastasis presenting a solitary or multiple nodules from primary thyroid cancer, and the infiltrative pattern of metastatic disease from thyroiditis based on SUV. As we are a tertiary referral center for cancer, subspecializing in certain types of cancers, many patients are initially diagnosed at outside institutions. In none of the 24 patients was the initial SUV of the lesion at the primary site available to us. An interesting addition to future studies could include this comparison.
5. Conclusions
In any patient with a previous or current history of an extrathyroid malignancy, an 18FDG avid solitary nodule, multiple discrete nodules, or diffuse infiltration of the thyroid on 18F-PET/CT should be considered a potential intrathyroid metastasis until proven otherwise. Knowledge of an intrathyroid metastasis may impact patient management, especially if the thyroid or neck are the only sites of metastatic disease as this may be mistaken on imaging as a primary thyroid cancer.
Footnotes
Conflicts of Interest Disclosure
The authors declare that there is no conflict of interest statement.
References
- 1.Berge T, Lundberg S. Cancer in Malmo 1958-1969; An autopsy study. Acta Pathol Microbiol Scand Suppl. 1977;260:1–235. [PubMed] [Google Scholar]
- 2.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma: analysis of 1000 autopsied cases. Cancer. 1950 Jan;3(1):74–85. doi: 10.1002/1097-0142(1950)3:1<74::AID-CNCR2820030111>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Thorpe JD. Metastatic cancer in the thyroid gland: report of four cases. Surg Obstet Gynecol. 1954 Nov;62(11):574–6. [PubMed] [Google Scholar]
- 4.Elliott RH, Jr, Frantz VK. Metastatic carcinoma masquerading as primary thyroid cancer: A report of authors’ 14 cases. Ann Surg. 1960 Apr;151:551–61. doi: 10.1097/00000658-196004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimaoka K, Sokal JE, Pickren JW. Metastatic neoplasms in the thyroid gland: pathological and clinical findings. 1962 May-Jun;15:557–65. doi: 10.1002/1097-0142(196205/06)15:3<557::aid-cncr2820150315>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Wychulis AR, Beahrs OH, Woolner LB. Metastasis of carcinoma to the thyroid gland. Ann Surg. 1964 Aug;160:169–77. doi: 10.1097/00000658-196408000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverberg SG, Vidone RA. Metastatic tumors in the thyroid. Pacific Med Surg. 1966 Aug;164(2):291–9. doi: 10.1097/00000658-196608000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillay SP, Angorn IB, Baker LW. Tumour metastasis to the thyroid gland. S Afr Med J. 1977 Apr;51(15):9. 509–12. [PubMed] [Google Scholar]
- 9.Ericsson M, Biorklund A, Cederquist E, et al. Surgical treatment of metastatic disease in the thyroid gland. J Surg Oncol. 1981;17(1):15–23. doi: 10.1002/jso.2930170104. [DOI] [PubMed] [Google Scholar]
- 10.Czech JM, Lichtor TR, Carney JA, et al. Neoplasms metastatic to the thyroid gland. Surg Gynecol Obstet. 1982 Oct;155(4):503–5. [PubMed] [Google Scholar]
- 11.Ivy HK. Cancer metastatic to the thyroid: A diagnostic problem. Mayo Clin Proc. 1984 Dec;59(12):856–9. doi: 10.1016/S0025-6196(12)65622-5. [DOI] [PubMed] [Google Scholar]
- 12.McCabe DP, Farrar WB, Petkov TM, et al. Clinical and pathologic correlations in disease metastatic to the thyroid gland. Am J Surg. 1985 Oct;150(4):519–23. doi: 10.1016/0002-9610(85)90167-9. [DOI] [PubMed] [Google Scholar]
- 13.Haugen BR, Nawaz S, Cohn A, et al. Secondary malignancy of the thyroid gland: a case report and review of the literature. Thyroid. 1994 Fall;4(3):297–300. doi: 10.1089/thy.1994.4.297. [DOI] [PubMed] [Google Scholar]
- 14.Nakhjavani MK, Gharib H, Goellner JR, et al. Metastasis to the thyroid gland. A report of 43 cases. Cancer. 1997 Feb;79(3):1. 574–8. doi: 10.1002/(SICI)1097-0142(19970201)79:3<574::AID-CNCR21>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Hsu CH, Wu CH, Chu JS. Metastatic Cancer in Thyroid Detected by FDG PET. Clin Nucl Med. 2006 Jan;31(1):51–2. doi: 10.1097/01.rlu.0000191787.58078.a3. [DOI] [PubMed] [Google Scholar]
- 16.Basu S, Alavi A. Metastatic Malignant Melanoma to the Thyroid Gland Detected by FDG-PET Imaging. Clin Nucl Med. 2007 May;32(5):388–9. doi: 10.1097/01.rlu.0000259613.28127.f8. [DOI] [PubMed] [Google Scholar]
- 17.Jackson RT, Sinha P, Conrad GR. Demonstration of Thyroidal Metastasis From Lung Cancer by F-18 FDG PET Scan. Clin Nucl Med. 2008 Jul;33(7):505–6. doi: 10.1097/RLU.0b013e318177938d. [DOI] [PubMed] [Google Scholar]
- 18.Osmany S, Padhy AK, Ng DC. Detection of Thyroid Metastases From Nasopharyngeal Carcinoma With F-18. FDG PET/CT. 2008 Mar;33(3):224–5. doi: 10.1097/RLU.0b013e31815976ca. [DOI] [PubMed] [Google Scholar]
- 19.Kaushik A, Ho L. Thyroid Metastasis From Primary Renal Cell Cancer on FDG PET/CT. Clin Nucl Med. 2011 Jan;36(1):56–8. doi: 10.1097/RLU.0b013e3181fef00e. [DOI] [PubMed] [Google Scholar]
- 20.Manohar K, Mittal BR, Kashyap R, et al. Renal Cell Carcinoma Presenting as Isolated Thyroid Metastasis 13 Years After Radical Nephrectomy, Detected on F-18 FDG PET/CT. Clin Nucl Med. 2010 Oct;35(10):818–9. doi: 10.1097/RLU.0b013e3181ef0b05. [DOI] [PubMed] [Google Scholar]
- 21.Treglia G, Del Ciello A, Fadda G, et al. Usefulness of 18F-FDG PET/CT in a Rare Case of Malignant Pseudothyroiditis. Clin Nucl Med. 2014 Feb;39(2):e163–5. doi: 10.1097/RLU.0b013e31827a27c8. [DOI] [PubMed] [Google Scholar]
- 22.Nakamoto Y, Tatsumi M, Hammoud D, et al. Normal FDG distribution patterns in the head and neck: PET/CT evaluation. Radiology. 2005 Mar;234(3):879–85. doi: 10.1148/radiol.2343030301. [DOI] [PubMed] [Google Scholar]
- 23.Bogsrud TV, Lowe VJ. Normal variants and pitfalls in whole-body PET imaging with 18F-FDG. Appl Radiol. 2006;35(6):16–30. [Google Scholar]
- 24.Willis RA. Metastatic tumors in the thyroid gland. American Journal of Pathology. 1931 May;7(3):187–208. [PMC free article] [PubMed] [Google Scholar]
- 25.Rice CO. Microscopic metastases in the thyroid gland. American Journal of Pathology. 1934 May;10(3):407–12.1. [PMC free article] [PubMed] [Google Scholar]
- 26.Mortensen JD, Woolner LB, Bennett WA. Secondary malignant tumors of the thyroid gland. Cancer. 1956 Mar-Apr;9(2):306–9. doi: 10.1002/1097-0142(195603/04)9:2<306::AID-CNCR2820090217>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 27.Choi JY, Lee KS, Kim HJ, et al. Focal Thyroid Lesions Incidentally Identified by Integrated 18F-FDG PET/CT: Clinical Significance and Improved Characterization. J Nucl Med. 2006 Apr;47(4):609–15. [PubMed] [Google Scholar]
- 28.Karantanis D, Bogsrud TV, Wiseman GA, et al. Clinical Significance of Diffusely Increased 18F-FDG Uptake in the Thyroid Gland. As an incidental finding on PET/CT. J Nucl Med. 2007 Jun;48(6):896–901. doi: 10.2967/jnumed.106.039024. [DOI] [PubMed] [Google Scholar]
- 29.Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in non-palpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002 May;87(5):1941–6. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 30.Yeh HC, Futterweit W, Gilbert P. Micronodulation: ultrasonographic sign of Hashimoto thyroiditis. J Ultrasound Med. 1996 Dec;15(12):813–9. doi: 10.7863/jum.1996.15.12.813. [DOI] [PubMed] [Google Scholar]
- 31.Simeone JF, Daniels GH, Mueller PR, et al. High-resolution real-time sonography of the thyroid. Radiology. 1982 Nov;145(2):431–5. doi: 10.1148/radiology.145.2.7134448. [DOI] [PubMed] [Google Scholar]
- 32.Butch RJ, Simeone JF, Mueller PR. Thyroid and parathyroid ultrasonography. Radiol Clin North Am. 1985 Mar;23(1):57–71. [PubMed] [Google Scholar]
- 33.Pedersen OM, Aardal NP, Larssen TB, et al. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid. 2000 Mar;10(3):251–9. doi: 10.1089/thy.2000.10.251. [DOI] [PubMed] [Google Scholar]


