Abstract
The development of novel nanoparticles consisting of both diagnostic and therapeutic components has increased over the past decade. These “theranostic” nanoparticles have been tailored toward one or more types of imaging modalities and have been developed for optical imaging, magnetic resonance imaging, ultrasound, computed tomography, and nuclear imaging comprising both single-photon computed tomography and positron emission tomography. In this review, we focus on state-of-the-art theranostic nanoparticles that are capable of both delivering therapy and self-reporting/tracking disease through imaging. We discuss challenges and the opportunity to rapidly adjust treatment for individualized medicine.
Keywords: nanotechnology, characterization, fabrication, nanobiotech
Introduction
The use of nanoparticles in medicine has boomed in the past two decades, and several new nano-based therapeutic and diagnostic imaging strategies have been developed for a variety of diseases and organs. Historically, the concept of nanoparticles can be traced back to Paul Ehrlich, a German immunologist known for his work in bacterial staining, winner of the Nobel Prize in Physiology or Medicine in 1908, and physician-scientist who coined the term magic bullets and initiated the concept of targeted chemotherapy and drug delivery.1 Then, in 1959, American physicist and Nobel laureate Richard Feynman gave an after-dinner speech at the American Physics Society conference providing visionary examples and benefits of nanotechnology, providing inspiration and igniting the field of nanomedicine.2 These concepts were then popularized by K. Eric Drexler through the 1981 article in the Proceedings of the National Academy of Sciences article, “Molecular Engineering: An Approach to the Development of General Capabilities for Molecular Manipulation,”3 as well as through other published books.
In the 1960s, liposomes were first reported and studied as a nanoparticle drug delivery platform to more efficiently and safely diagnose and treat diseases.4 These efforts, led by Alberto Gabizon and Yechezkel Barenholz, took many reiterations and trials but came into fruition with Doxil, the first Food and Drug Administration (FDA)–approved nano-drug (1995) used to treat certain types of cancer, including ovarian cancer, AIDS-related Kaposi’s sarcoma, and multiple myeloma.5 Since the 1960s, a myriad nanometer-sized particles that range and vary in composition and physiochemical properties such as size, shape, surface charge, stability, and biodegradability, physiological properties including clearance and biodistribution, as well as targeting moieties and strategies has been designed for both therapeutic and diagnostic function.6–9 One feature of nanoparticles is their high surface-to-volume ratio, which enables the surface to be co-functionalized with simultaneous diagnostic and therapeutic formulations. Moreover, many nanoparticles, such as iron oxide, have intrinsic imaging properties themselves and can be further modified for drug delivery and possess multifunctional characteristics. On the flip side, nanomaterials can also have intrinsic therapeutic properties, such as the case with gold nanoshells and their potential to deliver photothermal therapy.10 The unique opportunities to use nanoparticles for both diagnostic and therapeutic purposes, or “theranostics,” will be the focus of this review.
The most basic definition of a “theranostic” nanoparticle is a nanoparticle that has both therapeutic and diagnostic agents on a single platform. Additional meanings of theranostics in general include efforts in the clinic using diagnostic testing for individual patients to develop personalized therapies and can include image-guided surgery and post-surgery evaluation.11 This can be achieved in 2 ways: (1) therapy followed by diagnostics to test reactions to treat and identify patients in which therapy has an effect or (2) diagnostics followed by therapy to first identify disease type with the ultimate goal of providing personalized therapy for individual patients. Given the recent focus on precision and personalized medicine as well as the advances in nanochemistry and nanoparticle formulations, efforts in the field of dual therapy and imaging have risen, as evidenced by the word theranostics in publication titles totaling just 3 in 2006 to more than 260 in 2016 (Fig. 1).
Figure 1.
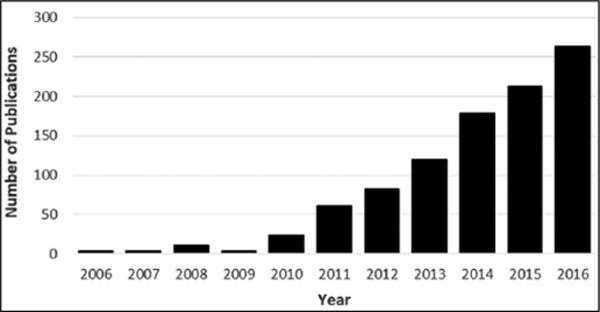
The growing number of theranostic publications in recent years.
Theranostic nanoparticles have been designed for a variety of imaging modalities including optical imaging, magnetic resonance imaging (MRI), ultrasound, computed tomography (CT), and nuclear imaging comprising of both single-photon computed tomography (SPECT) and positron emission tomography (PET). Moreover, therapeutic strategies include (1) combining nanoparticles with various imaging modalities to convert light-sensitive compounds into toxic agents upon exposure of selective wavelengths (e.g., photosensitizers),10 (2) using the intrinsic properties of the nanoparticle to induce photothermal therapeutic effects, or (3) incorporating small-molecule drugs or biologics into the nanoparticle entity. In this review, we will highlight theranostic nanoparticles of the latter two, in which an imaging modality is combined with either the intrinsic therapeutic properties of the nanoparticle itself or the incorporation of a therapeutic drug into the nanoparticle (Table 1). Moreover, we pay particular attention to and emphasize those platforms in which self-reporting and disease tracking is possible in real time. For a broader understanding of theranostic nanoparticles, we suggest the following reviews by Janib et al.,12 Ryu et al.,13 and Kelkar et al.14 Moreover, nanotherapeutics using small interfering RNA (siRNA),15 polymeric materials,16 photon upconversion,11 for photothermal therapy17 and cancer,10,18,19 can be found in other reviews.
Table 1.
Summary of Featured Studies and Technologies.
| Nanoparticle | Application | Therapeutic Component |
Targeting Element |
Additional Features |
Reference | |
|---|---|---|---|---|---|---|
| Optical imaging | PLGA | Cancer | Camptothecin | Folate | Caspase-3 activatable peptide Proton sponge effect Monitoring through FRET |
20 |
| Poly(isobutylene-alt-maleic anhydride) | Cancer | Paclitaxel | Caspase-3 activatable peptide Monitoring through FRET |
21 | ||
| Gold | Cancer | Survivin antisense detection | 22 | |||
| NIRF imaging | PEG-hyaluronic acid | Colon cancer | Irinotecan | Hyaluronic acid | 23,24 | |
| Silica-hyaluronic acid | Tumor | Hyaluronic acid | Mesenchymal celllabeling, PET, MRI | 25 | ||
| Silicon quantum dot | Cancer | Chlorambucil | Photo-triggered release of drug, drug release monitoring | 26 | ||
| Raman spectroscopy | Gold | Cancer | EpCAM antibody | Image-guided tumor ablation | 27 | |
| Gold | Cancer | Doxorubicin | 64Cu for PET, photoacoustic imaging | 28 | ||
| MRI | SPION | Acute allograft rejection | Diacylglycerol kinase alpha (DGKa) pDNA | CD3 antibody (T-cell) | 29 | |
| SPIO | Immune response detection | Dendritic cell tracking | 30 | |||
| Perfluorocarbon emulsion, CS-1000 | Cell tracking | 19F for MRI | 31 | |||
| Ferumoxytol | Cancer | Taxol, doxorubicin | Drug release monitoring at pH 6.8 and 6.0 | 32 | ||
| Ultrasound | PEG-PCL micelle/perfluoropentane | Cancer | Doxorubicin | Nanoemulsion convert to microbubbles for enhanced cell membrane permeabilization | 33 | |
| Hyaluronic acid encapsulated with MnO2 | Cancer | Photodynamic therapy using indocyanin green As NIR laser-activated photosensitizer |
Hyaluronic acid | 34 | ||
| Mesoporous silica | Cardiac stem cell therapy | Insulin-like growth factor (IGF) | Gd for MRI | 35 | ||
| Perfluoropentane-chitosan-deoxycholic acid | Cancer | siRNA | Liquid nano-droplets convert to microbubbles | 36 | ||
| SPECT | Liposome | Ovarian cancer | Rhenium-186 half-life 90.7 h, 18F-FDG for PET | 37 | ||
| PET | T7 phage nanoparticle | Cancer | RGD | Bifunctional chelator AmBaSar | 38 | |
| Reduced graphene oxide-based iron oxide | Peripheral arterial disease | 64Cu, nanoparticles also absorb light at NIR, photoacoustic imaging | 39 | |||
| CT | Glycol-chitosan-coated gold | Cerebrovascular thrombi detection | tPA | Fibrin-binding peptide | 40 | |
| Bismuth sulfide | Cancer | Photothermal therapy | Multispectral optoacoustic tomography | 41 |
PLGA = poly(lactic-co-glycolic acid); FRET = fluorescence resonance energy transfer; PEG = poly(ethylene glycol); PET = positron emission tomography; MRI = magnetic resonance imaging; EpCAM = epithelial cell adhesion molecule; SPION = superparamagnetic iron oxide nanoparticles; DGKα = diacylglycerol kinase alpha; SPIO = superparamagnetic iron oxide; PCL = polycaprolactone; NIR = near infrared; siRNA = small interfering RNA; SPECT = single-photon computed tomography; 18F-FDG = 18F fludeoxyglucose; RGD = arginylglycylaspartic acid; CT = computed tomography; tPA = tissue plasminogen activator.
Optical Imaging
Optical imaging is a newly emerging clinical imaging technique for noninvasively looking inside the patient’s body and obtaining images that can offer cellular resolution. This technique detects photons emitted in the visible and near-infrared (NIR) range from bioluminescent, fluorescent, or even Raman probes. Unlike x-rays, which use ionizing radiation, optical imaging is considered a safer nonionizing radiation imaging technique that is ideal for repeat imaging procedures and is also relatively cost-effective. Moreover, optical imaging is very amenable to multimodal imaging and extends over a wide resolution and wavelength range, and it is often used in combination with other imaging techniques. In addition, emerging optical imaging techniques such as fluorescence lifetime imaging microscopy can be used to monitor theranostic nanoparticle uptake and drug release.42 Unfortunately, the drawbacks of optical imaging includes superficial tissue penetration (up to 2 cm) and high background noise due to tissue autofluorescence, absorption by proteins (257–280 nm), heme groups (up to 560 nm), and water (above 900 nm).12 To bypass these limitations, the NIR range (700–900 nm) has been used for in vivo imaging in many cases in theranostic nanomedicine.43–45
Luo et al.20 reported on self-reporting and self-adapting theranostic nanoparticles based on poly(lactic-co-glycolic acid) for cancer applications. These stimuli-responsive nanomaterials also consist of camptothecin, an anticancer drug; folate for targeting; as well as a caspase-3 activatable fluorescent peptide, dabcyl-KFFFDEVDK-FAM. The fluorescence is turned off due to the fluorescence resonance energy transfer (FRET) effect and is turned on upon reaction with caspase-3, a protease that can monitor the apoptosis effect upon administering the theranostic nanoparticle. HeLa tumor-bearing mice after intravenous injection confirmed the ability for therapeutic monitoring and allowed for semiquantitative analysis for up to 48 h. The authors confirmed these theranostic nanoparticles inhibited tumor volume for up to 15 d, providing the initial report of one single nanoparticle system for cancer cell eradication and rapid response to activated caspase-3 in the apoptosis process to achieve visualized therapeutic self-reporting. Similar FRET approaches have been reported46,47 for cancer therapy including monitoring of paclitaxel release from poly(isobutylene-alt-maleic anhydride) nanoparticles by caspase-3. Upon cleaving the peptide sequence Asp-Glu-Val-Asp (DEVD) during chemotherapy-mediated apoptosis, caspase-3 releases fluorescein isothiocyanate that was quenched through FRET by QSY 7, providing feedback and a nanoreporter approach.21
Using optical imaging, others have reported on nanoparticles capable of mRNA detection and regulation.48 Prigodich and colleagues22 developed nanoflare gold nanoparticles with antisense oligonucleotides that can target, bind, and detect mRNA of survivin, a gene used in cancer diagnosis and treatment. The gold nanoparticle surface is functionalized with the antisense strand, and complementary Cy5-labeled oligonucleotides are added. In the absence of the target, nanoconjugate solutions exhibit low fluorescence. The addition of the survivin target increases the fluorescence signal as the quenching effect is reversed, and even just one mismatch base pair was found to decrease the signal dramatically, speaking to the precision and specificity of this system. Survivin mRNA detection was semiquantitated in live HeLa cells, showing the development of mRNA-directed theranostics has potential to prevent translation of survivin mRNA.
As mentioned, NIR fluorescence (NIRF) has frequently been used as a strategy to obtain higher spatial resolution and sensitivity, with less absorption and autofluorescence.49–52 For example, Choi et al.23,24 recently developed theranostic nanoparticles based on poly(ethylene glycol) (PEG)–conjugated hyaluronic acid for colon cancer, previously shown to selectively accumulate in tumor tissue through strong receptor binding to the hyaluronic acid receptor, CD44, which is overexpressed in various cancer cell types. These nanoparticles were labeled with Cy5.5 and loaded with the anticancer drug irinotecan and were found to bind to early-stage colon tumors in orthotopic mouse models. They can provide diagnostic as well as therapeutic monitoring capabilities for up to 125 d. In addition, NIRF has been combined with cell-based or cell-inspired theranostic particles for enhanced therapeutic efficacy and self-monitoring.53 Huang et al.25 developed silica-hyaluronic acid nanoparticles capable of multimodal imaging to label mesenchymal stem cells for tumor targeting. The authors show that nanoparticle delivery is enhanced when internalized within mesenchymal stem cells (MSCs) compared with free nanoparticles because of the tropic and migratory properties of MSCs. Injected intravenously in a glioblastoma model, PET, MR, and optical imaging confirmed homing to tumors for up to 24 h, providing proof-of-concept for future drug-loaded nanoparticle delivery using MSCs.
Because of the photoluminescent properties, quantum dots have also been used as a promising nanomaterial for theranostics using optical imaging.54 Paul et al.26 used silicon quantum dots to design a photoresponsive nanocarrier that used o-nitrobenzyl as a phototrigger for controlled release of the anticancer drug chlorambucil. O-nitrobenzyl was chosen as it initially cages the drug and quenches the fluorescence of the silicon quantum dots through a photoinduced electron transfer process, providing an “off” state. Upon irradiation, the drug is uncaged and the fluorescence switched “on,” allowing for real-time monitoring of the drug release. Paul et al.26 showed this ability in vitro using HeLa cells for up to 30 min.
A relatively new optical imaging strategy uses the light-scattering principles as seen in Raman spectroscopy. Raman imaging detects the inelastically scattered photons produced after illumination with a light source (i.e., laser). This Raman effect is rather weak and tends to require a metallic surface to create a plasmon resonance that increases the incident electromagnetic field and in turn enhances the Raman signal by several orders of magnitude (Fig. 2). This phenomenon is known as the surface-enhanced Raman scattering (SERS) effect.56 As a result, researchers developed a new metallic nanoparticle intended as a new imaging probe that offered ultrasensitive detection and multiplexing capabilities.57,58 The nanoparticle was composed of a unique Raman active layer adsorbed onto a 60 nm gold core with a 30 nm silica shell totaling a diameter of 120 nm.57 There have been several reports on this new nano-based Raman imaging strategy with varying Raman nanoparticle constructs, showing its vast potential for sensitive cancer imaging since its inception in 2008.59–68 More recently, there has been interest in further developing these nanoparticles into theranostic agents by using the intrinsic properties of the gold core to induce photothermal therapy. Sun et al.27 created a SERS gold nanorod construct that possessed strong Raman signal and high photothermal conversion efficiency. The group functionalized the nanoparticles with epithelial cell adhesion molecule antibodies and showed specific tumor targeting and effective cell killing in human-derived prostate and glioblastoma cell lines. Their “see and treat” strategy involved using the SERS component for imaging of the tumor-targeted nanoparticles and then immediately ablating the area, resulting in image-guided real-time photothermal therapy. Sun et al.27 created a SERS gold nanorod construct that possessed strong Raman signal and high photothermal conversion efficiency. The group functionalized the nanoparticles with epithelial cell adhesion molecule antibodies and showed specific tumor targeting therapy approach.28 The chemotherapeutic agent doxorubicin was loaded into the porous nanoparticles, and laser irradiation was shown to trigger drug release. The nanoparticles were further modified with 64Cu to harness the advantages of imaging with PET. Furthermore, they demonstrated imaging using the photoacoustic properties of the gold. Photoacoustic imaging uses light to excite an area of interest, where the absorption of light causes thermal expansion of the material and as a result acoustic waves that can be detected with an ultrasound transducer. This novel nanoparticle construct clearly demonstrates the multimodal imaging potential for theranostic nanoparticles integrating Raman, PET, and photo-acoustic imaging all together. The therapeutic effect using the combined chemophotothermal therapy approach was effective as well. The doxorubicin-loaded gold nanoshells revealed an increased survival rate in mice bearing human-derived glioblastoma xenografts.28
Figure 2.
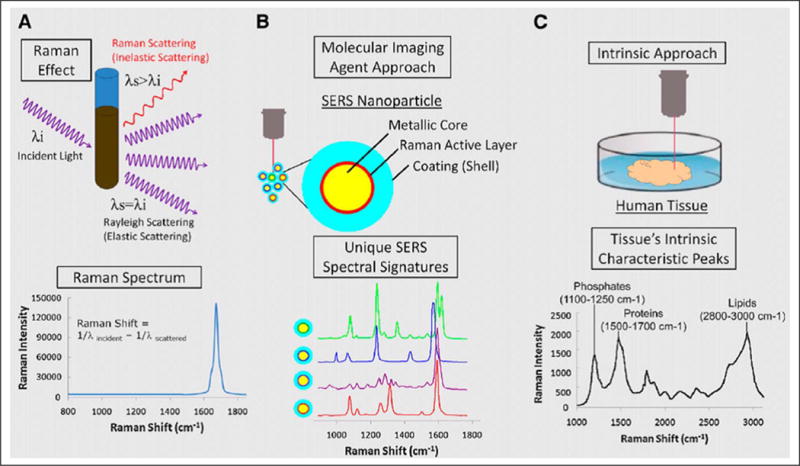
(A) Schematic of the Raman effect. (B) Molecular imaging agent showing surface-enhanced Raman scattering nanoparticles. (C) Intrinsic approach showing human tissue being irradiated with a laser source.55 This figure was originally published in JNM. Zavaleta et al. 2011;52:1839–1844. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.
MRI
MRI is a relatively safe medical imaging modality that generates images based on the relaxation properties of the water hydrogen nuclei within an applied magnetic field. Paramagnetic molecules such as gadolinium (Gd) can be functionalized onto nanoparticles to shorten the relaxation parameters (T1 and T2) of water, or as mentioned above, inherently superparamagnetic iron oxide nanoparticles can act as a drug delivery vehicle for theranostic applications.69–71 MRI has relatively good spatial resolution but is limited in sensitivity.12 Nonetheless, this imaging modality has been applied in theranostic applications including tracking cell therapy as well as monitoring environment-responsive drug release.
PEG-grafted-poly(ethylenimine) nanoparticles functionalized with the cluster of differentiation 3, or CD3, singlechain antibody, a T-cell marker, and superparamagnetic iron oxide nanoparticles (SPION) was developed by Guo et al.29 as a T-cell–targeted theranostic nanosystem to detect acute allograft rejection after organ transplantation. In addition, nanoparticles were complexed with pDNA containing the therapeutic immunosuppressive gene diacylglycerol kinase alpha (DGKα) encoding for the reporter gene, enhanced green fluorescent protein. In rats with cardiac transplantation, the T-cell–targeted theranostic nanosystem localization to T cells was detected using MRI, which reflected the allograft rejection level. Over time, the MRI signal intensity stayed consistent, strongly demonstrating that the therapeutic DGKα gene delivered by the T-cell–targeted nanosystem had effectively suppressed the acute immune rejection in the allograft. Thus, T-cell accumulation significantly declined. These results were confirmed under histology: Phosphate-buffered saline or nontargeted nanoparticle-treated rats had immunorejections featured as significant lymphocyte infiltration in both the myocardium and endocardium, coagulative myocyte necrosis, scattered hemorrhage, and severe vasculopathy compared with targeted therapeutic nanoparticles. The function of the treated hearts was superior, as measured by the heart beating score, providing a novel approach to detecting, treating, and monitoring allograft rejection after cardiac transplantation. Moreover, the potential to monitor loading and packaging of DNA through changes in SPION T2 relaxivity provides an additional advantage to MRI-based theranostics.72
In addition to suppressing the immune system, theranostic approaches have also been used to stimulate the immune system. For instance, cell therapy is emerging as a strategy to stimulate the immune response against tumors and other diseases. The success of these treatments partially depends on the accurate delivery of the transplanted cells to target organs. MRI-based cell tracking has typically used contrast agents or tracer agents containing Gd, which causes a decrease in the spin-lattice relaxation time T1 and thus a localized region of hyperintensity in T1-weighted images.31 In addition, another common approach is the use of iron-based contrast agents such as SPIO that result in a drop in apparent spin-spin relaxation time T2* around the agents and creates a region of hypointensity. Using MRI-based cell tracking, monocyte-derived dendritic cells (DCs) were detected as a measure of monitoring cell therapeutics.30,31 Immunotherapy uses antigen-loaded DCs to stimulate T cells in an effort to modulate the immune response and has been proposed as a vaccination strategy. Functionality of DCs and effective migration from the site of injection to the lymphoid organs is a major requirement to stimulate the immune system. To follow DC migration, Crisci et al.30 labeled monocyte-derived DCs with SPIO particles and tested their ability to elicit functional immune responses in pigs upon exposure to virus-like particles (VLPs) from rabbit hemorrhagic disease virus (RHDV) harboring a T-cell epitope derived from foot and mouth disease virus 3A protein. SPIO-labeled DCs were found to be viable, and their morphology and CD172a, SLAII, CD163, and CD80/86 patterns were similar to non–SPIO-labeled cells, confirming functionality in vitro. Upon exposure to lipopolysaccharide or viral particles, tumor necrosis factor–α levels were also found to be similar to unlabeled cells. DCs were then injected subcutaneously in vivo, and using MRI, DCs were tracked in the lymph nodes. Notably, RHDV-VLP pulsed DCs were found to induce higher numbers of interferon-γ–secreting inguinal lymph node cells against RHDV-3A-VLP compared with unpulsed animals. These results provide one of the first proof-of-principle studies to describe swine DC tracking in vivo and the efficacy of DC-based immunization.
Bonetto et al.31 also reported the use of MRI for DC tracking, but they used 19F MRI and showed the ability to directly quantify the number of human DCs using this approach. The benefits of 19F MRI include higher sensitivity and negligible endogenous 19F in vivo, allowing for minimal background and noise. Human monocyte-derived DCs were labeled using CS-1000, an aqueous colloidal nanoemulsion of perfluorocarbon polymer, achieved by cell internalization. Cell quantification and a standard curve was derived from T1- or T2*-weighted sequences and calibrated with 19F as a reference. With this tool, the authors confirm successful in vivo detection of labeled human DCs in a xenograft mouse model upon subcutaneous injection.
In addition to monitoring cells in vivo, stimuli-responsive nanotheranostics that are able to monitor treatment have been used with MRI.73 Kaittanis and colleagues32 developed iron oxide “nanophores” derived from nanoparticles that are clinically approved (ferumoxytol) with various chemotherapeutics, such as taxol and doxorubicin. The loading of drugs increases the nanophores’ T2 and T1 nuclear magnetic resonance proton relaxation times, which is directly related to the amount of loaded cargo, and at acidic pH 6.8 and 6.0, a rapid decrease in T2 and T1 was observed in accordance with the loss of drug release. In the prostate cancer cell line LNCaP, release of doxorubicin or model fluorophores from ferumoxytol dequenched and recovered ferumoxytol’s supraparamagentic properties, similar to empty nanoparticles. These findings demonstrated that measurement of relaxation can be used as a measurement of payloads carried by iron oxide nanoparticles. This ability to monitor drug release from nanophores in vivo was confirmed in nude mice bearing tumors. Similarly, Park et al.72 developed hollow mesoporous Prussian Blue–manganese (Mn) nanoparticles as smart pH-responsive T1-weighted MRI contrast agents with ultrahigh longitudinal relativity. A positive correlation between pH-responsive MRI intensity and doxorubicin release was determined, allowing for efficient drug release monitoring via MRI.
Ultrasound
Ultrasound is a safe, cost-effective, and relatively fast imaging modality in which a transducer that emits high-frequency sound waves is placed against the skin, and the echoes from the sound waves reflected back from the internal organs are recorded.74 To obtain the resolution required to image tissues that are located deeper in the body, intravascular ultrasound can be performed in which an ultrasound unit is mounted on the tip of a catheter and guided into the body. This is an invasive approach, and instead, ultrasound contrast agents that generate gas can be used to improve imaging as they have either different acoustic properties from that of the surrounding tissues themselves or can stimulate an environment with different acoustic properties.12
To that end, Mohan et al.33 tested the therapeutic efficacy of doxorubicin-loaded PEG-polycaprolactone micelles stabilized in perfluoro-15-crown-5-ether (PFCE) or perfluoropentane nanoemulsions in combination with sonication. Without ultrasound, micelles accumulated in the cytoplasm of A2780 ovarian cancer cells but did not penetrate the cell nuclei. With the addition of ultrasound, the nanoemulsions converted into microbubbles, allowing for enhanced permeabilization of both plasma and nuclear membranes. Hence, doxorubicin was found to be triggered into the cell nuclei in vitro to a greater effect. Moreover, because micro-bubbles are several thousand times more reflective than the normal body, the researchers confirmed that this theranostic tool can be used to enhance imaging as well as intracellular drug trafficking.
Gao et al.34 reported on the use of ultrasound for monitoring oxygen-generating MnO2 nanoparticles in the context of photodynamic therapy. In photodynamic therapy, an excited photosensitizer produces reactive oxygen species, particularly H2O2, to induce apoptosis to exposed cells. Because cancer cells normally produce more H2O2, the authors exploited the high reactivity of MnO2 nanoparticles to H2O2 and developed oxygen-generating, tumor-targeted nanoparticles to first observe sufficient oxygen generation in the tumor area before initiating photodynamic therapy. The nanoparticles consisted of hyaluronic acid encapsulated with MnO2 nanoparticles and functionalized with indocyanine green (ICG) used to achieve an NIR laser-activated photosensitizer. Hyaluronic acid was used to improve biocompatibility, tumor targeting via binding with CD44-expression tumor cells, and the release of MnO2 nanoparticles upon hyaluronidase degradation. Oxygen generation was found with continuous NIR laser irradiation for up to 10 h and enhanced cytotoxicity in the squamous cell carcinoma cell line, SCC7 cells. In SCC7 tumor-bearing mice, an NIR laser was applied 6 h postinjection when nanoparticle accumulation peaked within tumors. Through ICG, oxygen was converted into cytotoxic singlet oxygen, ablating the tumor and showing the potential of these hybrid nanoparticles for monitoring nanoparticles in vivo and image-guided cancer therapy.
Like in MRI, ultrasound has also been used in combination with nanoparticle contrast agents to image cell therapy. Kempen and colleagues35 developed mesoporous silica nanoparticles loaded with the prosurvival drug insulin-like growth factor to develop a theranostic nanoparticle that could increase cardiac stem cells for treating heart disease. Like microbubbles, the interface between tissue and the silica nanoparticle offers an impedance mismatch and, hence, enhanced cell tracking and molecular imaging in vivo. Moreover, the nanoparticles were also loaded with Gd for use in dual-imaging modalities and for higher resolution in follow-up studies. After nanoparticles were internalized by human bone marrow mesenchymal stem cells, 500,000 nanoparticle-loaded stem cells were implanted into the left ventricle wall of nude mice. Ultrasound images confirmed enhanced backscatter compared with vehicle-only groups, and the authors calculated that cell counts down to 10,000 could be detectable using ultrasounds and 100,000 using MRI. Importantly, the silica nanoparticles degraded within cells within 1 mo and increased stem cell viability and survival up to 40% after 1 wk.
Another novel theranostic approach developed by the Stride group at Oxford University involves using a nano-droplet to microbubble conversion strategy to deliver siRNA therapy.36 The nano-droplets’ core consists of liquid perfluoropentane and magnetic nanoparticles with a shell comprising chitosan-deoxycholic acid conjugates and siRNA, totaling a size of ~257 nm. After exposure to ultra-sound, the liquid droplets undergo phase transition to form gas microbubbles on the sizes of 3822 nm. This clever strategy allows for the nanoparticles to circulate for longer periods of time and even extravasate before they take on their larger microbubble form. The magnetic nanoparticles within the complex were used to enhance localization through an externally applied magnetic field. Delivery of siRNA and silencing were successfully demonstrated with a fourfold reduction in cell viability in human-derived breast and lung cancer cell lines. During the ultrasound exposure period, acoustic imaging was also used to estimate the acoustic power of cavitation emissions and monitor the correlation between the energy of these acoustic emissions from the microbubbles and the treatment efficacy.36
Nuclear Imaging
SPECT and PET are nuclear medicine imaging techniques in which radiolabeled tracers, or radionuclides, are injected into the patient to observe physiological processes within the body. Gamma rays emitted by the injected radionuclides are captured using a gamma camera to obtain three-dimensional images. SPECT imaging has been the workhorse of nuclear medicine clinics around the world for the past several decades. It uses relatively inexpensive radionuclides such as technetium-99m (99mTc) that can be freshly extracted from molybdenum-based technetium generators, which are delivered to clinics on a weekly basis. 99mTc has several favorable properties for nuclear imaging, including its 6 h half-life and its gamma ray emission at 140 keV, which is ideal for the scintillation crystals that comprise the gamma cameras in most clinics. Researchers have stably incorporated 99mTc radionuclides into liposomal nanoparticles for imaging of cancer and detection of sentinel lymph nodes.75–82 Furthermore, Goins and colleagues77 developed a strategy to encapsulate rhenium-186 (186Re) into a liposomal nanoparticle (Fig. 3). 186Re is a great theranostic radionuclide as it has a useful half-life of ~90 h, which is ideal to administer and follow therapy. It also has a therapeutic beta emission of ~1 MeV and a gamma ray emission at 137 keV, which is ideal for nuclear SPECT imaging. The therapeutic beta emission travels a distance of ~1.8 mm, making it possible for the nanoparticles to deliver effective treatment without having to incorporate itself into the cell or even the nucleus. The longer half-life allows for monitoring of the therapy with SPECT imaging for several days postadministration. Researchers have evaluated this nano-based theranostic approach by using a novel intraperitoneal delivery strategy developed to prolong nanoparticle retention in the peritoneal cavity of an ovarian cancer model with encouraging results.37 This approach is also currently being evaluated in a clinical trial led by Dr. Andrew Brenner83 to evaluate the maximum tolerated dose, safety, and efficacy of rhenium nanoliposomes in recurrent glioblastoma.
Figure 3.
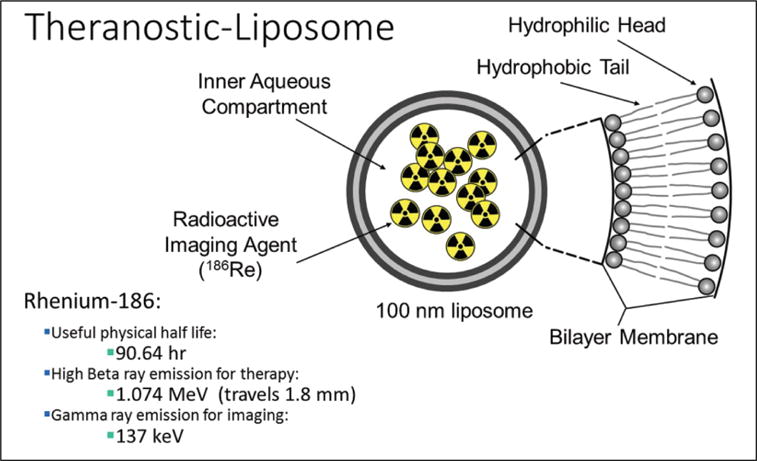
Theranostic liposome formulation with rhenium-186 encapsulated in the aqueous core. Rhenium-186 has several favorable properties for both therapy and imaging.
PET is another nuclear imaging technique that uses positron emission to create two 511 keV gamma ray photons that are emitted in opposite directions. These photons are detected simultaneously at 180° from one another within a PET scintillation ring. Although PET suffers from poor spatial resolution, PET is relatively sensitive and is quantitative, making it well suited to evaluate patient response to medical interventions over time. Because of its long half-life (12.7 h) of copper-64 (64Cu), many PET tracers have included this radionuclide because it is short enough to be compatible with rapid pharmacokinetics such as small molecules as well as compatible with the time scales required for optimal biodistribution of slower compounds such as nanoparticles and antibodies.84
To that end, Li et al.38 developed integrin αvβ3-targeted T7 phage nanoparticles expressing arginylglycylaspartic acid peptides on its surface and conjugated with a novel bifunctional chelator, 4-((8-amino-3,6,10,13,16,19-hexaazabicyclo [6.6.6] ico-sane-1-ylamino) methyl) benzoic acid (AmBaSar) for 64Cu chelation with the NIRF dye Cy5.5. The T7 nanoparticles were confirmed to bind integrin αvβ3 via enzyme-linked immunosorbent assays and enhanced internalization to SKOV3 ovarian cancer cells. In mice bearing tumors with integrin αvβ3 expression, radiolabeled phage nanoparticles showed enhanced tumor uptake using microPET over nanoparticles with 1,4,7,10-tetraazadodecane-N,N′,N″,N″′-tetraacetic acid and reached a plateau after 4 h postinjection. Moreover, lower liver uptake was found for nanoparticles functionalized with AmBaSar, confirming that the choice of the chelator can significantly affect imaging results and theranostic capabilities.
Because of the high surface-to-volume ratio of nanoparticles and the advances and sophistication in surface modification, it has become increasingly common to incorporate multimodal imaging capabilities, as is the case with other imaging modalities. England and colleagues39 developed 64Cu-labeled reduced graphene oxide (RGO)–based iron oxide nanoparticles capable MRI, photo acoustic imaging, and PET imaging to monitor nanoparticle-mediated drug delivery through the enhanced permeability and retention effect in the context of peripheral arterial disease, specifically for critical limb ischemia. In a mouse model of critical limb ischemia, RGO nanoparticles were found to have the highest accumulation after nanoparticle injection 3 d after surgery, compared with administration at 10 and 17 d, and confirmed that nanoparticle accumulation depends on the extent of ischemia in the hind limb when angiogenesis is the highest. Because RGO nanoparticles absorb light in the NIR, photoacoustic imaging was performed by exciting the nanoparticles with an 808 nm laser. Using photoacoustic imaging, ultrasound waves were used to visualize the legs (brightness mode, B-mode), whereas the photoacoustic transducers and excitation lasers were used to produce a photoacoustic image (photoacoustic mode). The highest photoacoustic signal was found in the ischemic hind limb compared with minimal background single in preinjected hind limbs, further confirming RGO nanoparticle accumulation and its potential for multimodal imaging. The current study provided the groundwork regarding RGO nanoparticles to monitor nanoparticle-mediated drug delivery using PET and photoacoustic imaging. The authors suggest future studies with active targeting elements could further enhance localization of RGO nanoparticles in ischemic tissues.
CT
CT is often used to provide complementary anatomical information alongside other imaging modalities such as PET, which provides information about physiological processes. In CT, computer-processed x-rays are used to produce tomographic images, or “slices,” that are compiled to obtain images of anatomical structures. The difference in atomic number and electron density of the tissues results in varying degrees of x-ray attenuation, and hence, nanoparticles containing electron-dense elements with high atomic number such as iodine, bismuth, or gold have been studied as CT contrast agents.
Cy5.5-labeled, glycol-chitosan–coated gold nanoparticles were synthesized and conjugated with fibrin-targeting peptides as a CT contrast agent to visualize cerebrovascular thrombi and guide thrombolytic therapy using tissue plasminogen activator (tPA).40 tPA is the only FDA-approved therapy for acute ischemic stroke and is used to lyse thrombi. However, the dosing could be personalized such that thrombolysis is effective and hemorrhagic complications are minimized. To that end, Kim et al.40 used the fibrin-targeting peptides (tyrosine-D-glutamine-cysteine-hydroxyproline-L-3-chlorotyrosine-glysine-leucine-cysteine-tyrosineisoleucine-glutamine) on gold nanoparticles and found there were clear differences between targeted and nontargeted nanoparticles on cerebral CT imaging in an embolytic stroke mouse model. Not only was thrombi detected, but the amount of thrombus present was also quantified. Upon administration of tPA, reduced thrombus area was found and post-tPA residual thrombus burden after 24 h correlated with the final infarct size, confirming the success of quantitating thrombolytic therapy.
Bismuth sulfide nanorods were developed by Liu et al.41 for multispectral optoacoustic tomography and x-ray CT-guided photothermal therapy as a precision cancer nanomedicine. Photothermal therapy employs a light-harvesting agent for localized conversion of NIR light into heat to ablate cancer cells. Using multimodal imaging with the high resolution of CT in three-dimensional tissue reconstruction and the high spatial resolution for soft tissues of multispectral optoacoustic tomography, Liu et al. aimed to provide a theranostic nanoparticle capable of real-time disease diagnosis, guidance to procedures, therapeutic response monitoring, and disease treatment with greater specificity and sensitivity. In vitro, nanorods were steadily internalized for up to 48 h in the murine breast cancer cell line 4T1, and the photothermal effect and cell death increased with increasing nanorods or increasing laser power densities upon irradiation for 8 min. In vivo, tumor-bearing mice injected with nanorods showed enhanced contrast in tumors using multispectral optoacoustic tomography. CT also corroborated these results, and photothermal therapy was performed. The tumor surface temperature increased from 29 °C to 68 °C during the 15 min of laser exposure and stopped tumor growth. Although nanorods were also found to be present in the liver, no toxicity was found, confirming the use of bismuth-based nanorods as theranostic tools for CT and multispectral optoacoustic tomography and directed photothermal therapy.
Future Perspectives
The field of theranostics has inspired the development of several classes of promising therapeutic and diagnostic vehicles. However, to successfully bring these agents to the clinic, a major challenge that has confronted the conventional drug development process must also be addressed. Although a plethora of preclinical studies has demonstrated that nanoparticles can markedly improve treatment efficacy and safety for a wide spectrum of disease indications, the simultaneous functionalization of nanoparticles with multiple agents, such as therapeutic and imaging compounds, presents unique barriers to successful translation. It is important to note that combination therapy with or without the use of nanotechnology creates a drug and dose space that precludes global optimization when conventional drug development methodologies are used. Standard strategies include the use of disease targets to select drug compounds (e.g., siRNA, small molecules) and the use of dose escalation to select the adequate administration parameters in an unmodified or nanotechnology-modified fashion. Although these approaches may result in enhanced preclinical efficacy, there is room for optimization. A recent clinical study demonstrated that drug synergy and antagonism are dose dependent.85 This is a major issue that should be considered when designing drug combinations. Drug synergy at the in vitro and preclinical levels may not translate into the clinic. Furthermore, given this information, global optimization is actually a function of simultaneously selecting the right drugs and their respective dose ratios. Unfortunately, this need creates a been shown that agnostic optimization can result in the best possible treatment efficacy and safety in a mechanism-independent fashion that is applicable to all disease indications and patients.88 PPM has been successfully applied to nanomedicine drug delivery in the area of nanodiamond-based breast cancer treatment. This study demonstrated that PPM-optimized nanodiamond/drug combinations outperformed nanodiamond-modified and unmodified monotherapy, as well as arbitrarily designed nanodiamond/drug treatment. Importantly, the measure of efficacy for this study was the difference between the apoptosis of multiple breast cancer lines as well as the viability of multiple control cell lines, demonstrating the multiparametric optimization capabilities of PPM (Fig. 4). Because PPM has also been validated in the clinic, it represents a translationally relevant technology platform to optimize unmodified and nanotechnology-modified drug delivery and imaging.90–94 Moreover, in addition to reconciling the disparity of dosage requirement between diagnostic and therapeutic entities within a single nanoparticle platform, such emerging technologies can be used to reconcile the differences in circulation times that will be needed to realize the potential of theranostic nanoparticles.
Figure 4.
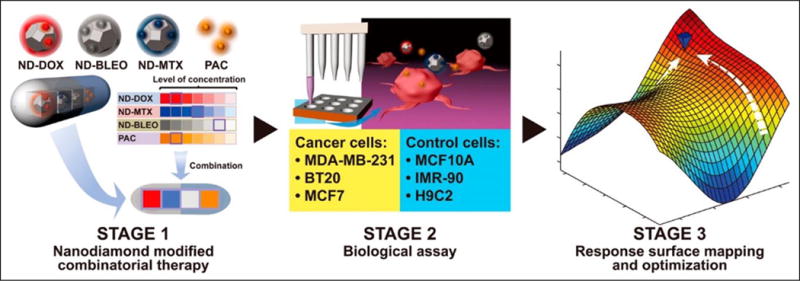
Phenotypic personalized medicine (PPM) was used to optimize nanomedicine drug combinations. PPM-mediated drug combination design markedly outperformed the efficacy and safety of monotherapy (nano and nonnano) and arbitrary nanomedicine drug combinations. Reprinted with permission from Wang et al.89 ACS Nano, 9(3):3322–3344. Copyright (2015) American Chemical Society.
Acknowledgments
The authors would like to acknowledge the financial support from the National Cancer Institute (National Institutes of Health) under award K22 CA160834 as well as R21 CA184608 granted to C.Z.; the National Science Foundation CAREER Award (CMMI-1350197), Center for Scalable and Integrated NanoManufacturing (DMI-0327077), CMMI-0856492, DMR-1343991, OISE-1444100, V Foundation for Cancer Research Scholars Award, Wallace H. Coulter Foundation Translational Research Award, National Cancer Institute grant U54CA151880 (the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health), Society for Laboratory Automation and Screening Endowed Fellowship, Beckman Coulter Life Sciences, and the American Academy of Implant Dentistry Research Foundation under grant number 20150460 awarded to D.H.; and the National Heart, Lung, and Blood Institute (National Institutes of Health) under award R00HL124279, Eli and Edythe Broad Innovation Award, and the L.K. Whittier Foundation Non-Cancer Translational Research Award granted to E.J.C.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kreuter J. Nanoparticles—A Historical Perspective. Int J Pharmaceut. 2007;331:1–10. doi: 10.1016/j.ijpharm.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Feynman R. Plenty of Room at the Bottom. http://www.its.caltech.edu/~feynman/plenty.html.
- 3.Drexler KE. Molecular Engineering: An Approach to the Development of General Capabilities for Molecular Manipulation. Proc Natl Acad Sci US A. 1981;78:5275–5278. doi: 10.1073/pnas.78.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sessa G, Weissmann G. Phospholipid Spherules (Liposomes) as a Model for Biological Membranes. J Lipid Res. 1968;9:310–318. [PubMed] [Google Scholar]
- 5.Barenholz Y. Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J Control Rel. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Acar H, Srivastava S, Chung EJ, et al. Self-Assembling Peptide-Based Building Blocks in Medical Applications. Adv Drug Deliv Rev. 2017;110–111:65–79. doi: 10.1016/j.addr.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung EJ. Targeting and Therapeutic Peptides in Nanomedicine for Atherosclerosis. Exp Biol Med. 2016;241:891–898. doi: 10.1177/1535370216640940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marciel AB, Chung EJ, Brettmann BK, et al. Bulk and Nanoscale Polypeptide Based Polyelectrolyte Complexes. Adv Colloid Interf Sci. 2017;239:187–198. doi: 10.1016/j.cis.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khodabandehlou K, Masehi-Lano JJ, Poon C, et al. Targeting Cell Adhesion Molecules with Nanoparticles Using In Vivo and Flow-Based In Vitro Models of Atherosclerosis. Exp Biol Med. 2017;242:799–812. doi: 10.1177/1535370217693116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy Chowdhury M, Schumann C, Bhakta-Guha D, et al. Cancer Nanotheranostics: Strategies, Promises and Impediments. Biomed Pharmacother. 2016;84:291–304. doi: 10.1016/j.biopha.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Qiu H, Prasad PN, et al. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem Rev. 2014;114:5161–5214. doi: 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janib SM, Moses AS, MacKay JA. Imaging Drug Delivery Using Theranostic Nanoparticles. Adv Drug Deliv Rev. 2010;62:1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryu JH, Lee S, Son S, et al. Theranostic Nanoparticles for Future Personalized Medicine. J Control Rel. 2014;190:477–484. doi: 10.1016/j.jconrel.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Kelkar SS, Reineke TM. Theranostics: Combining Imaging Therapy. Bioconjug Chem. 2011;22:1879–1903. doi: 10.1021/bc200151q. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder A, Levins CG, Cortez C, et al. Lipid-Based Nanotherapeutics for siRNA Delivery. J Intern Med. 2010;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Niu G, Chen X. Polymeric Materials for Theranostic Applications. Pharmaceut Res. 2014;31:1358–1376. doi: 10.1007/s11095-013-1103-7. [DOI] [PubMed] [Google Scholar]
- 17.Zou L, Wang H, He B, et al. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics. 2016;6:762–772. doi: 10.7150/thno.14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L, Staley C, Kooby D, et al. Current Status of Biomarker and Targeted Nanoparticle Development: The Precision Oncology Approach for Pancreatic Cancer Therapy. Cancer Lett. 2017;388:139–148. doi: 10.1016/j.canlet.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melancon MP, Stafford RJ, Li C. Challenges to Effective Cancer Nanotheranostics. J Control Rel. 2012;164:177–182. doi: 10.1016/j.jconrel.2012.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Y, Huang L, Yang Y, et al. A Programmed Nanoparticle with Self-Adapting for Accurate Cancer Cell Eradication and Therapeutic Self-Reporting. Theranostics. 2017;7:1245–1256. doi: 10.7150/thno.18187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar SK, Khater Y, Kulkarni A, et al. Feedback-Mediated Cancer Therapy: A FRET-Based Nanoreporter Approach. Proc SPIE. 2014:916607–916607-8. [Google Scholar]
- 22.Prigodich AE, Seferos DS, Massich MD, et al. Nano-Flares for mRNA Regulation and Detection. ACS Nano. 2009;3:2147–2152. doi: 10.1021/nn9003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi KY, Jeon EJ, Yoon HY, et al. Theranostic Nanoparticles Based on PEGylated Hyaluronic Acid for the Diagnosis, Therapy and Monitoring of Colon Cancer. Biomaterials. 2012;33:6186–6193. doi: 10.1016/j.biomaterials.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi KY, Chung H, Min KH, et al. Self-Assembled Hyaluronic Acid Nanoparticles for Active Tumor Targeting. Biomaterials. 2010;31:106–114. doi: 10.1016/j.biomaterials.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Huang X, Zhang F, Wang H, et al. Mesenchymal Stem Cell-Based Cell Engineering with Multifunctional Mesoporous Silica Nanoparticles for Tumor Delivery. Biomaterials. 2013;34:1772–1780. doi: 10.1016/j.biomaterials.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul A, Jana A, Karthik S, et al. Photoresponsive Real Time Monitoring Silicon Quantum Dots for Regulated Delivery of Anticancer Drugs. J Mater Chem B. 2016;4:521–528. doi: 10.1039/c5tb02045j. [DOI] [PubMed] [Google Scholar]
- 27.Sun C, Gao M, Zhang X. Surface-Enhanced Raman Scattering (SERS) Imaging-Guided Real-Time Photothermal Ablation of Target Cancer Cells Using Polydopamine-Encapsulated Gold Nanorods as Multifunctional Agents. Anal Bioanal Chem. 2017;409:4915–4926. doi: 10.1007/s00216-017-0435-2. [DOI] [PubMed] [Google Scholar]
- 28.Song J, Yang X, Yang Z, et al. Rational Design of Branched Nanoporous Gold Nanoshells with Enhanced Physico-Optical Properties for Optical Imaging and Cancer Therapy. ACS Nano. 2017;11:6102–6113. doi: 10.1021/acsnano.7b02048. [DOI] [PubMed] [Google Scholar]
- 29.Guo Y, Chen W, Wang W, et al. Simultaneous Diagnosis and Gene Therapy of Immuno-Rejection in Rat Allogeneic Heart Transplantation Model Using a T-Cell-Targeted Theranostic Nanosystem. ACS Nano. 2012;6:10646–10657. doi: 10.1021/nn3037573. [DOI] [PubMed] [Google Scholar]
- 30.Crisci E, Fraile L, Novellas R, et al. In Vivo Tracking Immunological Properties of Pulsed Porcine Monocyte-Derived Dendritic Cells. Mol Immunol. 2015;63:343–354. doi: 10.1016/j.molimm.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Bonetto F, Srinivas M, Heerschap A, et al. A Novel 19F Agent for Detection and Quantification of Human Dendritic Cells Using Magnetic Resonance Imaging. Int J Cancer. 2011;129:365–373. doi: 10.1002/ijc.25672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaittanis C, Shaffer TM, Ogirala A, et al. Environment-Responsive Nanophores for Therapy and Treatment Monitoring via Molecular MRI Quenching. Nat Comm. 2014;(5):3384. doi: 10.1038/ncomms4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohan P, Rapoport N. Doxorubicin as a Molecular Nanotheranostic Agent: Effect of Doxorubicin Encapsulation in Micelles or Nanoemulsions on the Ultrasound-Mediated Intracellular Delivery and Nuclear Trafficking. Mol Pharm. 2010;7:1959–1973. doi: 10.1021/mp100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao S, Wang G, Qin Z, et al. Oxygen-Generating Hybrid Nanoparticles to Enhance Fluorescent/Photoacoustic/Ultrasound Imaging Guided Tumor Photodynamic Therapy. Bomaterials. 2017;112:324–335. doi: 10.1016/j.biomaterials.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 35.Kempen PJ, Greasley S, Parker KA, et al. Theranostic Mesoporous Silica Nanoparticles Biodegrade after Pro-Survival Drug Delivery and Ultrasound/Magnetic Resonance Imaging of Stem Cells. Theranostics. 2015;(5):631. doi: 10.7150/thno.11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JY, Crake C, Teo B, et al. Ultrasound-Enhanced siRNA Delivery Using Magnetic Nanoparticle-Loaded Chitosan-Deoxycholic Acid Nanodroplets. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201601246. [DOI] [PubMed] [Google Scholar]
- 37.Zavaleta CL, Goins BA, Bao A, et al. Imaging of 186Re-Liposome Therapy in Ovarian Cancer Xenograft Model of Peritoneal Carcinomatosis. J Drug Target. 2008;16:626–637. doi: 10.1080/10611860802230372. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Jin Q, Huang C, et al. Trackable and Targeted Phage as Positron Emission Tomography (PET) Agent for Cancer Imaging. Theranostics. 2011;(1):371. doi: 10.7150/thno/v01p0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.England CG, Im HJ, Feng L, et al. Re-assessing the Enhanced Permeability and Retention Effect in Peripheral Arterial Disease Using Radiolabeled Long Circulating Nanoparticles. Biomaterials. 2016;100:101–109. doi: 10.1016/j.biomaterials.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JY, Ryu JH, Schellingerhout D, et al. Direct Imaging of Cerebral Thromboemboli Using Computed Tomography and Fibrin-Targeted Gold Nanoparticles. Theranostics. 2015;(5):1098. doi: 10.7150/thno.11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Zheng X, Yan L, et al. Bismuth Sulfide Nanorods as a Precision Nanomedicine for in Vivo Multimodal Imaging-Guided Photothermal Therapy of Tumor. ACS Nano. 2015;9:696–707. doi: 10.1021/nn506137n. [DOI] [PubMed] [Google Scholar]
- 42.Basuki JS, Duong HTT, Macmillan A, et al. Using Fluorescence Lifetime Imaging Microscopy to Monitor Theranostic Nanoparticle Uptake and Intracellular Doxorubicin Release. ACS Nano. 2013;7:10175–10189. doi: 10.1021/nn404407g. [DOI] [PubMed] [Google Scholar]
- 43.Chung EJ, Mlinar LB, Nord K, et al. Monocyte-Targeting Supramolecular Micellar Assemblies: A Molecular Diagnostic Tool for Atherosclerosis. Adv Healthc Mater. 2015;4:367–376. doi: 10.1002/adhm.201400336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung EJ, Mlinar LB, Sugimoto MJ, et al. In Vivo Biodistribution Clearance of Peptide Amphiphile Micelles. Nanomed Nanotechnol Biol Med. 2015;11:479–487. doi: 10.1016/j.nano.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Santra S, Kaittanis C, Perez JM. Cytochrome c Encapsulating Theranostic Nanoparticles: A Novel Bifunctional System for Targeted Delivery of Therapeutic Membrane-Impermeable Proteins to Tumors and Imaging of Cancer Therapy. Mol Pharm. 2010;7:1209–1222. doi: 10.1021/mp100043h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang X, Swierczewska M, Choi KY, et al. Multiplex Imaging of an Intracellular Proteolytic Cascade by Using a Broad-Spectrum Nanoquencher. Angewandte Chemie. 2012;51:1625–1630. doi: 10.1002/anie.201107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Liang YC, Lin X, et al. Self-Monitoring and Self-Delivery of Photosensitizer-Doped Nanoparticles for Highly Effective Combination Cancer Therapy In Vitro and In Vivo. ACS Nano. 2015;9:9741–9756. doi: 10.1021/acsnano.5b02513. [DOI] [PubMed] [Google Scholar]
- 48.Ding Q, Zhan Q, Zhou X, et al. Theranostic Upconversion Nanobeacons for Tumor mRNA Ratiometric Fluorescence Detection and Imaging-Monitored Drug Delivery. Small. 2016;12:5944–5953. doi: 10.1002/smll.201601724. [DOI] [PubMed] [Google Scholar]
- 49.He X, Gao J, Gambhir SS, et al. Near-Infrared Fluorescent Nanoprobes for Cancer Molecular Imaging: Status and Challenges. Trends Mol Med. 2010;16:574–583. doi: 10.1016/j.molmed.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Q, Du Y, Li Y, et al. Gx1-Conjugated Endostar Nanoparticle: A New Drug Delivery System for Anti-Colorectal Cancer In Vivo. Proc SPIE. 2014:895606–895606-7. [Google Scholar]
- 51.Bremer C, Tung C-H, Weissleder R. In Vivo Molecular Target Assessment of Matrix Metalloproteinase Inhibition. Nat Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 52.Yang L, Sajja HK, Cao Z, et al. uPAR-Targeted Optical Imaging Contrasts as Theranostic Agents for Tumor Margin Detection. Theranostics. 2014;4:106–118. doi: 10.7150/thno.7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo Z, Zheng M, Zhao P, et al. Self-Monitoring Artificial Red Cells with Sufficient Oxygen Supply for Enhanced Photodynamic Therapy. Sci Rep. 2016;6:23393. doi: 10.1038/srep23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan WB, Jiang S, Zhang Y. Quantum-Dot Based Nanoparticles for Targeted Silencing of HER2/neu Gene via RNA Interference. Biomaterials. 2007;28:1565–1571. doi: 10.1016/j.biomaterials.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 55.Zavaleta CL, Kircher MF, Gambhir SS. Raman’s “Effect” on Molecular Imaging. J Nucl Med. 2011;52:1839–1844. doi: 10.2967/jnumed.111.087775. [DOI] [PubMed] [Google Scholar]
- 56.Fleischmann M, Hendra PJ, McQuillan AJ. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem Phys Lett. 1974;26:163–166. [Google Scholar]
- 57.Keren S, Zavaleta C, Cheng Z, et al. Noninvasive Molecular Imaging of Small Living Subjects Using Raman Spectroscopy. Proc Natl Acad Sci USA. 2008;105:5844–5849. doi: 10.1073/pnas.0710575105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qian X, Zhou X, Nie S. Surface-Enhanced Raman Nanoparticle Beacons Based on Bioconjugated Gold Nanocrystals Long Range Plasmonic Coupling. J Am Chem Soc. 2008;130:14934–14935. doi: 10.1021/ja8062502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bohndiek SE, Wagadarikar A, Zavaleta CL, et al. A Small Animal Raman Instrument for Rapid, Wide-Area, Spectroscopic Imaging. Proc Natl Acad Sci USA. 2013;110:12408–12413. doi: 10.1073/pnas.1301379110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garai E, Sensarn S, Zavaleta CL, et al. A Real-Time Clinical Endoscopic System for Intraluminal, Multiplexed Imaging of Surface-Enhanced Raman Scattering Nanoparticles. PloS One. 2015;10:e0123185. doi: 10.1371/journal.pone.0123185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jokerst JV, Miao Z, Zavaleta C, et al. Affibody-Functionalized Gold-Silica Nanoparticles for Raman Molecular Imaging of the Epidermal Growth Factor Receptor. Small. 2011;7:625–633. doi: 10.1002/smll.201002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kircher MF, de la Zerda A, Jokerst JV, et al. A Brain Tumor Molecular Imaging Strategy Using a New Triple-Modality MRI-Photoacoustic-Raman Nanoparticle. Nat Med. 2012;18:829–834. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McVeigh PZ, Mallia RJ, Veillieux I, et al. Development of Widefield SERS Imaging Endoscope. Proc SPIE. 2012;8217 [Google Scholar]
- 64.Wang Y, Kang S, Khan A, et al. Quantitative Molecular Phenotyping with Topically Applied SERS Nanoparticles for Intraoperative Guidance of Breast Cancer Lumpectomy. Sci Rep. 2016;6:21242. doi: 10.1038/srep21242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang YW, Doerksen JD, Kang S, et al. Multiplexed Molecular Imaging of Fresh Tissue Surfaces Enabled by Convection-Enhanced Topical Staining with SERS-Coded Nanoparticles. Small. 2016;12:5612–5621. doi: 10.1002/smll.201601829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie HN, Stevenson R, Stone N, et al. Tracking Bisphosphonates Through a 20 mm Thick Porcine Tissue by Using Surface-Enhanced Spatially Offset Raman Spectroscopy. Angewandte Chemie. 2012;51:8509–8511. doi: 10.1002/anie.201203728. [DOI] [PubMed] [Google Scholar]
- 67.Zavaleta CL, Garai E, Liu JT, et al. A Raman-Based Endoscopic Strategy for Multiplexed Molecular Imaging. Proc Natl Acad Sci USA. 2013;110:E2288–E2297. doi: 10.1073/pnas.1211309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zavaleta CL, Smith BR, Walton I, et al. Multiplexed Imaging of Surface Enhanced Raman Scattering Nanotags in Living Mice Using Noninvasive Raman Spectroscopy. Proc Natl Acad Sci USA. 2009;106:13511–13516. doi: 10.1073/pnas.0813327106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang C, Ravi S, Garapati US, et al. Multifunctional Chitosan Magnetic-Graphene (CMG) Nanoparticles: A Theranostic Platform for Tumor-Targeted Co-Delivery of Drugs, Genes and MRI Contrast Agents. J Mater Chem B. 2013;1:4396–4405. doi: 10.1039/C3TB20452A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen O, Riedemann L, Etoc F, et al. Magneto-Fluorescent Core-Shell Supernanoparticles. Nat Comm. 2014;5:5093. doi: 10.1038/ncomms6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoo SP, Pineda F, Barrett JC, et al. Gadolinium-Functionalized Peptide Amphiphile Micelles for Multimodal Imaging of Atherosclerotic Lesions. ACS Omega. 2016;1:996–1003. doi: 10.1021/acsomega.6b00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park IK, Ng CP, Wang J, et al. Determination of Nanoparticle Vehicle Unpackaging by MR Imaging of a T2 Magnetic Relaxation Switch. Biomaterials. 2008;29:724–732. doi: 10.1016/j.biomaterials.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 73.Lee GY, Qian WP, Wang L, et al. Theranostic Nanoparticles with Controlled Release of Gemcitabine for Targeted Therapy and MRI of Pancreatic Cancer. ACS Nano. 2013;7:2078–2089. doi: 10.1021/nn3043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung EJ, Tirrell M. Recent Advances in Targeted Self-Assembling Nanoparticles to Address Vascular Damage due to Atherosclerosis. Adv Healthc Mater. 2015;4:2408–2422. doi: 10.1002/adhm.201500126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bao A, Goins B, Klipper R, et al. A Novel Liposome Radiolabeling Method Using 99mTc-”SNS/S” Complexes: In Vitro and In Vivo Evaluation. J Pharm Sci. 2003;92:1893–1904. doi: 10.1002/jps.10441. [DOI] [PubMed] [Google Scholar]
- 76.Bao A, Phillips WT, Goins B, et al. Potential Use of Drug Carried-Liposomes for Cancer Therapy via Direct Intratumoral Injection. Int J Pharm. 2006;316:162–169. doi: 10.1016/j.ijpharm.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 77.Goins B, Bao A, Phillips WT. Techniques for Loading Technetium-99m Rhenium-186/188 Radionuclides into Preformed Liposomes for Diagnostic Imaging Radionuclide Therapy. Methods Mol Biol. 2017;1522:155–178. doi: 10.1007/978-1-4939-6591-5_13. [DOI] [PubMed] [Google Scholar]
- 78.Goins B, Klipper R, Rudolph AS, et al. Biodistribution and Imaging Studies of Technetium-99m-Labeled Liposomes in Rats with Focal Infection. J Nucl Med. 1993;34:2160–2168. [PubMed] [Google Scholar]
- 79.Goins B, Klipper R, Rudolph AS, et al. Use of Technetium-99m-Liposomes in Tumor Imaging. J Nucl Med. 1994;35:1491–1498. [PubMed] [Google Scholar]
- 80.Phillips WT, Goins B. Assessment of Liposome Delivery Using Scintigraphic Imaging. J Liposome Res. 2002;12:71–80. doi: 10.1081/lpr-120004779. [DOI] [PubMed] [Google Scholar]
- 81.Phillips WT, Klipper R, Goins B. Use of (99m)Tc-Labeled Liposomes Encapsulating Blue Dye for Identification of the Sentinel Lymph Node. J Nucl Med. 2001;42:446–451. [PubMed] [Google Scholar]
- 82.Zavaleta CL, Phillips WT, Soundararajan A, et al. Use of Avidin/Biotin-Liposome System for Enhanced Peritoneal Drug Delivery in an Ovarian Cancer Model. Int J Pharm. 2007;337:316–328. doi: 10.1016/j.ijpharm.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 83.Brenner AJ. Maximum Tolerated Dose, Safety and Efficacy of Rhenium Nanoliposomes in Recurrent Glioblastoma. https://clinicaltrials.gov/ct2/results?term=Andrew+Brenner %2C+M.D.%2C+Ph.D&Search=Search (accessed Jun. 16, 2017)
- 84.Anderson CJ, Ferdani R. Copper-64 Radiopharmaceuticals for PET Imaging of Cancer: Advances in Preclinical Clinical Research. Cancer Biother Radiopharm. 2009;24:379–393. doi: 10.1089/cbr.2009.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zarrinpar A, Lee DK, Silva A, et al. Individualizing Liver Transplant Immunosuppression Using a Phenotypic Personalized Medicine Platform. Sci Transl Med. 2016;8:333ra49–333ra49. doi: 10.1126/scitranslmed.aac5954. [DOI] [PubMed] [Google Scholar]
- 86.Ho D, Zarrinpar A, Chow EK-H. Diamonds Digital Health and Drug Development: Optimizing Combinatorial Nanomedicine. ACS Nano. 2016;10:9087–9092. doi: 10.1021/acsnano.6b06174. [DOI] [PubMed] [Google Scholar]
- 87.Mohd Abdul Rashid MB, Toh TB, Silva A, et al. Identification and Optimization of Combinatorial Glucose Metabolism Inhibitors in Hepatocellular Carcinomas. J Lab Autom. 2015;20:423–437. doi: 10.1177/2211068215579612. [DOI] [PubMed] [Google Scholar]
- 88.Lee DK, Chang VY, Kee T, et al. Optimizing Combination Therapy for Acute Lymphoblastic Leukemia Using a Phenotypic Personalized Medicine Digital Health Platform. SLAS Technol. 2017;22:276–288. doi: 10.1177/2211068216681979. [DOI] [PubMed] [Google Scholar]
- 89.Wang H, Lee DK, Chen KY, et al. Mechanism-Independent Optimization of Combinatorial Nanodiamond and Unmodified Drug Delivery Using a Phenotypically Driven Platform Technology. ACS Nano. 2015;9:3332–3344. doi: 10.1021/acsnano.5b00638. [DOI] [PubMed] [Google Scholar]
- 90.Su CL. Finding the Discrepancy of MRI Images Using the Second Moment and Geometric Comparison Techniques. J Lab Autom. 2000;5(4):87–91. [Google Scholar]
- 91.Tsai N, Lee B, Kim A, et al. Nanomedicine for Global Health. J Lab Autom. 2014;19:511–516. doi: 10.1177/2211068214538263. [DOI] [PubMed] [Google Scholar]
- 92.Han S-J, Wang S. Magnetic Nanotechnology for Biodetection. J Lab Autom. 2010;15:93–98. [Google Scholar]
- 93.Chow EKH. Implication of Cancer Stem Cells in Cancer Drug Development and Drug Delivery. J Lab Autom. 2013;18:6–11. doi: 10.1177/2211068212454739. [DOI] [PubMed] [Google Scholar]
- 94.Fong ELS, Toh TB, Yu H, et al. 3D Culture as a Clinically Relevant Model for Personalized Medicine. SLAS Technol. 2017;22:245–253. doi: 10.1177/2472630317697251. [DOI] [PubMed] [Google Scholar]


