Abstract
Ultrasound that is widely used in medical diagnosis has drawn growing interests as a noninvasive means of neuromodulation. Focused pulsed ultrasound (FPUS) effectively modulates neural encoding and transmission in the peripheral nervous system (PNS) with unclear mechanism of action, which is further confounded by contradictory experimental outcomes from recordings of compound action potentials (CAP). To address that, we developed a novel in vitro set up to achieve simultaneous single-unit recordings from individual mouse sciatic nerve axon and systematically studied the neuromodulation effects of FPUS on individual axon. Unlike previous CAP recordings, our single-unit recordings afford superior spatial and temporal resolution to reveal the subtle but consistent effects of ultrasonic neuromodulation. Our results indicate that, 1) FPUS did not evoke action potentials directly in mouse sciatic nerve at all the tested intensities (spatial peak temporal average intensity, ISPTA of 0.91 to 28.2 W/cm2); 2) FPUS increases the nerve conduction velocity (CV) in both fast-conducting A- and slow-conducting C- type axons with effects more pronounced at increased stimulus duration and intensity; and 3) effects of increased CV is reversible and cannot be attributed to the change of local temperature. Our results support existing theories of non-thermal mechanisms underlying ultrasonic neuromodulation with low-intensity FPUS, including NICE, flexoelectricity, and solition models. This work also provides a solid experimental basis to further advance our mechanistic understandings of ultrasonic neuromodulation in the PNS.
Introduction
Ultrasound (US) has prevailed the field of medical diagnosis for long but yet to be established as a therapeutic paradigm. Neuromodulatory effects of US were first reported in 1929 when Harvey showed that innervated skeletal muscles from frogs and turtles responded to US stimulation in vitro (1). More recent researches focus on the US effects at the central nervous system (CNS), including the disruption of blood brain barrier, motor and sensory responses, and suppressed or evoked action potentials e.g., (2–12), which culminated in the approval of the Food and Drug Administration (FDA) to treat refractory patients with essential tremor using MRI-guided focused ultrasound (13). In addition, ex vivo studies on hippocampal slice cultures have shown that, low-intensity FPUS can elicit electrical activities as indicated by calcium imaging from mouse (14) and simultaneously enhance (at fiber volley) and suppress (at dendritic layers) compound action potentials (CAP) from rat hippocampal dentate gyrus (15).
In contrast, the effects of US on the peripheral nervous system (PNS) is comparatively understudied with inconsistent reports. US appears to evoke or enhance the peripheral neural activities in frog sciatic nerve in vitro (16), sensitize neuron in C. elegans (17), cause deqi sensations (i.e., tingling, numbness, heaviness, and fullness) by stimulation of an acupuncture point (LI4, He Gu) (18), and induce somatosensory sensations in human skin (19). In contrast, US also reportedly attenuate or block peripheral neural activities, including inhibition of rat rhythmic bladder contraction by posterior tibial nerve stimulation (20), conduction block or suppression of sciatic nerves in vitro by high intensity focused ultrasound in normal and neuropathic rats (21) and bullfrogs (22), suppressed conduction in rat vagal nerve by low-intensity FPUS (23). In addition, Mihran et al. reported both enhanced and suppressed effects from US stimulation of frog sciatic nerve in vitro (24). It is worth mentioning that, the above studies unanimously rely on CAP to assess the effects of US neuromodulation, i.e., a summation of extracellular neural activities from a population of peripheral nerve axons which is highly dependent upon the particular nerve-electrode configuration during the recording.
In this study, we revealed the neuromodulatory effects of FPUS on mouse sciatic nerves in vitro by single-unit recordings from individual nerve axon. To the best of our knowledge, this is the first ever attempt to systematically study the effects of ultrasonic stimulation on mammalian peripheral nerves at single neuron/axon resolution. Through single-unit recordings from individual peripheral nerve axon, we have observed the subtle but consistent increase of neural conduction velocity following FPUS stimulation, which returns to pre-stimulus state within minutes after terminating the FPUS stimulation.
Methods
All experimental procedures involving animals were performed in compliance with the standards set by the University of Connecticut Institutional Animal Care and Use Committee (IACUC).
In vitro setup to allow single-unit recoding and US stimulation
To achieve reliable in vitro single-unit recordings from sciatic nerve and robust delivery of focused ultrasonic stimulation, we developed a custom-built three-chamber setup as schemed in Fig. 1A, which consists of a tissue chamber perfused with Krebs solution, a nerve recording chamber covered with paraffin oil, and a cone-shaped water chamber underneath to ensure the alignment of the focal point of the disc-shaped ultrasonic transducer with the nerve segment to be modulated. The cone-shaped water chamber is separated from the tissue chamber by a 0.2mm-thick polystyrene film allowing a minimal attenuation of ultrasonic energy. The main sciatic nerve segment (~25mm) is placed in the tissue chamber perfused with Krebs solution (4–6 mL/min) which contains (in mM): 117.9 NaCl, 4.7 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4, 2.5 CaCl2, and 11.1 D-Glucose, is oxygenated by 95% O2 and 5% CO2 and maintained at 30–32°C by a heated circulating bath (1130-A, Poly Science, Niles, IL). The recording chamber is separated from the tissue chamber by a gate with a mouse hole at the bottom, allowing the ~5mm distal sciatic nerve to be pulled into the recording chamber and placed onto a glass mirror for nerve fiber splitting. The recording chamber is covered with mineral oil (Fisher Scientific, Waltham, MA) to enhance the recording single-to-noise ratio (25). The solution volume in the tissue chamber was limited to be no more than 6 mL to allow periodic replacement of chamber solution (within 1 min for 6mL/min flow). The nerve was laid on the surface with the proximal end in tissue chamber and the distal end in recording chamber. The bottom surface of the perfusion chamber was covered by a layer of silicone (Sylgard 182 Silicone Elastomer, Dow Corning, Midland, MI) for the ease of pinning down the nerve.
Figure 1.

Schematic diagram of the in vitro single-unit recording setup to assess the neuromodulatory effects of focused pulsed ultrasound (FPUS). (A) The setup consists of a tissue chamber perfused with Krebs solution, a recording chamber covered with paraffin oil and a water chamber for delivering FPUS. (B) Isometric view of the custom-built tissue chamber and recording chamber.
Mouse sciatic nerve harvesting and splitting
Sciatic nerves were harvested from male C57BL/6 mice aged 6–8 weeks, 20–30 g (Taconic, Germantown, NJ) following the procedure reported previously(25). A mouse was euthanized by overdose inhalation of isoflurane, perforation of the right atrium, and transcardiac perfusion with oxygenated Krebs solution via the tip of the left ventricle for proper exsanguination. Bilateral sciatic nerves, each ~30 mm, were harvested from their proximal projections to the L4 spinal cord to their distal branches innervating gastrocnemius muscles. The nerves were transferred to the custom-built tissue chamber perfused with oxygenated Krebs solution of 30–32°C. The proximal region of the sciatic nerve (~25 mm) was attached to the bottom of the tissue chamber by carefully pinning down the attached connective tissue, of which the ~2 mm nerve segment was placed on the polystyrene film separating the tissue chamber from the cone-shaped water chamber, i.e., the focal region of the disk-shaped ultrasonic transducer underneath (Figs 1A). Pulled through the mouse hole under the gate, separating the tissue and recording chambers, the distal end (~5 mm) of the sciatic nerve was laid onto a glass mirror in the recording chamber to allow splitting into fine nerve filaments. To achieve single-unit recordings with higher signal-to-noise ratio (SNR), the connective tissue layers consisting of epineurium and perineurium were removed by meticulous dissection to unwrap the nerve fascicles allowing further splitting into fine nerve filaments of ~20 μm thick. To facilitate the nerve splitting, nerve trunks were treated with a mild concentration of collagenase as reported previously: 2 mg/mL collagenase (Type 4, Worthington, NJ) at room temperature for 10 min (25).
Ultrasonic and electrical stimulation protocol
A focused US transducer (H-101G, Sonic Concepts, Washington, USA) with a center frequency of 1.1 MHz was used to deliver FPUS to the sciatic nerve in vitro. An arbitrary waveform generator (BK Precision 4054, Yorba Linda, CA) was used to deliver square pulses (200 ns pulse width, 200 kHz pulse repetition frequency [PRF]) to a radiofrequency (RF) power amplifier (A-300, Electronics & Innovation, Rochester, NY) viaan adjustable attenuator (50DR-046, JFW Industries, Indianapolis, IN). The RF power amplifier drives the US transducer through a fundamental resonance impedance matching network to ensure optimal energy transfer to the transducer. The ultrasonic energy is transmitted through degassed water in the cone-shaped water chamber with an ellipsoidal focal region which is 1.3 mm wide, transversely and 9.7 mm long, axially (at −6 dB attenuation range). The US intensity was validated and tracked by a needle hydrophone (HNR-1000, Onda Corp., Sunnyvale, CA) during the alignment process, which also was used for creating the US stimulus intensity profile at the focal region during the in vitro experiments. The sciatic nerve was electrically stimulated at the proximal end by a liquid suction electrode fabricated with quartz glass capillary providing monopolar cathodal current pulses (0.5 Hz, 2 mA amplitude, 0.2 ms pulse width) via a stimulus isolator (A365, World Precision Instruments, Sarasota, FL).
To assess the effects of US stimulation on individual sciatic nerve axon, we employed an electrical stimulus protocol that evokes action potentials at the proximal end of the sciatic nerve. The protocol consists of 90 consecutive electrical pulses at 0.5 Hz, which were divided as pre-US stimulation pulses (1–20), during US stimulation pulses (21–40) and post-US stimulation pulses (41–90). For each 90-pulse protocol, the US stimulation intensity remained the same. For each nerve axon, 3–5 protocols with different US stimulation intensities were assessed (ISPTA, spatial peak temporal average in W/cm2: 0 as control, 0.91, 2.99, 14.5 and 28.2). To avoid nerve fatigue, a gap of at least 5 minutes was ensured between successive protocols.
Single-unit recording with custom-built multi-wire electrode array
To achieve simultaneous single-unit recordings from the split nerve fibers, a customized 5-channel electrode array was fabricated with a PCB board and micro-wires (Nichrome, 65 μm thick, A-M Systems Inc., Sequim, WA) as reported previously (25). One end of the PCB board was configured with the connectors compatible with the recording device while the other end was connected to micro-wires through metal pads. Micro-wires were organized in parallel and set 100–150 μm apart from each other. The insulation (Formvar) of the micro-wires were peeled off at the ~2mm distal end to expose the conducting tips and the shaft of the electrode array was insulated by a thin layer of silicone for proper electrical insulation and mechanical support. Single-unit action potentials recorded from the multi-channel electrodes were digitized at 25 kHz, bandpass filtered (300–3000 Hz), and stored in a PC using an integrated neural stimulation and recording system (RZ5D and PZ5-32, Tucker Davis Technologies, FL) (25).
Temperature measurement at the US focal region
The temperature of the oxygenated Krebs solution flowing through the perfusion chamber was maintained at 30–32°C using a heated circulating bath (1130-A, Poly Science, Niles, IL). The temperature of the solution was monitored by a digital thermometer (Durac, H-B Instrument, Trappe, PA). In addition, the instantaneous temperature at the focal zone of the US transducer during US stimulation was measured using an infrared imaging camera (FLIR One, FLIR Systems, Wilsonville, Oregon) with 0.1°C detection resolution and 8.7 Hz imaging frame rate. Change of focal temperature was measured at all four US stimulus intensities during the ultrasonic and electrical stimulation protocol described above (in W/cm2: 0.91, 2.99, 14.5 and 28.2).
Histological assessment
Histologi cal assessments were conducted on the sciatic nerve tissues to see if there is any deleterious effect of FPUS. For this purpose, 4 sciatic nerves were harvested, and each were subjected to the electrical and US stimulation protocol described above at the following US intensities (in W/cm2): 0 as control, 2.99, 28.2 and 102.31 (a significantly higher stimulus intensity). Methods were reported previously in details (25). Briefly, the specimens were then collected and fixed at 4°C for 60 min in a 0.12 M phosphate buffer solution (PB, pH 7.2) containing 2.5% glutaraldehyde, 2% paraformaldehyde, and 3 mM MgCl2. Following two rinses in PB, the tissues were further fixed with 1% Osmium tetroxide in 0.12 M PB at room temperature for 2 hours in the dark. After an additional rinse in PB, the tissues were dehydrated through a series of 30, 50, 70, 95 and 100% ethyl ethanol for 10 min each followed by two exposures to 100% propylene oxide for 10 min each. Tissues were then flat-embedded in an epoxy resin mixture and polymerized at 60°C for 48 hours. Blocks were sectioned on an ultra-microtome (Leica, Bannockburn, IL) and collected on grids. Grids were then stained in 2% uranyl acetate and 2.5% Sato’s lead citrate, washed with water, and dried at room temperature. The images were taken with an FEI Tecnai T12 transmission electron microscope equipped with an AMT 2K XR40 CCD (4 megapixel) camera at an accelerating voltage of 80 KV.
Data processing and statistical analysis
For quantifying US intensity, an oscilloscope (BK Precision 2540B Series, Yorba Linda, CA) was used to acquire the impulse responses of the transducer at varying US stimulation parameters, which were digitized, loaded into a PC via Comsoft2 software (B&K Precision Corp.) and post-hoc processed using MATLAB (Mathworks R2016b). Following a previous report (26), the spatial peak temporal average intensity (ISPTA) of ultrasonic stimulation was calculated according to the following set of equations:
| (1) |
| (2) |
| (3) |
In which, PRF (in Hz) is the pulse repetition frequency, and ESP (in J/cm2) stands for spatial peak pulse intensity integral which is the final value of pulse intensity integral E(t) (in J/cm2) defined by equation (2). The v(τ) stands for the response of the US transducer to an impulse voltage stimulus which is several cycles in duration or ~6 μs for the transducer we used. The intensity response factor Kf2 (in V2 cm2/W) was related to the conventional free-field voltage sensitivity M according to equation (3), where, ρ (in kg/m3) and c (in m/s) stand for density of water and speed of acoustic wave in water, respectively (26).
The data from single-unit recordings were processed offline using customized programs in MATLAB (Mathworks R2016b). Before delivering any electrical stimuli, data were recorded for 5ms to determine the root mean square (RMS) value of the noise corresponding to that particular recording configuration which was later used to set the detection threshold as 5 times the RMS value. The time at which a spike first exceeds the detection threshold was defined as the onset of an action potential. The time difference between the onset of a stimulus artifact and the onset of the action potential was defined as the conduction delay of that spike. The distance between the stimulating and recording electrodes was measured to compute the conduction velocity (CV). Data are presented as means ± SE. One-way ANOVA with proper repeated measure was performed using SigmaPlot v9.0 (Systat Software, San Jose, CA). Differences were considered significant when p < 0.05.
Results
US stimulation up to 28.2 W/cm2 did not directly activate mouse sciatic nerve in vitro
First, single-unit recordings from peripheral nerve axons were achieved as shown in Fig. 3A in which action potentials were evoked by electrically stimulating the proximal end of the sciatic nerve in the tissue chamber via a suction electrode (cathodal monopolar, 0.5 Hz, 2 mA amplitude, 0.2ms duration). Spontaneous firing activities were not detected in the in vitro sciatic nerve. FPUS of 40 seconds alone at all four intensities (0.91, 2.99, 14.5 and 28.2 W/cm2) did not evoke any neural action potential in the sciatic nerve with examples of the lowest and highest intensity as shown in Figs. 3B and 3C, respectively. Our single-unit recordings were unaffected by the brief FPUS stimulation as indicated by identical responses to electrical stimulation 20 mins after the FPUS stimulation (Fig. 3D) when compared to the pre-FPUS response (Fig. 3A). Consistent with our previous report (25), in vitro single-unit recordings from sciatic nerves are robust and repeatable for at least 20 mins.
Figure 3.

FPUS alone did not evoke action potentials in sciatic nerve in vitro. (A) Electrical stimulation evoked action potentials as revealed by single-unit recordings. FPUS alone for 40 sec did not evoke action potentials at either 0.91 (B) or 28.2 W/cm2 (C). (D) Single-unit recordings evoked by electrical stimulation 20 mins after the FPUS is almost identical to recordings in (A). Arrow: electrical stimulus artifact; arrow heads: single-unit action potentials from individual axon.
FPUS enhanced the conduction velocity of both A- and C- type sciatic nerve axons
The effects of US stimulation on action potential transmission were assessed by concurrent electrical stimulation at the proximal nerve ending (to evoke action potentials) and FPUS stimulation at the ~2mm middle segment of the sciatic nerve. Displayed in Fig. 4A are typical single-unit recordings of electrically evoked action potentials before (pre), during and after (post) FPUS stimulation. As described in the Methods, in total, 90 electrical stimulus pulses (0.5Hz) were delivered and US stimulation was applied from the 21stto the 40th stimulus pulses. For consistent comparison, a snippet (120ms) of data was recorded triggered by each electrical stimulation as shown in Fig. 4A. Consistent decrease of conduction delay or increase of conduction velocity is observed in both slow-conducting C- fibers and fast-conducting A- fibers. Displayed in Fig. 4B are plots of conduction delays from a C- fiber and an A- fiber which were tested with 4 different FPUS intensities (in W/cm2: 0.91, 2.99, 14.5 and 28.2) and a control without FPUS. Apparent decrease of conduction delay was noticed immediately after the onset of FPUS stimulation (21st pulse) which continues to decrease progressively with FPUS stimulation till the 41st pulse. Higher FPUS intensity results in more pronounced decrease in conduction delay. As soon as the FPUS terminates, a progressive elevation of conduction delay takes place during the post stimulus recovery phase.
Figure 4.
FPUS increases conduction velocities (i.e., decrease in conduction delay) of both C- and A- type sciatic nerve axons. (A) Representative single-unit recordings following the 20-20-50 protocol of FPUS, including recordings from the same nerve filaments pre-, during, and post-FPUS. The top most bar represents 20-20-50 stimulation protocol. The conduction delays from a slow-conducting C- fiber (CV < 1m/s) and a fast-conducting A- fiber (CV > 1m/s) were labeled to indicate the subtle but consistent increase of CV following FPUS. (B) Representative conduction delays recorded from a C- fiber (upper) and an A- fiber (lower) undergoing five protocols of FPUS (0, 0.91, 2.99, 14.5, 28.2 W/cm2) at randomized sequence.
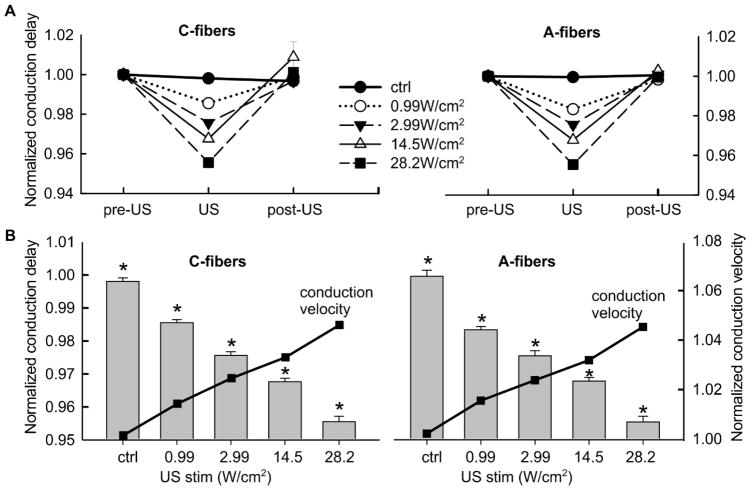
A total of 62 fibers recorded from 15 mice were tested with the 20-20-50 protocol; each fiber was assessed with at least one of the 4 FPUS stimulus intensities along with a control (zero FPUS stimuli); each intensity was tested in at least 20 fibers. For each protocol, the conduction delays of the pre-US, US, and post-US stimulation were calculated as the average conduction delays from the 11th–20th, 35th–40th, and 81st–90th stimulus pulses, respectively, which were normalized by their respective pre-US controls and summarized in Fig. 5A. FPUS significantly reduced the conduction delay in C- fibers at all 4 intensities (repeated measure one-way ANOVA, F2, 62 = 61.5, p<0.001 for 0.99W/cm2; F2,62 = 84.4, p<0.001 for 2.99W/cm2; F2,62 = 22.6, p<0.001 for 14.5W/cm2; F2,62 = 329, p<0.001 for 28.2W/cm2). Similarly, FPUS significantly reduced the conduction delay of A- fibers (F2, 61 = 109.3, p<0.001 for 0.99W/cm2; F2,62 = 105.4, p<0.001 for 2.99W/cm2; F2,62 = 220.7, p<0.001 for 14.5W/cm2; F2,61 = 164.7, p<0.001 for 28.2W/cm2). The conduction delays return to control level 80 seconds after terminating the FPUS stimuli (post-hoc comparison, p>0.05 for pre-US vs. post-US). As shown in Fig. 5B, the effects of FPUS are more pronounced at higher intensities as indicated by significant difference between the conduction delays during US stimulation at all five intensities (including zero intensity as control): one-way ANOVA, F4,104 = 192, p<0.05 for all pairwise post-hoc comparisons for C- fibers and F4,102 = 118.8, p<0.05 for all pairwise post-hoc comparisons for A- fibers.
Figure 5.
(A) Summarized conduction delays recorded from 31 C-fibers (left) and 31 A-fibers (right) undergoing FPUS stimulation protocols. Each nerve fiber was subjected to 1–4 FPUS stimulus intensities plus a control protocol (no FPUS stimulation). Each measured conduction delay was normalized by the pre-US control value. (B) The conduction delays at all five FPUS intensities, including zero as a control were significantly different from one another (bar plots, left Y axis). The corresponding normalized conduction velocity (line plots, right Y axis) was increased following FPUS.
Minimum local temperature effects by the 20-20-50 FPUS stimulus protocol
The change of local temperature at the US focal region during the 20-20-50 FPUS stimulation was measured by an infrared camera with 0.1°C temperature sensitivity (FLIR One, FLIR Systems, Wilsonville, Oregon) as shown in Fig. 6A. The maximum change of temperature during the FPUS stimulation (40 sec) was less than 1°C which is comparable to the temperature variation pre- and post-US stimulation. Lack of apparent temperature change during US stimulation was also confirmed by a digital thermometer placed in the tissue chamber next to the US focal region (Durac, H-B Instrument, Trappe, PA)
Figure 6.
FPUS stimulation didn’t cause apparent temperature changes at US focal region in the in vitro preparation. (A) A representative temperature distribution in the tissue and recoding chamber recorded by an infrared camera. (B) Temperature at the US focal region measured during the 20-20-50 FPUS stimulus protocols.
Histological Assessment on FPUS Effects
Transmission electron microscopy was employed to assess any potential structural damage following FPUS protocols. For this purpose, four sciatic nerves were used and each subjected to a low level FPUS stimulations of zero as control, 0.91, 2.99 and 102.31 W/cm2, respectively. The cross-sectional views of those four nerves are presented in Fig. 7A to D. Anatomically, there is no apparent difference among all four groups, which is in stark contrast to the histological damage reported in previous studies with high-intensity focused ultrasound of over 1200 W/cm2, e.g., distorted fascicles and change in tissue orientation (21, 27).
Figure 7.

Histological assessments by transmission electron microscopic images of nerve cross sections undergoing the 20-20-50 FPUS stimulation protocol, at (A) no FPUS stimulation, (B) 2.99 W/cm2, (C) 28.2 W/cm2, and (D) 102.31 W/cm2.
Discussion
The promise of therapeutic ultrasound (US) in medical applications has been marked by numerous research activities for ages and rejuvenated as an emerging modality for treating neurologic disorders like essential tremor (28), depression (29), obsessive-compulsive disorder (30), dystonia (31), epilepsy, Parkinson’s-, Alzheimer’s- disease alongside multifarious neuropathic pains (32, 33). Though detailed guidelines for US stimulation are yet to be published, in preliminary level, FDA has approved the application of therapeutic US allowing a maximum intensity of (0.72 W/cm2 ISPTA) (20). US stimulation with intensity < 1 W/cm2 ISPTA was reported by several studies (4, 9–11, 14) to show apparent effects especially on neurons in the central nervous systems. But from our current study on peripheral nerves, no appreciable effects of focused ultrasound with intensity < 1 W/cm2 ISPTA were observed in vitro.
Between 1 and 100 W/cm2 ISPTA, effects of US stimulation on the PNS appear to have no apparent thermal effects from studies by us and others. We used non-contact thermometry and continuously tracked the temperature at the US focal region; the extent of temperature change during US stimulation is comparable to the temperature variation without US stimulation in our in vitro setup. We also tested the effects at higher US intensities (65.2 W/cm 2 and 102.31 W/cm2 ISPTA) and did not find any apparent temperature change (data not shown). These findings agree with previous studies showing that low intensity US produces very little or no change in the temperature, especially in the pulsed stimulation mode (34–36), which collectively support the non-thermal mechanisms underlying ultrasonic neuromodulation at low intensity range (<100 W/cm2 ISPTA). US stimulation with intensity from 1 to 100 W/cm2 ISPTA targeting CNS was reported to elicit various motor responses (6, 7, 9) which agree with our observations of increased nerve conduction velocities.
High-intensity focused ultrasound (HIFU) (> 100 W/cm2 ISPTA) that are approved by the FDA for ablating cancer cells in patients (37, 38) reportedly suppressed CAP amplitude in a portion of the nerves that recovered from the stimulus (21, 22, 27). These phenomena are generally attributed to the thermal effects of US causing permanent degeneration of nerve fibers. In our in vitro study, we observed irreversible elimination of action potential spikes when intensity exceeds 65.5 W/cm2 (ISPTA) and henceforth, limited the intensity to be below 28.2 W/cm2 to ensure a reversible modulation. The therapeutic potential of HIFU to irreversibly alter the neural encoding of the peripheral nerves deserve further consideration and future systematic investigations.
Unlike the CNS neurons, PNS nerves do not have the protection of bony skull or vertebrae but are tightly wrapped by multiple layers of connective tissues, which function as electrical insulators to challenge direct neural recordings from individual nerve axon. Consequently, the major metrics to assess US neuromodulation on the PNS are mostly compound action potentials (CAP) (16, 21–24). The shape of CAP (peak amplitude, temporal location) depends on the temporal summation of a population of action potentials and has a distorted representation of nerve axon population, which is evidenced by the disproportionally large peaks from fast-conducting A- fibers even though C- fibers are the clear majority in peripheral nerves. The CAP shape and amplitude can also be affected by the changes in recording conditions, e.g., change of access impedance of recording electrode due to altered moisture conditions or changes in electrode locations. Thus, CAP amplitude and shape are incapable of providing precise assessment of the subtle modulatory effects by US stimulation. It is thus not unexpected that studies relying on CAP assessments reported contradictory effects of US stimulation on peripheral nerves with intensity between 1 and 100 W/cm2, with some reporting suppressed action potentials (21, 22) while others reporting increased excitability (16)or mixed results (24).
In contrast, single-unit recordings from peripheral nerves capture electrophysiological properties from a single neuron or axon via surgical removal of the epineurium followed by intrafascicular splitting to ~20 μm thick fiber bundles consisting of a limited number of myelinated axon and/or Remak bundles of unmyelinated axons. To overcome the limitations of CAP, severalearlier studies successfully used single-unit recordings to assess the effects of various neuromodulation schemes (39–47). The advantages of single-unit recording over CAP includes, its reliance on temporal information rather than amplitude, repeatability, robustness, and very high sensitivity to subtle temporal change. To the best of our knowledge, this is the first systematic study to use single-unit recordings for assessing the neuromodulatory effects of focused ultrasound on peripheral nerves. We have observed the progressive decrease in conduction delay (CD) right after the onset of the FPUS stimulation (i.e., increase in conduction velocity[CV]), following almost a linear trend with increase of stimulus duration (Fig. 4), in agreement with a prior study based upon CAP recordings (16). The neuromodulatory effects of FPUS are comparable between fast-conducting A- fibers and slow-conducting C- fibers, showing similar linear trend line of CD reduction and almost identical maximum extent of CD reduction at all four stimulus intensities. Due to the contradictory nature of the reported US effects on peripheral nerves, our results did not support the following studies in which the decrease in CV was reported (23, 24, 48).
We observed that low-intensity FPUS can reliably and reversibly modulate nerve conduction. The modulation effects, presented in our study, were evoked by the US intensities (0.91, 2.99, 14.5 and 28.2 W/cm2 ISPTA) covering an optimally diversified intensity range. The combined anatomic evidence showing no appreciable nerve tissue damage and functional data indicating full recovery of nerve CV following FPUS strongly suggests that the US intensities, even up to 28.2 W/cm2 ISPTA might be applicable to vertebrate peripheral nerve without causing any anatomical, physiological and functional loss. We also observed similar modulatory effects with 65.5 W/cm2 ISPTA, which however caused total elimination of action potentials in a portion of the nerve fibers tested. To avoid potential structural damage, we also did not extend the continuous stimulus duration beyond 40 sec (20 pulses at 0.5 Hz). It remains undetermined whether the conduction velocity will continue to increase following extended stimulus duration. Thus, it is possible to use the current in vitro setup to determine the safety limit (in terms of intensity and duration) for reversible US neuromodulation, a most wanted yet an unaddressed issue, in future studies.
Our current experimental data from high-resolution single-unit recordings will provide a reliable experimental basis to assess and refine existing theories of ultrasonic neuromodulation, especially models of non-thermal effects of US neuromodulation including the soliton model (49), the flexoelectricity hypothesis (50), and the recent NICE model (51). For example, the NICE model appears to interpret our experimental findings of increased nerve conduction velocity by attributing to the change of membrane capacitance following US stimulation, which leads to a capacitive current to alter axonal excitability. Thus, single-unit recordings have great potential in advancing our mechanistic understandings of peripheral neuromodulation.
Conclusions
In this study we have developed a robust single-unit recording setup and used it to explore the neuromodulatory effects of FPUS stimulation on mouse peripheral nerves in vitro. We discovered that, FPUS stimulation alone cannot evoke action potentials in mouse sciatic nerves even at 28.2 W/cm2. On the other hand, FPUS significantly increases the conduction velocity of electrically-evoked action potentials at all four intensities (0.99, 2.99, 14.5, 28.2 W/cm2). The conduction velocity increases gradually and consistently with increasing US stimulus intensity and duration for both C- and A- type fibers. The change in nerve conduction velocity by US stimulation is reversible up to 28.2 W/cm2 and cannot be attributed to local thermal effects of US. Our single-unit recording setup allows an objective assessment of the safety threshold of US neuromodulation as a non-invasive therapeutic process. In addition, outcomes of current study will provide a solid experimental foundation to assess and refine existing theories of non-thermal effects of ultrasounds and advance our mechanistic understanding of ultrasonic neuromodulation.
Figure 2.
Custom-built three-chamber setup for single-unit recordings from individual sciatic nerve axon while conducting FPUS on nerve segment. (A) A picture of the whole setup. (B) Sciatic nerve before (left) and after (right) splitting into fine filaments. (C) Magnified view of the split nerve and fine axon filaments of ~20 μm thick. (D) Custom-built microelectrode array for extracellular single-unit recordings.
Acknowledgments
This study is supported by NIH grant DK100460 and NSF grant CMMI-1727185 awarded to BF. We acknowledge Dr. Maritza Abril for the technical support of tissue preparation and electron microscopy imaging at the Biosciences Electron Microscopy Laboratory (BEML) of the University of Connecticut.
References
- 1.Harvey EN. The effect of high frequency sound waves on heart muscle and other irritable tissues. American Journal of Physiology--Legacy Content. 1929;91(1):284–90. [Google Scholar]
- 2.Kobus T, Vykhodtseva N, Pilatou M, Zhang Y, McDannold N. Safety Validation of Repeated Blood-Brain Barrier Disruption Using Focused Ultrasound. Ultrasound Med Biol. 2016 Feb;42(2):481–92. doi: 10.1016/j.ultrasmedbio.2015.10.009. Epub 2015/12/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamimura HA, Wang S, Chen H, Wang Q, Aurup C, Acosta C, et al. Focused ultrasound neuromodulation of cortical and subcortical brain structures using 1.9 MHz. Medical physics. 2016 Oct;43(10):5730. doi: 10.1118/1.4963208. Epub 2016/10/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H, Park MY, Lee SD, Lee W, Chiu A, Yoo SS. Suppression of EEG visual-evoked potentials in rats through neuromodulatory focused ultrasound. Neuroreport. 2015 Mar 04;26(4):211–5. doi: 10.1097/WNR.0000000000000330. Epub 2015/02/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu PC, Liu HL, Lai HY, Lin CY, Tsai HC, Pei YC. Neuromodulation accompanying focused ultrasound-induced blood-brain barrier opening. Scientific reports. 2015 Oct 22;5:15477. doi: 10.1038/srep15477. Epub 2015/10/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehic E, Xu JM, Caler CJ, Coulson NK, Moritz CT, Mourad PD. Increased anatomical specificity of neuromodulation via modulated focused ultrasound. PloS one. 2014;9(2):e86939. doi: 10.1371/journal.pone.0086939. Epub 2014/02/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H, Lee SD, Chiu A, Yoo SS, Park S. Estimation of the spatial profile of neuromodulation and the temporal latency in motor responses induced by focused ultrasound brain stimulation. Neuroreport. 2014 May 07;25(7):475–9. doi: 10.1097/WNR.0000000000000118. Epub 2014/01/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H, Chiu A, Lee SD, Fischer K, Yoo SS. Focused ultrasound-mediated non-invasive brain stimulation: examination of sonication parameters. Brain stimulation. 2014 Sep-Oct;7(5):748–56. doi: 10.1016/j.brs.2014.06.011. Epub 2014/08/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King RL, Brown JR, Newsome WT, Pauly KB. Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med Biol. 2013 Feb;39(2):312–31. doi: 10.1016/j.ultrasmedbio.2012.09.009. Epub 2012/12/12. eng. [DOI] [PubMed] [Google Scholar]
- 10.Yoo SS, Kim H, Min BK, Franck E, Park S. Transcranial focused ultrasound to the thalamus alters anesthesia time in rats. Neuroreport. 2011 Oct 26;22(15):783–7. doi: 10.1097/WNR.0b013e32834b2957. Epub 2011/08/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010 Jun 10;66(5):681–94. doi: 10.1016/j.neuron.2010.05.008. Epub 2010/06/16. eng. [DOI] [PubMed] [Google Scholar]
- 12.Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. NeuroImage. 2005 Jan 01;24(1):12–20. doi: 10.1016/j.neuroimage.2004.06.046. Epub 2004/12/14. eng. [DOI] [PubMed] [Google Scholar]
- 13.Fishman PS, Frenkel V. Focused Ultrasound: An Emerging Therapeutic Modality for Neurologic Disease. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2017 Apr;14(2):393–404. doi: 10.1007/s13311-017-0515-1. Epub 2017/03/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PloS one. 2008;3(10):e3511. doi: 10.1371/journal.pone.0003511. Epub 2008/10/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachtold MR, Rinaldi PC, Jones JP, Reines F, Price LR. Focused ultrasound modifications of neural circuit activity in a mammalian brain. Ultrasound Med Biol. 1998 May;24(4):557–65. doi: 10.1016/s0301-5629(98)00014-3. Epub 1998/07/04. eng. [DOI] [PubMed] [Google Scholar]
- 16.Tsui PH, Wang SH, Huang CC. In vitro effects of ultrasound with different energies on the conduction properties of neural tissue. Ultrasonics. 2005 Jun;43(7):560–5. doi: 10.1016/j.ultras.2004.12.003. Epub 2005/06/14. eng. [DOI] [PubMed] [Google Scholar]
- 17.Ibsen S, Tong A, Schutt C, Esener S, Chalasani SH. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat Commun. 2015 Sep 15;6:8264. doi: 10.1038/ncomms9264. Epub 2015/09/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo SS, Lee W, Kim H. Pulsed application of focused ultrasound to the LI4 elicits deqi sensations: pilot study. Complementary therapies in medicine. 2014 Aug;22(4):592–600. doi: 10.1016/j.ctim.2014.05.010. Epub 2014/08/26. eng. [DOI] [PubMed] [Google Scholar]
- 19.ab Ithel Davies I, Gavrilov LR, Tsirulnikov EM. Application of focused ultrasound for research on pain. Pain. 1996 Sep;67(1):17–27. doi: 10.1016/0304-3959(96)03042-4. Epub 1996/09/01. eng. [DOI] [PubMed] [Google Scholar]
- 20.Casella DP, Dudley AG, Clayton DB, Pope JCt, Tanaka ST, Thomas J, et al. Modulation of the rat micturition reflex with transcutaneous ultrasound. Neurourology and urodynamics. 2017 Mar 27; doi: 10.1002/nau.23241. Epub 2017/03/28. eng. [DOI] [PubMed] [Google Scholar]
- 21.Lee YF, Lin CC, Cheng JS, Chen GS. High-intensity focused ultrasound attenuates neural responses of sciatic nerves isolated from normal or neuropathic rats. Ultrasound Med Biol. 2015 Jan;41(1):132–42. doi: 10.1016/j.ultrasmedbio.2014.08.014. Epub 2014/12/03. eng. [DOI] [PubMed] [Google Scholar]
- 22.Colucci V, Strichartz G, Jolesz F, Vykhodtseva N, Hynynen K. Focused ultrasound effects on nerve action potential in vitro. Ultrasound Med Biol. 2009 Oct;35(10):1737–47. doi: 10.1016/j.ultrasmedbio.2009.05.002. Epub 2009/08/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juan EJ, Gonzalez R, Albors G, Ward MP, Irazoqui P. Vagus Nerve Modulation Using Focused Pulsed Ultrasound: Potential Applications and Preliminary Observations in a Rat. International journal of imaging systems and technology. 2014 Mar 01;24(1):67–71. doi: 10.1002/ima.22080. Epub 2014/08/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mihran RT, Barnes FS, Wachtel H. Temporally-specific modification of myelinated axon excitability in vitro following a single ultrasound pulse. Ultrasound Med Biol. 1990;16(3):297–309. doi: 10.1016/0301-5629(90)90008-z. Epub 1990/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Ilham S, Guo T, Emadi S, Feng B. In vitro multichannel single-unit recordings of action potentials from the mouse sciatic nerve. Biomedical Physics & Engineering Express. 2017;3(4):045020. doi: 10.1088/2057-1976/aa7efa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris GR. A discussion of procedures for ultrasonic intensity and power calculations from miniature hydrophone measurements. Ultrasound in Medicine & Biology. 1985 Nov 01;11(6):803–17. doi: 10.1016/0301-5629(85)90074-2. [DOI] [PubMed] [Google Scholar]
- 27.Foley JL, Little JW, Vaezy S. Image-guided high-intensity focused ultrasound for conduction block of peripheral nerves. Annals of biomedical engineering. 2007 Jan;35(1):109–19. doi: 10.1007/s10439-006-9162-0. Epub 2006/10/31. eng. [DOI] [PubMed] [Google Scholar]
- 28.Shaw KD, Johnston AS, Rush-Evans S, Prather S, Maynard K. Nursing Management of the Patient Undergoing Focused Ultrasound: A New Treatment Option for Essential Tremor. The Journal of neuroscience nursing: journal of the American Association of Neuroscience Nurses. 2017 Oct;49(5):307–10. doi: 10.1097/JNN.0000000000000301. Epub 2017/08/18. eng. [DOI] [PubMed] [Google Scholar]
- 29.Kim M, Kim CH, Jung HH, Kim SJ, Chang JW. Treatment of Major Depressive Disorder via Magnetic Resonance-Guided Focused Ultrasound Surgery. Biological psychiatry. 2017 May 12; doi: 10.1016/j.biopsych.2017.05.008. Epub 2017/06/12. eng. [DOI] [PubMed] [Google Scholar]
- 30.Jung HH, Kim SJ, Roh D, Chang JG, Chang WS, Kweon EJ, et al. Bilateral thermal capsulotomy with MR-guided focused ultrasound for patients with treatment-refractory obsessive-compulsive disorder: a proof-of-concept study. Molecular psychiatry. 2015 Oct;20(10):1205–11. doi: 10.1038/mp.2014.154. Epub 2014/11/26. eng. [DOI] [PubMed] [Google Scholar]
- 31.Fasano A, Llinas M, Munhoz RP, Hlasny E, Kucharczyk W, Lozano AM. MRI-guided focused ultrasound thalamotomy in non-ET tremor syndromes. Neurology. 2017 Aug 22;89(8):771–5. doi: 10.1212/WNL.0000000000004268. Epub 2017/07/28. eng. [DOI] [PubMed] [Google Scholar]
- 32.Abe K, Taira T. Focused Ultrasound Treatment, Present and Future. Neurologia medico-chirurgica. 2017 Aug 15;57(8):386–91. doi: 10.2176/nmc.ra.2017-0024. Epub 2017/07/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hersh DS, Eisenberg HM. Current and future uses of transcranial focused ultrasound in neurosurgery. Journal of neurosurgical sciences. 2017 Nov 03; doi: 10.23736/S0390-5616.17.04230-8. Epub 2017/11/07. eng. [DOI] [PubMed] [Google Scholar]
- 34.Tyler WJ. The mechanobiology of brain function. Nature reviews Neuroscience. 2012 Dec;13(12):867–78. doi: 10.1038/nrn3383. Epub 2012/11/21. eng. [DOI] [PubMed] [Google Scholar]
- 35.Patel PR, Luk A, Durrani A, Dromi S, Cuesta J, Angstadt M, et al. In vitro and in vivo evaluations of increased effective beam width for heat deposition using a split focus high intensity ultrasound (HIFU) transducer. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2008 Nov;24(7):537–49. doi: 10.1080/02656730802064621. Epub 2008/07/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalecki D. Mechanical bioeffects of ultrasound. Annual review of biomedical engineering. 2004;6:229–48. doi: 10.1146/annurev.bioeng.6.040803.140126. Epub 2004/07/17. eng. [DOI] [PubMed] [Google Scholar]
- 37.Zhou YF. High intensity focused ultrasound in clinical tumor ablation. World journal of clinical oncology. 2011 Jan 10;2(1):8–27. doi: 10.5306/wjco.v2.i1.8. Epub 2011/05/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Copelan A, Hartman J, Chehab M, Venkatesan AM. High-Intensity Focused Ultrasound: Current Status for Image-Guided Therapy. Seminars in interventional radiology. 2015 Dec;32(4):398–415. doi: 10.1055/s-0035-1564793. Epub 2015/12/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffer JA, Loeb GE, Pratt CA. Single unit conduction velocities from averaged nerve cuff electrode records in freely moving cats. Journal of neuroscience methods. 1981 Oct;4(3):211–25. doi: 10.1016/0165-0270(81)90033-9. Epub 1981/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 40.Sharkey KA, Cervero F. An in vitro method for recording single unit afferent activity from mesenteric nerves innervating isolated segments of rat ileum. Journal of neuroscience methods. 1986 Apr;16(2):149–56. doi: 10.1016/0165-0270(86)90047-6. Epub 1986/04/01. eng. [DOI] [PubMed] [Google Scholar]
- 41.Schalow G, Lang G. Recording of single unit potentials in human spinal nerve roots: a new diagnostic tool. Acta neurochirurgica. 1987;86(1–2):25–9. doi: 10.1007/BF01419500. Epub 1987/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 42.Guiloff RJ, Modarres-Sadeghi H. Preferential generation of recurrent responses by groups of motor neurons in man. Conventional and single unit F wave studies. Brain: a journal of neurology. 1991 Aug;114(Pt 4):1771–801. doi: 10.1093/brain/114.4.1771. Epub 1991/08/01. eng. [DOI] [PubMed] [Google Scholar]
- 43.Wu G, Ekedahl R, Hallin RG. Consistency of unitary shapes in dual lead recordings from myelinated fibres in human peripheral nerves: evidence for extracellular single-unit recordings in microneurography. Experimental brain research. 1998 Jun;120(4):470–8. doi: 10.1007/s002210050420. Epub 1998/07/09. eng. [DOI] [PubMed] [Google Scholar]
- 44.Kagitani F, Uchida S, Hotta H, Aikawa Y. Manual acupuncture needle stimulation of the rat hindlimb activates groups I, II, III and IV single afferent nerve fibers in the dorsal spinal roots. The Japanese journal of physiology. 2005 Jun;55(3):149–55. doi: 10.2170/jjphysiol.R2120. Epub 2005/07/05. eng. [DOI] [PubMed] [Google Scholar]
- 45.Schmelz M, Schmidt R. Microneurographic single-unit recordings to assess receptive properties of afferent human C-fibers. Neuroscience letters. 2010 Feb 19;470(3):158–61. doi: 10.1016/j.neulet.2009.05.064. Epub 2009/06/02. eng. [DOI] [PubMed] [Google Scholar]
- 46.Gaunt RA, Bruns TM, Crammond DJ, Tomycz ND, Moossy JJ, Weber DJ. Single- and multi-unit activity recorded from the surface of the dorsal root ganglia with non-penetrating electrode arrays. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2011;2011:6713–6. doi: 10.1109/IEMBS.2011.6091655. Epub 2012/01/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srinivasan A, Tipton J, Tahilramani M, Kharbouch A, Gaupp E, Song C, et al. A regenerative microchannel device for recording multiple single-unit action potentials in awake, ambulatory animals. The European journal of neuroscience. 2016 Feb;43(3):474–85. doi: 10.1111/ejn.13080. Epub 2015/09/16. eng. [DOI] [PubMed] [Google Scholar]
- 48.Young RR, Henneman E. Functional effects of focused ultrasound on mammalian nerves. Science. 1961 Nov 10;134(3489):1521–2. doi: 10.1126/science.134.3489.1521. Epub 1961/11/10. eng. [DOI] [PubMed] [Google Scholar]
- 49.Heimburg T, Jackson AD. On soliton propagation in biomembranes and nerves. Proc Natl Acad Sci U S A. 2005 Jul 12;102(28):9790–5. doi: 10.1073/pnas.0503823102. Epub 2005/07/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrov AG, Mircevova L. Is flexoelectricity the coupling factor between chemical energy and osmotic work in the pump? A model of pump. General physiology and biophysics. 1986 Aug;5(4):391–403. Epub 1986/08/01. eng. [PubMed] [Google Scholar]
- 51.Plaksin M, Shoham S, Kimmel E. Intramembrane cavitation as a predictive bio-piezoelectric mechanism for ultrasonic brain stimulation. Physical review X. 2014;4(1):011004. [Google Scholar]