Abstract
Objectives:
To evaluate the impact of moderate to severe aortic insufficiency (AI) on continuous flow left ventricular assist device (CF-LVADs) outcomes.
Background:
The development of worsening AI is a common complication of prolonged CFLVAD support and portends poor prognosis in single-center studies. Predictors of worsening AI and its impact on clinical outcomes have not been examined in a large cohort.
Methods:
We conducted a retrospective analysis of CF-LVAD patients in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). Development of significant AI was defined as the first instance of at least moderate AI. Primary outcomes of interest were survival after development of significant AI and time to adverse events including device complications and rehospitalizations.
Results:
Among 10603 eligible patients, 1399 developed moderate to severe AI on CF-LVAD support. Prevalence of significant AI progressively increased over time. Predictors of worsening AI included older age, female gender, smaller BMI, mild pre-implant AI, and destination therapy strategy. Moderate to severe AI was associated with significantly higher left ventricular enddiastolic diameter, reduced cardiac output, and higher brain natriuretic peptide levels. Significant AI was associated with higher rates of rehospitalization (32.1% vs 26.6% at 2-years, p=0.015) and mortality (77.2% vs 71.4% at 2-years, p=0.005) conditional upon survival to 1 year.
Conclusion:
Development of moderate to severe AI negatively impacts hemodynamics, hospitalizations, and survival on CF-LVAD support. Pre- and post- implant management strategies should be developed to prevent and treat this complication.
Keywords: mechanical circulatory support, left ventricular assist device, aortic insufficiency, INTERMACS
Introduction
The use of CF-LVADs is rapidly expanding in patients with advanced heart failure, both as bridge to transplant and as destination therapy1. The development of aortic insufficiency during CF-LVAD support has been well documented, affecting 15% to 52% of patients after 1 year of support2–5. Proposed mechanisms for the etiology of worsening AI during CF-LVAD utilization include leaflet deterioration and/or commissural fusion, aortic sinus dilatation, and increased transvalvular gradients: all of which may have implications for device function, thromboembolism, and device explant for ventricular recovery6–8. With the anticipation that those patients with underlying valvular pathology will have more rapidly progressive aortic valve disease on CF-LVAD support, current consensus guidelines recommend that moderate or greater AI at the time of implant should be treated surgically (Class I, Level of Evidence C)9. Concomitant surgical repair can be achieved by oversewing the AV with Park’s stitch or modified Park’s stitch, closure of the ventriculo-aortic junction with a surgical patch, or valve replacement using a bioprosthesis10–12.
In the post-implantation period, transthoracic echocardiography is usually performed every 6 – 12 months to assess frequency of AV opening, severity of AI, and AV structure. In conjunction with the aforementioned surveillance, multiple device management strategies have been proposed to promote AV opening and prevent AI development or progression via optimization of CF-LVAD parameters under TTE guidance9. To date, single center studies have been unable to demonstrate an effect of post-implant AI on short- or long-term survival on CFLVAD support, leading some to question of whether or not methods to optimize AV opening and device parameters are clinically indicated. Moreover, the impact of AI on secondary clinical outcomes on LVAD support such as worsening heart failure, hospital readmission, and device complications, remains largely unknown. In the current study, we utilized the INTERMACS registry to 1) characterize prevalence and the natural history of AI during CF-LVAD support, 2) identify predictors of the development of moderate to severe AI on device support, and 3) assess the impact of significant AI on worsening heart failure, adverse events, quality of life, and mortality on CF-LVAD support.
Methods
Study Design, Variables, and Definitions
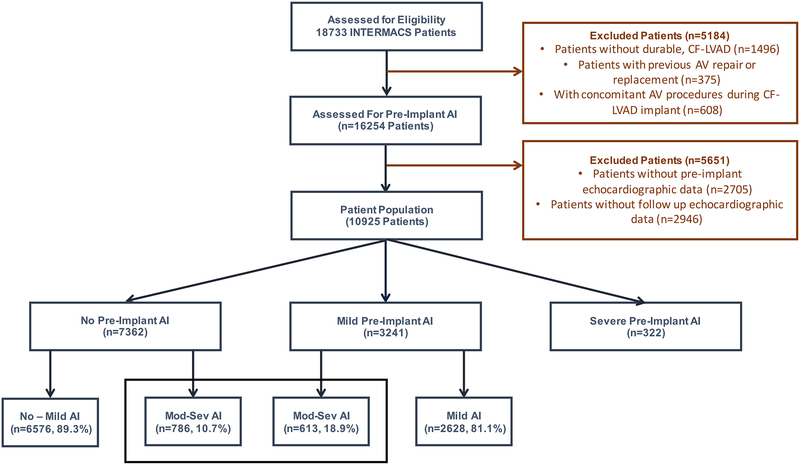
The INTERMACS registry was queried to identify patients who received a durable, CFLVAD between 2006 and 2016. Excluded patients were those without a pre-implant assessment of AI, those without follow up echocardiograms, and those who received a pre-implant or concomitant AV procedure (Figure 1). Echocardiographic assessment of AI was available at serial time points from 1-week post-implant to 8-years post-implant; AI at these time points was graded as none, mild, moderate, or severe. Significant AI was defined as the first instance of moderate or severe AI during the follow-up period in patients with no or mild pre-implant AI.
Figure 1:
Patient Population
Statistical Analysis
Descriptive analyses were conducted for all baseline variables and are presented as means and standard deviations for continuous variables and numbers and percentages for categorical variables. Non-normally distributed variables are presented as median and interquartile range. Differences between those who would go on to develop significant AI and those who did not were assessed with Student’s t-test and Kruskal-Wallis tests. Freedom from significant AI was assessed using Kaplan-Meier survival estimates with log-rank tests for comparison among subgroups. Univariate and multivariate Cox proportional hazard regression analysis was used to identify predictors of significant AI among patients with no or mild pre-implant AI. Proportional hazards assumptions were tested by visual assessments of Kaplan-Meier estimates. All variables in the final model were tested for interactions. The impact of AI on NYHA class, LV ejection fraction, and LV end diastolic diameter, and quality of life was assessed at serial time points during the study period and compared between patients with and without moderate to severe AI as assessed at that time point. All p-values were reported as two-sided tests with p<0.05 considered statistically significant. The effect of AI on survival and adverse events was assessed utilizing Kaplan-Meier survival methods conditional upon survival to one year with comparisons between those patients with and without a diagnosis of significant AI in the first year of support. STATA version 13.1 (Stata Corp., College Station, TX) was used to perform statistical analysis.
Results
Baseline Clinical Characteristics Based on Pre-Implant Aortic Insufficiency
A total of 10.925 patients were identified as being eligible for analysis in the current study. Among them, 10603 had no AI (n=7362, 69.4%) or mild AI (n=3241, 30.6%) at the time of device implantation. Three hundred and twenty-two patients had moderate or severe pre-implant AI and did not undergo concomitant AV procedure during the index operation (Figure 1). A total of 2296 patients died during the follow up period and 2671 patients were transplanted with a mean follow up time of 13.4 months.
Baseline demographics and medical histories of patients with no or mild pre-implant AI were compared between those who did and did not develop moderate to severe AI (Table 1). Patients who developed moderate to severe AI on CF-LVAD support were older and were more likely to be female. Development of moderate to severe AI was more prevalent in those with a lower BSA and those with ischemic cardiomyopathy and peripheral vascular disease. Patients who developed worsening AI were twice as likely to have mild AI as opposed to no AI at the time of CF-LVAD implant. Pulmonary hypertension, peripheral vascular disease, and chronic kidney disease were more common in patients with moderate to severe AI. Significant AI was more common in patients who received a CF-LVAD as destination therapy, with moderate to severe AI patients spending more time on device support. Overall, 80% had NYHA Class IV heart failure symptoms at the time of CF-LVAD implant, and 16% percent of patients were INTERMACS profile 1. Eighty-three percent of the devices utilized were axial, and 96% were used in an isolated LVAD configuration. In terms of device strategy, 57.1% of patients were implanted as BTT while 42.2% were candidates only for destination therapy.
Table 1:
Patient Characteristics
| Variable | Overall (n=10603) |
Mod-Severe AI (n=1399) |
No - Mild AI (n=9204) |
p-value |
|---|---|---|---|---|
| Age > 60, (years) | 4725 (44.6%) | 845 (60.4%) | 3880 (42.2%) | <0.001 |
| Female Gender | 2357 (22.2%) | 375 (26.8%) | 1982(21.5%) | <0.001 |
| African American | 2742 (25.9%) | 316(22.6%) | 2426 (26.4%) | 0.003 |
| BSA<2 | 4617(43.5%) | 739(52.8%) | 3878(42.1%) | <0.001 |
| Blood Type O | 5071 (48.4%) | 678 (49.1%) | 4393 (48.3%) | 0.587 |
| Ischemic Diagnosis | 4738 (45.0%) | 665 (47.8%) | 4073 (44.6%) | 0.026 |
| Mild Pre-Implant AI | 3241 (30.6%) | 613 (43.8%) | 2628 (28.6%) | <0.001 |
| Comorbid Conditions | ||||
| Diabetes | 389 (3.8%) | 45 (3.3%) | 344 (3.8%) | 0.381 |
| Pulmonary HTN | 1022(10.0%) | 153 (11.5%) | 869 (9.7%) | 0.045 |
| PVD | 229(2.2%)' | 43 (3.2%) | 186(2.1%) | 0.009 |
| Prior CVA | 90 (0.9%) | 12 (0.9%) | 78 (0.9%) | 0.924 |
| Atrial Arrhythmia | 1548(20.8%) | 190(22.5%) | 1358(20.6%) | 0.196 |
| Active Smoker | 538 (7.1%)' | 62 (7.1%) | 476(7.1%)' | 0.016 |
| CKD | 1937(25.0%) | 258 (28.4%) | 1679(24.5%) | 0.012 |
| NYHA Class | 0.665 | |||
| Class I & II | 103 (1.0%) | 14(1.1%) | 89 (1.0%) | |
| Class III | 1840(18.55) | 232 (17.6%) | 1608(18.6%) | |
| Class IV | 8018(80.5%) | 1074(81.4%) | 6944 (80.4%) | |
| INTERMACS Profile | 0.005 | |||
| INTERMACS 1 | 1644(15.6%) | 188 (13.5%) | 1456(15.9%) | |
| INTERMACS 2 | 3806(36.1%) | 516(37.1%) | 3290 (36.0%) | |
| INTERMACS 3 | 3420 (32.4%) | 429 (30.9%) | 2991 (32.7%) | |
| INTERMACS 4–7 | 1672(15.9%) | 257(18.5%) | 1415(15.5%) | |
| IV Inotropes | 8713(82.5%) | 1153(82.7%) | 7560 (82.4%) | 0.806 |
| Events During Index Hospitalization | ||||
| ECMO | 321 (3.0%) | 28 (2.0%) | 293 (3.2%) | 0.016 |
| IABP | 2124(20.0%) | 238 (17.0%) | 1886(20.5%) | 0.002 |
| Cardiac Arrest | 472 (4.5%)' | 60 (4.3%) | 412 (4.5%)' | 0.751 |
| Dialysis | 247 (2.3%) | 31 (2.2%) | 216(2.4%) | 0.762 |
| Intubated | 1082(10.2%) | 135 (9.7%) | 947 (10.3%) | 0.462 |
| Device Configuration | 0.428 | |||
| CF-LVAD Alone | 10279 (96.9%) | 1361 (97.3%) | 8918(96.9%) | |
| BiVAD | 324(3.1%) ' | 38 (2.7%) | 286(3.1%)' | |
| CF-LVAD Type | <0.001 | |||
| Axial | 8857(83.5%) | 1256 (89.8%) | 7601 (82.6%) | |
| Centrifugal | 1746(16.5%) | 143 (10.2%) | 1603(17.4%) | |
| Time on Device (mos), median | 13.4(6.0–26.3) | 25.6(13.6–40.9) | 12.1 (5.5–23.6) | <0.001 |
| Device Strategy | <0.001 | |||
| Bridge to Recovery | 44 (0.4%) | 6 (0.4%) | 38 (0.4%) | |
| Bridge to Transplant | 6047 (57.0%) | 676(48.3%) | 5371 (58.4%) | |
| Destination Therapy | 4474 (42.2%) | 716(51.2%) | 3759 (40.8%) | |
| Other/Rescue | 37 (0.4%) | 1 (0.1%) | 36 (0.4%) | |
BSA, body surface area; AI, aortic insufficiency; HTN, hypertension; PVD, peripheral vascular disease; CVA, cerebrovascular accident; CKD, chronic kidney disease; ECMO, extracorporeal membranous oxygenation; IABP, intraaortic balloon pump; CF-LVAD, continuous flow left ventricular assist device; BiVAD, biventricular assist device.
Pre-implant laboratory values and hemodynamics are summarized in Table 2. Patients who developed significant AI had a higher BUN and creatinine and a lower albumin prior to implant. In addition to having a higher BNP, patients with moderate to severe AI had larger LVEDDs, lower blood pressures, and lower cardiac output at baseline.
Table 2:
Pre-Implant Laboratory and Hemodynamic Values
| Variable | Overall (n=10603) |
Mod-Severe AI (n=1399) |
No - Mild AI (n=9204) |
p-value |
|---|---|---|---|---|
| BUN (mg/dL), mean ± SD | 24 (17 – 35) | 25 (18 – 36) | 24 (17 – 35) | 0.030 |
| Creatinine (mg/dL), mean ±SD | 1.27 (1.00 – 1.60) | 1.30 (1.00 – 1.60) | 1.27 (1.00 – 1.60) | 0.837 |
| Total Bilirubin (mg/dL), mean ± SD | 1.0 (0.6 – 1.6) | 1.0 (0.7 – 1.7) | 1.0 (0.6 – 1.5) | <0.001 |
| AST, median(IQR) | 29 (22 – 44) | 29 (22 – 44) | 29 (21 – 44) | 0.520 |
| ALT, median(IQR) | 29 (19 – 49) | 28 (18 – 48) | 29 (19 – 49) | 0.038 |
| Albumin (g/dL), mean ± SD | 3.39 ± 0.66 | 3.33 ± 0.66 | 3.40 ± 0.66 | <0.001 |
| Hemoglobin (g/dL), mean ±SD |
11.29 ± 2.09 | 11.17 ± 2.05 | 11.31 ± 2.09 | 0.025 |
| Platelets (x109/L), mean ± SD | 198.22 ±79.88 | 193.14±78.28 | 198.98 ±80.09 | 0.010 |
| BNP (ng/L), median(IQR) | 784 (393 – 1461) | 915 (489 – 1783) | 756 (382 – 1421) | 0.001 |
| HR (bpm), mean ± SD | 89.0 ± 17.6 | 87.3 ± 16.9 | 89.3 ± 17.7 | <0.001 |
| SBP (mmHg), mean ± SD | 104.6 ± 16.0 | 103.3 ± 15.4 | 104.8 ± 16.0 | 0.002 |
| DBP (mmHg), mean ± SD | 64.7 ± 11.3 | 63.3 ± 11.3 | 64.9 ± 11.3 | <0.001 |
| LVEDD (cm), mean ± SD | 6.84 ± 1.14 | 6.91 ± 1.18 | 6.83 ± 1.13 | 0.030 |
| CVP (mmHg), mean ± SD | 13.3 ± 8.5 | 13.9 ± 9.2 | 13.2 ± 8.3 | 0.023 |
| PCWP (mmHg), mean ± SD | 24.7 ± 9.0 | 24.4 ± 8.9 | 24.7 ± 9.0 | 0.379 |
| Cardiac Output (L/min), mean ± SD | 2.27 ± 0.92 | 2.30 ± 0.94 | 2.26 ± 0.92 | 0.305 |
BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BNP, brain natriuretic peptide; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEDD, left ventricular end diastolic diameter; CVP, central venous pressure; PCWP, pulmonary capillary wedge pressure.
Natural History of Worsening Aortic Insufficiency on CF-LVAD Support
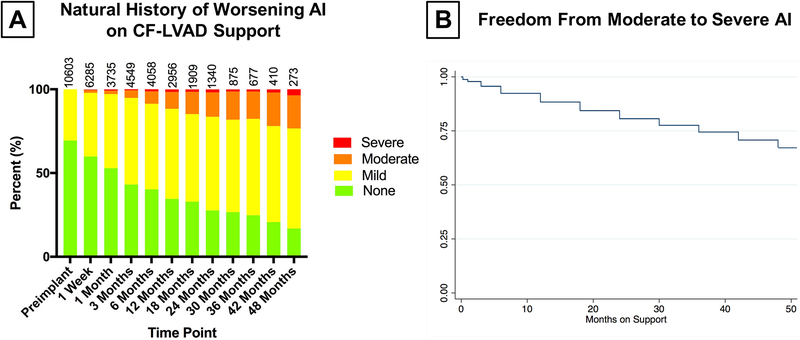
Among patients with no or mild pre-implant AI, a total of 31571 echocardiograms were performed during the follow up period. The distribution of AI at each time point during the first two post-operative years is displayed in Figure 2A. Among patients with no or mild pre-implant AI and follow up echocardiographic data, the proportion of patients with mild AI increased dramatically during the study period, such that by 6 months of follow up 55% of patients with echocardiograms had at least mild AI. Similarly, the proportion of patients with moderate AI increased from 1% at 1 week follow up to 10% at 1 year and 14% at 2 years. Kaplan-Meier estimates of freedom from moderate or severe AI are shown in Figure 2B.
Figure 2:
A) Natural History of Worsening AI on CF-LVAD Support
B) Kaplan Meier Estimates: Freedom From Moderate - Severe AI on CF-LVAD Support
Clinical Predictors of Worsening Aortic Insufficiency on CF-LVAD Support
Clinically-relevant patient and device characteristics were entered into univariate and multivariate Cox proportional hazard regression analyses to determine predictors of progression to moderate or severe AI among patients with no or mild pre-implant AI (Table 3). Multivariate analysis identified age >60 years (HR 1.75, CI 1.50 – 2.04, p<0.001), female sex (HR 1.29, CI 1.09 – 1.52, p=0.002), and body surface area < 2.0 m2 (HR: 1.30, CI: 1.13 – 1.49, p<0.001) and mild pre-implant AI (HR: 1.87, CI: 1.64 – 2.13, p<0.001) as significant predictors of worsening AI on CF-LVAD support.
Table 3:
Cox Proportional Hazards Modeling: Predictors of Moderate to Severe AI (n=10603)
| Variable | HR (95% CI) | P-Value | HR (95% CI) | P-value |
|---|---|---|---|---|
| Age > 60 yr. | 1.87 (1.68 – 2.09) | <0.001 | 1.75 (1.50 – 2.04) | <0.001 |
| Female Gender | 1.29 (1.14 – 1.45) | <0.001 | 1.29 (1.09 – 1.52) | 0.002 |
| African American | 0.83 (0.73 – 0.94) | <0.001 | 0.81 (0.69 – 0.97) | 0.014 |
| Blood Type 0 | 1.02 (0.92 – 1.14) | 0.629 | ||
| BSA < 2 | 1.49 (1.34 – 1.66) | <0.001 | 1.30 (1.13 – 1.49) | <0.001 |
| Ischemic Dx | 1.12 (1.01 – 1.24) | 0.036 | 0.93 (0.81 – 1.08) | 0.275 |
| Mild Pre-Implant AI | 2.87 (1.69 – 2.08) | <0.001 | 1.87 (1.64 – 2.13) | <0.001 |
| Diabetes | 0.87 (0.65 – 1.18) | 0.382 | ||
| Pulmonary HTN | 1.18 (1.00 – 1.40) | 0.050 | ||
| Prior CVA | 1.01 (0.57 – 1.78) | 0.969 | ||
| Atrial Arrhytlimia | 1.11 (0.94 – 1.30) | 0.209 | ||
| Smoking History | 1.00 (0.77 – 1.29) | 0.961 | ||
| CKD | 1.19 (1.04 – 1.38) | 0.014 | 0.99 (0.85 – 1.15) | 0.848 |
| NYHA | 0.667 | |||
| NYHA 1 – 2 | (Reference) | |||
| NYHA 3 | 0.91 (0.53 – 1.56) | |||
| NYHA 4 | 0.91 (0.57 – 1.64) | |||
| INTERMACS Profile | 0.008 | |||
| INTERMACS 1 | 0.81 (0.70 – 0.95) | |||
| INTERMACS 2 | 0.88 (0.76 – 1.02) | |||
| INTERMACS 3 | 0.74 (0.61 – 0.89) | |||
| INTERMACS 4 – 7 | (Reference) | |||
| IV Inotropes | 1.02 (0.89 – 1.17) | 0.810 | ||
| BiVAD | 0.87 (0.63 – 1.21) | 0.415 | ||
| Destination Therapy | 1.46 (1.32 – 1.63) | <0.001 | 1.00 (0.87 – 1.16) | 0.973 |
| TBili > 2.5 | 1.02 (0.85 – 1.23) | 0.828 | ||
| Albumin < 3 | 1.13 (1.00 – 1.29) | 0.053 | ||
| Hemoglobin < 10 | 1.17 (1.04 – 1.31) | 0.009 | ||
| Platelets < 150 | 1.27 (1.12 – 1.41) | <0.001 | ||
| BNP > 500 | 1.48 (1.23 – 1.77) | <0.001 | ||
| HR > 100 | 0.85 (0.74 – 0.96) | 0.009 | ||
| SBP < 100 | 1.17 (1.05 – 1.30) | 0.005 | ||
| LVEDD > 6.8 | 0.99 (0.88 – 1.11) | 0.817 | ||
| CVP > 12 | 1.02 (0.89 – 1.17) | 0.752 | ||
| PCWP> 18 | 1.04 (0.88 – 1.24) | 0.635 |
BSA, body surface area; AI, aortic insufficiency; HTN, hypertension; PVD, peripheral vascular disease; CVA, cerebrovascular accident; CKD, chronic kidney disease; ECMO, extracorporeal membranous oxygenation; IABP, intraaortic balloon pump; CF-LVAD, continuous flow left ventricular assist device; BiVAD, biventricular assist device; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BNP, brain natriuretic peptide; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEDD, left ventricular end diastolic diameter; CVP, central venous pressure; PCWP, pulmonary capillary wedge pressure.
Impact of Aortic Insufficiency on Ventricular Remodeling During CF-LVAD Support
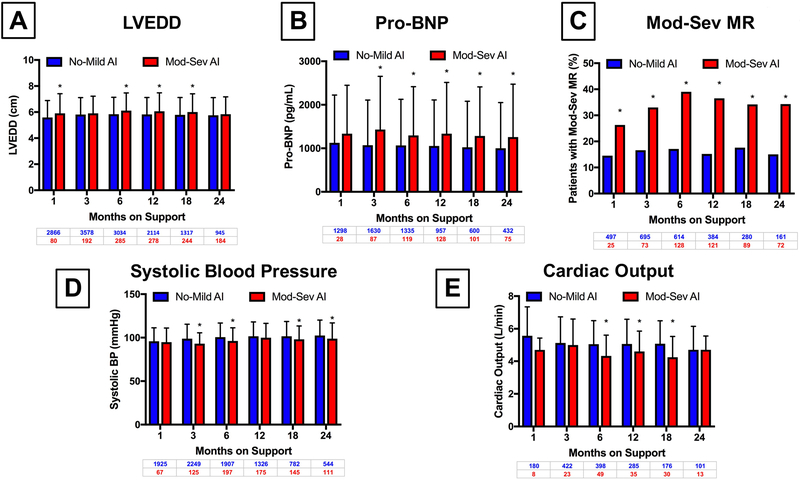
In order to try to quantify the impact of significant AI on ventricular structure and function, we analyzed serial echocardiograms and invasive hemodynamic testing of patients during the study period. As displayed in Figure 3A and 3B, LVEDD and Pro-BNP was higher at multiple time points during the study when compared between those with no or mild AI and moderate to severe AI at a given time point. In addition, the percentage of patients with at least moderate mitral regurgitation was significantly higher in patients with moderate to severe AI at all time points assessed in the first two years of support (Figure 3C). This translated into lower systolic blood pressures (Figure 3D) and lower cardiac output (Figure 3E). There were no significant differences in mean right atrial pressure, pulmonary vascular resistance, or pulmonary capillary wedge pressures between the two groups at serial time points (Supplemental Figure 1). Six-minute walk distance and KCCQ-12 scores were lower in those with moderate to severe AI, though not to a significant degree (Supplemental Figure 2).
Figure 3:
Cross-Sectional Comparison of the Prevalence of Indices of Left Ventricular Remodeling Based Upon AI Status
A) Left Ventricular End Diastolic Diameter
B) Pro-BNP
C) Moderate to Severe Mitral Regurgitation
D) Systolic Blood Pressure
E) Cardiac Output
Impact of Aortic Insufficiency on Survival on CF-LVAD Support
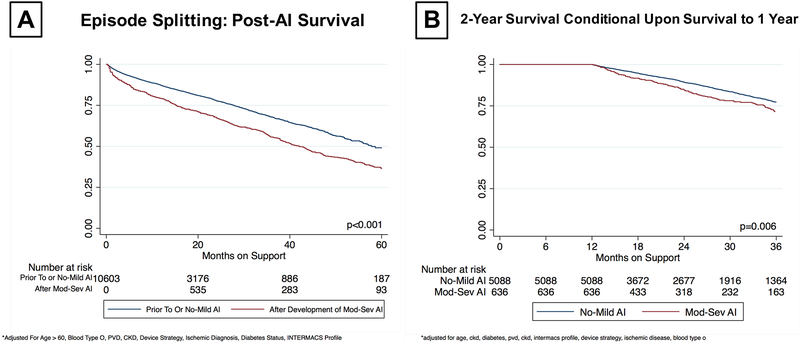
In the overall cohort, on-device survival at 5 years was 46.9%. The impact of moderate to severe AI on survival on CF-LVAD support was first evaluated using episode splitting. In this approach, patients with significant AI remained in the control arm until the time they were first diagnosed with moderate to severe AI and were then switched to the significant AI arm. Using this approach, survival on CF-LVAD support was significantly lower in patients with significant AI vs no or mild AI (49.1% vs 36.5% at 5-year, p<0.001) even after adjusting for age at implant, INTERMACS profile, and chronic kidney disease (Figure 4A). As an alternative approach, we analyzed freedom from death conditional on survival to 1 year on CF-LVAD based on the presence or absence of moderate to severe AI within the first year of support. Even when adjusted for covariates, conditional survival analysis also suggested a significant difference in survival (77.2% vs. 71.4% at 2 years, p=0.005) (Figure 4B). These trends persisted in a sensitivity analysis of destination therapy patients (Supplemental Figure 3).
Figure 4:
Kaplan Meier Survival Estimates for On-Device Survival
A) Episode Splitting at the Time of Moderate - Severe AI Development
B) Conditional Survival to 2-Years Based Upon On-Device AI Status At 1-Year
Impact of Worsening AI on Device Complications and Rehospitalizations
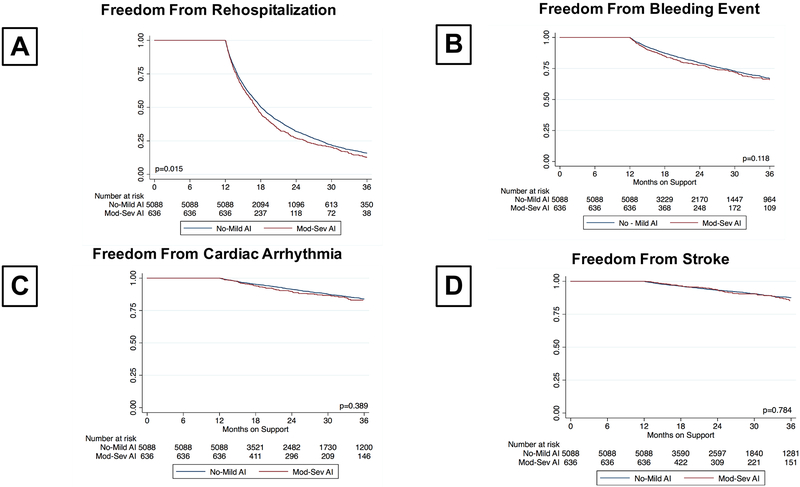
Freedom from rehospitalization, device malfunction, arrhythmia, and stroke by presence or absence of moderate to severe AI within the first year of CF-LVAD support, conditional on survival to 1 year, was represented in Figure 5. As shown, patients who develop significant AI within the first year of CF-LVAD support have significantly lower freedom of rehospitalization (32.1 % vs. 26.6% at 2-year, p=0.015, Figure 5A). No significant differences were observed between rates of bleeding, arrhythmia, and stroke (Figure 5B–D).
Figure 5:
Freedom From Device Complications Conditional Upon Survival to 1-Year
A) Rehospitalization
B) Bleeding Event
C) Cardiac Arrhythmia
D) Stroke
Discussion
The current study investigates the incidence and impact of moderate to severe AI during CF-LVAD support. Important findings include 1) AI as a progressive disease that develops during CF-LVAD support with well over 50% developing mild disease at two years of support and 15% developing moderate to severe disease, 2) Old age, female sex, small body size, and presence of mild AI at the time of CF-LVAD implantation predict development of moderate to severe AI on CF-LVAD support., 3) development of moderate to severe AI is associated with adverse left ventricular remodeling on CF-LVAD support 4) our data suggests that CF-LVAD patients with moderate to severe AI are at higher risk for rehospitalizations and mortality after one year of pump support.
Multiple prior studies have addressed the natural history of AI during CF-LVAD support. In a single center study from 2005 – 2013, Holley et al. identified 210 CF-LVAD patients among whom 32 (15.2%) developed moderate to severe AI in a median time of 482 days. At 5 years after implantation, 35% of patients had developed de novo AI5. Similarly, Cowger, et al. analyzed 166 patients over 291 person years and discovered 36 patients who developed moderate to severe AI (0.17 persons per year). In a meta-analysis of 7 observational studies including 657 patients, the rate of development of AI was 4% per month of support13. In the current study, we report a total of 1399 patients (n=13.2%) who developed significant AI in the current study. When all echocardiograms available at each time point were combined, we demonstrate a progressive increase both in the number of patients with mild, moderate or severe AI while on CF-LVAD support. Thus, our study supports the observations of previously published, small, single-center studies which suggest that AI on CF-LVAD support is a progressive disease.
In those patients with no or mild AI at the time of implant, our study confirms the findings of multiple previous studies which have suggested that older age, female gender, and smaller body size are risk factors for progression to moderate or severe disease13, 14. Although the current study focused only on CF-LVADs, previous studies that have included both continuous and pulsatile devices have identified CF-LVADs as conferring higher relative risk for AI15. Similarly, while not available in the INTERMACS data set, much attention has been paid to the opening status of the AV and its impact on AI2, 14. In addition to the aforementioned risk factors which were confirmed in the present study, we identified mild AI, and elevated BNP at the time of implant as an independent predictors of progression to moderate or severe AI. It is likely that structural/anatomic factors (BMI, e.g.) as well as factors that contribute to prolonged CF-LVAD support (DT indication, e.g.) contribute to increased risk of AV disease progression.
In order to assess the impact of AI on heart failure, both structurally and functionally, we analyzed the impact of AI development on LVEDD, LVEF, PCWP, CVP, and cardiac output. We found that, when compared among patients who would go on to develop moderate to severe AI, LVEDD was higher in those patients in whom AI had already developed. This trend persisted when LVEF was assessed. This translated into lower systolic blood pressures as well as lower cardiac output. Recently, Sayer et al. compared invasive hemodynamics and echocardiographic assessment of AI on CF-LVAD support and reported similar elevations in CVP and PCWP among patients with AI16. Overall, it does appear that AI has both hemodynamic consequences that must be managed aggressively – whether medically or surgically – prior to and during CF-LVAD support in order to prevent worsening clinical status. Additional studies assessing the impact of AI on LV recovery are warranted.
The effect of AI on device adverse events was also assessed in the current study utilizing AI status and conditional survival to one year to assess freedom from bleeding, arrhythmia, stroke, and rehospitalizations. We demonstrate that after 1 year of support, patients with moderate to severe AI experience decreased freedom from rehospitalizations. Taken together with the data presented regarding LV size, moderate MR, blood pressure, and cardiac output, it is conceivable that the changes in LV structure and function may predispose patients to rehospitalization for worsening heart failure symptoms.
Lastly, the effect of moderate or severe AI on survival was assessed. All prior single center studies have failed to demonstrate a difference in survival based upon the development of AI5, 14, 17. Rather than stratifying patients by the development of AI and assessing their survival from the time of implantation, we chose to compare survival before and after development of significant AI as well as conditional upon 1-year survival. In this way, we demonstrate decreased survival in patients after the development of AI when censored for transplant or device exchange as well as decreased survival after 1 year of support after the development of AI. Although the reason for death is not directly analyzed in this study, we hypothesize that, in part, worsening heart failure secondary to worsening AI may play a role.
Given the significant impact of moderate to severe AI on clinical end-points in CFLVAD patients, prevention and management strategies need to be developed for patients with this condition. In particular, the dilemma of mild pre-implant AI must be addressed. Our analysis suggests that patients with mild AI at the time of implant have both an increased risk of AV disease progression and a shorter time to development of significant AI when compared to those without pre-existing AI (Supplemental Figure 3B). Given its associated risks, consideration may be given to concomitant AV repair high risk patients – particularly those with expected prolonged time on device support, i.e. destination therapy patients. At our institution, our current practice is to concomitantly repair mild AI using Park’s stitch in patients who are implanted for DT indication. In BTT patients, decision to repair mild AI is made case-by-case based on likelihood of prolonged support such as high-level HLA sensitization and becoming a destination therapy patient after implant due to worsening renal function, aging, etc. We avoid repairing AI in patients who have possibility of myocardial recovery and device explantation, particularly patients with young age, non-ischemic etiology (including myocarditis), and shorter-duration of heart failure. In addition, early interventions such as speed adjustment echocardiograms allowing for aortic valve opening whenever feasible may potentially the reduce risk of worsening AI in high-risk individuals. Transplant candidates who are severely symptomatic from AI on CFLVAD support should be considered for status upgrade. Those who are ineligible for transplant may potentially benefit from rapidly evolving percutaneous therapies such as transcatheter aortic valve replacement (TAVR) and occluder devices. However, safety, durability, and efficacy of these approaches requires further investigation.
This study has many inherent limitations. First, because the data was collection from a large national registry, it is subject to error in entry as well as missingness of data. Secondly, all patients did not have echocardiographic data at all time points during the study. In addition, echocardiographic data was limited and we were thus not able to assess aortic valve and aortic root dilatation, device settings, and other important details for each patient at each time point. We also recognize that there is significant inter-observer variability both within and among centers in terms of interpreting echocardiograms. Because of the large numbers of patients included in the current registry based study, many comparisons between groups have reached statistical significance. Caution should be taken, however, to acknowledge these differences only when clinically relevant. Importantly, we recognize that AI is a time-dependent phenomenon and many analyses are subject to influence by time on device support.
In conclusion, we demonstrate that AI following CF-LVAD implantation is a progressive disease that contributes to worsening heart failure, increased rehospitalizations, and decreased survival. Those patients who are older, with a smaller body size, and with a CF-LVAD placed for destination therapy are at higher risk of AV disease progression, particularly if they have mild AI prior to CF-LVAD implant. In this patient population, AV intervention at the time of CF-LVAD implant may be warranted.
Supplementary Material
Clinical Perspectives.
The current study highlights worsening AI as a common, progressive disease of CFLVAD support with significant subclinical and clinical implications. AI is a time-dependent phenomenon and thus disproportionately affects patients receiving CF-LVADs as destination therapy. Patients with mild AI prior to implant – those in whom concomitant repair is not currently recommended – are at higher risk. Futures studies should aim to identify high risk patients and evaluate the efficacy of concomitant repair of mild AI in this particularly sub-population. Development of AI may limit survival and indications of aortic valve repair on CFLVAD support may expand as the percutaneous technologies continue to evolve.
Translational Outlook.
The translation of this research to the care of the individual patient may help cardiologists and surgeons identify those patients at high risk for the development of worsening AI during device support, and help to facilitate a discussion of the risks and benefits of concomitant aortic valve repair. In addition, on a more global scale, the results of the study suggest that a prospective, randomized control trial of concomitant aortic valve repair in those with mild pre-implant disease is warranted – particularly in the destination therapy population.
Acknowledgements/Disclosures:
We thank the INTERMACS investigators, coordinators, and participating institutions for the data they have provided for this registry. The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) or the National Institutes of Health. This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant No. UL1TR001873 (V.K.T.) and KL2TR001874 (A.R.G.) and the Lisa and Mark Schwartz and the Program to Reverse Heart Failure at New York Presbyterian Hospital/Columbia University.
Dr. Naka received consulting fees from Thoratec and Heartware. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations:
- CF-LVAD
Continuous Flow Left Ventricular Assist Device
- INTERMACS
Interagency Registry for Mechanically Assisted Circulatory Support
- BTT
Bridge to Transplant
- AI
Aortic Insufficiency
- AV
Aortic Valve
- LVEDD
Left Ventricular End Diastolic Diameter
- LVEF
Left Venticular Ejection Fraction
- PVR
Pulmonary Vascular Resistance
- PCWP
Pulmonary Capillary Wedge Pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT and Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:1495–504. [DOI] [PubMed] [Google Scholar]
- 2.Jorde UP, Uriel N, Nahumi N, Bejar D, Gonzalez-Costello J, Thomas SS, Han J, Morrison KA, Jones S, Kodali S, Hahn RT, Shames S, Yuzefpolskaya M, Colombo P, Takayama H and Naka Y. Prevalence, significance, and management of aortic insufficiency in continuous flow left ventricular assist device recipients. Circulation Heart failure. 2014;7:310–9. [DOI] [PubMed] [Google Scholar]
- 3.Pak SW, Uriel N, Takayama H, Cappleman S, Song R, Colombo PC, Charles S, Mancini D, Gillam L, Naka Y and Jorde UP. Prevalence of de novo aortic insufficiency during long-term support with left ventricular assist devices. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29:1172–6. [DOI] [PubMed] [Google Scholar]
- 4.Soleimani B, Haouzi A, Manoskey A, Stephenson ER, El-Banayosy A and Pae WE. Development of aortic insufficiency in patients supported with continuous flow left ventricular assist devices. ASAIO journal. 2012;58:326–9. [DOI] [PubMed] [Google Scholar]
- 5.Holley CT, Fitzpatrick M, Roy SS, Alraies MC, Cogswell R, Souslian L, Eckman P and John R. Aortic insufficiency in continuous-flow left ventricular assist device support patients is common but does not impact long-term mortality. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2017;36:91–96. [DOI] [PubMed] [Google Scholar]
- 6.John R, Mantz K, Eckman P, Rose A and May-Newman K. Aortic valve pathophysiology during left ventricular assist device support. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29:1321–9. [DOI] [PubMed] [Google Scholar]
- 7.Mudd JO, Cuda JD, Halushka M, Soderlund KA, Conte JV and Russell SD. Fusion of aortic valve commissures in patients supported by a continuous axial flow left ventricular assist device. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2008;27:1269–74. [DOI] [PubMed] [Google Scholar]
- 8.May-Newman K, Hillen B and Dembitsky W. Effect of left ventricular assist device outflow conduit anastomosis location on flow patterns in the native aorta. ASAIO journal. 2006;52:132–9. [DOI] [PubMed] [Google Scholar]
- 9.Cowger J, Rao V, Massey T, Sun B, May-Newman K, Jorde U and Estep JD. Comprehensive review and suggested strategies for the detection and management of aortic insufficiency in patients with a continuous-flow left ventricular assist device. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:149–57. [DOI] [PubMed] [Google Scholar]
- 10.Park SJ, Liao KK, Segurola R, Madhu KP and Miller LW. Management of aortic insufficiency in patients with left ventricular assist devices: a simple coaptation stitch method (Park's stitch). The Journal of thoracic and cardiovascular surgery. 2004;127:264–6. [DOI] [PubMed] [Google Scholar]
- 11.Morgan JA and Brewer RJ. Modified central closure technique for treatment of aortic insufficiency in patients on left ventricular assist device support. ASAIO journal. 2012;58:626–8. [DOI] [PubMed] [Google Scholar]
- 12.Cohn WE, Demirozu ZT and Frazier OH. Surgical closure of left ventricular outflow tract after left ventricular assist device implantation in patients with aortic valve pathology. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30:59–63. [DOI] [PubMed] [Google Scholar]
- 13.Deo SV, Sharma V, Cho YH, Shah IK and Park SJ. De novo aortic insufficiency during long-term support on a left ventricular assist device: a systematic review and meta-analysis. ASAIO journal. 2014;60:183–8. [DOI] [PubMed] [Google Scholar]
- 14.Cowger J, Pagani FD, Haft JW, Romano MA, Aaronson KD and Kolias TJ. The development of aortic insufficiency in left ventricular assist device-supported patients. Circulation Heart failure. 2010;3:668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajagopal K, Daneshmand MA, Patel CB, Ganapathi AM, Schechter MA, Rogers JG and Milano CA. Natural history and clinical effect of aortic valve regurgitation after left ventricular assist device implantation. The Journal of thoracic and cardiovascular surgery. 2013;145:1373–9. [DOI] [PubMed] [Google Scholar]
- 16.Sayer G, Sarswat N, Kim GH, Adatya S, Medvedofsky D, Rodgers D, Kruse E, Ota T, Jeevanandam V, Lang R and Uriel N. The Hemodynamic Effects of Aortic Insufficiency in Patients Supported with Continuous-Flow Left Ventricular Assist Devices. Journal of cardiac failure. 2017. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal A, Raghuvir R, Eryazici P, Macaluso G, Sharma P, Blair C, Tatooles AJ, Pappas PS and Bhat G. The development of aortic insufficiency in continuous-flow left ventricular assist device-supported patients. The Annals of thoracic surgery. 2013;95:493–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.