Abstract
Objective
There is uncertainty about the direction and magnitude of the associations between parity, breastfeeding and the risk of coronary heart disease (CHD). We examined the separate and combined associations of parity and breastfeeding practices with the incidence of CHD later in life among women in a large pan-European cohort study.
Methods
Data were used from EPIC-CVD, a case-cohort study nested within the EPIC prospective study of 520,000 participants from 10 countries. Information on reproductive history was available for 14,917 women, including 5,138 incident cases of CHD. Using Prentice-weighted Cox regression separately for each country followed by random-effects meta-analysis, we calculated hazard ratios (HRs) and 95% confidence intervals (CIs) for CHD, after adjustment for age, study centre, and several socioeconomic and biological risk factors.
Results
Compared with nulliparous women, the adjusted HR was 1.19 (95% CI: 1.01-1.41) among parous women; HRs were higher among women with more children (e.g., adjusted HR: 1.95, 1.19-3.20, for women with ≥5 children). Compared with women who did not breastfeed, the adjusted HR was 0.71 (0.52-0.98) among women who breastfed. For childbearing women who never breastfed, the adjusted HR was 1.58 (1.09, 2.30) compared with nulliparous women, whereas for childbearing women who breastfed the adjusted HR was 1.19 (0.99, 1.43).
Conclusion
Having more children was associated with a higher risk of CHD later in life, whereas breastfeeding was associated with a lower CHD risk. Women who both had children and breastfed did not have a non-significantly higher risk of CHD.
Introduction
Pregnancy is associated with profound changes in the maternal metabolic system, including weight gain, accumulation of abdominal fat, increased insulin resistance, and higher circulating lipid levels.1, 2 While these metabolic changes of pregnancy support the growth of the foetus and prepare the mother's body for breastfeeding in the short-term, they may also have a prolonged effect on maternal risk of cardiovascular diseases (CVD). However, previous studies have reported conflicting associations on parity (i.e., the number of live children to whom a woman has given birth) and risk of CVD later in life.3–10
Conversely, since the metabolic changes in pregnancy appear to reverse more quickly and more completely with breastfeeding, it has been proposed that breastfeeding could reduce maternal risk of cardiometabolic diseases.11 Studies have reported that, compared with women who have never breastfed, women who have breastfed have favourable cardiometabolic profiles,12, 13 and exhibit a lower burden of subclinical cardiovascular disease.14 There may be a lower risk of developing metabolic syndrome,15 hypertension,16, 17 and type 2 diabetes18–21 among women who have breastfed for longer cumulative durations. However, it is uncertain whether there is an association between breastfeeding and incident CVD outcomes.10, 22–24 Also, while lifetime duration of breastfeeding will be affected by the number of children, it is unknown whether extended duration of breastfeeding for one child is associated with the same extent of inverse association with CHD risk as multiple periods of shorter breastfeeding across several pregnancies. Moreover, whether breastfeeding could compensate for the potential effect of parity on CHD risk has not been examined.
The EPIC (European Prospective Investigation into Cancer and Nutrition)-CVD study provides an opportunity to address these outstanding issues and to evaluate the separate and combined associations of parity and breastfeeding on the risk of incident CHD in a large sample of women from diverse European countries.
Methods
EPIC-CVD is a large, prospective, case-cohort study nested within the European Prospective Investigation into Cancer and Nutrition (EPIC) study. 25, 26 Briefly, the EPIC study involves 366,521 women and 153,457 men, mostly aged 35–70 years, recruited by 23 centres in 10 European countries (Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom) between 1991 and 1999. Participants completed questionnaires on their diet, lifestyle, and medical history, and data were centralized at the International Agency for Research on Cancer (IARC) in Lyon, France. A representative random subcohort of 18,249 participants (62% women), stratified by centre, was selected for the EPIC-CVD project.27 After exclusion of 609 participants with a prior history of myocardial infarction or stroke at baseline, 17,640 subcohort members remained. This study complied with the Declaration of Helsinki; ethical review boards of IARC and all local institutions where participants had been recruited gave approval for the study, and all participants gave written informed consent.
Definition and ascertainment of CHD events
First-time CHD events, whether non-fatal or fatal, as defined by codes 410-414 of the International Classification of Diseases Ninth Edition (ICD-9), and codes I20-I25 of the Tenth Edition (ICD-10) were the primary study endpoint. Individual centres used different methods to ascertain first-time non-fatal CHD events, including self-report and linkage with morbidity or hospital registries. Non-fatal CHD events were further validated by additional review of medical records and/or linkage with registries.26 Fatal CHD events were generally ascertained through mortality registries. End of follow-up for CHD events varied between centres and ranged between 2003 and 2010.
Study population and measurement
Of the 16,504 women in EPIC-CVD who did not have a known history of CHD or stroke at baseline, 14,917 women provided data on reproductive history. Two EPIC centres (Bilthoven, the Netherlands, and Umea, Sweden) did not assess parity and breastfeeding history and thus did not contribute to the analyses. Reproductive history, socioeconomic and lifestyle factors, and medical history were assessed once using a self-administrated questionnaire at study baseline. Trained health professionals measured blood pressure, weight, height, and waist circumference during a visit to each study centre, except in the France and Oxford centres where anthropometry was self-reported. Blood pressure measurements were not available for the Norway, Asturias, and Navarra centres. High blood pressure was defined as self-reported hypertension at baseline, systolic blood pressure>140 or diastolic blood pressure >90, or self-reported use of hypertension medication. Body mass index was calculated as weight divided by the square of height in meters. HDL cholesterol and total cholesterol levels, measured in baseline serum samples using a Roche MODULAR ANALYTICS EVO analyser, were available in all centres except Norway.
Statistical analyses
Baseline characteristics for the subcohort by parity were presented as means (standard deviation) or medians (interquartile range) for continuous variables and as percentages for categorical variables. Cox proportional hazards models, modified for the case-cohort design using the Prentice method28, were used to estimate hazard ratios and 95% confidence intervals for first-time CHD by parity and breastfeeding history. Participants contributed only to the first CHD outcome (whether non-fatal or fatal) experienced during follow-up, so fatal events that followed non-fatal events were not included. Given the multilevel structure of the data, models were first fitted separately within each country before pooling the country-specific estimates by multivariate random-effects meta-analysis using inverse variance weights. Age was used as the underlying time variable, with entry time defined as the participant’s age at recruitment, and exit time as the age of first-time CHD, loss-to-follow-up or censoring at the end of the follow-up, whichever came first. The I2 statistic was used to quantify the percentage of total variability between countries due to between-country heterogeneity. Parity, defined as the number of live births, was categorized as nulliparous (reference), 1 child, 2 children, 3 children, 4 children, or 5 or more children. Breastfeeding history was examined among parous women, comparing women who ever breastfed to women who had never breastfed, and categorized into groups of lifetime duration of breastfeeding (never [reference], >0-<3 months, 3-<6 months, 6-<12 months, 12-<23 months, and 23 months or more), and into groups of mean duration of breastfeeding per live-born child (never [reference], >0-<1 months, 1-<3 months, 3-<6 months, and 6 months or more). Group-specific 95% confidence intervals were estimated only from the variances that correspond to the amount of information underlying each group (including the reference group).29 Models were adjusted for age at study entry and centre (Model I), and then additionally for level of attained education, smoking status, and number of livebirths (for breastfeeding history only) (Model II), followed by further adjustment for other confounders and potential mediators (history of high blood pressure, HDL cholesterol, total cholesterol, history of diabetes mellitus, and BMI) (Model III). Model III was used as our primary, most conservative, analyses model. To account for the impact of missing covariate data on our results, we restricted our main analyses to individuals with complete data for all models. Secondary analyses allowed the set of individuals to vary between models and used all individuals with non-missing values for the covariates separately for each model. To investigate whether age or number of live births modified the association between parity or breastfeeding with CHD risk, we calculated the HRs for CHD in women <55 years versus ≥ 55 years of age, and in women with 1 or 2 children versus those with 3 children or more. Statistical interaction was evaluated by adding a cross-product term to the country-specific regression models and pooling these using random-effects meta-analysis. In a sensitivity analysis, we excluded women younger than 45 in whom reproductive history may not yet be complete. To assess the combined effect of parity and breastfeeding on CHD risk, we also examined the association between parity, history of breastfeeding and risk of first-time CHD in models including nulliparous women as the reference group. All statistical analyses were performed using STATA, version 12.0 (Stata, College Station, TX).
Results
The baseline characteristics of the 9,985 women in the subcohort with information on reproductive history are shown in Table 1. The mean (SD) age at entry was 52.7 (9.1) years. Parous women who had ever breastfed had more children than parous women who had never breastfed. Supplementary Table 1 shows the descriptive statistics for main characteristics by centre. Overall, 88% of women were parous, with rates ranging from 83% in the UK to 93% in Norway. Of these 87% had ever breastfed, ranging from 77% of women in France to over 92-96% of women in Sweden, Norway, and Denmark. During a median follow-up of 11.1 years (interquartile range 8.0-13.4), 5,138 of 14,890 women developed CHD, of whom 206 were also in the subcohort (27 women without follow-up data available were excluded). The number of participants contributing to the main analyses are shown in Supplementary table 2.
Table 1. Baseline characteristics of women in the EPIC-CVD subcohort by parity and breastfeeding history.
| Total | Nulliparous | Parous - never breastfed | Parous - ever breastfed | |
|---|---|---|---|---|
| % of overall subcohort* | - | 12.4 | 11.2 | 76.4 |
| Age at study entry, years | 52.7 (9.1) | 52.7 (10.1) | 52.0 (8.4) | 52.7 (9.0) |
| Education level, % | ||||
| None | 11.3 | 7.0 | 9.1 | 12.4 |
| Primary | 34.3 | 23.9 | 34.9 | 35.6 |
| Secondary | 15.4 | 18.2 | 19.4 | 14.5 |
| Tertiary | 39.0 | 50.9 | 36.6 | 37.5 |
| Current smoker, % | 21.7 | 25.4 | 20.4 | 21.1 |
| History of diabetes, % | 2.6 | 2.3 | 3.5 | 2.5 |
| BMI, kg/m2 | 26.1 (4.7) | 24.9 (4.3) | 26.4 (4.9) | 26.2 (4.7) |
| Systolic blood pressure, mmHg | 130.8 (20.0) | 129.6 (20.4) | 129.3 (19.9) | 131.0 (19.9) |
| Diastolic blood pressure, mmHg | 80.3 (10.6) | 79.2 (10.4) | 80.2 (11.2) | 80.5 (10.5) |
| History of high blood pressure, % | 34.3 | 30.1 | 33.2 | 35.0 |
| Total cholesterol, mmol/L | 6.0 (1.1) | 6.0 (1.2) | 6.0 (1.1) | 6.0 (1.1) |
| HDL cholesterol, mmol/L | 1.6 (0.4) | 1.7 (0.4) | 1.6 (0.4) | 1.6 (0.4) |
| Postmenopausal, % | 52.7 | 53.9 | 47.7 | 52.9 |
| Age at menopauseƗ | 48.5 (4.9) | 48.1 (5.3) | 48.2 (5.0) | 48.7 (4.9) |
| Number of children, % | ||||
| 1 child | - | - | 25.6 | 13.1 |
| 2 children | - | - | 49.7 | 47.5 |
| ≥3 children | - | - | 24.8 | 39.4 |
| Lifetime duration of breastfeeding, months | - | - | - | 9.7 (10.4) |
| Duration of breastfeeding per child, months | - | - | - | 4.2 (3.7) |
Values are mean (standard deviation) for continuous variables.
131 of 9,985 women with incomplete information about breastfeeding history were excluded.
postmenopausal women only.
Parity and risk of coronary heart disease
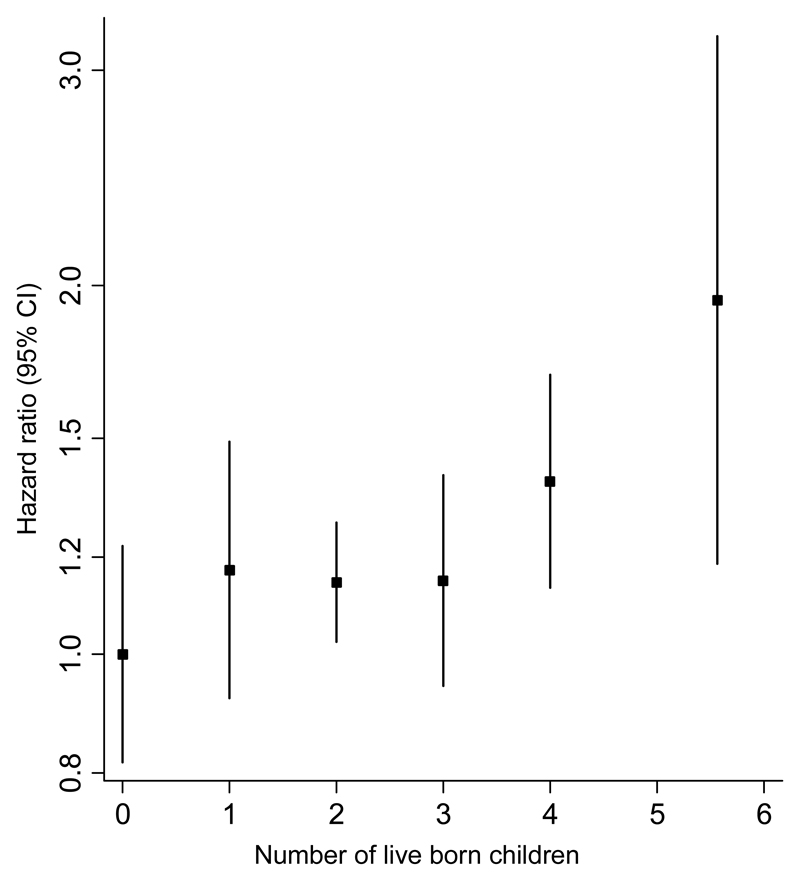
Of 12,319 women with complete data, 3,336 developed CHD during follow-up. The HR (95% CI) for CHD in parous versus nulliparous women was 1.27 (1.09, 1.47) in the age- and centre adjusted model and attenuated to 1.19 (1.01, 1.41) following adjustment for potential confounders and mediators (Table 2). The I2 statistic for the multiple-adjusted analyses was 0% (0%-67%), indicating that there was minimal heterogeneity between countries. Compared to nulliparous women, the multiple-adjusted HRs for CHD were 1.17 (0.92, 1.49), 1.15 (1.02, 1.28), 1.15 (0.94, 1.40), 1.39 (1.14, 1.70), and 1.95 (1.19, 3.20) for women with 1, 2, 3, 4, or 5 or more children, respectively (Table 2 and Figure 1). Results were similar in analyses including the largest set of women with complete data available, irrespective of incomplete data in subsequent models, or when restricting the analyses to women aged 45 years or older at study entry (Supplementary Tables 3 and 4).
Table 2. Hazard ratios (95% confidence intervals) for incident coronary heart disease associated with parity in all women and history of breastfeeding in parous women only.
| Model I | Model II | Model III | |
|---|---|---|---|
| Parity, n | 12,319 | 12,319 | 12,319 |
| Parous vs. not | 1.27 (1.09, 1.47) | 1.24 (1.07, 1.44) | 1.19 (1.01, 1.41) |
| I2 for heterogeneity (95% CI) | 7% (0%, 67%) | 0% (0%, 65%) | 0% (0%, 65%) |
| Number of children, n | 9,701 | 9,701 | 9,701 |
| None | 1.00 (0.82, 1.22) | 1.00 (0.82, 1.22) | 1.00 (0.82, 1.23) |
| 1 child | 1.21 (1.00, 1.45) | 1.16 (0.95, 1.42) | 1.17 (0.92, 1.49) |
| 2 children | 1.21 (1.10, 1.33) | 1.17 (1.10, 1.25) | 1.15 (1.02, 1.28) |
| 3 children | 1.21 (1.05, 1.39) | 1.20 (1.02, 1.40) | 1.15 (0.94, 1.40) |
| 4 children | 1.46 (1.20, 1.77) | 1.43 (1.18, 1.75) | 1.39 (1.14, 1.70) |
| 5 or more children | 2.19 (1.41, 3.38) | 2.02 (1.27, 3.20) | 1.95 (1.19, 3.20) |
| Breastfeeding, n | 8,044 | 8,044 | 8,044 |
| Ever vs. never | 0.71 (0.59, 0.85) | 0.69 (0.57, 0.85) | 0.71 (0.52, 0.98) |
| I2 for heterogeneity | 8% (0%, 73%) | 16% (0%, 59%) | 58% (2%, 82%) |
| Lifetime duration of breastfeeding, n | 8,012 | 8,012 | 8,012 |
| Never breastfed | 1.00 (0.84, 1.19) | 1.00 (0.83, 1.21) | 1.00 (0.75, 1.34) |
| >0 to >3 months | 0.69 (0.57, 0.83) | 0.69 (0.57, 0.83) | 0.73 (0.60, 0.89) |
| ≥3 to <6 months | 0.71 (0.55, 0.91) | 0.73 (0.60, 0.88) | 0.68 (0.56, 0.83) |
| ≥6 to <12 months | 0.73 (0.61, 0.87) | 0.70 (0.58, 0.84) | 0.69 (0.55, 0.87) |
| ≥12 to <23 months | 0.65 (0.57, 0.74) | 0.63 (0.53, 0.74) | 0.63 (0.51, 0.76) |
| ≥23 months | 0.66 (0.50, 0.87) | 0.60 (0.46, 0.78) | 0.62 (0.45, 0.86) |
| Duration of breastfeeding per child, n | 8,012 | 8,012 | 8,012 |
| Never breastfed | 1.00 (0.85, 1.17) | 1.00 (0.84, 1.20) | 1.00 (0.73, 1.37) |
| >0 to <1 months | 0.79 (0.66, 0.94) | 0.73 (0.61, 0.88) | 0.77 (0.63, 0.94) |
| ≥1 to <3 months | 0.72 (0.62, 0.85) | 0.71 (0.61, 0.83) | 0.69 (0.61, 0.78) |
| ≥3 to <6 months | 0.66 (0.56, 0.78) | 0.65 (0.55, 0.75) | 0.67 (0.57, 0.77) |
| ≥6 months | 0.63 (0.51, 0.78) | 0.66 (0.53, 0.82) | 0.67 (0.56, 0.80) |
Analyses of parity are conducted in all women. Analyses of breastfeeding are conducted in parous women only.
Model I: Adjusted for age at study entry and centre; Model II: model I + level of attained education, smoking status, and number of livebirths (for breastfeeding history only); Model III: model II + high blood pressure, HDL cholesterol, total cholesterol, history of diabetes mellitus, and BMI.
Figure 1. Adjusted hazard ratios (with group-specific 95% confidence intervals) for incident coronary heart disease associated with number of live born children.
The highest category of live born children (5 or more) is plotted at 5.5. Adjusted for age at study entry, centre, level of attained education, smoking status, high blood pressure, HDL cholesterol, total cholesterol, history of diabetes mellitus, and BMI.
Breastfeeding history and risk of coronary heart disease
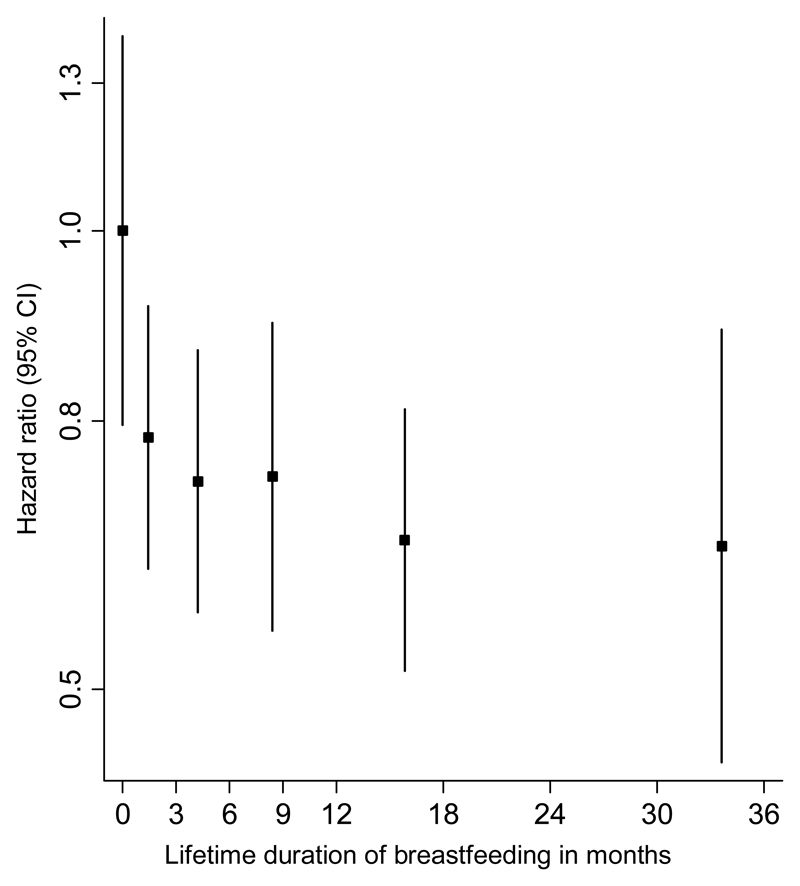
Complete data on breastfeeding history was available on 8,044 parous women with 2,404 CHD events. Parous women who had ever breastfed had a multiple-adjusted HR for CHD of 0.71 (95% CI: 0.52, 0.98) compared with parous women who never breastfed (Table 2). There was substantial heterogeneity between countries; the I2 statistic was 58% (2%-82%). Compared with parous women who had never breastfed, women with a lifetime duration of breastfeeding of >0-<3 months, 3-<6 months, 6-<12 months, 12-<23 months, or 23 months or more had a multiple-adjusted HR for CHD of 0.73 (0.60, 0.89), 0.68 (0.56, 0.83), 0.69 (0.55, 0.87), 0.63 (0.51, 0.76), and 0.62 (0.45, 0.86), respectively (Figure 2 and Table 2). Similar results were obtained in the analyses on the mean duration of breastfeeding per child and CHD risk (Figure 3 and Table 2). Analyses restricted to women 45 years or older or including women with missing data on some covariables did not change the findings materially (Supplementary Tables 3 and 4).
Figure 2. Adjusted hazard ratios (with group-specific 95% confidence intervals) for incident coronary heart disease associated with lifetime duration of breastfeeding in parous women.
Categories are never, 0-<3 months, 3-<6 months, 6-<12 months, 12-<23 months, and 23 months or more. On the x-axis, results are placed on the mean lifetime duration of breastfeeding within category. Adjusted for age at study entry, centre, level of attained education, smoking status, number of livebirths, high blood pressure, HDL cholesterol, total cholesterol, history of diabetes mellitus, and BMI.
Figure 3. Adjusted hazard ratios (with group-specific 95% confidence intervals) for incident coronary heart disease associated with mean duration of breastfeeding per live born child.
Categories are never, 0-<1 months, 1-<3 months, 3-<6 months, and 6 months or more. On the x-axis, results are placed on the mean duration of breastfeeding per child within category. Adjusted for age at study entry, centre, level of attained education, smoking status, number of livebirths, high blood pressure, HDL cholesterol, total cholesterol, history of diabetes mellitus, and BMI.
Combination of parity and breastfeeding and risk of coronary heart disease
Analyses of the combination of parity and breastfeeding indicated that parous women who had never breastfed were at a significantly higher risk of CHD compared to nulliparous women (HR: 1.58 [1.09, 2.30]; Table 3). There was no significant evidence for a higher risk of CHD in parous women who had ever breastfed (HR: 1.19 [0.99, 1.43]), although power to detect weak effects was limited.
Table 3. Hazard ratios (95% confidence intervals) for incident coronary heart disease associated with the combined effects of parity and a history of breastfeeding.
| N | Nulliparous | Parous – never breastfed | Parous – ever breastfed | |
|---|---|---|---|---|
| Model I | 9,511 | 1.00 [reference] | 1.73 (1.30, 2.30) | 1.24 (1.05, 1.46) |
| Model II | 9,511 | 1.00 [reference] | 1.72 (1.34, 2.20) | 1.22 (1.02, 1.46) |
| Model III | 9,511 | 1.00 [reference] | 1.58 (1.09, 2.30) | 1.19 (0.99, 1.43) |
Model I: Adjusted for age at study entry and centre; Model II: model I + level of attained education and smoking status. Model III: model II + high blood pressure, HDL cholesterol, total cholesterol, history of diabetes mellitus, and BMI. Model V: Model IV + number of live births
Subgroup analyses
Analyses in subgroups of women younger than 55 years versus those 55 years or above at study entry provided no evidence for a different effect of parity by age, and neither did the association between number of children and risk of CHD differ between the age groups (Table 4). A history of breastfeeding was associated with similar reductions in risk of CHD in younger as in older women, and in women with 1 or 2 children as in those with 3 or more children. The associations between lifetime duration of breastfeeding and duration of breastfeeding per child and CHD risk also did not differ between age groups or by parity (Table 4).
Table 4. Hazard ratios (95% confidence intervals) for incident coronary heart disease by parity and history of breastfeeding in subgroups.
| Age | Parity | ||||
|---|---|---|---|---|---|
| <55 years | ≥55 years | 1-2 children | ≥3 children | ||
| Parous (yes vs. no) | 1.14 (0.83, 1.55) | 1.20 (0.99, 1.46) | - | - | |
| Number of children | |||||
| None | 1.00 (0.77, 1.31) | 1.00 (0.78, 1.28) | - | - | |
| 1 child | 1.37 (0.79, 2.38) | 1.07 (0.63, 1.80) | - | - | |
| 2 children | 1.12 (0.64, 1.96) | 1.67 (0.79, 3.53) | - | - | |
| 3 children | 1.15 (0.75, 1.77) | 1.36 (0.94, 1.96) | - | - | |
| 4 children | 1.13 (0.81, 1.57) | 1.53 (0.45, 5.19) | - | - | |
| 5 or more children | 1.30 (0.91, 1.87) | 1.84 (1.20, 2.82) | - | - | |
| Ever breastfed (yes vs. no) | 0.71 (0.52, 0.95) | 0.77 (0.54, 1.08) | 0.69 (0.51, 0.94) | 0.76 (0.54, 1.08) | |
| Lifetime duration of breastfeeding | |||||
| Never breastfed | 1.00 (0.69, 1.45) | 1.00 (0.72, 1.38) | 1.00 (0.71, 1.41) | 1.00 (0.71, 1.41) | |
| >0 to <3 months | 0.74 (0.49, 1.11) | 0.67 (0.41, 1.10) | 0.67 (0.45, 0.99) | 0.87 (0.53, 1.41) | |
| ≥3 to <6 months | 0.51 (0.35, 0.74) | 0.68 (0.40, 1.15) | 0.63 (0.42, 0.94) | 0.79 (0.51, 1.22) | |
| ≥6 to <12 months | 0.75 (0.46, 1.22) | 0.61 (0.38, 1.01) | 0.64 (0.36, 1.14) | 0.72 (0.45, 1.17) | |
| ≥12 to <23 months | 0.54 (0.34, 0.86) | 0.60 (0.34, 1.05) | 0.61 (0.37, 1.01) | 0.65 (0.37, 1.14) | |
| ≥23 months | 0.55 (0.29, 1.04) | 0.55 (0.37, 0.81) | 1.09 (0.48, 2.48) | 0.62 (0.43, 0.89) | |
| Duration of breastfeeding per child | |||||
| Never breastfed | 1.00 (0.77, 1.31) | 1.00 (0.70, 1.42) | 1.00 (0.72, 1.39) | 1.00 (0.72, 1.39) | |
| >0 to <1 months | 0.79 (0.54, 1.14) | 0.71 (0.44, 1.15) | 0.65 (0.42, 1.01) | 0.96 (0.66, 1.40) | |
| ≥1 to <3 months | 0.59 (0.42, 0.82) | 0.66 (0.43, 1.03) | 0.67 (0.46, 0.97) | 0.70 (0.44, 1.10) | |
| ≥3 to <6 months | 0.62 (0.40, 0.98) | 0.63 (0.40, 0.99) | 0.63 (0.40, 0.99) | 0.70 (0.41, 1.17) | |
| ≥6 months | 0.64 (0.44, 0.93) | 0.59 (0.36, 0.97) | 0.67 (0.43, 1.03) | 0.63 (0.39, 1.01) |
Analyses of parity are conducted in all women. Analyses of breastfeeding are conducted in parous women only.
Models are adjusted for age at study entry, centre, level of attained education, smoking status, and number of livebirths (for breastfeeding history only), high blood pressure, HDL cholesterol, total cholesterol, history of diabetes mellitus, and BMI.
Discussion
In this case-cohort analysis, nested within the 10-country EPIC prospective cohort study, we examined the separate and combined associations of parity and breastfeeding on the risk of incident CHD. Our main findings are threefold.
First, we report that parous women are at higher risk of CHD as compared to nulliparous women; with the highest risk seen among women who had the most offspring. These findings, therefore, add to the accumulating evidence that repeated pregnancies could result in an accumulation of cardiometabolic changes, including elevated pro-atherogenic lipid levels, accumulation of abdominal fat, endothelial dysfunction, atherosclerosis, and increased systemic inflammation,1, 30, 31 that may have permanent effects on the cardiovascular system, leading to a higher risk of CHD later in life.3–7
Second, in agreement with previous observations that breastfeeding is associated with lower risk of the metabolic syndrome,15 hypertension,16, 17 and diabetes,18–21 our study supports the existence of inverse associations between breastfeeding and CHD risk. Previous studies have reported that prolonged periods of breastfeeding could have beneficial effects on maternal cardiovascular risk factors, including on lipids, blood pressure, insulin and glucose homeostasis, and body mass index.12, 32 Despite the biological plausibility, our results are more consistent with a threshold effect between never breastfeeding and any duration of breastfeeding and indicate that possible inverse dose-response curve between longer cumulative breastfeeding duration and CHD risk is relatively weak. Results from the Nurses’ Health Study also suggested that the association between lifetime duration of breastfeeding and risk of incident CHD was characterized by a threshold effect; only women with lifetime cumulative breastfeeding duration of two years or more had a significantly lower risk of incident CHD compared to women who had never breastfed.23 Moreover, while a history of breastfeeding was associated with a slightly lower risk of fatal CHD in a cohort of 267,400 women from Shanghai, increasing duration of breastfeeding did not strengthen the association.10 In contrast, a previous study of more than 20,000 Norwegian women reported that breastfeeding was associated with a lower risk of CVD mortality, potentially in a U-shaped fashion, but only among parous women younger than 65 years at study baseline.22
Third, our study constitutes one of the few available analyses of the combined associations of parity and breastfeeding on the incidence of CHD. Despite the higher number of children among women who had ever breastfed, we found that childbearing only raised the risk of CHD among women who had never breastfed. This is consistent with the notion that the physiological changes in pregnancy could reverse more quickly and more completely with breastfeeding, which in turn may confer cardiovascular protection later in life.11
Our observations are consistent with the conclusions of previous studies that attempted to disentangle biological processes related to pregnancy from lifestyle factors related to childrearing by comparing results from men and women within the same cohort. For example, a study among men from prospective cohorts in the US found no relationship between the number of children and the paternal risk of CHD,33 whereas there was a positive association between having 6 or more pregnancies and CHD risk among women from the same cohort.5 A cross-sectional analysis of men and women from the British Women's Heart and Health Study and the British Regional Heart Study reported that having more offspring was associated with higher body mass index for both male and female parents, and with more adverse lipid profiles and diabetes in women only.6 However, as there was a positive association between offspring and CHD among women (but not men) in these cohorts, the authors concluded that the biological effects of pregnancy in women persist into later life.
The strengths and potential limitations of our study merit consideration. Our analysis maximized power and efficiency by conducting a case-cohort analysis of incident CHD in the large prospective EPIC cohort, thereby focusing measurement of lipids and other biochemical risk factors on the most relevant subset of the cohort. The validity of our findings was enhanced by our ability to adjust for a range of relevant covariates, and by the robustness of our results to a variety of sensitivity and subgroup analyses. The generalisability of our findings was enhanced by the inclusion of women from 10 diverse European countries. However, we cannot preclude the possibility that the associations observed in this study were, at least partly, due to unmeasured or residual confounding. For example, confounding is a major concern in studies of breastfeeding and health outcomes, because mothers who breastfeed are more likely to engage in other health-promoting behaviours such as high fruit and vegetable consumption and abstinence from smoking.34–36 Nevertheless, we found that adjustment in our study for several relevant factors did not materially affect the relationships we observed. Our study had insufficient data to account for CHD risk factors before or during pregnancy that determine breastfeeding initiation and duration as well as future CHD risk, leaving our results potentially liable to “reverse causality”. For example, women with pre-existing CHD risk factors such as obesity, type 1 diabetes, preeclampsia, or polycystic ovary syndrome, might be less likely to initiate breastfeeding and or could breastfeed for shorter durations than women without these CHD risk factors. Because our study involved self-reported information on parity and breastfeeding, information that was sometimes recalled and recorded decades after childbirth and weaning (with the added limitation that duration of breastfeeding was recorded in EPIC only for a woman’s first three children and final child), the true strength of any associations we observed could have been underestimated. Finally, data on the number of children in men was not available, so we were not able to dissect whether the association between parity and CHD was due to biological effects of childbearing or factors related to childrearing.
In conclusion, this analysis of women from 10 European countries found that having more children was associated with a higher risk of CHD later in life, whereas breastfeeding was associated with a lower CHD risk. Women who both had children and breastfed did have a non-significantly higher risk of CHD, suggesting the need for studies to determine whether breastfeeding can compensate for the CHD risk associated with greater parity.
Supplementary Material
Key messages.
What is already known about this subject?
-
-
Pregnancy poses a substantial challenge to cardiovascular system of the mother and breastfeeding might reverse some of these changes.
-
-
The direction and magnitude of the associations between parity, breastfeeding, and their combined effects, and the risk of coronary heart disease (CHD) are uncertain.
What does this study add?
-
-
Compared with nulliparous women, parous women were at a 20% increased risk of CHD and that the excess risks increased with increasing number of children.
-
-
Breastfeeding was associated with a reduced the risk of CHD of 30%, compared with parous women who did not breastfeed.
-
-
Compared with nulliparous women, childbearing was only associated with an increased risk of CHD among women who had never breastfed. This is despite the higher number of children among women who had ever breastfed.
How might this impact on clinical practice?
-
-
Breastfeeding might reverse the physiological changes in pregnancy more quickly and more completely.
-
-
If causal, promotion of prolonged breastfeeding in parous women may confer long-term cardiovascular benefit.
Acknowledgements
We thank all EPIC participants and staff for their contribution to the study. We thank staff from the EPIC-CVD and EPIC-InterAct Coordinating Centres for carrying out sample preparation and data-handling work, particularly Sarah Spackman (EPIC-CVD Data Manager).
Funding
EPIC-CVD has been supported by the European Union Framework 7 (HEALTH-F2-2012-279233), the European Research Council (268834), the UK Medical Research Council (G0800270 and MR/L003120/1), the British Heart Foundation (SP/09/002 and RG/08/014 and RG13/13/30194), and the UK National Institute of Health Research. EPIC Asturias was also supported by the Regional Government of Asturias. EPIC-Greece is also supported by the Hellenic Health Foundation. EPIC-Oxford was also supported by the UK Medical Research Council (MR/M012190/1) and Cancer Research UK (570/A16491). EPIC-Ragusa was also supported by the Sicilian Government, AIRE ONLUS Ragusa, and AVIS Ragusa. EPIC-Sweden was also supported by Swedish Cancer Society, Swedish Scientific Council, and Regional Government of Skåne and Västerbotten (Sweden).
EPIC-Turin was also supported also by the Compagnia di San Paolo and the Human Genetics Foundation-Torino (HuGeF).
Footnotes
Conflicts of interest
None
Author contributions: SP, YT, AW, KM, MS, EW, and AB were involved in the concept and design of the study. SP and MS conducted the statistical analyses. SP prepared the first draft of the manuscript. All authors were involved in the acquisition and/or interpretation of the data, made critical revision of the manuscript for important intellectual content, and provided final approval of the version to be published. SP, YT, and AB are responsible for the integrity of the work as a whole.
References
- 1.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clinical obstetrics and gynecology. 2007;50(4):938–48. doi: 10.1097/GRF.0b013e31815a5494. [DOI] [PubMed] [Google Scholar]
- 2.Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130(12):1003–8. doi: 10.1161/CIRCULATIONAHA.114.009029. [DOI] [PubMed] [Google Scholar]
- 3.Parikh NI, Cnattingius S, Dickman PW, Mittleman MA, Ludvigsson JF, Ingelsson E. Parity and risk of later-life maternal cardiovascular disease. American heart journal. 2010;159(2):215–21.e6. doi: 10.1016/j.ahj.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe DH, Eisenbach Z, Manor O. The effect of parity on cause-specific mortality among married men and women. Maternal and child health journal. 2011;15(3):376–85. doi: 10.1007/s10995-010-0591-x. [DOI] [PubMed] [Google Scholar]
- 5.Ness RB, Harris T, Cobb J, et al. Number of pregnancies and the subsequent risk of cardiovascular disease. The New England journal of medicine. 1993;328(21):1528–33. doi: 10.1056/NEJM199305273282104. [DOI] [PubMed] [Google Scholar]
- 6.Lawlor DA, Emberson JR, Ebrahim S, et al. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Findings from the British Women's Heart and Health Study and the British Regional Heart Study. Circulation. 2003;107(9):1260–4. doi: 10.1161/01.cir.0000053441.43495.1a. [DOI] [PubMed] [Google Scholar]
- 7.Koski-Rahikkala H, Pouta A, Pietilainen K, Hartikainen AL. Does parity affect mortality among parous women? Journal of epidemiology and community health. 2006;60(11):968–73. doi: 10.1136/jech.2005.044735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steenland K, Lally C, Thun M. Parity and coronary heart disease among women in the American Cancer Society CPS II population. Epidemiology (Cambridge, Mass) 1996;7(6):641–3. doi: 10.1097/00001648-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. A prospective study of age at menarche, parity, age at first birth, and coronary heart disease in women. American journal of epidemiology. 1987;126(5):861–70. doi: 10.1093/oxfordjournals.aje.a114723. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher LG, Davis LB, Ray RM, et al. Reproductive history and mortality from cardiovascular disease among women textile workers in Shanghai, China. International journal of epidemiology. 2011;40(6):1510–8. doi: 10.1093/ije/dyr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuebe AM, Rich-Edwards JW. The reset hypothesis: lactation and maternal metabolism. American journal of perinatology. 2009;26(1):81–8. doi: 10.1055/s-0028-1103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunderson EP, Lewis CE, Wei GS, Whitmer RA, Quesenberry CP, Sidney S. Lactation and changes in maternal metabolic risk factors. Obstetrics and gynecology. 2007;109(3):729–38. doi: 10.1097/01.AOG.0000252831.06695.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstetrics and gynecology. 2009;113(5):974–82. doi: 10.1097/01.AOG.0000346884.67796.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClure CK, Catov JM, Ness RB, Schwarz EB. Lactation and maternal subclinical cardiovascular disease among premenopausal women. American journal of obstetrics and gynecology. 2012;207(1):46.e1–8. doi: 10.1016/j.ajog.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunderson EP, Jacobs DR, Jr, Chiang V, et al. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: a 20-Year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults) Diabetes. 2010;59(2):495–504. doi: 10.2337/db09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SY, Kim MT, Jee SH, Yang HP. Does long-term lactation protect premenopausal women against hypertension risk? A Korean women's cohort study. Preventive medicine. 2005;41(2):433–8. doi: 10.1016/j.ypmed.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Stuebe AM, Schwarz EB, Grewen K, et al. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. American journal of epidemiology. 2011;174(10):1147–58. doi: 10.1093/aje/kwr227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jager S, Jacobs S, Kroger J, et al. Breast-feeding and maternal risk of type 2 diabetes: a prospective study and meta-analysis. Diabetologia. 2014;57(7):1355–65. doi: 10.1007/s00125-014-3247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA : the journal of the American Medical Association. 2005;294(20):2601–10. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- 20.Villegas R, Gao YT, Yang G, et al. Duration of breast-feeding and the incidence of type 2 diabetes mellitus in the Shanghai Women's Health Study. Diabetologia. 2008;51(2):258–66. doi: 10.1007/s00125-007-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aune D, Norat T, Romundstad P, Vatten LJ. Breastfeeding and the maternal risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2014;24(2):107–15. doi: 10.1016/j.numecd.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Natland Fagerhaug T, Forsmo S, Jacobsen GW, Midthjell K, Andersen LF, Ivar Lund Nilsen T. A prospective population-based cohort study of lactation and cardiovascular disease mortality: the HUNT study. BMC public health. 2013;13:1070. doi: 10.1186/1471-2458-13-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuebe AM, Michels KB, Willett WC, Manson JE, Rexrode K, Rich-Edwards JW. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. American journal of obstetrics and gynecology. 2009;200(2):138 e1–8. doi: 10.1016/j.ajog.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition; a cohort study. BMC medicine. 2015;13:252. doi: 10.1186/s12916-015-0484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public health nutrition. 2002;5(6b):1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 26.Danesh J, Saracci R, Berglund G, et al. EPIC-Heart: the cardiovascular component of a prospective study of nutritional, lifestyle and biological factors in 520,000 middle-aged participants from 10 European countries. European journal of epidemiology. 2007;22(2):129–41. doi: 10.1007/s10654-006-9096-8. [DOI] [PubMed] [Google Scholar]
- 27.Langenberg C, Sharp S, Forouhi NG, et al. Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia. 2011;54(9):2272–82. doi: 10.1007/s00125-011-2182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73(1):1–11. [Google Scholar]
- 29.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Statistics in medicine. 1991;10(7):1025–35. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 30.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. The Journal of clinical endocrinology and metabolism. 2007;92(3):969–75. doi: 10.1210/jc.2006-2083. [DOI] [PubMed] [Google Scholar]
- 31.Sanghavi M, Kulinski J, Ayers CR, et al. Association between number of live births and markers of subclinical atherosclerosis: The Dallas Heart Study. European journal of preventive cardiology. 2016;23(4):391–9. doi: 10.1177/2047487315571891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Natland ST, Nilsen TI, Midthjell K, Andersen LF, Forsmo S. Lactation and cardiovascular risk factors in mothers in a population-based study: the HUNT-study. International breastfeeding journal. 2012;7(1):8. doi: 10.1186/1746-4358-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ness RB, Cobb J, Harris T, D'Agostino RB. Does number of children increase the rate of coronary heart disease in men? Epidemiology (Cambridge, Mass) 1995;6(4):442–5. doi: 10.1097/00001648-199507000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Beck LF, Morrow B, Lipscomb LE, et al. Prevalence of selected maternal behaviors and experiences, Pregnancy Risk Assessment Monitoring System (PRAMS), 1999. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002) 2002;51(2):1–27. [PubMed] [Google Scholar]
- 35.Pesa JA, Shelton MM. Health-enhancing behaviors correlated with breastfeeding among a national sample of mothers. Public health nursing (Boston, Mass) 1999;16(2):120–4. doi: 10.1046/j.1525-1446.1999.00120.x. [DOI] [PubMed] [Google Scholar]
- 36.Yngve A, Sjostrom M. Breastfeeding determinants and a suggested framework for action in Europe. Public health nutrition. 2001;4(2b):729–39. doi: 10.1079/phn2001164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.