Abstract
The skin is in daily contact with environmental pollutants, but the long-term effects of such exposure remain underinvestigated. Many of these toxins bind and activate the pregnane X receptor (PXR), a ligand-activated transcription factor that regulates genes central to xenobiotic metabolism. The objective of this work was to investigate the effect of constitutive activation of PXR in the basal layer of the skin to mimic repeated skin exposure to noxious molecules. We designed a transgenic mouse model that overexpresses the human PXR gene linked to the herpes simplex VP16 domain under the control of the keratin 14 promoter. We show that transgenic mice display increased transepidermal water loss and elevated skin pH, abnormal stratum corneum lipids, focal epidermal hyperplasia, activated keratinocytes expressing more thymic stromal lymphopoietin, a T helper type 2/T helper type 17 skin immune response, and increased serum IgE. Furthermore, the cutaneous barrier dysfunction precedes development of the T helper type 2/T helper type 17 inflammation in transgenic mice, thereby mirroring the time course of atopic dermatitis development in humans. Moreover, further experiments suggest increased PXR signaling in the skin of patients with atopic dermatitis when compared with healthy skin. Thus, PXR activation by environmental pollutants may compromise epidermal barrier function and favor an immune response resembling atopic dermatitis.
Introduction
During the last decade, much progress has been made in dissecting the mechanisms by which foreign compounds elicit alterations in xeno- and endobiotic metabolism. In the liver, many toxic compounds, including drugs, pesticides, and endocrine disruptors, bind to a transcription factor called pregnane X receptor (PXR or NR1I2). PXR regulates numerous genes encoding detoxification-related proteins such as phase I (e.g., cytochrome P450 [CYP450]) and phase II (e.g., sulfotransferases, glucuronosyltransferases, and glutathione S-transferases) enzymes, and transporters involved in xenobiotic elimination (e.g., multidrug resistance-associated proteins, organic anion-transporting polypeptides) (Kliewer et al., 1998). PXR is primarily expressed in the gastrointestinal tract and liver, with some expression in the testis and embryonic tissues (Kliewer et al., 2002). Furthermore, low but consistent PXR expression is detectable in immune cells such as T and B lymphocytes and dendritic cells (DCs) (Dubrac et al., 2010; Elentner et al., 2015; Jeannesson et al., 2011; Schote et al., 2007; Siest et al., 2008) as well as in the skin (Elentner et al., 2015; Haslam et al., 2013) of both mice and humans.
The skin is in daily contact with various noxious chemicals able to trigger PXR activation (Bickers and Athar, 2006; Kleiner et al., 2004; Kliewer et al., 2002; Lille-Langøy et al., 2015). Cutaneous barrier defects, such as those caused by loss-of-function mutations in the Filaggrin gene, could potentially facilitate penetration by xenobiotics into the skin, thereby predisposing to contact dermatitis (de Jongh et al., 2008; Novak et al., 2008; Thyssen et al., 2012). In skin cells such as keratinocytes and Langerhans cells, chemicals undergo biological activation via CYP450 enzymes (Modi et al., 2012). The reactive products in turn can activate the skin immune system, potentially leading to the production of a major T helper type 2 (Th2) cytokine called thymic stromal lymphopoietin (TSLP) (Kim et al., 2013; Takai, 2012). We recently showed that, following topical application with the polycyclic aromatic hydrocarbon 7,12-dimethylbenz[a] anthracene, PXR becomes upregulated in the skin, especially in Langerhans cells (Elentner et al., 2015). Moreover, several studies have shown that PXR activation interferes with immune responses (Dubrac et al., 2010; Zhou et al., 2006), including those of the skin (Elentner et al., 2015).
Basal keratinocytes are a major target of topically applied chemicals (Simonsson et al., 2011) and therefore are a consequential cell type for PXR activation. To investigate the effect of constitutive expression of PXR in the basal layer of the skin, we designed a transgenic mouse model that over-expresses the human PXR gene linked to the herpes simplex VP16 domain under the control of the keratin 14 (K14) promoter (Figure 1a). These mice named K14-VPPXR display congenital skin barrier defects associated with abnormal cutaneous homeostasis, evolving into a Th2/Th17 skin immune response and with elevated serum IgE. It is notable that the impaired epidermal barrier function precedes the development of overt inflammation in K14-VPPXR newborn mice, as a similar temporal relationship for these pathologies was recently reported in babies and young children (Cole et al., 2014; Kelleher et al., 2015). Here, we demonstrate that sustained activation of human PXR in the epidermis, mimicking daily contact with environmental noxious agents, triggers pathophysiologic pathways leading to the development of atopic dermatitis (AD)-like symptoms in mice. Extrapolating these results to humans, we suggest that chronic skin exposure to pollutants might be a significant contributor to the increased incidence of AD observed worldwide over the past few decades (Ahn, 2014; Kim et al., 2016; Silverberg, 2016). Additional experiments carried out with human skin show increased PXR signaling in AD skin when compared with healthy skin, corroborating this hypothesis.
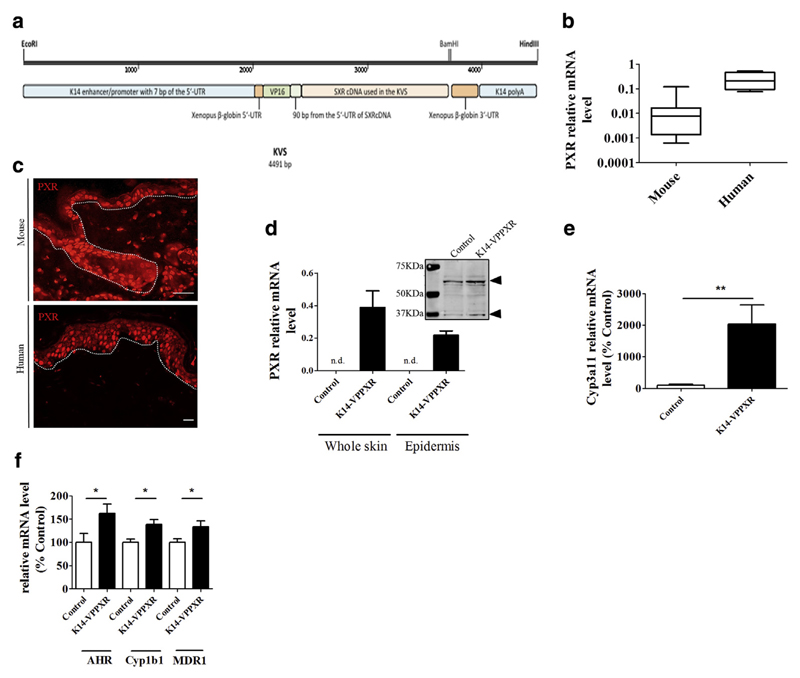
Figure 1. PXR locates to basal keratinocytes in mouse skin, and constitutive activation of PXR in the epidermis leads to an overall upregulation of xenobiotic metabolism.
(a) The KVS transgene (drawn to scale) depicting the human SXR cDNA attached to the VP16 domain sequence as subcloned in the K14 cassette. The VP16 domain is from herpes simplex virus, and the K14 sequences are from the human K14 gene. The EcoRI-HindIII linear fragment was used to generate transgenic mice (see also Supplementary Materials and Methods and Supplementary Figure S1). (b) Quantitative PCR showing relative expression of PXR mRNA levels relative to housekeeping genes in mouse (n = 10) and human (n = 4) skin. (c) Immunohistochemistry showing PXR staining in mouse (upper panel) and human (lower panel) skin. Bar = 50 μm. Dotted lines separate the epidermis from the dermis. (d) Quantitative PCR showing relative expression of human PXR mRNA levels in the skin or the epidermis of control (n = 5) and K14-VPPXR (n = 12) mice. Western blot analysis shows mouse PXR in control mouse skin and mouse + transgenic PXR expression in the K14-VPPXR mouse skin (pools of three skins). Arrows indicate the PXR. Quantitative PCR shows relative expression of (e) Cyp3A11 (n = 5) and (f) Ahr, Cyp1B1, and Mdr1/Abcb1 (n = 10–19) in the K14-VPPXR and control mouse skin. Data were analyzed with a Student’s t-test. *P <0.05, **P < 0.01. K14, keratin 14; n.d., not detectable; PXR, pregnane X receptor.
Results
Constitutive activation of human PXR in the epidermis leads to an overall upregulation of xenobiotic metabolism in the skin
Both human and mouse skin express low but consistent levels of PXR mRNA (Figure 1b) (Elentner et al., 2015; Haslam et al., 2013). In contrast, the PXR protein is markedly expressed in the epidermis (Figure 1c), where it mainly localizes to basal keratinocytes in mouse skin and to basal and suprabasal keratinocytes in human skin (Figure 1c). PXR is also expressed in some dermal cells in both human and mouse skin (Figure 1c).
To study the role of PXR in the epidermis, we created mice overexpressing constitutively activated human PXR under the control of the K14 promoter (K14-VPPXR) (Figure 1a, Supplementary Materials and Methods online, and Supplementary Figure S1 online). We used quantitative PCR and western blot analyses to measure the expression of human PXR in the whole skin and in the epidermis of these K14-VPPXR mice (Figure 1d). We found that overexpression of human PXR is accompanied by increased expression of well-known mouse PXR-downstream genes such as Cyp3A11, Ahr, Mdr1/Abcb1, and Cyp1B1 (Maglich et al., 2002) (Figure 1e–f) demonstrating that human PXR is functional in mouse epidermis. Thus, constitutive activation of the human PXR in mouse epidermis causes an overall upregulation of xenobiotic metabolism-related genes in the skin.
Constitutive activation of human PXR in the epidermis leads to a skin barrier defect and alters local homeostasis
Although adult (10–16 weeks old) K14-VPPXR mice exhibit neither gross skin abnormalities nor scratching behavior, they do display increased transepidermal water loss (TEWL) associated with increased skin surface pH when compared with control mice (Figure 2a), traits consistent with impaired cutaneous barrier function. Histologically, the skin from K14-VPPXR mice exhibits mild-to-severe epidermal hyperplasia (Figure 2b, Supplementary Figure S2 online) in association with increased keratinocyte proliferation, as reflected by increased numbers of Ki67+ cells and mRNA levels of the proliferation marker Pcna in the whole skin and epidermis of K14-VPPXR mice when compared with controls (Figure 2c, 2d). Moreover, expression of keratin 16 (Krt16) is borderline increased in the skin of K14-VPPXR mice (Figure 2e). Expression of Egfr and IL22 is associated with keratinocyte proliferation (Cavani et al., 2012; Kolev et al., 2008; Sääf et al., 2012). We found higher expression of Egfr in the epidermis of transgenic mice when compared with control mice (Figure 2f). IL22 mRNA was not detected in any skin samples (data not shown). Expression of the differentiation marker Filaggrin is enhanced in the skin of transgenic mice (Supplementary Figure S3 online), but not the expression of other differentiation markers such as keratin 1 (Krt1), keratin 10 (Krt10), and transglutaminase 1 (Tgm1) (data not shown). We evaluated apoptosis by measuring the levels of Fas and Fas-lg mRNAs in the mouse skin, and found no significant change in the expression of these genes (Figure 2g).
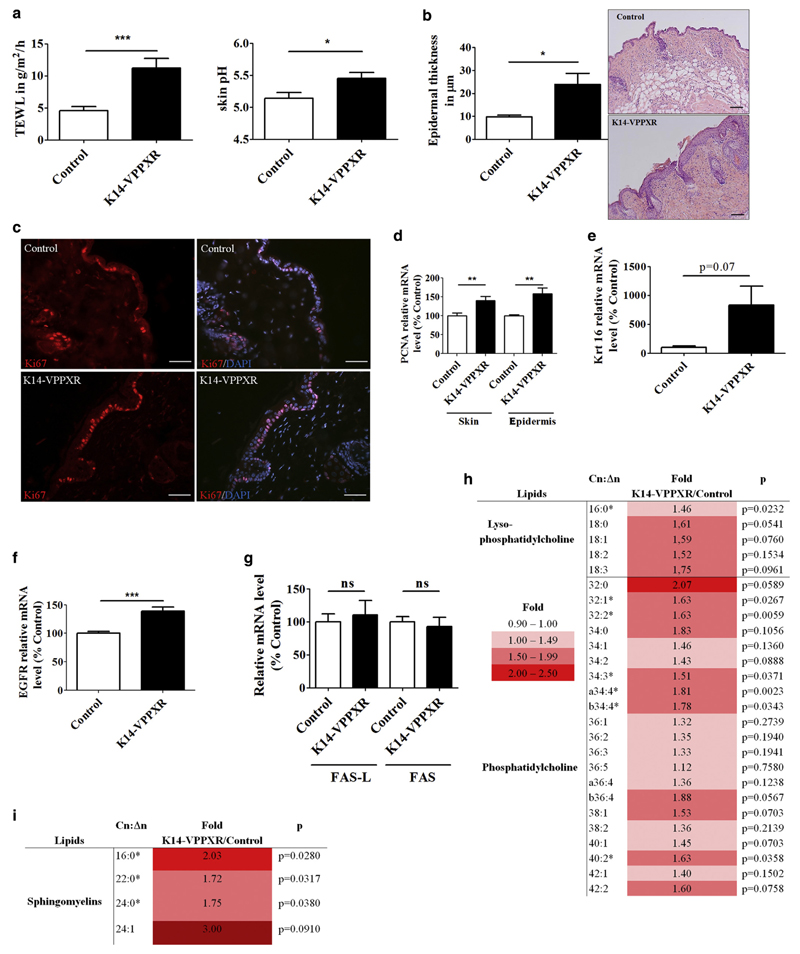
Figure 2. Constitutive activation of PXR in the epidermis leads to skin barrier defects and alters local homeostasis.
(a) TEWL (n = 29) and skin surface pH (n = 9–13) in adult K14-VPPXR and control mice. (b) Epidermal thickness and representative pictures of hematoxylin and eosin staining from the K14-VPPXR and control mouse back skin. Bar = 100 μm. (c) Representative images of Ki67 staining from K14-VPPXR and control mouse skin. Nuclei are counterstained with DAPI. Bar = 50 μM. Quantitative PCR showing relative expression levels of (d) Pcna, (e) Krt16, (f) Egfr, and (g) Fas and Fas-L in K14-VPPXR and control mouse epidermis or whole skin (n = 5–19). Fold increase and P values for epidermal (h) lysophosphatidylcholine and (i) sphingomyelin lipid species. Means of lipid amounts in K14-VPPXR mouse epidermis were divided by those in controls, n = 10–11. Data were analyzed with a Student's t-test: *P < 0.05, **P < 0.01, ***P < 0.001. K14, keratin 14; ns, nonsignificant; PXR, pregnane X receptor; TEWL, transepidermal water loss.
Liquid chromatography-mass spectrometry analyses of epidermal lipids revealed increased proportions of short-chain fatty acid nonhydroxy ceramides (NS 16:0–20:0) and decreased proportions of nonhydroxy ceramides with longer-chain fatty acids (NS 24:0–26:0), which are the most abundant nonhydroxy ceramide species in mouse epidermis (Supplementary Table S1 online) (Grond et al., 2017). Moreover, the epidermis of K14-VPPXR mice exhibits an increased content of the lysophosphatidylcholine species 16:0 and 18:0 as well as the phosphatidylcholine species with C32 and C34 (Figure 2h), and long and very long chain sphingomyelin lipid species (Figure 2i) when compared with controls. All together, these results demonstrate abnormal homeostasis in the epidermis and the stratum corneum of K14-VPPXR mice.
Constitutive activation of PXR in the epidermis triggers Th2/Th17-mediated skin inflammation
Abnormal metabolism and proinflammatory features of K14-VPPXR keratinocytes are demonstrated by their ectopic expression of major histocompatibility complex class II antigen (Figure 3a) (Auböck et al., 1986). Accordingly, we found that the proportions of CD45+ cells are increased in the skin of K14-VPPXR mice when compared with control mice (Figure 3b), and involve all leukocyte populations, that is, lymphocytes, DCs (Figure 3c, 3d), and granulocytes such as eosinophils (Figure 3e and data not shown). Both CD4+ and CD8+ T-cell populations are enlarged in the skin of transgenic mice, with a specific increase in dermal T cells, regardless of TCR repertoire (Figure 3c). All populations of skin DCs are increased as well (Figure 3d). In contrast, mast cell numbers are similar in both mouse groups (data not shown). Expression of the inflammatory cytokine IL1B is elevated in the skin of K14-VPPXR mice when compared with controls, in contrast to TGFB1 and TNFA (Figure 3f). Expression of the Th2 cytokines IL13, TSLP, and CCL27 is enhanced in K14-VPPXR skin when compared with controls (Figure 3g), whereas expression of IL5, IL25, IL4, and IL31 is very low or absent (37<CT<40) in all skin samples (data not shown). In contrast, expression of IL33 is reduced in the skin of transgenic mice when compared with controls (Figure 3g). Moreover, expression of IL13 is increased in the epidermis of transgenic mice when compared with controls (Figure 3h). Expression of CCL17 is unchanged in the K14-VPPXR mouse skin, and IFNG and IL21 mRNAs (38<CT<40) were not detectable in the skin of either genotype (data not shown). Interestingly, IL17A expression is induced in the skin of transgenic mice, whereas it remains very low or absent in the skin of control mice (Figure 3i). This correlates with an upregulation of IL6 mRNA levels in the skin of K14-VPPXR mice when compared with control mice (Figure 3i). We next investigated if innate immunity is triggered in K14-VPPXR mice. We found that type 2 innate lymphoid cells (ILCs2) are consistently increased in the skin of K14-VPPXR mice when compared with controls (Figure 3j). Thus, constitutive activation of human PXR in mouse epidermis tunes the skin immune system toward a Th2/Th17 polarization involving both adaptive and innate immunity.
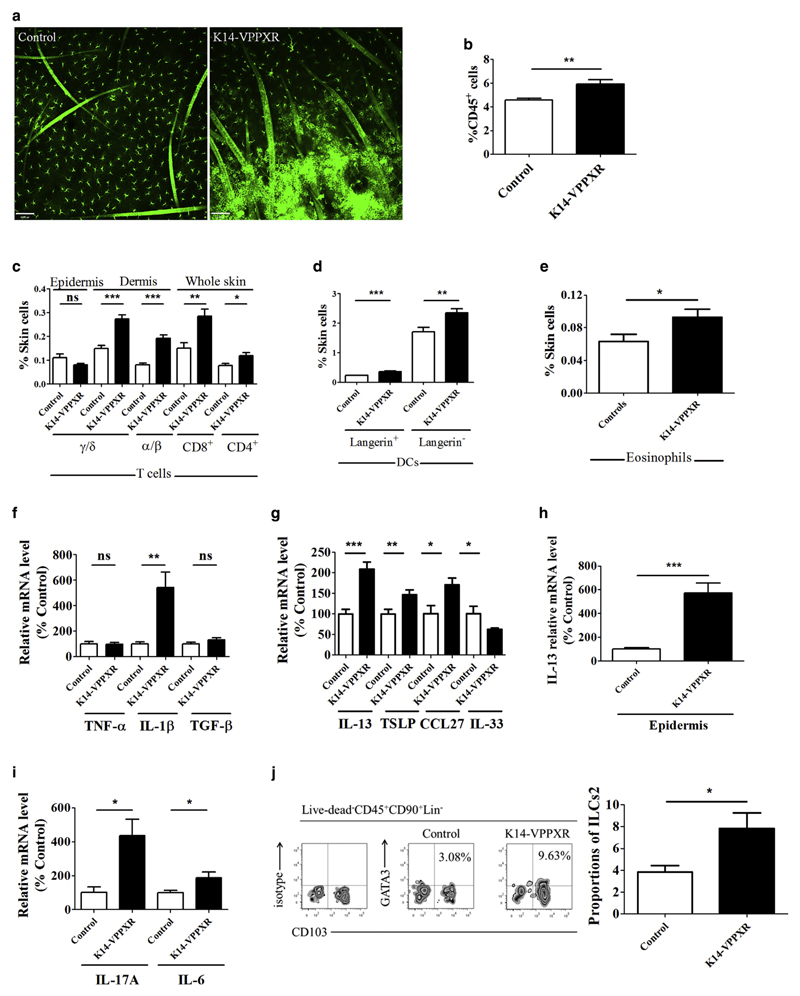
Figure 3. Constitutive activation of PXR in the epidermis triggers Th2/Th17-mediated skin inflammation.
(a) Representative confocal microscopy images showing major histocompatibility complex class II staining in epidermal sheets from K14-VPPXR and control mouse skin (n = 5). Bar = 60 μm. Percentages of (b) leukocytes (CD45+ cells) (n = 10), (c) T lymphocytes (n = 5), (d) DCs (n = 10), and (e) eosinophils (CD45+CD11b+CD193+F4/80+ [CD4−CD8−B220−CD19−]) (n = 6–8) in the skin of K14-VPPXR and control mice. Quantitative PCR showing relative expression of (f) TNFA, IL1B, and TGFB; (g) IL13, TSLP, CCL27, and IL33 (n = 7–19), (h) epidermal IL-13 (n = 10), and (i) IL17A and IL6 (n = 8–16) in the K14-VPPXR and control mouse skin. (j) Representative FACS and percentages of ILCs2 (CD45+Lin−CD90+CD103+GATA3+) in the K14-VPPXR and control mouse skin, n = 5. Data were analyzed with a Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001. DC, dendritic cells; ILC, innate lymphoid cell; K14, keratin 14; ns, nonsignificant; PXR, pregnane X receptor; Th2, T helper type 2; Th17, T helper type 17.
Keratinocytes and skin-derived T cells are a source of proinflammatory cytokines and growth factors in the skin of K14-VPPXR transgenic mice
In an effort to identify the immune cells responsible for the Th2/Th17 immune response in the skin of transgenic mice, we next analyzed cytokine production in the various subsets of skin T cells in K14-VPPXR and control mice. The percentages of IL-13-producing epidermal TCRγ/δ+ T cells are enhanced in the skin of K14-VPPXR mice when compared with control mice (Figure 4a), confirming results obtained at the mRNA level in the epidermis (Figure 3h). The percentages of IL-17A-producing α/β dermal T lymphocytes are increased but not those of IL-17A-producing γ/δ T lymphocytes (Figure 4b). Production of IFN-γ (Figure 4a), IL-2, and IL-10 remains very low and unchanged in K14-VPPXR mouse T lymphocytes when compared with controls (data not shown). We next assessed the production of IL-13 by ILCs2. We found that approximately one-third of ILCs2 produce IL-13 and that, on a single-cell basis, ILCs2 from transgenic mice produce similar amounts of IL-13 as those isolated from wild-type mice (data not shown). We next assessed the production of cytokines and growth factors by keratinocytes. The secretion of the growth factor GM-CSF is increased in K14-VPPXR keratinocytes when compared with control keratinocytes (Figure 4c). In contrast, secretion of IL-1β is abolished in K14-VPPXR keratinocytes when compared with control keratinocytes (Figure 4c). Moreover, we found that K14-VPPXR keratinocytes exhibit increased expression of TSLP and decreased expression of IL33 (Figure 4d), as found in vivo (Figure 3g). These results show that constitutive PXR activation intrinsically triggers TSLP and represses IL33 in keratinocytes. Thus, constitutive activation of human PXR in mouse epidermis is sufficient to modify the skin microenvironment and promote local inflammation.
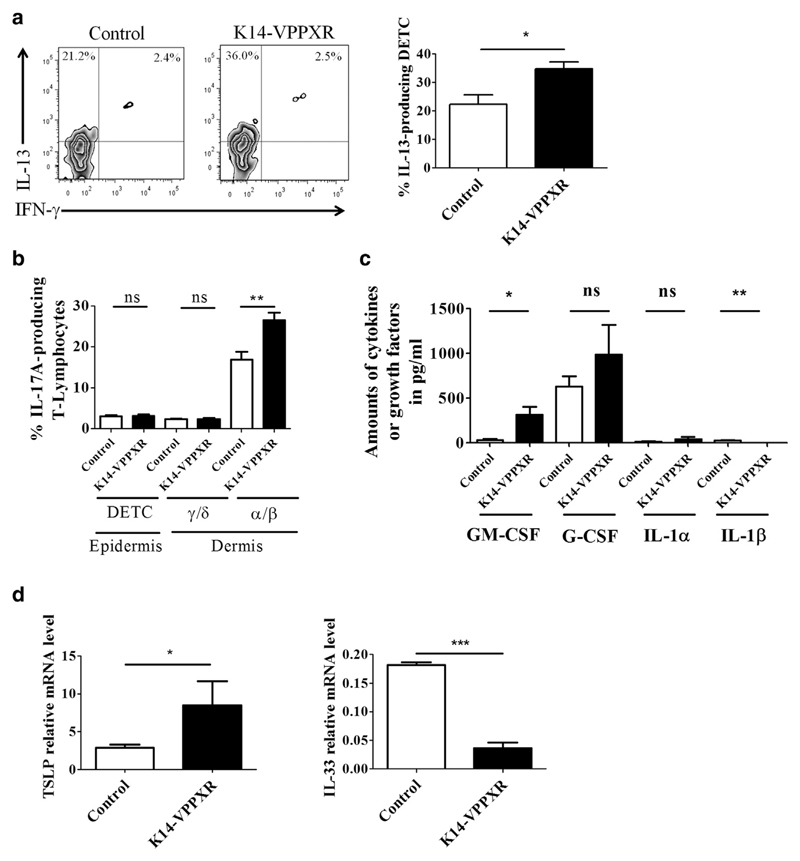
Figure 4. Sources of cytokines and growth factors in the skin of K14-VPPXR transgenic mice.
Epidermal and dermal cell suspensions were prepared as described in the Materials and Methods section and stained for flow cytometric analyses. All analyses used a pregate on CD45+ viable cells. (a) Representative FACS and percentages of IL-13-producing DETCs (CD4+TCRγ/δ+) in K14-VPPXR and control mouse epidermis, n = 5. (b) Percentages of IL-17A-producing skin-derived T lymphocytes (CD4+TCRγ/δ+ or CD4+TCRα/β+) in K14-VPPXR and control mouse skin, n = 5. (c) Production of cytokines by second-passage K14-VPPXR and control mouse keratinocytes analyzed by multi-analytical ELISA (n = 3). (d) Quantitative PCR showing relative expression of TSLP and IL33 in second-passage K14-VPPXR and control mouse keratinocytes. Three independent experiments were performed. Data were analyzed with a Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.0001. DETC, dendritic epidermal TCRγ/δ+ T cells; K14, keratin 14; ns, nonsignificant; PXR, pregnane X receptor.
Constitutive activation of PXR in the epidermis triggers atopy
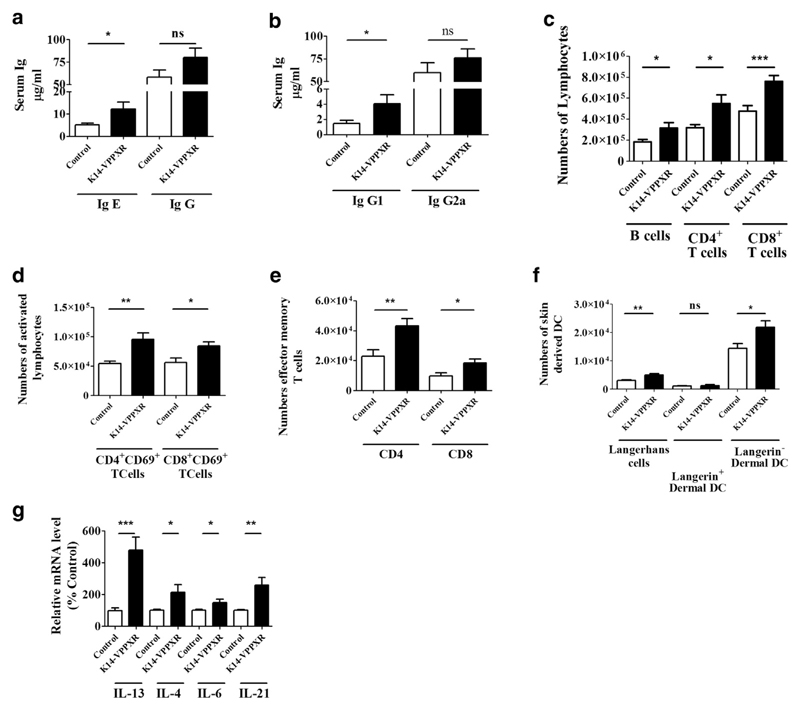
To investigate if proatopic effects of constitutive activation of human PXR in the epidermis extend beyond the skin, we measured various parameters in the blood and in skin draining lymph nodes of mice. Basal levels of serum IgE are significantly higher in K14-VPPXR mice when compared with control mice (approximately +87%, P < 0.05) (Figure 5a), in contrast to basal levels of serum IgG (approximately +38%, P > 0.05) (Figure 5a). However, we found a 2.8-fold increase in the levels of serum IgG1 in K14-VPPXR mice when compared with controls, in contrast to the levels of serum IgG2a (Figure 5b). Furthermore, K14-VPPXR mice have slightly enlarged skin draining lymph nodes (data not shown) containing increased numbers of lymphocytes (Figure 5c) and activated CD4+ and CD8+ lymphocytes (Figure 5d). We additionally found increased numbers of effector memory T cells (in both the CD4+ and CD8+ subsets) in skin draining lymph nodes of K14-VPPXR mice when compared with controls (Figure 5e). The migration of skin-derived DCs is increased in K14-VPPXR mice, particularly migration of Langerhans cells and Langerin− dermal DCs (Figure 5f). These data confirm a key role of the Langerhans cell subset in the priming of Th2 cells (Elentner et al., 2009; Nakajima et al., 2012). In contrast, the numbers of Langerin+ dermal DCs (Figure 5f) are not significantly changed, and the numbers of plasmacytoid DCs are borderline increased in skin draining lymph nodes of K14-VPPXR mice when compared with controls (Supplementary Figure S4 online). The numbers of regulatory T cells and expression of IL10 and Foxp3 are similar in skin draining lymph nodes of K14-VPPXR and control mice (data not shown). Moreover, the expression of IL13, IL4, IL6, and IL21 (Figure 5g) is increased in skin draining lymph nodes of K14-VPPXR mice when compared with control mice, in contrast to the expression of IL17A that is reduced by twofold (data not shown). In peripheral blood, K14-VPPXR mice exhibit increased percentages of leukocytes and TCRγ/δ+ T cells (Supplementary Figure S5 online). Moreover, the percentages of IL-13-producing TCRγ/δ+ T cells are enhanced in the blood of transgenic mice when compared with controls (Supplementary Figure S5). Therefore, constitutive activation of human PXR in the epidermis triggers a humoral Th2 immune response associated with increased serum IgE.
Figure 5. Constitutive activation of PXR in the epidermis triggers atopy.
Levels of serum (a) IgE and IgG, and (b) IgG1 and IgG2a in K14-VPPXR and control mice (n = 13–15). Numbers of (c) lymphocytes, (d) activated T lymphocytes, (e) effector memory T lymphocytes (CD44hiCD62Llow), and (f) emigrated skin-derived DCs (Langerhans cells, Langerin+CCR7+CD11c+CD103−, Langerin+ dermal DCs, Langerin+CCR7+CD11c+CD103+, Langerin− dermal DCs, Langerin+CCR7+CD11c+CD103+/−) in skin draining lymph nodes of K14-VPPXR and control mice (n = 10–18). Quantitative PCR showing relative expression of IL13, IL4, IL6, and IL21 in skin draining lymph nodes of K14-VPPXR and control mice (n = 10). Data were analyzed with a Student's t-test: *P < 0.05, **P < 0.01, ***P < 0.001. DC, dendritic cells; K14, keratin 14; ns, nonsignificant; PXR, pregnane X receptor.
Constitutive activation of human PXR in the epidermis results in impaired epidermal barrier function that precedes skin inflammation
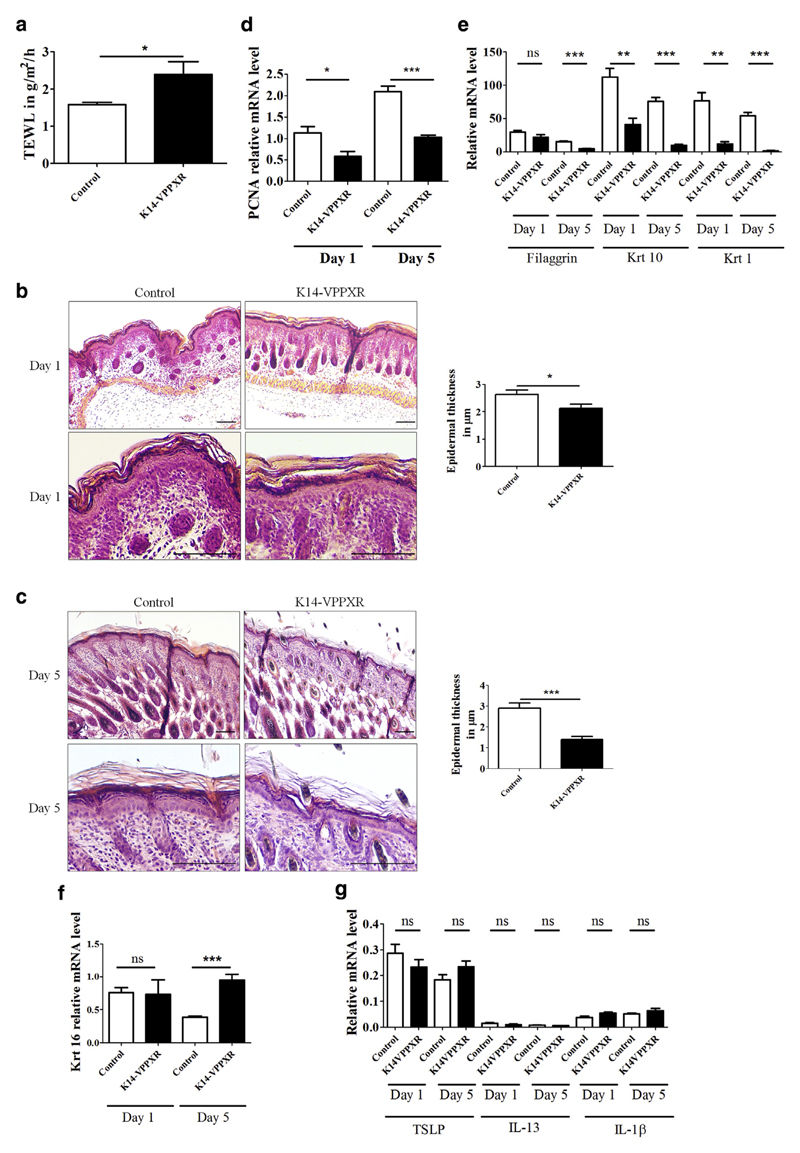
We next sought to decipher the sequence of events leading to impaired cutaneous barrier function and inflammation in K14-VPPXR mice. We observed that K14-VPPXR mice can exhibit transient dry and scaly skin in the first days after birth (Supplementary Figure S6 online) associated with an increased TEWL (Figure 6a). Histological analysis of the skin of K14-VPPXR mice revealed a slight but significant epidermal thinning at birth (Figure 6b) that is accentuated 5 days after birth (Figure 6c). In K14-VPPXR newborns, the expression of Pcna and most differentiation markers is reduced, whereas the expression of Krt16 remains unchanged when compared with controls (Figure 6d–f). In 5-day-old K14-VPPXR mice, the reduced expression of Pcna and differentiation markers is further evident and is associated with significantly increased expression of Krt 16 (Figure 6d–f). We next assessed the expression of key inflammatory mediators in the skin of 1- and 5-day-old mice. We found that the expression of both TSLP and IL13 remains unaltered in K14-VPPXR and control mice 1 and 5 days after birth, similar to IL1B (Figure 6g). Furthermore, IL-17A is not yet induced in the skin of K14-VPPXR pups (data not shown). Thus, when PXR is constitutively activated in the epidermis, a primary cutaneous barrier defect precedes the development of the Th2/Th17 skin immune response.
Figure 6. Constitutive activation of PXR in the epidermis leads to impaired barrier function that precedes skin inflammation.
(a) TEWL in newborn K14-VPPXR and control mice (n = 5–6). Representative hematoxylin and eosin staining at two different magnifications and epidermal thickness of (b) day 1 and (c) day 5 K14-VPPXR and control mice (n = 5–6). Bar = 50 μm. Quantitative PCR showing relative expression of (d) Pcna; (e) Flg, Krt1, Krt10; (f) Krt16; (g) TSLP, IL13, and ILB in day 1 and day 5 K14-VPPXR and control mice (n = 5–6). Data were analyzed with a Student's t-test: *P < 0.05, **P < 0.01, ***P < 0.001. PXR, pregnane X receptor; K14, keratin 14; ns, nonsignificant; TEWL, transepidermal water loss.
PXR signaling is increased in the skin of patients with AD
We finally studied PXR signaling in the skin of patients with AD and of healthy donors. We first assessed the relative expression and distribution of PXR and of its downstream target, CYP3A4 in skin biopsies. PXR localizes in both the nucleus and the cytoplasm of basal and suprabasal keratinocytes in healthy human skin (Figure 1b, Supplementary Figure S7 online). In AD skin, PXR expression changes to a very dotty pattern suggesting an increased transcriptional activity (Supplementary Figure S7). In line with this, the expression of CYP3A4 is markedly increased in the suprabasal layers of epidermis from patients with AD when compared with healthy controls. Moreover, amounts of CYP3A4 and UGT1A1 mRNAs are significantly higher in AD skin when compared with control skin, despite similar amounts of PXR mRNAs (Supplementary Figure S7). Thus, PXR signaling is triggered in AD skin.
Discussion
Since the industrial revolution, the environment has been continuously fouled with various xenobiotics such as pesticides, herbicides, and endocrine disruptors that, in large part, are harmful to living organisms. Recent work has shown that they can have potent adverse effects by contributing to cancer, asthma, type 1 diabetes, and atopy, although the underlying mechanisms remain to be fully understood (Bodin et al., 2015; Miller and Peden, 2014). It is now clear that some of these molecules are capable of inducing a Th2/Th17 immune response, elevating serum IgE levels, increased numbers of activated DCs in epithelial tissues such as the lungs, and causing epigenetic modifications that promote a deregulated immune response (Acciani et al., 2013; Kim, 2015b; Miller and Peden, 2014). The skin is in daily contact with many such molecules not only because they are present in air and water, but also because they are constituents of cosmetics, shampoos, and skin care products; however, the pathological effects of long-term skin exposure to low doses of these molecules remain unclear.
AD is characterized by relapsing eczematous lesions, and skin dryness, and involves immune hyper-responsiveness of the skin, epidermal barrier abnormalities, genetic susceptibility, and environmental factors. Nonlesional AD skin, despite a normal appearance, shows epidermal hyperplasia and increased TEWL, and is associated with deregulated keratinocyte differentiation, higher skin surface pH, and subclinical signs of Th2/Th17 inflammation (Gruber et al., 2015; Suárez-Fariñas et al., 2011). In addition, acute lesions exhibit increased expression of TSLP by damaged epidermal cells, mostly keratinocytes (Hammad and Lambrecht, 2015) that drives type 2 inflammation (Clausen et al., 2013; Dhingra et al., 2013; Goo et al., 2010; Kim, 2015a). In our study, mice overexpressing constitutively activated human PXR in the epidermis exhibit a clear Th2 (TSLP, IL-13, CCL27)/Th17 (IL-17A, IL-6) skin immune response associated with skin infiltration by lymphocytes, DCs, granulocytes such as eosinophils, and ILCs2. K14-VPPXR keratinocytes express more TSLP and secrete more GM-CSF, whereas the percentages of skin-derived T lymphocytes producing IL-13 and IL-17A are increased in mice when compared with controls, similar to what is observed in AD (Hamid et al., 1994; Kim, 2015a; Leung and Guttman-Yassky, 2014; Werfel, 2009). Excess IL-13 produced in transgenic mouse skin originates from epidermal TCRγ/δ+ T cells. Increased production of IL-13 by γ/δ+ T cells, including epidermal TCRγ/δ+ T cells, although uncommon, has already been reported in Th2 skewed immunity (Han et al., 2014; Korematsu et al., 2007; Naiki et al., 2000). Interestingly, lysophosphatidylcholines are well-known chemoattractants for T lymphocytes (Han et al., 2004; Ryborg et al., 1994) and their amounts are significantly increased in the epidermis of K14-VPPXR mice in comparison to controls. In inflammatory skin diseases, keratinocytes upregulate the expression of major histocompatibility complex class II as a sign of activation (Auböck et al., 1986), as we observed in K14-VPPXR mice. In their activated state, K14-VPPXR keratinocytes produce increased amount of GM-CSF, which is involved in the maintenance of AD (Hamid et al., 1994). Thus, the skin microenvironment observed in K14-VPPXR mice displays several key hallmarks of the immunological features observed in nonlesional AD with additional increased TEWL, eosinophils, and epidermal TSLP (Kim, 2015a) (Supplementary Table S2).
The identification of both loss-of-function mutations in the human Filaggrin gene and genetic abnormalities in the epidermal differentiation complex region of chromosome 1 of patients with AD strongly supports the hypothesis that skin barrier defects are a driving force in AD pathogenesis (Hoffjan and Stemmler, 2007; Leung and Guttman-Yassky, 2014). This hypothesis is further strengthened by recent work showing that increased TEWL in newborns precedes AD (Kelleher et al., 2015). We showed here that mice over-expressing constitutively activated human PXR in the epidermis exhibit abnormal epidermal barrier function. Indeed, at birth, K14-VPPXR mice feature dry and scaly skin associated with increased TEWL that persists into adulthood, and also higher surface pH, phenotypes consistent with a skin barrier defect (Kelleher et al., 2015). In young K14-VPPXR mice, the abnormal barrier function likely originates from abnormal epidermal homeostasis and altered expression of Krt 16, which can impair epidermal barrier function by disturbing keratin filaments, thereby altering the organization and adhesion of keratinocytes (Wawersik et al., 2001). Moreover, the epidermal barrier abnormality precedes the onset of inflammation in young K14-VPPXR mice, because expression of the inflammatory markers TSLP, IL13, and IL1B is not yet increased in the skin of those mice, whereas TEWL is already enhanced. Thus, our data demonstrate that impaired epidermal barrier function precedes inflammation in K14-VPPXR mice, which is temporarily similar to AD onset in very young children (Kelleher et al., 2015).
AD prevalence underwent a marked increase during the past 30 years, potentially resulting from a higher pollution burden (Ahn, 2014; Miller and Peden, 2014). Furthermore, the levels of endocrine disruptors, such as phthalates, are increased in the dust collected from the bedrooms of children with AD (Ahn, 2014). Many environmental toxicants target AHR that might be one of the mechanisms eliciting AD (Hidaka et al., 2017; Kabashima et al., 2016). However, a large number of environmental pollutants bind and activate PXR (Bickers and Athar, 2006; Julliard et al., 2014; Kleiner et al., 2004; Kliewer et al., 2002). Accordingly, PXR signaling is triggered in the skin of patients with AD as shown by the increased expression of two PXR target genes that possess PXR responsive element in the regulatory region of their gene (Hariparsad et al., 2009). In K14-VPPXR transgenic mice, which provide a model of chronic skin exposure to pollutants, we observed increased expression of Ahr, Cyp3a11, and Cyp1b1 in the skin, demonstrating upregulation of several genes involved in xenobiotic metabolism. The present work strengthens the findings of other studies focusing on AHR, which showed skin inflammation in mice overexpressing Ahr (Hidaka et al., 2017; Tauchi et al., 2005). If these findings can be extrapolated to humans, they might explain why the percentage of young patients with AD who experience full remission later in life has decreased in recent years (Harrop et al., 2007; Shaw et al., 2011) (http://isaac.auckland.ac.nz/). Collectively, all these observations emphasize the contribution of environmental pollutants to AD development and maintenance, and the potential contribution of PXR in the skin as target of pollutants. Compromised barrier function as observed in AD might lead to increased penetration by lipophilic pollutants, thereby triggering PXR in keratinocytes and sustaining a vicious circle of immune hyper-responsiveness and impaired barrier function. Hence, triggering of AD features may arise as a side effect of the upregulation of pollutant metabolism owing to the role of xenobiotic receptors not only in toxin clearance, but also in inflammation and lipid metabolism. This hypothesis is further supported by previous work showing the presence of phase II enzyme metabolites in the skin, including glucuronides of drugs and pollutants (Manevski et al., 2015), and increased susceptibility of patients with AD to chemical irritation (Nutten, 2015).
Materials and Methods
All experimental protocols were approved by the Austrian Federal Ministry of Science and Research and performed according to institutional guidelines. Detailed information on techniques used in this study is provided in Supplementary Materials and Methods online.
TEWL and surface skin pH measurements
The back skin from adult mice was shaved 3 days before measurements. Measurements for TEWL and surface skin pH were carried out under light anesthesia with ketamine using a multiprobe 6 device from Courage + Khasaka (MP6, Cologne, Germany).
Quantification of gene expression
Total RNAwas extracted from mouse ears using TRIZOL (Gibco BRl, Life Technologies, Vienna, Austria). Random primed cDNA was prepared (Superscript II RNase H-reverse transcriptase; Life Technologies) from total RNA. Genomic DNA was removed from samples by DNase treatment (Ambion, Austin, TX). Quantitative PCR analysis was performed by real-time PCR (real-time PCR detection system CFX96; Bio-Rad, Vienna, Austria) using a Brilliant III Ultra-Fast Quantitative PCR Kit from Agilent Technologies (Vienna, Austria). Some sequences for probes and primers specific for mouse and human mRNA molecules were selected using the Primer Express software (Applied Biosystems, Foster City, CA) and synthesized by Microsynth (Balgach, Switzerland), whereas others were purchased from Applied Biosystems (Foster City, CA) (Supplementary Table S3 online). The housekeeping gene used for relative gene expression was TATA binding protein, which shows minimal variations in all sample groups. Two other housekeeping genes were used to verify relative gene expression, that is, cyclophilin and beta-2 microglobulin.
Cell preparations
Mouse epidermal cells were isolated by trypsinization and further cultured in keratinocyte medium (CnT-07, CellnTec, Bern, Switzerland). Second-passage keratinocytes were used for the experiments.
Western blot analysis
The skin was lysed in modified radioimmunoprecipitation assay (RIPA) lysis buffer (10 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, pH 7.4) in the presence of protease inhibitor (Pierce, Rockford, IL). Endogenous proteins (100 μg) were detected with primary and secondary antibodies as listed in the Supplementary Materials and Methods online. Blots were then scanned with a LI-COR Biosciences analyzer, and band intensity was analyzed with the Odyssey software (Lincoln, NE). Specificity of a PXR antibody was evaluated previously with human PXR recombinant protein (Elentner et al., 2015) (see Supplementary Figure S8 online).
Supplementary Material
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at http://dx.doi.org/10.1016/j.jid.2017.07.846.
Acknowledgments
This work was supported by a grant from the Austrian Science Fund to SD (FWF P-21449 and FWF-P-28039) and to FPWR (FWF project P25944). We are indebted to Birgit Moser and Viktoria Gapp for their excellent technical assistance. We thank Dr Michael Downes and Dr Wen Xie for kindly providing the plasmid pCMV-VPSXR.
Abbreviations
- AD
atopic dermatitis
- CYP450
cytochrome P450
- DC
dendritic cell
- ILC
innate lymphoid cell
- ILCs2
type 2 innate lymphoid cells
- K14
keratin 14
- PXR
pregnane X receptor
- TEWL
transepidermal water loss
- Th
T helper
- TSLP
thymic stromal lymphopoietein
Footnotes
ORCID
Sandrine Dubrac: orcid.org/0000-0002-2936-8488
Conflict of Interest
The authors state no conflict of interest.
Author Contributions
AE performed experiments, NY designed the transgenic mice and contributed to manuscript writing, TOE and FPWR performed liquid chromatography-mass spectrometry and analyzed related data, RG recruited patients and performed biopsies, MH performed confocal microcopy, MS contributed to project design and to manuscript writing, BDF provided human biological materials, and SD designed experiments, analyzed data, and wrote the manuscript.
References
- Acciani T, Brandt E, Khurana Hershey G, Le Cras T. Diesel exhaust particle exposure increases severity of allergic asthma in young mice. Clin Exp Allergy. 2013;43:1406–18. doi: 10.1111/cea.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol. 2014;134:993–9. doi: 10.1016/j.jaci.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Auböck J, Romani N, Grubauer G, Fritsch P. HLA-DR expression on keratinocytes is a common feature of diseased skin. Br J Dermatol. 1986;114:465–72. doi: 10.1111/j.1365-2133.1986.tb02851.x. [DOI] [PubMed] [Google Scholar]
- Bickers D, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–75. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- Bodin J, Stene LC, Nygaard UC. Can exposure to environmental chemicals increase the risk of diabetes type 1 development? Biomed Res Int. 2015;2015:208947. doi: 10.1155/2015/208947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavani A, Pennino D, Eyerich K. Th17 and Th22 in skin allergy. Chem Immunol Allergy. 2012;96:39–44. doi: 10.1159/000331870. [DOI] [PubMed] [Google Scholar]
- Clausen M, Jungersted JM, Andersen PS, Slotved HC, Krogfelt KA, Agner T. Human β-defensin-2 as a marker for disease severity and skin barrier properties in atopic dermatitis. Br J Dermatol. 2013;169:587–93. doi: 10.1111/bjd.12419. [DOI] [PubMed] [Google Scholar]
- Cole C, Kroboth K, Schurch NJ, Sandilands A, Sherstnev A, O’Regan GM, et al. Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. J Allergy Clin Immunol. 2014;134:82–91. doi: 10.1016/j.jaci.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jongh C, Khrenova L, Verberk MM, Calkoen F, van Dijk FJ, Voss H, et al. Loss-of-function polymorphisms in the filaggrin gene are associated with an increased susceptibility to chronic irritant contact dermatitis: a case-control study. Br J Dermatol. 2008;159:621–7. doi: 10.1111/j.1365-2133.2008.08730.x. [DOI] [PubMed] [Google Scholar]
- Dhingra N, Suárez-Fariñas M, Fuentes-Duculan J, Gittler JK, Shemer A, Raz A, et al. Attenuated neutrophil axis in atopic dermatitis compared to psoriasis reflects TH17 pathway differences between these diseases. J Allergy Clin Immunol. 2013;132:498–501.e3. doi: 10.1016/j.jaci.2013.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrac S, Elentner A, Ebner S, Horejs-Hoeck J, Schmuth M. Modulation of T lymphocyte function by the pregnane X receptor. J Immunol. 2010;184:2949–57. doi: 10.4049/jimmunol.0902151. [DOI] [PubMed] [Google Scholar]
- Elentner A, Finke D, Schmuth M, Chappaz S, Ebner S, Malissen B, et al. Langerhans cells are critical in the development of atopic dermatitis-like inflammation and symptoms in mice. J Cell Mol Med. 2009;13:2658–72. doi: 10.1111/j.1582-4934.2009.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elentner A, Ortner D, Clausen B, Gonzalez FJ, Fernández-Salguero PM, Schmuth M, et al. Skin response to a carcinogen involves the xenobiotic receptor pregnane X receptor. Exp Dermatol. 2015;24:835–40. doi: 10.1111/exd.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goo J, Ji JH, Jeon H, Kim MJ, Jeon SY, Cho MY, et al. Expression of antimicrobial peptides such as LL-37 and hBD-2 in nonlesional skin of atopic individuals. Pediatr Dermatol. 2010;27:341–8. doi: 10.1111/j.1525-1470.2010.01122.x. [DOI] [PubMed] [Google Scholar]
- Grond S, Radner FP, Eichmann TO, Kolb D, Grabner GF, Wolinski H, et al. Skin barrier development depends on CGI-58 protein expression during late-stage keratinocyte differentiation. J Invest Dermatol. 2017;137:403–13. doi: 10.1016/j.jid.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Börnchen C, Rose K, Daubmann A, Volksdorf T, Wladykowski E, et al. Diverse regulation of claudin-1 and claudin-4 in atopic dermatitis. Am J Pathol. 2015;185:2777–89. doi: 10.1016/j.ajpath.2015.06.021. [DOI] [PubMed] [Google Scholar]
- Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–6. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, Lambrecht B. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Han J, Lee E, Kim E, Yeom MH, Kwon O, Yoon TH, et al. Role of epidermal γδ T-cell-derived interleukin 13 in the skin-whitening effect of Ginsenoside F1. Exp Dermatol. 2014;23:860–2. doi: 10.1111/exd.12531. [DOI] [PubMed] [Google Scholar]
- Han K, Hong KH, Ko J, Rhee KS, Hong MK, Kim JJ, et al. Lysophosphatidylcholine up-regulates CXCR4 chemokine receptor expression in human CD4 T cells. J Leukoc Biol. 2004;76:195–202. doi: 10.1189/jlb.1103563. [DOI] [PubMed] [Google Scholar]
- Hariparsad N, Chu X, Yabut J, Labhart P, Hartley DP, Dai X, et al. Identification of pregnane-X receptor target genes and coactivator and corepressor binding to promoter elements in human hepatocytes. Nucleic Acids Res. 2009;37:1160–73. doi: 10.1093/nar/gkn1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrop J, Chinn S, Verlato G, Olivieri M, Norbäck D, Wjst M, et al. Eczema, atopy and allergen exposure in adults: a population-based study. Clin Exp Allergy. 2007;37:526–35. doi: 10.1111/j.1365-2222.2007.02679.x. [DOI] [PubMed] [Google Scholar]
- Haslam I, Pitre A, Schuetz JD, Paus R. Protection against chemotherapy-induced alopecia: targeting ATP-binding cassette transporters in the hair follicle? Trends Pharmacol Sci. 2013;34:599–604. doi: 10.1016/j.tips.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Hidaka T, Ogawa E, Kobayashi EH, Suzuki T, Funayama R, Nagashima T, et al. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat Immunol. 2017;18:64–73. doi: 10.1038/ni.3614. [DOI] [PubMed] [Google Scholar]
- Hoffjan S, Stemmler S. On the role of the epidermal differentiation complex in ichthyosis vulgaris, atopic dermatitis and psoriasis. Br J Dermatol. 2007;157:441–9. doi: 10.1111/j.1365-2133.2007.07999.x. [DOI] [PubMed] [Google Scholar]
- Jeannesson E, Siest G, Herbeth B, Albertini L, Shahabi P, Pfister M, et al. Biological and genetic factors associated with ABCB1 and pregnane-X-receptor expressions in peripheral blood mononuclear cells in the STANISLAS cohort. Drug Metabol Drug Interact. 2011;26:27–32. doi: 10.1515/DMDI.2011.102. [DOI] [PubMed] [Google Scholar]
- Julliard W, Fechner JH, Mezrich JD. The aryl hydrocarbon receptor meets immunology: friend or foe? A little of both. Front Immunol. 2014;5:458. doi: 10.3389/fimmu.2014.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K, Otsuka A, Nomura T. Linking air pollution to atopic dermatitis. Nat Immunol. 2016;18:5–6. doi: 10.1038/ni.3615. [DOI] [PubMed] [Google Scholar]
- Kelleher M, Dunn-Galvin A, Hourihane JO, Murray D, Campbell LE, McLean WH, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135:930–935.e1. doi: 10.1016/j.jaci.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim BS. Innate lymphoid cells in the skin. J Invest Dermatol. 2015a;135:673–8. doi: 10.1038/jid.2014.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JP, Chao I, Simpson E, Silverberg J. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681–687.e11. doi: 10.1016/j.jaad.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. Influences of environmental chemicals on atopic dermatitis. Toxicol Res. 2015b;31:89–96. doi: 10.5487/TR.2015.31.2.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner H, Vulimiri SV, Hatten WB, Reed MJ, Nebert DW, Jefcoate CR, et al. Role of cytochrome p4501 family members in the metabolic activation of polycyclic aromatic hydrocarbons in mouse epidermis. Chem Res Toxicol. 2004;17:1667–74. doi: 10.1021/tx049919c. [DOI] [PubMed] [Google Scholar]
- Kliewer S, Goodwin B, Willson T. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- Kliewer S, Moore J, Wade L, Staudinger J, Watson M, Jones S, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Kolev V, Mandinova A, Guinea-Viniegra J, Hu B, Lefort K, Lambertini C, et al. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nat Cell Biol. 2008;10:902–11. doi: 10.1038/ncb1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korematsu S, Tanaka Y, Nagakura T, Minato N, Izumi T. Human gammadelta T cells modulate the mite allergen-specific T-helper type 2-skewed immunity. Clin Exp Allergy. 2007;37:1681–7. doi: 10.1111/j.1365-2222.2007.02826.x. [DOI] [PubMed] [Google Scholar]
- Leung D, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134:769–79. doi: 10.1016/j.jaci.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lille-Langøy R, Goldstone JV, Rusten M, Milnes MR, Male R, Stegeman JJ, et al. Environmental contaminants activate human and polar bear (Ursus maritimus) pregnane X receptors (PXR, NR1I2) differently. Toxicol Appl Pharmacol. 2015;284:54–64. doi: 10.1016/j.taap.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich J, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–46. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- Manevski N, Swart P, Balavenkatraman KK, Bertschi B, Camenisch G, Kretz O, et al. Phase II metabolism in human skin: skin explants show full coverage for glucuronidation, sulfation, N-acetylation, catechol methylation, and glutathione conjugation. Drug Metab Dispos. 2015;43:126–39. doi: 10.1124/dmd.114.060350. [DOI] [PubMed] [Google Scholar]
- Miller R, Peden D. Environmental effects on immune responses in patients with atopy and asthma. J Allergy Clin Immunol. 2014;134:1001–8. doi: 10.1016/j.jaci.2014.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi BG, Neustadter J, Binda E, Lewis J, Filler RB, Roberts SJ, et al. Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science. 2012;335:104–8. doi: 10.1126/science.1211600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiki Y, Nishimura H, Itohara S, Yoshikai Y. Gammadelta T cells may dichotomously modulate infection with avirulent Salmonella choleraesuis via IFN-gamma and IL-13 in mice. Cell Immunol. 2000;201:61–9. doi: 10.1006/cimm.2000.1659. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Igyártó BZ, Honda T, Egawa G, Otsuka A, Hara-Chikuma M, et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J Allergy Clin Immunol. 2012;129:1048–1055.e6. doi: 10.1016/j.jaci.2012.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak N, Baurecht H, Schäfer T, Rodriguez E, Wagenpfeil S, Klopp N, et al. Loss-of-function mutations in the filaggrin gene and allergic contact sensitization to nickel. J Invest Dermatol. 2008;128:1430–5. doi: 10.1038/sj.jid.5701190. [DOI] [PubMed] [Google Scholar]
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(Suppl 1):8–16. doi: 10.1159/000370220. [DOI] [PubMed] [Google Scholar]
- Ryborg A, Deleuran B, Thestrup-Pedersen K, Kragballe K. Lysophosphatidylcholine: a chemoattractant to human T lymphocytes. Arch Dermatol Res. 1994;286:462–5. doi: 10.1007/BF00371572. [DOI] [PubMed] [Google Scholar]
- Sääf A, Pivarcsi A, Winge MC, Wahlgren CF, Homey B, Nordenskjöld M, et al. Characterization of EGFR and ErbB2 expression in atopic dermatitis patients. Arch Dermatol Res. 2012;304:773–80. doi: 10.1007/s00403-012-1242-4. [DOI] [PubMed] [Google Scholar]
- Schote A, Turner JD, Schiltz J, Muller CP. Nuclear receptors in human immune cells: expression and correlations. Mol Immunol. 2007;44:1436–45. doi: 10.1016/j.molimm.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Shaw T, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. J Invest Dermatol. 2011;131:67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siest G, Jeannesson E, Marteau JB, Samara A, Marie B, Pfister M, et al. Transcription factor and drug-metabolizing enzyme gene expression in lymphocytes from healthy human subjects. Drug Metab Dispos. 2008;36:182–9. doi: 10.1124/dmd.107.017228. [DOI] [PubMed] [Google Scholar]
- Silverberg N. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267–71. [PubMed] [Google Scholar]
- Simonsson C, Andersson SI, Stenfeldt AL, Bergström J, Bauer B, Jonsson CA, et al. Caged fluorescent haptens reveal the generation of cryptic epitopes in allergic contact dermatitis. J Invest Dermatol. 2011;131:1486–93. doi: 10.1038/jid.2010.422. [DOI] [PubMed] [Google Scholar]
- Suárez-Fariñas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, Cardinale I, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127:954–64. e1-4. doi: 10.1016/j.jaci.2010.12.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T. TSLP expression: cellular sources, triggers, and regulatory mechanisms. Allergol Int. 2012;61:3–17. doi: 10.2332/allergolint.11-RAI-0395. [DOI] [PubMed] [Google Scholar]
- Tauchi M, Hida A, Negishi T, Katsuoka F, Noda S, Mimura J, et al. Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol Cell Biol. 2005;25:9360–8. doi: 10.1128/MCB.25.21.9360-9368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyssen J, Linneberg A, Engkilde K, Menné T, Johansen JD. Contact sensitization to common haptens is associated with atopic dermatitis: new insight. Br J Dermatol. 2012;166:1255–61. doi: 10.1111/j.1365-2133.2012.10852.x. [DOI] [PubMed] [Google Scholar]
- Wawersik M, Mazzalupo S, Nguyen D, Coulombe PA. Increased levels of keratin 16 alter epithelialization potential of mouse skin keratinocytes in vivo and ex vivo. Mol Biol Cell. 2001;12:3439–50. doi: 10.1091/mbc.12.11.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werfel T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J Invest Dermatol. 2009;129:1878–91. doi: 10.1038/jid.2009.71. [DOI] [PubMed] [Google Scholar]
- Zhou C, Tabb MM, Nelson EL, Grün F, Verma S, Sadatrafiei A, et al. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006;116:2280–9. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.