Abstract
Objective
To investigate predictors of flare in rheumatoid arthritis (RA) patients with low disease activity (LDAS) and to evaluate the impact of flare on 12-month clinical outcomes.
Methods
RA patients on DMARDs with a stable DAS28<3.2 were eligible for inclusion. At baseline and every 3 months, clinical (DAS28), functional (HAQ-DI, EQ-5D, FACIT-F, SF36), serum biomarkers (MBDA, calprotectin, CXCL10) and imaging data were collected. Flare was defined as an increase in DAS28>1.2 or >0.6 if concurrent DAS28≥3.2. Cox regression analyses were used to identify baseline predictors of flare. Biomarkers were cross-sectionally correlated at time of flare. Linear regressions were performed to compare clinical outcomes after 1 year.
Results
Of 152 patients, 46 (30%) experienced a flare. Functional disability at baseline was associated with flare; HAQ-DI unadjusted HR 1.82 (95% CI:1.20-2.72) and EQ-5D HR 0.20 (95% CI:0.07-0.57). In multivariate analyses only HAQ-DI remained a significant independent predictor of flare HR 1.76 (95% CI:1.05-2.93). At time of flare, DAS28 and its components significantly correlated with MBDA and calprotectin, but correlation coefficients were low at 0.52 and 0.49 respectively. Two thirds of flares were not associated with a rise in biomarkers. Patients who flared had significantly worse outcomes at 12 months (HAQ-DI, EQ-5D, FACIT-F, SF36 and radiographic progression).
Conclusion
Flares occurs frequently in RA patients with LDAS and are associated with worse disease activity, quality-of-life and radiographic progression. Higher baseline HAQ-DI was modestly predictive of flare, whilst biomarker correlation at the time of flare suggests a non-inflammatory component in a majority of events.
Key Indexing Term: Rheumatoid arthritis, Low disease activity, Flare, Quality of life, Serum biomarkers, Multi-biomarker disease activity (MBDA) score
Introduction
Guidelines for the treatment of rheumatoid arthritis have emphasised a ‘treat-to-target’ approach with the explicit aim of low disease activity states [1, 2]). However, disease activity in RA can fluctuate. Episodic worsening of disease activity, described as “flare”, is common. Flare was originally defined by the Outcome Measures in Rheumatology Clinical Trials (OMERACT 9) group as a cluster of symptoms of sufficient duration and intensity to require initiation, change or increase in therapy [3]. These definitions focused on the more severe end of the flare continuum for evaluation of flares in randomised controlled trials (RCTs). In daily practice, flare can vary in duration, intensity, frequency and manageability [4] with approximately half of RA patients in remission experiencing a disease flare within 2 years [5]. This has important clinical implications because flares in patients with apparently low disease activity states are associated with radiographic progression [6, 7], functional deterioration [7] and worsening cardiovascular comorbidity [8].
Predicting flare is therefore of direct relevance to clinical practice. Saleem et al demonstrated that functional disability (Health Assessment Questionnaire Disability Index HAQ-DI) and ultrasound power Doppler (PD) positivity at baseline were independently associated with flare in RA patients in remission [9]. Furthermore, a recent meta-analysis revealed an association between ultrasound PD positivity and flare in RA patients in remission [10].
The finding of PD positivity despite clinical remission provides evidence that flares may be related to incomplete suppression of inflammation. Based on this hypothesis, serum biomarkers may detect subclinical disease activity and consequently predict flare. In contrast to ultrasound, biomarkers may have smaller measurement error and may be less operator dependent, costly and time-consuming. In recent years, the predictive value of the multi-biomarker disease activity (MBDA) score, calprotectin (S100A8/A9) and CXCL10 for treatment response in RA has been investigated. In the DRESS study, baseline MBDA score was predictive of flare and major flare in patients with low disease activity who did not taper treatment (usual care group) [11]. To our knowledge, calprotectin and CXCL10 have not been investigated as predictors of flare in DMARD treated RA patients in a low disease activity state. Calprotectin was found to be more strongly associated with ultrasound-detected synovitis than ESR or CRP [12] and baseline calprotectin appeared to be predictive of clinical response to methotrexate [13]. However, its predictive role as a marker of response to biologic DMARDs is conflicting [14], [15]. CXCL10 was correlated with multiple disease activity measures in early RA [16] whilst elevated baseline levels of CXCL10 were associated with favourable response to TNF inhibitor therapy in RA [10].
The aims of our study were three-fold. Firstly, to describe the frequency of flares in a cohort of prospective RA patients in stable low disease activity states (including remission) over 1 year. Secondly to explore the predictive value of a wide range of biomarkers (including clinical, functional, serum and imaging variables) for flare. And thirdly to evaluate the impact of flare in RA patients with low disease activity states.
Materials and Methods
Study design and patients
The REMIRA study is a prospective cohort study investigating RA patients with stable low disease activity states including clinical remission. Clinical outcomes have been reported recently [17]. Adult RA patients diagnosed according to the 1987 revised ACR criteria with a disease duration < 10 years, stable DMARD treatment for > 6 months and DAS28 < 3.2 for at least 1 month apart, were eligible for inclusion. Three centres across south London participated: Guy’s and St Thomas’ Hospital, King’s College Hospital and University Hospital Lewisham NHS Foundation Trusts. Patient were managed as part of routine care. The study was approved by the local ethics committee and conducted according to the guidelines of the Declaration of Helsinki (REC:09/H0803/154). Written informed consent was obtained from all patients.
Clinical assessments
At baseline, demographic, disease and treatment characteristics were collected. Clinical assessments were carried out every 3 months for 1 year and included pain and fatigue (both on visual analogue scale 0-100), DAS28, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Questionnaires were used to assess function and quality of life: HAQ-DI, EQ-5D-3L, 36-Item Short Form Survey (SF-36: including physical component score (PCS) and mental component score (MCS)) and Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-F). Flare was defined according to previously validated criteria: a DAS28 increase of >1.2 compared with baseline or a DAS28 increase of >0.6 compared with baseline and concurrent DAS28 ≥3.2 [18]. For patients with multiple flares, only the first flare was considered in analyses.
Serum biomarker measurements
Serum samples were obtained at each time point and stored at −80 °C until being shipped frozen to the Crescendo Bioscience Clinical Laboratory (South San Francisco, CA, USA) for MBDA score, calprotectin and CXCL10 measurement. The MBDA test (Vectra® DA, Crescendo Bioscience) combines the serum concentrations of 12 protein biomarkers (interleukin-6, tumour necrosis factor receptor type I, vascular cell adhesion molecule 1, epidermal growth factor, vascular endothelial growth factor A, YKL-40, matrix metalloproteinase 1, MMP-3, CRP, serum amyloid A, leptin and resistin) in an algorithm to provide a score that quantifies RA disease activity on a scale of 1-100 with validated categories for low (≤30), moderate (30 to 44) and high disease activity (>44) [19]. Calprotectin and CXCL10 were measured by ELISA (Buhlmann MRP 8/14 ELISA Product Code EK-MRP8/14m; R&D Systems Human CXCL10/IP-10 Quantikine ELISA Product Code DIP100).
Imaging assessments
Ultrasonography of hands and wrists and conventional radiographs of hands and feet were carried out at baseline and 12 months. Erosive progression was defined as new or larger erosions over 1 year on radiographs. All sonographic assessments were performed using high-sensitivity ultrasound equipment (GE Logiq 9) with a 2D M12L transducer. A single experienced sonographer (TG), blinded to clinical or laboratory data, scanned 10 metacarpophalangeal joints and 2 wrists from a dorsal aspect for grey-scale synovial hypertrophy (GSUS) and intra-articular power Doppler signals (PDUS) [20]. GSUS and PDUS were graded on a scale of 0-3 using a validated semi-quantitative scoring system [21]. The composite GSUS and PDUS scores were the sum scores of the 12 individual joints.
Statistical analysis
Descriptive statistics were provided with mean (+/- standard deviation (SD)), median (interquartile ranges, IQR) or frequencies depending on data distribution.
Cross-sectional correlations between all measurements (biomarkers and DAS28 components) at time of flare were assessed by Spearman’s correlation coefficient (rs), and interpreted according to commonly used classification: rs<0.20: very weak, rs=0.20–0.39: weak, rs =0.40–0.59: moderate, rs=0.60–0.79: strong and rs>0.80 very strong correlation [22].
To identify predictors of time to flare, we performed univariate Cox regression in which time to flare was the dependent variable and clinical, functional, serum and imaging measurements the independent variables. Multivariate analyses were performed to identify factors that were independently associated with flare, adjusting for age, gender, DAS28, VAS-pain, CRP, ESR and US scores (for HAQ model only) and MBDA score (for EQ-5D model only).
Linear regression was used to determine the impact of flare on 12-month clinical outcomes (i.e. disease activity and functional status). A multivariate linear regression model was applied adjusting for baseline age, gender, disease duration, erosive status, baseline DAS28, HAQ and baseline variable of interest. A P value ≤ 0.05 was regarded as being significant. As this was an exploratory study, no correction for multiple hypothesis testing was performed. Missing data were addressed using a multiple imputation module (Annex 1). All analyses were performed with STATA 14.1 statistical software.
Results
Patient characteristics
In total, 152 patients were enrolled in the REMIRA study. Baseline characteristics are depicted in Table 1. The majority of patients were on DMARD monotherapy (n=70, 46%) and the median (IQR) disease duration was 3 (2-6) years. Ninety-seven patients (66%) fulfilled DAS28 remission criteria (DAS28 <2.6). All patients had synovial hypertrophy (GSUS>1) and 90% had detectable power Doppler activity on ultrasound at baseline.
Table 1. Patient characteristics.
| Patients (n=152) | |
|---|---|
| Age, years* | 57 (14) |
| Female gender | 101 (66%) |
| Disease duration, years † | 3 (2-6) |
| Treatment | |
| csDMARD monotherapy | 69 (45%) |
| csDMARD combination therapy | 59 (39%) |
| bDMARD therapy | 24 (16%) |
| Prednisolone | 3 (2%) |
| Seropositive (RF and/or ACPA) | 103 of 137 (75%) |
| Erosive | 67 (45%) |
| TJC28† | 0 (0-1) |
| SJC28† | 0 (0-2) |
| PGA (0-100mm) † | 19 (10-36) |
| ESR†, | 7 (4-13) |
| CRP†, (mg/L) | 5 (1-31) |
| DAS28-ESR* | 2.1 (0.9) |
| DAS28 remission | 97 of 148 (66%) |
| VAS pain (0-100mm) † | 15 (3-34) |
| HAQ-DI† | 0.25 (0-0.86) |
| EQ-5D† | 0.76 (0.69-1.00) |
| SF-36 PCS* | 46 (11) |
| SF-36 MCS* | 51 (10) |
| FACIT-F† | 42 (34-47) |
| MBDA score (1-100) † | 31 (18-39) |
| Calprotectin† (ng/ml) | 2358 (1487-3358) |
| CXCL10† (pg/ml) | 198 (143-291) |
| Number of patients with GSUS >0 | 104 of 104 (100%) |
| Total GSUS score (/36) † | 12 (8-14) |
| Number of patients with PDUS >0 | 93 (90) |
| Total PDUS score (/36) † | 2 (1-4) |
All values are gives as number (%) unless otherwise specified. * Mean (SD). † Median (IQR) Abbreviations: csDMARD, conventional synthetic DMARD; bDMARD, biological DMARD; RF, rheumatoid factor; ACPA, anticitrullinate protein antibody; TJC28 tender 28-joint count; SJC28, swollen 28- joint count; VAS, visual analog scale for pain; HAQ-DI, Health Assessment Questionnaire disability Index; EQ-5D, EuroQol five dimensions questionnaire; SF-36 MCS, Short Form 36 Health Survey Physical Component Summary; SF-36 MCS, Short Form 36 Health Survey Mental Component Summary; FACIT-F, Functional Assessment of Chronic Illness Therapy –Fatigue; MBDA, multi-biomarker disease activity; GSUS, grey-scale synovial hypertrophy; PDUS, intra-articular power Doppler signals.
Characteristics of flare
Forty-six patients (30%) experienced at least one flare. Twelve patients had first flare by 3 months, 10 by 6 months, 11 by 9 months, and 13 by 12 months. Seventeen patients experienced multiple flares; 11 patients flared at 2 visits, 5 patients at 3 visits and 1 patient at all 4 visits after baseline. When limiting the cohort to patients who were in remission defined by DAS28 <2.6 at baseline, 24 patients of a total 97 (25%) experience at least one flare.
Serum biomarkers at time of flare
There were 70 individual flares events. Seventeen percent (n=12) of flares were driven solely by increases in patient global assessment (PGA) and tender joint count (TJC), without any increase in swollen joint count (SJC) or ESR.
In total, 33% of flares (n=23) had a concurrent high MBDA score (>44), whilst 13% (n=44) of visits without flare had a high MBDA score. The levels of ESR, CRP, MBDA score and calprotectin were significantly higher at flare visits than at non-flare visits [median (IQR) ESR 14mm/hr (5-23) versus 6mm/hr (3-12), CRP 5mg/L (5-9) versus 5mg/L (5-5), MBDA 38 (25-50) versus 28 (18-38) and calprotectin 2916ng/ml (2002-4186) versus 2377ng/ml (1504 - 3358)].
DAS28 significantly correlated with MBDA score (rs =0.5, p=0.0002) at time of flare. The rs of 0.5 suggests that the MBDA values explain only 25% of the variation in DAS28. The correlation of MBDA was stronger with the components ESR and SJC, and non-significant for TJC and PGA. Similar findings were seen for calprotectin (rs =0.49, p=0.0007). CXCL10 did not correlate with DAS28 or its components at time of flare (Supplementary Table 1).
Prediction of flare
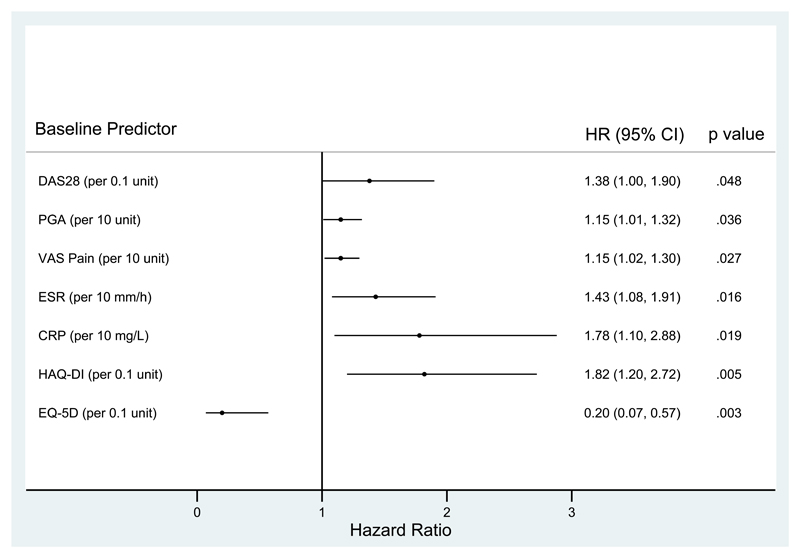
Univariate Cox regression showed that several baseline characteristics were associated with flare (DAS28, ESR, CRP, PGA, VAS pain, HAQ-DI and EQ-5D: Figure 1 and supplementary table 2). The strongest magnitude of association was seen with HAQ-DI and EQ-5D. Baseline ultrasound synovitis (GSUS or PDUS) and mental health (using the SF-36 mental component) were not associated with flare. Baseline MBDA scores were also not predictive of flare, although a sensitivity analysis limited to flares with a rise in MBDA score to >44 (high disease activity) did show a relationship between baseline MBDA value and flare risk, with each unit rise in baseline MBDA score associated with a 7% increase in flare risk (1.07, 95% CI 1.02 to 1.11; p=0.005) (Supplementary table 4 & 5). Analysing each component of the MBDA score identified serum amyloid A, leptin and high sensitivity CRP as the strongest predictors of flare. The remaining 9 components of the MBDA score did not individually predict flare.
Figure 1.
Univariate analyses of prediction of flare with baseline variables
The imputation model confirmed the association between flare and baseline HAQ-DI and EQ-5D but did not demonstrate any other associations. In multivariate analyses only baseline HAQ-DI remained a significant independent predictor of flare [HR 1.76 (95% CI: 1.05 to 2.93) p=0.03].
Outcomes in flare versus sustained remission group
Adjusting for baseline values, patients who had a flare experienced significantly worse clinical outcomes at 12 months than patients in sustained remission, reflected by higher disease activity, worse functional outcomes and higher radiographic progression scores (Table 2). Having a flare was associated with a larger than minimal clinically important difference (MCID) increase in HAQ-DI (β=0.32 (95% CI: 0.29 to 0.36) p<0.01) and EQ-5D (β= -0.11 (95% CI: -0.12 to -0.09) p<0.01). Both the physical and mental performance measures from SF-36 were significantly worse in patients who flared in the unadjusted model. This was more marked with the physical component and did not remain significant with the mental component in the adjusted model. Patients who flared were 3.6 times (95% CI 2.77 to 4.67; p= 0.00) more likely to have erosive progression defined as new or larger erosions over 1 year on radiographs.
Table 2. Outcomes at 1 year in patients who flare compared to patients who do not flare.
| β constant | 95% CI | P value | ||
|---|---|---|---|---|
| HAQ-DI | Unadjusted | 0.59 | 0.37 to 0.80 | <0.01 |
| Adjusted | 0.19 | 0.04 to 0.32 | 0.01 | |
| Imputed (Adjusted) | 0.32 | 0.29 to 0.36 | <0.01 | |
| EQ-5D | Unadjusted | -0.19 | -0.26 to -0.13 | <0.01 |
| Adjusted | -0.11 | -0.18 to -0.05 | <0.01 | |
| Imputed (Adjusted) | -0.11 | -0.12 to -0.09 | <0.01 | |
| SF-36 PCS | Unadjusted | -8.79 | -12.4 to -5.18 | <0.01 |
| Adjusted | -3.92 | -7.04 to -0.8 | 0.01 | |
| Imputed (Adjusted) | -5.17 | -5.81 to -4.53 | <0.01 | |
| SF-36 MCS | Unadjusted | -5.42 | -9.41 to -1.42 | 0.01 |
| Adjusted | -2.86 | -6.83 to 1.12 | 0.16 | |
| Imputed (Adjusted) | -2.94 | -3.7 to -2.18 | <0.01 | |
| FACIT-F | Unadjusted | -7.83 | -11.6 to -4.06 | <0.01 |
| Adjusted | -4.07 | -7.91 to -0.24 | 0.04 | |
| Imputed (Adjusted) | -5.09 | -5.77 to -4.42 | <0.01 | |
| DAS 28 | Unadjusted | 1.32 | 0.96 to 1.68 | <0.01 |
| Adjusted | 1.07 | 0.77 to 1.37 | <0.01 | |
| Imputed (Adjusted) | 1.00 | 0.94 to 1.06 | <0.01 | |
| Odd Ratio | 95% CI | P value | ||
| Erosive progression | Unadjusted | 2.33 | 0.87 to 6.27 | 0.09 |
| Adjusted | 3.51 | 1.06 to 11.7 | 0.04 | |
| Imputed (Adjusted) | 3.60 | 2.77 to 4.67 | <0.01 | |
HAQ-DI, Health Assessment Questionnaire disability Index; EQ-5D, EuroQol five dimensions questionnaire; SF-36 MCS, Short Form 36 Health Survey Physical Component Summary; SF-36 MCS, Short Form 36 Health Survey Mental Component Summary; FACIT-F, Functional Assessment of Chronic Illness Therapy –Fatigue;
Discussion
In this prospective study, one third of RA patients with LDAS experienced a flare during 12 months follow-up. This is similar to flare rates reported in cohort studies, although these only included patients in remission [5, 9] and in drug tapering studies, in patients who remain on stable therapy. In both the DRESS [23] and the POET [24] study, the rate of short lived flare was significantly higher in patients who tapered or stopped their anti-TNF therapy compared to those who continued treatment, although in the DRESS study, the rate of major flares was similar between the two groups.
In this study, we have shown that the occurrence of a flare is hard to predict, but undeniably associated with worse clinical outcomes at 12 months. Our study highlights that identification of predictors of flare in patients with LDAS is challenging. In accordance with a previous remission cohort study [9], we found that HAQ-DI, a measure of functional activity, reflected by difficulties in activities of daily living, was predictive for flare. It is plausible that patients with low disease activity and high functional disability are more likely to flare. Functional impairment can herald a flare with the onset of morning stiffness and fatigue. A high HAQ may reflect severe rheumatoid with disease-related damage and the likelihood of grumbling disease.
Serum biomarkers were only modestly correlated with DAS28 at the time of flare. This might be explained by the fact that a flare is defined by worsening of the DAS28 composite score, and an increase in tender joint count and patient global assessment alone may increase the DAS28 score to a sufficient level to define a flare. It is possible that a flare event is not solely the result of direct synovial inflammation but may be driven by other pathways, for example chronification of pain due to central sensitisation and abnormal regulatory mechanisms [25]. This heterogeneity may partly explain why identifying predictors of flare is challenging. The OMERACT RA flare group recognise the limitation of DAS-28 in defining flare events. They are developing a consensus-based core domain set to set to identify and measure flare in RA [26, 27]. It is likely that improving the definition of flare and establishing a scoring system may help interpret predictors of flare in the future.
We found that a higher baseline CRP and ESR were predictive of flare in the univariate analyses, whilst baseline MBDA score, calprotectin and CXCL10 were not. In the sensitivity analysis limited to flare events with an associated high MBDA score at the time of flare, a relationship between baseline MBDA value and flare risk was established. This may suggest that baseline MBDA score is only predictive of flares which are driven directly by inflammation. Interestingly, when each component of the MBDA score was analysed individually, only 3 of the 12 components (serum amyloid A (SAA), leptin and high sensitivity CRP) predicted flare. Studies suggests a close correlation between leptin levels and RA disease duration, activity and severity [28]. The rapid production of SAA and its exceptionally wide dynamic range has proved advantageous as a biomarker of disease activity, with superiority over CRP in early RA studies [29].
Ultrasound parameters, including power Doppler signal, had no predictive value in this study. This is likely a reflection of the high proportion of patients in our cohort who had ultrasound activity at baseline. In the POET study only 63% of patients had US sign of arthritis with positive power Doppler signal [30]. This is partly explained by our cohort, which included a greater proportion of patients, one third, with low disease activity state (LDAS) above the DAS-28 remission cut-off. A large number of patients were on DMARD monotherapy, and only three were prescribed oral corticosteroids, which may explain the difference in power Doppler compared to other cohorts which have achieved LDAS with combination DMARDs and corticosteroid therapy. Scoring of PD was also more stringent in our cohort compared to others [9] leading to a much higher proportion of patients with PD signal being reported. The major limitation of ultrasound is that it remains a user-dependent technique. It is increasingly sensitive at demonstrating evidence of incomplete suppression of inflammation. The joints of healthy volunteers has been shown to display power Doppler signal [31, 32] and treatment escalation studies have argued against very stringent ultrasound targets [33]. Others have also shown that low grade PD signal and synovial hypertrophy may not necessarily reflect the presence of active synovitis in RA joints [34]. In our cohort, a high proportion had power Doppler activity at baseline and did not go on to flare. It may be postulated that a binary power Doppler cut-off might be insensitive in discriminating patients who are likely to flare.
This study also found that patients who flare were more likely to have erosive progression, worse quality of life and higher disease activity over 1 year. These findings consistent with previous studies [7, 9, 35] and emphasize the importance of flare and its relationship with patient outcomes. What remains unclear is whether flares are causally implicated in clinical outcome or if they are merely a biomarker of persistent low-grade disease. A flare may imply persistent uncontrolled inflammation contributing to disease progression, or a transient episode of inflammation (e.g. a 6 weeks flare within a stable 6-month period) that is sufficient to impact on long term outcome, or signify negative patient experience, a lack of self-control and unpredictability of the disease, which undoubtedly have psychological health implications.
There were several strengths of this study. The cohort was selected from routine care which is far more representative than a highly selective clinical trial population. Using patients in low disease activity states rather than remission enables access to a broader range of patients and is more in keeping with routine clinical care. Furthermore, this was a deeply phenotyped cohort with extensive clinical and laboratory data at multiple time points across the study period.
There are potential limitations to this study. We must acknowledge the limitation of the REMIRA study sample size and the limited number of predictors identified could reflect a type two error. We also acknowledge issues with missing data, particularly with incomplete available ultrasound reports. However, we believe that the pattern of missing data met the assumptions of missing at random and we were able to successfully construct an imputation model to address this. We only registered flares during a visit to a rheumatologist and the actual flare rate might be higher. Potential flares in-between visits could have been detected by a flare questionnaire [36] or alternative tools that permit remote monitoring. However, we would have only missed short lived flares (< 3 months), and these are of less clinical importance since they are less likely to lead to worse clinical outcomes (e.g. no radiographic progression) [23]. REMIRA was an observational study and any modifications in medications were carried out according to the physicians' and patients' choices. Since treatment was not protocolised, this may have impacted on the rate of flares. A single failure model was used to identify predictors of flare, and thus changes in therapy after a flare event should not influence the analysis. It is however possible that treatment modifications, for example glucocorticoids during a flare may improve disease outcome at 12 months.
In conclusion, we have demonstrated that flares are common in RA patients with low disease activity states and are strongly associated with poor clinical outcomes. Therefore, preventing flares is clinically relevant yet relatively challenging. HAQ-DI, a measure of functional activity, was an important predictor of flare. However flares are complex events and not simply a reflection of inflammatory disease activity. It is possible that two distinct subtypes of flare might exist; an ‘inflammatory’ flare predominately driven by an increase in swollen joint count and ESR and a ‘non-inflammatory’ flare with a disproportionately elevated tender joint count and a high patient global assessment score. Differentiating these two flare types may identify potential predictors. Further research is needed to explore if distinct flares exist and to categorise the potential predictors of each.
Supplementary Material
Acknowledgement
We acknowledge the Crescendo Bioscience team, in particular Eric Sasso and Nadine Defranoux for processing the Remira blood samples and assisting in the completion of this manuscript. We would also like to acknowledge Dr Stephen Kelly for his advice on setting up the ultrasound protocols.
The sources of support (grants):
This represents independent research by Dr Katie Bechman part funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. Margaret Ma work was funded by the UK National Institute of Health Research (DRF-2009-02-86 to M.H.Y. Ma).
References
- 1.Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2016;68:1–25. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, Bijlsma JWJ, Breedveld FC, Boumpas D, Burmester G. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bingham CO, Pohl C, Woodworth TG, Hewlett SE, May JE, Rahman MU, et al. Developing a standardized definition for disease “flare” in rheumatoid arthritis (OMERACT 9 Special Interest Group) J Rheumatol. 2009;36:2335–2341. doi: 10.3899/jrheum.090369. [DOI] [PubMed] [Google Scholar]
- 4.Alten R, Pohl C, Choy EH, Christensen R, Furst DE, Hewlett SE. Developing a construct to evaluate flares in rheumatoid arthritis: a conceptual report of the OMERACT RA flare definition working group. J Rheumatol. 2011;38:1745–50. doi: 10.3899/jrheum.110400. [DOI] [PubMed] [Google Scholar]
- 5.Molenaar ET, Voskuyl AE, Dinant HJ, Bezemer PD, Boers M, Dijkmans BA. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 2004;50:36–42. doi: 10.1002/art.11481. [DOI] [PubMed] [Google Scholar]
- 6.Welsing PM, Landewe RB, van Riel PL, Boers M, van Gestel AM, van der Linden S, et al. The relationship between disease activity and radiologic progression in patients with rheumatoid arthritis: a longitudinal analysis. Arthritis Rheum. 2004;50:2082–2093. doi: 10.1002/art.20350. [DOI] [PubMed] [Google Scholar]
- 7.Markusse IM, Dirven L, Gerards AH, van Groenendael JH, Ronday HK, Kerstens PJ, et al. Disease flares in rheumatoid arthritis are associated with joint damage progression and disability: 10-year results from the BeSt study. Arthritis Res Ther. 2015;17:232. doi: 10.1186/s13075-015-0730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myasoedova E, Chandran A, Ilhan B, Major BT, Michet CJ, Matteson EL, et al. The role of rheumatoid arthritis (RA) flare and cumulative burden of RA severity in the risk of cardiovascular disease. Ann Rheum Dis. 2016;75:560–565. doi: 10.1136/annrheumdis-2014-206411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saleem B, Brown AK, Quinn M, Karim Z, Hensor EM, Conaghan P, et al. Can flare be predicted in DMARD treated RA patients in remission, and is it important? A cohort study. Ann Rheum Dis. 2012;71:1316–1321. doi: 10.1136/annrheumdis-2011-200548. [DOI] [PubMed] [Google Scholar]
- 10.Han J, Geng Y, Deng X, Zhang Z. Subclinical Synovitis Assessed by Ultrasound Predicts Flare and Progressive Bone Erosion in Rheumatoid Arthritis Patients with Clinical Remission: A Systematic Review and Metaanalysis. J Rheumatol. 2016;43:2010–2018. doi: 10.3899/jrheum.160193. [DOI] [PubMed] [Google Scholar]
- 11.Bouman CAM, van der Maas A, van Herwaarden N, Sasso EH, van den Hoogen FHJ, den Broeder AA. A multi-biomarker score measuring disease activity in rheumatoid arthritis patients tapering adalimumab or etanercept: predictive value for clinical and radiographic outcomes. Rheumatology. 2017;56:973–980. doi: 10.1093/rheumatology/kex003. [DOI] [PubMed] [Google Scholar]
- 12.Nordal HH, Brokstad KA, Solheim M, Halse AK, Kvien TK, Hammer HB. Calprotectin (S100A8/A9) has the strongest association with ultrasound-detected synovitis and predicts response to biologic treatment: results from a longitudinal study of patients with established rheumatoid arthritis. Arthritis Res Ther. 2017;19:3. doi: 10.1186/s13075-016-1201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patro PS, Singh A, Misra R, Aggarwal A. Myeloid-related Protein 8/14 Levels in Rheumatoid Arthritis: Marker of Disease Activity and Response to Methotrexate. J Rheumatol. 2016;43:731–737. doi: 10.3899/jrheum.150998. [DOI] [PubMed] [Google Scholar]
- 14.Nordal HH, Brun JG, Hordvik M, Eidsheim M, Jonsson R, Halse AK. Calprotectin (S100A8/A9) and S100A12 are associated with measures of disease activity in a longitudinal study of patients with rheumatoid arthritis treated with infliximab. Scand J Rheumatol. 2016;45:274–281. doi: 10.3109/03009742.2015.1107128. [DOI] [PubMed] [Google Scholar]
- 15.Choi IY, Gerlag DM, Herenius MJ, Thurlings RM, Wijbrandts CA, Foell D, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499–505. doi: 10.1136/annrheumdis-2013-203923. [DOI] [PubMed] [Google Scholar]
- 16.Pandya JM, Lundell AC, Andersson K, Nordstrom I, Theander E, Rudin A. Blood chemokine profile in untreated early rheumatoid arthritis: CXCL10 as a disease activity marker. Arthritis Res Ther. 2017;19:20. doi: 10.1186/s13075-017-1224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma M, Ibrahim F, Kingsley G, Cope A, Scott D. Variable Impacts of different remission states on health related quality of life in rheumatoid arthritis. Clin Exp Rheumatol. 2017 [PubMed] [Google Scholar]
- 18.van der Maas A, Lie E, Christensen R, Choy E, de Man YA, van Riel P, et al. Construct and criterion validity of several proposed DAS28-based rheumatoid arthritis flare criteria: an OMERACT cohort validation study. Ann Rheum Dis. 2013;72:1800–1805. doi: 10.1136/annrheumdis-2012-202281. [DOI] [PubMed] [Google Scholar]
- 19.Centola M, Cavet G, Shen Y, Ramanujan S, Knowlton N, Swan KA, et al. Development of a multi-biomarker disease activity test for rheumatoid arthritis. PLoS One. 2013;8:e60635. doi: 10.1371/journal.pone.0060635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohrndorf S, Backhaus M. Advances in sonographic scoring of rheumatoid arthritis. Ann Rheum Dis. 2013;72:69–75. doi: 10.1136/annrheumdis-2012-202197. [DOI] [PubMed] [Google Scholar]
- 21.Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D'Agostino MA, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–2487. [PubMed] [Google Scholar]
- 22.Swinscow T. BMJ Publishing Group; 1997. Statistics at Square One. Chapter 11 Correlation and regression. [Google Scholar]
- 23.van Herwaarden N, van der Maas A, Minten MJ, van den Hoogen FH, Kievit W, van Vollenhoven RF, et al. Disease activity guided dose reduction and withdrawal of adalimumab or etanercept compared with usual care in rheumatoid arthritis: open label, randomised controlled, non-inferiority trial. BMJ. 2015;350:h1389. doi: 10.1136/bmj.h1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghiti Moghadam M, Vonkeman HE, Ten Klooster PM, Tekstra J, van Schaardenburg D, Starmans-Kool M, et al. Stopping Tumor Necrosis Factor Inhibitor Treatment in Patients With Established Rheumatoid Arthritis in Remission or With Stable Low Disease Activity: A Pragmatic Multicenter, Open-Label Randomized Controlled Trial. Arthritis Rheumatol. 2016;68:1810–7. doi: 10.1002/art.39626. [DOI] [PubMed] [Google Scholar]
- 25.Schaible HG, von Banchet GS, Boettger MK, Bräuer R, Gajda M, Richter F, et al. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci. 2010;1193:60–69. doi: 10.1111/j.1749-6632.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- 26.Bykerk VP, Lie E, Bartlett SJ, Alten R, Boonen A, Christensen R, et al. Establishing a core domain set to measure rheumatoid arthritis flares: report of the OMERACT 11 RA flare Workshop. J Rheumatol. 2014;41:799–809. doi: 10.3899/jrheum.131252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bykerk VP, Bingham CO, Choy EH, Lin D, Alten R, Christensen R, et al. Identifying flares in rheumatoid arthritis: reliability and construct validation of the OMERACT RA Flare Core Domain Set. 2016;2:e000225. doi: 10.1136/rmdopen-2015-000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gómez-Reino JJ, et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 2017;13:100–109. doi: 10.1038/nrrheum.2016.209. [DOI] [PubMed] [Google Scholar]
- 29.Hwang YG, Balasubramani GK, Metes ID, Levesque MC, Bridges SL, Moreland LW. Differential response of serum amyloid A to different therapies in early rheumatoid arthritis and its potential value as a disease activity biomarker. Arthritis Res Ther. 2016;18:108. doi: 10.1186/s13075-016-1009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamers-Karnebeek FB, Luime JJ, Ten Cate DF, Teerenstra S, Swen N, Gerards AH, et al. Limited value for ultrasonography in predicting flare in rheumatoid arthritis patients with low disease activity stopping TNF inhibitors. Rheumatology. 2017;56:1560–1565. doi: 10.1093/rheumatology/kex184. [DOI] [PubMed] [Google Scholar]
- 31.Ellegaard K, Torp-Pedersen S, Holm CC, Danneskiold-Samsoe B, Bliddal H. Ultrasound in finger joints: findings in normal subjects and pitfalls in the diagnosis of synovial disease. Ultraschall Med. 2007;28:401–408. doi: 10.1055/s-2007-963170. [DOI] [PubMed] [Google Scholar]
- 32.Terslev L, Torp-Pedersen S, Qvistgaard E, von der Recke P, Bliddal H. Doppler ultrasound findings in healthy wrists and finger joints. Ann Rheum Dis. 2004;63:644–648. doi: 10.1136/ard.2003.009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dale J, Stirling A, Zhang R, Purves D, Foley J, Sambrook M, et al. Targeting ultrasound remission in early rheumatoid arthritis: the results of the TaSER study, a randomised clinical trial. Ann Rehum Dis. 2016;75:1043–50. doi: 10.1136/annrheumdis-2015-208941. [DOI] [PubMed] [Google Scholar]
- 34.Gartner M, Mandl P, Radner H, Supp G, Machold KP, Aletaha D, et al. Sonographic joint assessment in rheumatoid arthritis: associations with clinical joint assessment during a state of remission. Arthritis Rheum. 2013;65:2005–2014. doi: 10.1002/art.38016. [DOI] [PubMed] [Google Scholar]
- 35.Ometto F, Raffeiner B, Bernardi L, Bostsios C, Veronese N, Punzi L, et al. Self-reported flares are predictors of radiographic progression in rheumatoid arthritis patients in 28-joint disease activity score remission: a 24-month observational study. Arthritis Res Ther. 2016;18:89. doi: 10.1186/s13075-016-0986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bykerk VP, Shadick N, Frits M, Bingham CO, Jeffery I, Iannaccone C. Flares in rheumatoid arthritis: frequency and management. A report from the BRASS registry. J Rheumatol. 2014;41:227–3. doi: 10.3899/jrheum.121521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.