Abstract
Purpose
The authors have shown previously that a recombinant HSV-1 that constitutively expresses two copies of murine IL-2 (HSV-IL-2) induces demyelination by activated CD8+ T cells in the brain and spinal cord of ocularly infected female BALB/c mice. The present study was conducted to determine whether the ocular infection with this recombinant virus induces optic neuritis independent of virus dose, major histocompatibility complex (MHC) background, or sex.
Methods
Female BALB/c, C57BL/6, SJL/6, and 129SVE mice and male BALB/c mice were ocularly infected with different doses of recombinant HSV-IL-2 virus. Demyelination of optic nerves in infected mice was monitored histologically using Luxol fast blue staining and by measurement of visual-evoked cortical potentials (VECPs).
Results
Both focal and diffuse regions of demyelination of the optic nerves were observed in the HSV-IL-2–infected mice as early as day 10 after infection and as late as day 60 after infection (the final experimental time point) in all strains of mice tested. Optic nerve demyelination was not observed in control mice ocularly infected with HSV-IL-4 or wild-type HSV-1. VECP responses were delayed significantly in the HSV-IL-2–infected mice compared with mice infected with control viruses.
Conclusions
The results demonstrate for the first time that a combination of viral infection and constitutive expression of IL-2, but not IFN-γ or IL-4, can result in demyelination and visual impairment in the optic nerves of ocularly infected mice.
Visual disturbances are initial manifestations of multiple sclerosis (MS).1–5 Demyelination of the optic nerve, also called optic neuritis (ON), is a common cause of visual and neurologic dysfunction in young adults with MS.1–5 Early signatures of MS often involve degradation of visual acuity. Although such degradation can result in vision impairment and even blindness, the close relationship between ON and MS has made ON more important than its visual prognosis. Approximately 50% to 70% of patients with monosymptomatic ON have clinically silent MS-like lesions on magnetic resonance images (MRIs) of the brain,1 and 60% to 70% of patients with ON exhibit the oligoclonal IgG banding in cerebrospinal fluid (CSF) typically seen in MS.1 More recent studies have demonstrated clearly that on longer follow-up, MS will be diagnosed in at least half of patients with monosymptomatic ON.1–5
It has been established that the number of IL-2-secreting cells, as well as the amounts of IL-2, in the sera of patients with MS are elevated.6–9 In addition the levels of soluble IL-2 receptor (sIL-2R) also appear to be elevated in the sera of patients with MS8,10–13 and in the CSF.14,15 Supernatants harvested from T lymphocytes of patients with MS cause damage to myelin and glial cells in vitro,16,17 suggesting that the MS T lymphocytes produce demyelination factors and are activated in vivo. A positive correlation also has been found between the percentage of IL-2 receptor-bearing lymphocytes and the degree of supernatant-induced in vitro demyelination. In addition, an association between the presence of IL-2 and the disease state has been demonstrated in the experimental autoimmune encephalomyelitis (EAE) model.18–20 Thus, both epidemiologic and histochemical analyses indicate that IL-2 plays a role in exacerbating MS. The results of a recent phase I/II clinical trial suggested that a humanized anti-IL-2 receptor mAb (daclizumab) can be used to treat severe uveitis, which is a TH1-mediated autoimmune condition.21 Furthermore, daclizumab has been used with positive results for treatment of MS.
The clinical findings of elevated IL-2 levels (and decreased IL-4 levels) in the sera of MS patients,8,10–13 suggest a possible link between the presence of IL-2 and the onset of MS. Specifically, they suggest that IL-2 is a strong candidate as the factor that causes demyelination mediated by proliferating T cells. To determine whether these clinical correlations reflect function, we constructed a recombinant HSV-1 that constitutively expressed the murine IL-2 gene under the control of the latency-associated transcript (LAT) promoter (HSV-IL-2).22 The LAT promoter was used because it is the only viral promoter that is always found, with active promoting high levels of transcription, throughout primary and latent infection in neurons.23–26 Using this HSV-1/LAT promoter vector overcomes the problems associated with the stability of cytokines after in vivo administration.27,28 In addition, after ocular infection, HSV-1 naturally travels to the mouse brain. Therefore, this vector also eliminates the problem of delivering material across the blood–brain barrier. Using this approach, we have examined the effects of infection of mice with HSV-1 recombinant virus expressing IL-2 in vivo on optic nerve demyelination by HSV-IL-2 expressing recombinant virus. Control experiments included infection of mice with similarly constructed recombinant HSV-1 viruses expressing IFN-γ (HSV-IFN-γ) or IL-4 (HSV-IL-4) in vivo. Using this approach, we have previously demonstrated that infection with HSV-IL-2 resulted in demyelination in the brain and spinal cord of infected BALB/c mice.29 We have now extended these studies to examine the effects of ocular infection with HSV-IL-2 on demyelination of the optic nerve. The results presented here demonstrate that infection of mice with a recombinant HSV-1 constitutively expressing the murine IL-2 gene resulted in optic nerve demyelination in different strains of mice, as determined by histologic evidence and measurement of visually evoked cortical potentials (VECPs), whereas infection with HSV-1 constructs expressing IFN-γ or IL-4 did not. Infections with control viruses alone did not result in demyelination.
Materials and Methods
Viruses, Cells, and Mice
Plaque-purified HSV-1 strains, McKrae (wild-type), KOS (wild-type), or dLAT2903 (HSV-IL-2, HSV-IL-4, and HSV-IFN-γ parental virus), and HSV-1 recombinant viruses expressing IL-2, IL-4, or IFN-γ (HSV-IL-2, HSV-IL-4, and HSV-IFN-γ) were grown in rabbit skin (RS) cell monolayers in minimal essential medium (MEM) containing 5% fetal calf serum (FCS), as described previously.22,30,31 The construction and characterization of recombinant HSV-IL-2, -IL-4, and -IFN-γ viruses have been described previously.22,30,31 McKrae, dLAT2903, and HSV-IFN-γ viruses are virulent at an infectious dose of 2 × 105 plaque-forming units (PFU)/eye,31 whereas KOS, HSV-IL-2, and HSV-IL-4 viruses are attenuated.22,30 Inbred 6-week-old male or female BALB/c mice or female C57BL/6, SJL/6 (The Jackson Laboratory, Bar Harbor, ME), or 129SVE (Taconic Farms, Germantown, NY) mice were used. Animals were handled in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Ocular Infection
Mice were infected ocularly with 2 × 103, 2 × 104, 2 × 105, 2 × 106, or 2 × 107 PFU of each virus per eye. Each virus was suspended in 2 μL of tissue culture medium and administered as an eye drop.
Preparation of Optic Nerves for Histologic Analyses
The optic nerves of infected mice were removed at necropsy at various times after infection. Both optic nerves were collected from experimental and control mice, then snap frozen in an isopentane-liquid nitrogen bath and stored at −80°C. Transverse sections of the entire optic nerve, 8 to 10 μm thick, were cut, air-dried overnight, and fixed in acetone for 3 minutes at 25°C.32 Consecutive sections were used for the pathologic analyses described below and in the legends of each figure.
Analysis of Demyelination Using Luxol Fast Blue (LFB) Staining
The presence or absence of demyelination in infected mice was evaluated using LFB staining of formalin-fixed sections of optic nerve as we described previously.29 Every fifth section of optic nerve was stained for LFB. The number of visible, pronounced demyelinated areas per section was counted in 10 sections for each of the optic nerves of both eyes per mouse. The results were reported as focal and/or diffuse demyelination lesions, These pathologic classifications are defined as (1) focal demyelination, relatively small, often perivascular, lesions or (2) diffuse demyelination, widespread lesions, often covering over half the width of the optic nerve (when specific plaque was detected in any of the 10 optic nerve sections in the absence of diffuse demyelination).
Measurement of VECPs
Mice were anesthetized before VECP recording with an injection of a mixture of 10 mg/mL ketamine and 4 mg/mL xylazine (100 μL IP). The mice were placed on a heating pad to maintain body temperature at 38°C. For the VECP testing, the active electrode was a stainless steel subdermal electrode positioned 3 mm lateral to lambda with the tip of the electrode resting on the skull. The reference electrode was a gold wire placed inside the mouth, and the ground was a needle under the skin near the tail. The mouse was placed in a stereotaxic (Stoelting, Wood Dale, IL) apparatus that held the snout securely, thereby allowing easy positioning of the eye. The flash VECP was recorded by placing the eye of the mouse orthogonal to the opening in a Ganzfeld dome. The interior of the dome was illuminated by brief flashes of light that was varied over a 4.0 log unit range of intensities using combinations of neutral density filters and settings on the photostimulator. Flash temporal frequency was set to 1 Hz for all testing. Considerable signal averaging was used, with up 100 records averaged at the weakest intensities. The VECPs recorded in response to a flash of light is a complicated waveform consisting of several negative and positive waves as described.33 The amplitude and implicit times of the first four major components (P1, N1, P2, and N2) were used for analytic purposes.
Statistical Analysis
Fisher's exact tests were performed (Instat; GraphPad, San Diego, CA) to analyze demyelination in HSV-IL-2 infected mice versus the absence of demyelination in control groups. Results were considered statistically significant at P < 0.05.
Results
Demyelination of the Optic Nerve of Female BALB/C Mice Infected with HSV-IL-2
Female BALB/c (H-2d) mice were infected with 2 × 105 PFU/eye of HSV-IL-2, HSV-IFN-γ, HSV-IL-4, wild-type HSV-1 strain McKrae, avirulent HSV-1 strain KOS, or parental dLAT2903 virus. At day 14 postinfection (PI), the optic nerves were dissected, postfixed, and stained with LFB. Generally, mice with predominantly focal demyelination also showed some diffuse demyelination, whereas mice with predominantly diffuse demyelination did not show any focal demyelination. Representative photomicrographs of the optic nerve sections dissected from mice infected with HSV-IL-2 or control viruses on day 14 PI are shown in Figure 1. At this time point, demyelination was observed in the optic nerves of 16 of 19 of the HSV-IL-2–infected mice, with both focal and diffuse demyelination being observed (Figs. 1A–E, arrows). No such lesions or other signs of demyelination were observed at this time point in any of the 10 mice per group that were infected with HSV-1 strain KOS (Fig. 1F), HSV-IL-4 (Fig. 1G), HSV-IFN-γ (Fig. 1H), or mock-infected (Fig. 1I). Similarly, female BALB/c mice that were infected ocularly with wild-type HSV-1 strain McKrae or parental dLAT2903 also showed no signs of demyelination (not shown). These differences between HSV-IL-2–infected mice and mice infected with HSV-IL-4, KOS, HSV-IFN-γ, or other control viruses were highly significant (P < 0.0001, Fisher's exact test).
Figure 1.
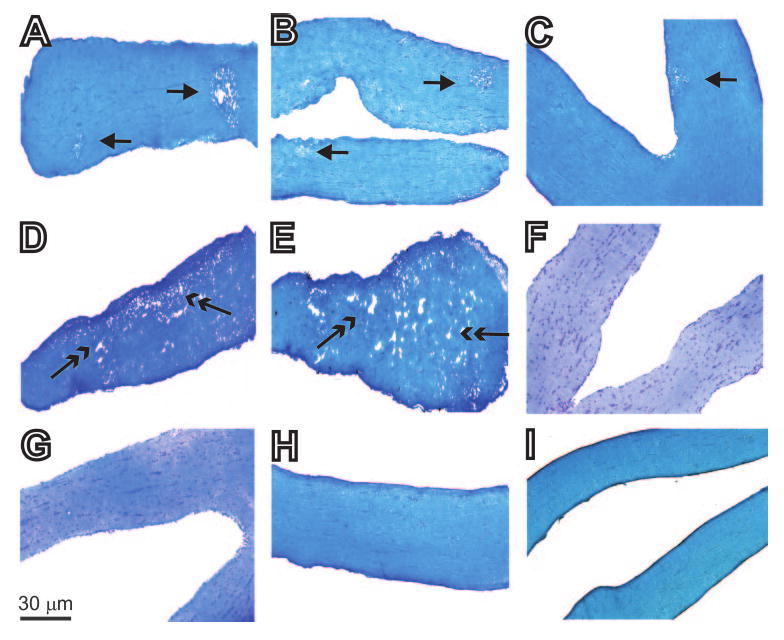
Histologic analyses of optic nerves obtained from female BALB/c mice 14 days after ocular infection with HSV-IL-2 or control viruses. Female BALB/c mice were infected ocularly with HSV-IL-2, HSV-IL-4, HSV-IFN-γ, KOS, or mock-infected, as described in the footnote to Table 1. The optic nerves were obtained 14 days later, fixed, sectioned, and stained with LFB. Representative photomicrographs are shown. In the photomicrograph of the section of the optic nerve from the HSV-IL-2–infected mouse, the single-headed arrow indicates focal demyelination, and the two-headed arrow indicates diffuse demyelination. (A–C) In HSV-IL-2–infected optic nerve areas of focal demyelination were apparent as plaque areas with loss of LFB staining (single-headed arrow). (D, E) In HSV-IL-2–infected optic nerve, areas of diffuse demyelination were apparent as plaque areas with loss of LFB staining (two-headed arrow). In contrast to (A–E), no demyelination was detected in mice infected with HSV-1 strain KOS (F), HSV-IL-4 (G), HSV-IFN-γ (H), or mock-infected (I) as shown by dense LFB blue staining in myelinated regions, and no detectable focal or diffuse positive material.
Time Course of Appearance of Demyelination in Optic Nerve of Infected Mice
To determine the time course of demyelination in the optic nerves of HSV-IL-2–infected mice, female BALB/c mice were infected with 2 × 105 PFU/eye of HSV-IL-2 or -IL-4, and the optic nerves were harvested at 3, 7, 10, 30, and 60 days PI. Demyelination was not detected in any of the HSV-IL-2–infected mice on days 3 or 7 PI (Table 1); however, demyelination was initially detected on day 10 PI in which the upper lesion is diffuse, whereas the other lesion is focal (Fig. 2A). Demyelination also was detected on days 30 and 60 PI (Table 1). Overall, the patterns of focal and diffuse demyelination plaques on days 10, 30, and 60 PI were similar to those observed on day 14 (Fig. 1). Generally, both focal and diffuse plaques were found in the optic nerve of the same animal infected with HSV-IL-2. Demyelination was not detected in the optic nerves of any of the HSV-IL-4–infected control mice on day 10 PI (Fig. 2B) and on days 3, 7, 30, or 60 PI (not shown). Thus, our results suggest that a combination of virus infection and constitutive expression of IL-2 induced optic neuritis in HSV-IL-2–infected mice as early as day 10 PI.
Table 1.
Demyelination of Optic Nerves of Female BALB/c Mice at Various Times after Infection with HSV-IL-2
| Pattern of Optic Nerve Demyelination | |||
|---|---|---|---|
| Days PI | Focal | Diffuse | Total |
| 3 | 0/5 (0) | 0/5 (0) | 0/5 (0) |
| 7 | 0/5 (0) | 0/5 (0) | 0/5 (0) |
| 10 | 2/5 (40) | 3/5 (60) | 3/5 (60) |
| 30 | 2/5 (40) | 3/5 (60) | 5/5 (100) |
| 60 | 1/5 (20) | 3/5 (60) | 4/5 (80) |
Female BALB/c mice were infected with 2 × 105 PFU/eye of HSV-IL-2, and the optic nerves were dissected at the indicated time points and fixed, sectioned, and stained with LFB. Ten sections per mouse were examined for evidence of demyelination. Data are presented as the number of mice showing optic nerve demyelination/total mice tested (%). Control animals were female BALB/c mice similarly infected with HSV-IL-4, HSV-IFN-γ, KOS, McKrae, or dLAT2903 parental virus (five mice per group). No areas of demyelination or plaque were detected in the optic nerves of mice infected with control viruses.
Figure 2.
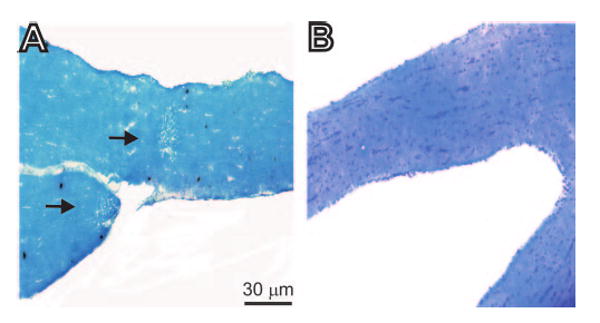
Histologic analyses of optic nerves obtained from female BALB/c mice 10 days after ocular infection with HSV-IL-2 or control HSV-IL-4. Female BALB/c mice were infected ocularly with HSV-IL-2 or -IL-4, as described in the footnote to Table 1. The optic nerves were obtained 10 days later, fixed, sectioned, and stained with LFB. Representative photomicrographs are shown. (A) In HSV-IL-2–infected optic nerve, areas of focal demyelination were apparent as plaque areas (arrows). (B) No demyelination was detected in mice infected with HSV-IL-4.
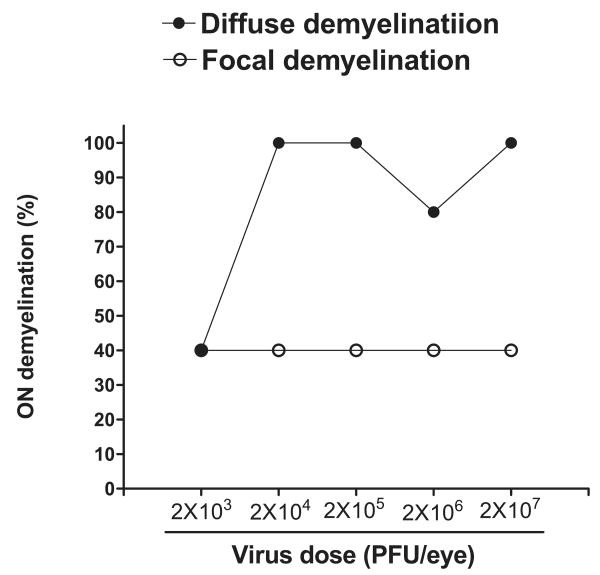
Dose Dependency of Demyelination in HSV-IL-2–Infected Mice
Administration of a high concentrations of IL-2 both in vitro34 and in vivo35 causes lysis of a broad array of cells. As IL-2 plays a major role in cytotoxic T-lymphocyte (CTL) activity in vitro,36 it is possible that the observed demyelination of the optic nerve could simply be a nonspecific cytotoxic effect due to the gross overexpression of IL-2 by HSV-1 in the central nervous system (CNS). The experiments were performed with mice that were infected with 2 × 105 PFU/eye of HSV-IL-2. To determine whether the effects are due to nonspecific cytotoxicity associated with overexpression of IL-2 by HSV-IL-2, we infected some mice with 10-fold (2 × 104) or 100-fold (2 × 103) lower doses of HSV-IL-2 or 10-fold (2 × 106) or 100-fold (2 × 107) higher doses of HSV-IL-2. Control mice were infected with 2 × 105 PFU of HSV-IL-2. Demyelination of the optic nerve was assessed on day 14 PI. The mice infected with 10- or 100-fold higher doses of HSV-IL-2 virus or 10- or 100-fold lower doses of virus exhibited levels of optic nerve demyelination similar to that of mice infected with 2 × 105 PFU/eye of HSV-IL-2 (Fig. 3, P > 0.1, Fisher's exact test). The results suggest that the observed demyelination is not dose-dependent and is not a cytotoxic effect of IL-2 overexpression. Similar to the above results, reinfection (also called superinfection) once on day 15 PI with 2 × 105 PFU/eye of HSV-IL-2 virus did not result in greater demyelination, when measured 14 days after the second infection (not shown).
Figure 3.
Demyelination of the optic nerve in female BALB/c mice infected with different doses of HSV-IL-2. Female BALB/c mice were infected ocularly with 2 × 103, 2 × 104, 2 × 105, 2 × 106, or 2 × 107 PFU/eye of HSV-IL-2 (five mice/group). The optic nerves were dissected 14 days later, fixed, sectioned, and stained with LFB. The presence of focal and/or diffuse demyelination in multiple sections was determined.
Optic Nerve Demyelination in HSV-IL-2–Infected Male BALB/c Mice
Females with MS outnumber males by approximately 2:1.37 Moreover, animal studies show that male mice, which are more resistant to EAE, produce more TH2 cytokines than do female mice, whereas female mice, which are more susceptible, produce more TH1 cytokines than do male mice.38 Recently, it was shown that vaccine efficacy against HSV-1 also is gender-dependent.39 These differences between male and female mice were attributed to differential IL-2 production in the absence of differences in IL-4. The experiments (Figs. 1, 2, 3, Table 1) were performed with female BALB/c mice. Thus, to establish whether the HSV-IL-2 virus is less efficient at causing demyelination in male mice, we infected male BALB/c mice with 2 × 105 PFU/eye of HSV-IL-2. In male BALB/c mice, 10% (1/10) of the HSV-IL-2–infected mice showed focal plaques compared with 42% (8/19) of female BALB/c mice (Table 2). Furthermore, male BALB/c mice had a lower number of optic nerves with diffuse demyelination (5/10) than did female mice (18/19; Table 2). Overall, 50% of male mice (5/10) showed demyelination compared with 95% (18/19) female mice. These differences between male and female BALB/c mice were statistically significant (P = 0.01, Fisher's exact test). Thus, this result suggests that the male mice are more resistant to HSV-IL-2-induced demyelination than are female mice.
Table 2.
Optic Nerve Demyelination in Male and Female BALB/c Mice at 14 Days after Infection With HSV-IL-2
| Pattern of Optic Nerve Demyelination | |||
|---|---|---|---|
| Sex | Focal | Diffuse | Total |
| Male | 1/10 (10) | 5/10 (50) | 5/10 (50) |
| Female | 8/19 (42) | 13/19 (68) | 18/19 (95) |
| Fisher's exact test (male vs. female) | P = 0.42 | P = 0.43 | P = 0.01 |
Male and female BALB/c mice were infected with 2 × 105 PFU/eye of HSV-IL-2, and the optic nerves were dissected at 14 days after infection, fixed, sectioned, and stained with LFB. Data for female mice are combined from four separate experiments. Data are presented as the number of mice showing optic nerve demyelination/total mice tested (%).
Effect of HSV-IL-2 on Demyelination in Different Strains of Mice
Animal studies have shown that different mouse strains have different degrees of susceptibility to demyelination.40 Some studies have suggested that susceptibility to demyelination is associated with the major histocompatibility complex (MHC) background.41 However, other studies have shown that demyelination-resistant mice strains have elevated levels of TH2 cytokine, whereas susceptible strains of mice have elevated levels of TH1 cytokines, and resistance or susceptibility to demyelination is not associated with their MHC background.42 To further examine the effect of the MHC background on the optic nerve demyelination associated with HSV-IL-2 infection, we infected female C57BL/6 (H-2b), 129SVE (H-2b), and SJL/6 (H-2s) mice (five mice per group) ocularly with 2 × 105 PFU/eye of HSV-IL-2. Control mice were infected with HSV-IL-4. Demyelination was assessed on day 14 PI. At 14 days PI, both focal and diffuse demyelination was observed in the optic nerves of the HSV-IL-2–infected female C57BL/6 mice (Figs. 4A–C, arrows), female 129SVE (Figs. 4E–G, arrows), and female SJL/6 (Figs. 4I–K, arrows) mice as was observed for the HSV-IL-2–infected female BALB/c mice. No sign of demyelination was observed at this time point for C57BL/6 (Fig. 4D), 129SVE (Fig. 4H), and SJL/6 (Fig. 4L) mice infected with HSV-IL-4. For each strain of mice, the differences between HSV-IL-2–infected mice and their corresponding control mice infected with HSV-IL-4 were statistically significant (P = 0.008, Fisher's exact test).
Figure 4.
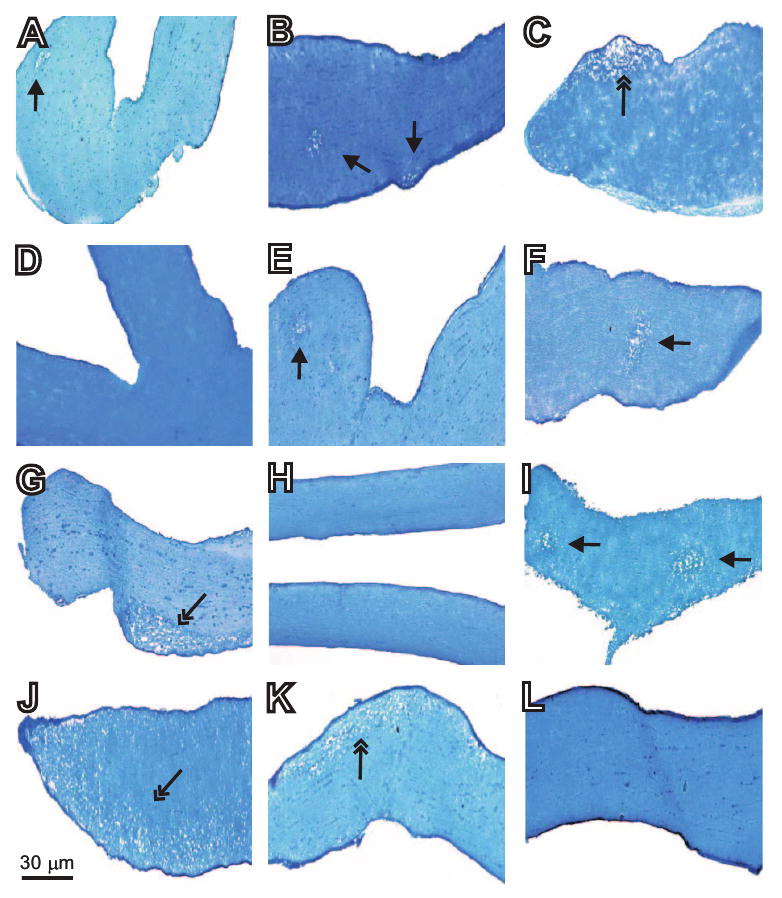
Effect of ocular HSV-IL-2 infection on optic nerve demyelination in different strains of mice. Female C57BL/6, 129SVE, or SJL/6 mice were infected ocularly with HSV-IL-2 (2 × 105 PFU/eye). Control mice were infected similarly with HSV-IL-4. The optic nerves were dissected 14 days later, fixed, sectioned, and stained with LFB. Representative micrographs are shown. In the photomicrograph of the section of the optic nerve from the HSV-IL-2–infected mouse, the single-headed arrow indicates focal demyelination, whereas the two-headed arrow indicates diffuse demyelination. (A–C) C57BL/6 mice infected with HSV-IL-2; (D) C57BL/6 mice infected with HSV-IL-4; (E–G) 129SVE mice infected with HSV-IL-2; (H) 129SVE mice infected with HSV-IL-4; (I–K) SJL/6 mice infected with HSV-IL-2; and (L) SJL/6 mice infected with HSV-IL-4.
The demyelination patterns observed in the optic nerves of the female C57BL/6, 129SVE, and SJL/6 mice on day 14 after HSV-IL-2 infection were similar to those observed in the optic nerves of the female HSV-IL-2–infected BALB/c mice at this time point. These results suggest that BALB/c, C57BL/6, 129SVE, and SJL/6 mice are equivalently susceptible to HSV-IL-2 demyelination. Thus, our results suggest that the nature of the immune responses, rather than the MHC background of infected mice are a more important factor in HSV-IL-2-induced demyelination.
Assessment of HSV-IL-2-Associated Optic Nerve Demyelination by Measurement of VECPs
An early sign of MS is often degradation of visual acuity, resulting in impaired vision or even blindness. Measurement of VECPs is widely used to assess patients with suspected MS because of the high sensitivity of this technique and its ability to detect even clinically silent lesions of the visual pathway. We therefore measured VECPs of female BALB/c mice 11 days after they were infected with 2 × 105 PFU/eye of HSV-IL-2. Control mice were infected with HSV-IL-4, McKrae, KOS, or parental virus 2903, or were mock-infected. Representative VECP responses to flashes of different intensity for mice infected with parental 2903 and HSV-IL-2 viruses are shown in Figure 5. As expected, the control dLAT2903-infected mice did not show any latency (time from the flash of light to the peak of the waveform in milliseconds) of P1, N1, and P2, or in the amplitude of the response (the size of the waveform in micro-volts from the N1 to P2; Fig. 5, top). Although recordable responses were observed in the HSV-IL-2–infected mice, they were clearly of smaller amplitude and, most important, they were significantly delayed in terms of the timing (latency) of peak components (Fig. 5, bottom). In the mouse infected with parental virus (Fig. 5, top), the latencies of the P1, N1, and P2 response to the most intense flash (top tracing of upper graph) were approximately 48, 72, and 96 ms, respectively. The amplitude from N1 to P2 was 82 μV. In the HSV-IL-2–infected mouse, the latencies of the P1, N1, and P2 response to the most intense flash (Fig. 5, top tracing, bottom lower graph) were approximately 53, 75, and 140 ms from N1 to P2, respectively. The amplitude from N1 to P2 was 33.5 μV. Mice infected with McKrae, KOS, or HSV-IL-4 virus did not show any signs of VECP abnormality (not shown). Thus, these experiments provide further evidence that a combination of virus and elevated levels of IL-2 can cause optic nerve disease.
Figure 5.
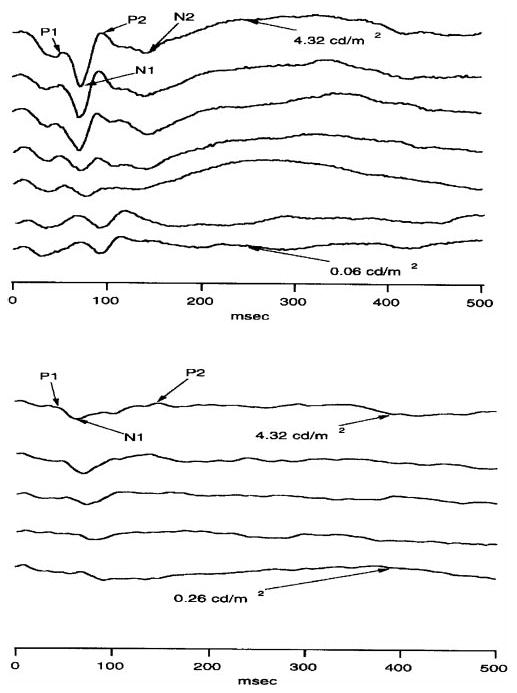
Effect of ocular infection with HSV-IL-2 on visual evoked cortical potentials of female BALB/c mice. Mice were infected ocularly with HSV-IL-2 (2 × 105 PFU/eye) or parental dLAT2903 (2 × 105 PFU/eye). After 11 days, the VECPs were recorded. Representative VECP recordings from a mice infected with (top) dLAT2903 or (bottom) HSV-IL-2.
Discussion
IL-2 has been implicated in the pathologic course of MS through the clinical observation that patients with MS have elevated levels of IL-2 in their CSF and sera6,8 and experimentally, as IL-2-deficient mice are more resistant to EAE than are their heterozygote and wild-type counterparts.19 This resistance is also demonstrated through our previous work where we show that demyelination of brain and spinal cord occurred in mice that were infected ocularly with HSV-IL-2 recombinant virus,29 but not with control recombinant viruses expressing IL-4 or IFN-γ instead of IL-2.30,31 Our present study provides further support for a role for IL-2 in MS, as four different strains of mice having three different MHC backgrounds all displayed demyelination of the optic nerve when infected with HSV-IL-2 virus. The observed demyelination in these animals was identified by both histologic examination and the functional VECP responses, whereas animals infected with HSV-IL-4 or -IFN-γ viruses did not exhibit demyelination. Furthermore, ocular infection of these mice with wild-type HSV-1 or parental virus did not demonstrate demyelination. Recently, transgenic mice that overexpress different cytokine genes in the CNS have been used as animal models to study demyelination. Transgenic mice that overexpress IL-3,43 IL-6,44 IL-12,45 IFN-γ,46 TNF-α,47 or IFN-α48 in their brain have been shown to develop neuroinflammatory disorders and demyelination disease; however, transgenic mice that overexpress IL-2 or -4 have not been examined. In our model we have shown that, in combination with HSV-1 virus, the expression of IL-2 causes CNS demyelination, whereas expression of IL-4 or IFN-γ does not.
There is a general belief that multiple factors are involved in the etiology of MS and that there may be a requirement for an environmental stimulus, a genetic propensity, and an overactive inflammatory and immune system that is triggered by an unknown event. Evidence has accumulated that infectious agents, particularly viral, may be involved,49,50 although the possibility remains controversial.51–53 If an infectious agent is involved, it alone may not be sufficient to initiate MS. Our observation that ocular infection of these mice with wild-type HSV-1 or parental virus did not cause demyelination of the CNS29 or the optic nerve in the absence of enhanced levels of IL-2 is consistent with this concept.
Serologic studies suggest that up to 90% of the adults in United States are HSV seropositive and HSV-1 infection of the eye is common.54–56 Although the data show that ocular infection with HSV-1 alone is not sufficient to trigger MS, the possibility exits that that HSV infection in combination with other factors, such as high levels of IL-2, contributes to the development of MS in humans. This possibility is evidenced by PCR analyses that fail to detect HSV-1 in the demyelinated regions of the brains taken from MS patients.52,53 A similar approach has implicated other herpes viruses.49,50 Our current studies demonstrate that ocular infection with HSV-1, in the context of high local levels of IL-2, can cause demyelination of the optic nerve, and raises the possibility that an ocular infection with HSV-1 may be an early trigger that then develops into MS involving the CNS, independent of the presence of an active infection.
Previously, we have shown that CD8+ T cells of the CD62LHighCD45RBLow phenotype appear to be directly involved in the demyelination process.29 However, IL-2 alone in the absence of viral infection does not cause demyelination (not shown). Thus, in our model, when the virus or cytokine is administered individually, disease does not occur, although when administered together, these agents cause demyelination and clinical abnormalities similar to those observed in MS. The numerous animal models of MS that have been developed use generally either viral models57 or direct autoimmune models58 to initiate disease. Our present study provides evidence that our novel model for demyelination, which incorporates both a viral and an immune component, is a model of the early events that are characteristic of MS, as well as the later events, as we have described previously. Finally, since HSV-1 is the leading cause of eye disease by an infectious agent in developed countries59 and visual disturbances are the initial manifestation of ON and MS,1–5 our results suggest that VECPs should be used as an early prognostic test for detection of ON and MS in individuals with a history of ocular HSV infection.
Acknowledgments
Supported by National Eye Institute Grant EY15557 (HG).
Footnotes
Disclosure: M. Zandian, None; R. Belisle, None; K.R. Mott, None; S. Nusinowitz, None; F.M. Hofman, None; H. Ghiasi, None
References
- 1.Soderstrom M, Ya-Ping J, Hillert J, Link H. Optic neuritis: prognosis for multiple sclerosis from MRI, CSF, and HLA findings. Neurology. 1998;50:708–714. doi: 10.1212/wnl.50.3.708. [DOI] [PubMed] [Google Scholar]
- 2.Sandberg-Wollheim M, Bynke H, Cronqvist S, Holtas S, Platz P, Ryder LP. A long-term prospective study of optic neuritis: evaluation of risk factors. Ann Neurol. 1990;27:386–393. doi: 10.1002/ana.410270406. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez M, Siva A, Cross SA, O'Brien PC, Kurland LT. Optic neuritis: a population-based study in Olmsted County, Minnesota. Neurology. 1995;45:244–250. doi: 10.1212/wnl.45.2.244. [DOI] [PubMed] [Google Scholar]
- 4.O'Riordan JI, Losseff NA, Phatouros C, et al. Asymptomatic spinal cord lesions in clinically isolated optic nerve, brain stem, and spinal cord syndromes suggestive of demyelination. J Neurol Neurosurg Psychiatry. 1998;64:353–357. doi: 10.1136/jnnp.64.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghezzi A, Martinelli V, Torri V, et al. Long-term follow-up of isolated optic neuritis: the risk of developing multiple sclerosis, its outcome, and the prognostic role of paraclinical tests. J Neurol. 1999;246:770–775. doi: 10.1007/s004150050453. [DOI] [PubMed] [Google Scholar]
- 6.Lu CZ, Fredrikson S, Xiao BG, Link H. Interleukin-2 secreting cells in multiple sclerosis and controls. J Neurol Sci. 1993;120:99–106. doi: 10.1016/0022-510x(93)90032-t. [DOI] [PubMed] [Google Scholar]
- 7.Gallo P, Piccinno M, Pagni S, Tavolato B. Interleukin-2 levels in serum and cerebrospinal fluid of multiple sclerosis patients. Ann Neurol. 1988;24:795–797. doi: 10.1002/ana.410240618. [DOI] [PubMed] [Google Scholar]
- 8.Gallo P, Piccinno MG, Pagni S, et al. Immune activation in multiple sclerosis: study of IL-2, sIL-2R, and gamma-IFN levels in serum and cerebrospinal fluid. J Neurol Sci. 1989;92:9–15. doi: 10.1016/0022-510x(89)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Trotter JL, Clifford DB, McInnis JE, et al. Correlation of immunological studies and disease progression in chronic progressive multiple sclerosis. Ann Neurol. 1989;25:172–178. doi: 10.1002/ana.410250211. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg SJ, Marcon L, Hurwitz BJ, Waldmann TA, Nelson DL. Elevated levels of soluble interleukin-2 receptors in multiple sclerosis. N Engl J Med. 1988;319:1019–1020. doi: 10.1056/NEJM198810133191517. [DOI] [PubMed] [Google Scholar]
- 11.Hartung HP, Hughes RA, Taylor WA, Heininger K, Reiners K, Toyka KV. T cell activation in Guillain-Barre syndrome and in MS: elevated serum levels of soluble IL-2 receptors. Neurology. 1990;40:215–218. doi: 10.1212/wnl.40.2.215. [DOI] [PubMed] [Google Scholar]
- 12.Bansil S, Troiano R, Cook SD, Rohowsky-Kochan C. Serum soluble interleukin-2 receptor levels in chronic progressive, stable and steroid-treated multiple sclerosis. Acta Neurol Scand. 1991;84:282–285. doi: 10.1111/j.1600-0404.1991.tb04955.x. [DOI] [PubMed] [Google Scholar]
- 13.Traugott U. Multiple sclerosis: relevance of class I and class II MHC-expressing cells to lesion development. J Neuroimmunol. 1987;16:283–302. doi: 10.1016/0165-5728(87)90082-8. [DOI] [PubMed] [Google Scholar]
- 14.Adachi K, Kumamoto T, Araki S. Interleukin-2 receptor levels indicating relapse in multiple sclerosis. Lancet. 1989;1:559–560. doi: 10.1016/s0140-6736(89)90103-7. [DOI] [PubMed] [Google Scholar]
- 15.Kittur SD, Kittur DS, Soncrant TT, et al. Soluble interleukin-2 receptors in cerebrospinal fluid from individuals with various neurological disorders. Ann Neurol. 1990;28:168–173. doi: 10.1002/ana.410280209. [DOI] [PubMed] [Google Scholar]
- 16.Selmaj K, Nowak Z, Tchorzewski H. Multiple sclerosis: effect of myelin basic protein on interleukin 1, interleukin 2 production and interleukin 2 receptor expression in vitro. Clin Exp Immunol. 1988;72:428–433. [PMC free article] [PubMed] [Google Scholar]
- 17.Selmaj K, Nowak Z, Tchorzewski H. Interleukin-1 and interleukin-2 production by peripheral blood mononuclear cells in multiple sclerosis patients. J Neurol Sci. 1988;85:67–76. doi: 10.1016/0022-510x(88)90036-6. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Lindsberg PJ, Hukkanen V, Seljelid R, Gahmberg CG, Meri S. Differential expression of cytokines (IL-2, IFN-gamma, IL-10) and adhesion molecules (VCAM-1, LFA-1, CD44) between spleen and lymph nodes associates with remission in chronic relapsing experimental autoimmune encephalomyelitis. Scand J Immunol. 2002;56:286–293. doi: 10.1046/j.1365-3083.2002.01132.x. [DOI] [PubMed] [Google Scholar]
- 19.Petitto JM, Streit WJ, Huang Z, Butfiloski E, Schiffenbauer J. Interleukin-2 gene deletion produces a robust reduction in susceptibility to experimental autoimmune encephalomyelitis in C57BL/6 mice. Neurosci Lett. 2000;285:66–70. doi: 10.1016/s0304-3940(00)00996-4. [DOI] [PubMed] [Google Scholar]
- 20.McCombe PA, Nickson I, Pender MP. Cytokine expression by inflammatory cells obtained from the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis induced by inoculation with myelin basic protein and adjuvants. J Neuroimmunol. 1998;88:30–38. doi: 10.1016/s0165-5728(98)00068-x. [DOI] [PubMed] [Google Scholar]
- 21.Nussenblatt RB, Thompson DJ, Li Z, et al. Humanized anti-interleukin-2 (IL-2) receptor alpha therapy: long-term results in uveitis patients and preliminary safety and activity data for establishing parameters for subcutaneous administration. J Autoimmun. 2003;21:283–293. doi: 10.1016/s0896-8411(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 22.Ghiasi H, Osorio Y, Perng GC, Nesburn AB, Wechsler SL. Overex-pression of interleukin-2 by a recombinant herpes simplex virus type 1 attenuates pathogenicity and enhances antiviral immunity. J Virol. 2002;76:9069–9078. doi: 10.1128/JVI.76.18.9069-9078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perng GC, Dunkel EC, Geary PA, et al. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J Virol. 1994;68:8045–8055. doi: 10.1128/jvi.68.12.8045-8055.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wechsler SL, Nesburn AB, Watson R, Slanina SM, Ghiasi H. Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. J Virol. 1988;62:4051–4058. doi: 10.1128/jvi.62.11.4051-4058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zwaagstra J, Ghiasi H, Nesburn AB, Wechsler SL. In vitro promoter activity associated with the latency-associated transcript gene of herpes simplex virus type 1. J Gen Virol. 1989;70:2163–2169. doi: 10.1099/0022-1317-70-8-2163. [DOI] [PubMed] [Google Scholar]
- 26.Zwaagstra JC, Ghiasi H, Slanina SM, et al. Activity of herpes simplex virus type 1 latency-associated transcript (LAT) promoter in neuron-derived cells: evidence for neuron specificity and for a large LAT transcript. J Virol. 1990;64:5019–5028. doi: 10.1128/jvi.64.10.5019-5028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddehase MJ, Mutter W, Koszinowski UH. In vivo application of recombinant interleukin 2 in the immunotherapy of established cytomegalovirus infection. J Exp Med. 1987;165:650–656. doi: 10.1084/jem.165.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel JP, Puri RK. Interleukin-2 toxicity. J Clin Oncol. 1991;9:694–704. doi: 10.1200/JCO.1991.9.4.694. [DOI] [PubMed] [Google Scholar]
- 29.Osorio Y, La Point SF, Nusinowitz S, Hofman FM, Ghiasi H. CD8+-dependent CNS demyelination following ocular infection of mice with a recombinant HSV-1 expressing murine IL-2. Exp Neurol. 2005;193:1–18. doi: 10.1016/j.expneurol.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Ghiasi H, Osorio Y, Perng GC, Nesburn AB, Wechsler SL. Recombinant herpes simplex virus type 1 expressing murine interleukin-4 is less virulent than wild-type virus in mice. J Virol. 2001;75:9029–9036. doi: 10.1128/JVI.75.19.9029-9036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghiasi H, Osorio Y, Hedvat Y, Perng GC, Nesburn AB, Wechsler SL. Infection of BALB/c mice with a herpes simplex virus type 1 recombinant virus expressing IFN-g driven by the LAT promoter. Virology. 2002;302:144–154. doi: 10.1006/viro.2002.1609. [DOI] [PubMed] [Google Scholar]
- 32.Ghiasi H, Wechsler SL, Kaiwar R, Nesburn AB, Hofman FM. Local expression of tumor necrosis factor alpha and interleukin-2 correlates with protection against corneal scarring after ocular challenge of vaccinated mice with herpes simplex virus type 1. J Virol. 1995;69:334–340. doi: 10.1128/jvi.69.1.334-340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nusinowitz S, Ridder W, 3rd, Heckenlively JR. Electrodiagnostic Techniques for Visual Function Testing in Mice. Amsterdam: Kluwer; 2002. [Google Scholar]
- 34.Tilden AB, Itoh K, Balch CM. Human lymphokine-activated killer (LAK) cells: identification of two types of effector cells. J Immunol. 1987;138:1068–1073. [PubMed] [Google Scholar]
- 35.Rosenberg SA, Mule JJ, Spiess PJ, Reichert CM, Schwarz SL. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985;161:1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 37.Duquette P, Pleines J, Girard M, Charest L, Senecal-Quevillon M, Masse C. The increased susceptibility of women to multiple sclerosis. Can J Neurol Sci. 1992;19:466–471. [PubMed] [Google Scholar]
- 38.Kim S, Voskuhl RR. Decreased IL-12 production underlies the decreased ability of male lymph node cells to induce experimental autoimmune encephalomyelitis. J Immunol. 1999;162:5561–5568. [PubMed] [Google Scholar]
- 39.Zhang X, Castelli FA, Zhu X, Wu M, Maillere B, BenMohamed L. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin Vaccine Immunol. 2008;15:1436–1449. doi: 10.1128/CVI.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 41.Maatta JA, Nygardas PT, Hinkkanen AE. Enhancement of experimental autoimmune encephalomyelitis severity by ultrasound emulsification of antigen/adjuvant in distinct strains of mice. Scand J Immunol. 2000;51:87–90. doi: 10.1046/j.1365-3083.2000.00686.x. [DOI] [PubMed] [Google Scholar]
- 42.Binder TA, Greiner DL, Grunnet M, Goldschneider I. Relative susceptibility of SJL/J and B10.S mice to experimental allergic encephalomyelitis (EAE) is determined by the ability of prethymic cells in bone marrow to develop into EAE effector T cells. J Neuroimmunol. 1993;42:23–32. doi: 10.1016/0165-5728(93)90208-g. [DOI] [PubMed] [Google Scholar]
- 43.Chiang CS, Powell HC, Gold LH, Samimi A, Campbell IL. Macrophage/microglial-mediated primary demyelination and motor disease induced by the central nervous system production of interleukin-3 in transgenic mice. J Clin Invest. 1996;97:1512–1524. doi: 10.1172/JCI118574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell IL, Abraham CR, Masliah E, et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pagenstecher A, Lassmann S, Carson MJ, Kincaid CL, Stalder AK, Campbell IL. Astrocyte-targeted expression of IL-12 induces active cellular immune responses in the central nervous system and modulates experimental allergic encephalomyelitis. J Immunol. 2000;164:4481–4492. doi: 10.4049/jimmunol.164.9.4481. [DOI] [PubMed] [Google Scholar]
- 46.LaFerla FM, Sugarman MC, Lane TE, Leissring MA. Regional hypomyelination and dysplasia in transgenic mice with astrocyte-directed expression of interferon-gamma. J Mol Neurosci. 2000;15:45–59. doi: 10.1385/JMN:15:1:45. [DOI] [PubMed] [Google Scholar]
- 47.Campbell IL, Stalder AK, Akwa Y, Pagenstecher A, Asensio VC. Transgenic models to study the actions of cytokines in the central nervous system. Neuroimmunomodulation. 1998;5:126–135. doi: 10.1159/000026329. [DOI] [PubMed] [Google Scholar]
- 48.Akwa Y, Hassett DE, Eloranta ML, et al. Transgenic expression of IFN-alpha in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol. 1998;161:5016–5026. [PubMed] [Google Scholar]
- 49.Challoner PB, Smith KT, Parker JD, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman JE, Lyons MJ, Cu G, et al. The association of the human herpesvirus-6 and MS. Mult Scler. 1999;5:355–362. doi: 10.1177/135245859900500509. [DOI] [PubMed] [Google Scholar]
- 51.Boman J, Roblin PM, Sundstrom P, Sandstrom M, Hammerschlag MR. Failure to detect Chlamydia pneumoniae in the central nervous system of patients with MS. Neurology. 2000;54:265. doi: 10.1212/wnl.54.1.265. [DOI] [PubMed] [Google Scholar]
- 52.Martin C, Enbom M, Soderstrom M, et al. Absence of seven human herpesviruses, including HHV-6, by polymerase chain reaction in CSF and blood from patients with multiple sclerosis and optic neuritis. Acta Neurol Scand. 1997;95:280–283. doi: 10.1111/j.1600-0404.1997.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 53.Mirandola P, Stefan A, Brambilla E, Campadelli-Fiume G, Grimaldi LM. Absence of human herpesvirus 6 and 7 from spinal fluid and serum of multiple sclerosis patients. Neurology. 1999;53:1367–1368. doi: 10.1212/wnl.53.6.1367-a. [DOI] [PubMed] [Google Scholar]
- 54.Pavan-Langston D. Ocular viral infections. Med Clin North Am. 1983;67:973–990. doi: 10.1016/s0025-7125(16)31162-2. [DOI] [PubMed] [Google Scholar]
- 55.Corey L. The current trend in genital herpes: progress in prevention. Sex Transm Dis. 1994;21:S38–S44. [PubMed] [Google Scholar]
- 56.Koelle DM, Ghiasi H. Prospects for developing an effective vaccine against ocular herpes simplex virus infection. Cur Eye Res. 2005;30:929–942. doi: 10.1080/02713680500313153. [DOI] [PubMed] [Google Scholar]
- 57.Bureau JF, Drescher KM, Pease LR, et al. Chromosome 14 contains determinants that regulate susceptibility to Theiler's virus-induced demyelination in the mouse. Genetics. 1998;148:1941–1949. doi: 10.1093/genetics/148.4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cua DJ, Groux H, Hinton DR, Stohlman SA, Coffman RL. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J Exp Med. 1999;189:1005–1010. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]