Abstract
Background:
Human milk immunoglobulins (Ig) are an important support for the naïve infant immune system, yet the extent to which these proteins survive within the infant digestive tract, particularly for preterm infants, is poorly studied.
Objectives:
Our objective was to evaluate the survival of human milk Igs in the preterm stomach across postprandial time.
Methods:
Human milk and infant gastric samples were collected from 11 preterm (23–32 wk gestational age) mother-infant pairs within 7–98 days postnatal age. Preterm gastric samples were collected 1, 2 and 3 h after the beginning of the feeding. Samples were analyzed for concentration of total IgA (secretory IgA (SIgA)/IgA), total secretory component (SC/SIgA/SIgM), total IgM (SIgM/IgM) and IgG via ELISA. Ig-chain fragment peptides were determined using peptidomic analysis. One-way ANOVA with repeated measures followed by Tukey’s multiple comparison tests were applied.
Results:
Concentrations of total IgA were lower in the gastric contents at 3 h postprandial compared with human milk and gastric contents at 1 and 2 h. Human milk SC/SIgA/SIgM, IgG and total IgM concentrations remained stable in the preterm stomach across postprandial time. Peptide counts from the Ig alpha-chain and the Ig gamma-chain increased in gastric contents from 1 to 2 h postprandial. Peptide counts from the human milk Ig mu-chain, Ig J-chain and SC were stable across postprandial time. These peptides from Ig-chains were not present in human milk but were released in the stomach due to their partial degradation.
Conclusions:
Human milk total SC (SIgA/SC/SIgM), total IgM and IgG survived mostly intact through the preterm infant stomach, while total IgA was partially digested.
Keywords: Digestion, Gastric contents, Premature infants, Breast milk, Peptidomic antibodies, Polymeric immunoglobulin receptor, Passive immunity
Introduction
Breastfeeding protects against bacterial and viral infection and promotes development of the newborn infant’s immature immune system [1]. This protection derives, in part, from the transfer of immune components from mother to the newborn [2]. Human milk contains many immunoglobulins (Ig), including IgA, secretory IgA (SIgA), IgM, secretory IgM (SIgM) and IgG [3,4]. In human milk, IgA is the predominant Ig, followed by IgG and IgM [5]. Neonatal Fc receptors (FcRn), which can transport IgG, were identified in normal human mammary epithelial cells (MEC) [6]. Thus, IgG from maternal blood likely binds to FcRn on the basolateral membrane of MEC and is transported via vesicles to the alveolar lumen, similar to the transport mechanism found in the placenta (fig. 2) [7]. IgM and IgA (both joined by J-chain polypeptides, fig. 1), on the other hand, are secreted by plasma cells in the interstitial fluid of the mammary gland tissues [8] and bound to polymeric immunoglobulin receptor (PIgR) on the basolateral membrane of the MEC, which mediates their transport across the MEC into the lumen [9] (fig. 2). To cross the apical membrane to enter the lumen, PIgR is cleaved, releasing the secretory component (SC) bound to the Ig (called SIgA or SIgM) [10]. Of note, the paracellular transfer of Ig from blood or interstitial fluid into milk is unlikely because the paracellular pathway is closed in lactation [11]. Plasma cells with the potential to secrete IgG, IgA and IgM have been identified in human milk, likely migrating in from the mammary tissue [12]. Additional IgA and IgM in milk could, thus, derive from secretion by plasma cells in the milk, which would not require binding to PIgR and, thus, would not have a SC (fig. 2).
Fig. 2.
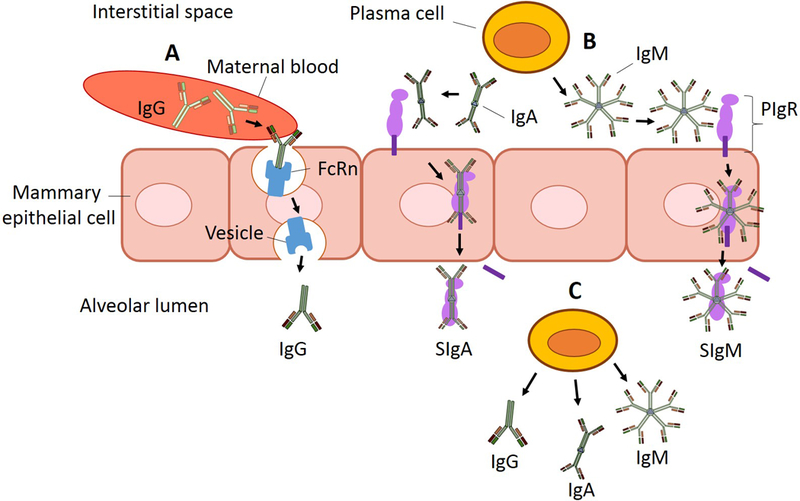
Schematization of the origin of IgG, secretory IgA (SIgA), IgA, secretory IgM (SIgM), and IgM in human milk. (A) Monomeric IgG is likely transported from maternal blood after binding to neonatal Fc receptors (FcRn) on the basolateral membrane of mammary epithelial cells and transported via vesicles to the alveolar lumen. (B) Plasma cells produce dimeric IgA and pentamer IgM in the interstitial spaces of the mammary gland. IgA and IgM bind to the secretory component (SC) of polymeric immunoglobulin receptor (PIgR) on the basolateral membrane of the mammary epithelial cell and the IgA-PIgR complex and IgM-PIgR complex travel to the apical membrane. PIgR is cleaved by a protease (unknown), releasing SC, which covalently binds to IgA or IgM, creating the complexes secretory IgA (SIgA) and secretory IgM (SIgM), which are secreted across the apical membrane. (C) Milk plasma cells can produce monomeric IgG, dimeric IgA and polymeric IgM in human milk inside the alveolar lumen and contribute to production of IgG, as well as IgA and IgM (without the SC).
Fig. 1.
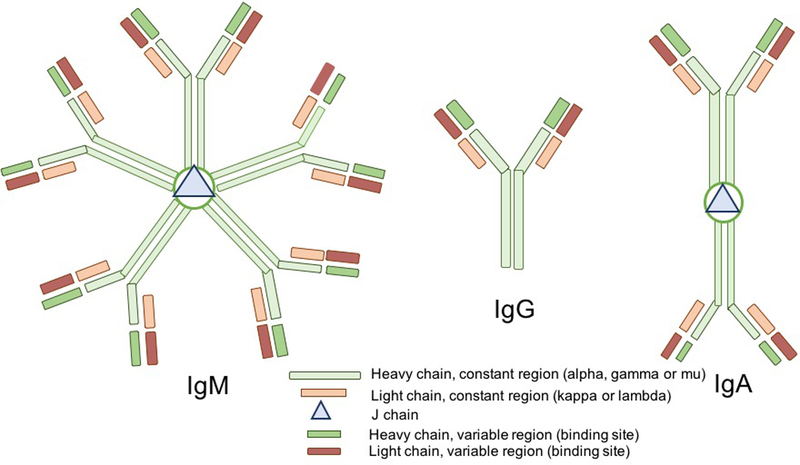
Form of immunoglobulins (Ig) secreted into human milk by plasma cells. IgA is mainly secreted as a dimer IgA joined by the J-chain polypeptide and contains alpha heavy constant chains; IgG is secreted as a monomer (without a J-chain) and possesses gamma heavy constant chains; IgM is secreted as a pentamer joined by the J-chain and contains mu heavy constant chains. All Igs contain at least two light constant chains (kappa-chain or lambda-chain), two light variable region chains and two heavy variable region chains, which form the specific antigen binding site.
Few studies have investigated the resistance of Igs to digestion. Previous studies have demonstrated that the binding of SC to IgA (creating the SIgA complex) increases the IgA subunit’s resistance to proteolysis compared with IgA alone [13]. Extracted human colostrum SIgA was more resistant to in vitro digestion (by either trypsin, chymotrypsin or human duodenal fluid) than colostrum IgA and serum IgG [14]. However, two oral supplementation studies (in adults fed bovine colostrum SIgA/IgA, IgM and IgG [15] and in preterm infants fed serum IgA and IgG [16]) demonstrated that IgG and IgM survive intact to the stool, whereas SIgA/IgA does not. Thus, the relative stability of human milk SIgA compared with total IgM or IgG during digestion remains unclear due to these conflicting results. The resistance of milk Igs to gastric digestion in preterm infants remains unknown. The aim of the present study was to determine the survival of human milk total IgA (SIgA/IgA), total SC (SC/SIgA/SIgM), total IgM (SIgM/IgM) and IgG concentrations in the preterm infant stomach across postprandial time.
Methods
Study Design and Sample Collection
This study was approved by the Institutional Review Boards of the University of California, Davis and Oregon State University. Inclusion criteria were preterm infants (born <37 completed weeks of gestation) with an admission to the neonatal intensive care unit, an indwelling nasogastric or orogastric feeding tube and tolerance of full enteral feeding. Exclusion criteria were anatomic or functional gastrointestinal disorders or gastrointestinal infections. Samples were collected from 11 mother-infant pairs following preterm birth ranging in gestational age (GA) at birth from 23–32 weeks during a range of postnatal age of 7–98 days at the University of California, Davis Children’s Hospital neonatal intensive care unit in Sacramento, California. Human milk samples were collected as described in our previous study [17]. The preterm infants were fed their mother’s milk (raw, not pasteurized) with fortification (Similac Human Milk Fortifier Powder, Abbott Park, IL, USA). The powdered fortifier contained intact bovine milk proteins. The protein concentration was 25 ± 2 mg/mL in the fortified milk samples. The human milk feedings were delivered via the nasogastric tubes over 30 min. From the same feed, a volume (0.5–2 mL) of each preterm infant’s gastric contents was collected at 1, 2 and 3 h after the initiation of feeding in a 3-mL syringe back through the feeding tube via suction. Gastric samples were placed into sterile vials and stored at –20 °C as described previously [18,19]. Sampling for each infant was successful at all time points. Human milk and gastric samples were transported to Oregon State University on dry ice and stored at –80 °C.
General Sample Preparation
Samples were thawed at 4 °C, pH was determined and samples were centrifuged at 4,226 x g for 10 min at 4 °C. The infranate was collected, separated into aliquots and stored at –80 °C.
ELISAs
The spectrophotometric ELISAs were recorded with a microplate reader (Spectramax M2, Molecular Devices, Sunnyvale, CA, USA) with two replicates of blanks, standards and samples. All ELISAs were performed according to the methods described by the manufacturers (supplementary table 1). The specific Ig concentrations in the samples were determined with antibody specificities as follows: human anti-alpha-chain antibody for total IgA (SIgA/IgA), anti-SC antibody for total SC (SC/SIgA/SIgM), gamma-chain antibody for IgG; and mu-chain antibody for total IgM (SIgM/IgM). Concentrations of total IgA, total SC, total IgM and IgG were determined in human milk, gastric samples and the fortifier alone.
Peptidomic Analysis
Peptides were extracted from human milk and gastric samples as described previously [20]. Mass spectrometric parameters were as described previously [21]. Spectra were analyzed by database searching in Thermo Proteome Discoverer (v2.1.0.81) using an in-house human milk protein sequence database. The tandem spectra were used to determine the counts and abundance of total Ig alpha-chain (from IgA or SIgA), total Ig gamma-chain (from IgG), Ig mu-chain (from IgM or SIgM), Ig J-chain (from IgA, SIgA, IgM or SIgM), Ig kappa-chain and total Ig lambda-chain (from IgA, SIgA, IgM, SIgM or IgG), SC (f19–603 of PIgR), total PIgR (f19–764) and FcRn. Peptide sequences with multiple post-translational modifications were grouped into a single peptide for counts. Peptide counts measured the number of unique peptides identified in a sample while peptide abundance measured the ion abundance (relative amount).
Statistical Methods
One-way ANOVA with repeated measures followed by Tukey’s multiple comparisons test (GraphPad Prism software, version 7.03) were applied to compare human milk and gastric samples at the three times postprandial. Linear regression models were applied to determine if the concentration of Igs in samples changed across postnatal age, GA, postmenstrual age (PMA), body weight at birth (BWb), body weight at sampling (BWs) or feed volume. Student t tests were used to compare the immunoglobulin concentrations between infant delivered by caesarian section (n = 9) and those delivered vaginally (n = 5). Differences were designated significant at p < 0.05. The sample size of preterm (n = 11) paired milk and gastric samples was selected based on our previous studies [18,19] and proved to be adequately powered to detect differences based on the results.
Results
Infant Demographics and Clinical Conditions
Demographic details for the mother-infant pairs are presented in table 1. Ig concentrations did not differ between infants delivered by caesarian section and those delivered vaginally in their mother’s milk or their gastric contents at 1, 2 or 3 h postprandial.
Table 1.
Demographics of preterm-delivering mother-infant pairs sampled for milk and gastric contentsa
| Preterm-delivering mother-infant pairs | |
|---|---|
| GA, weeks | 26 ± 3 (23–32) |
| Postnatal age, days | 48 ± 27 (7–98) |
| Postmenstrual age, weeks | 33 ± 2 (28–37) |
| Body weight at birth, kg | 0.9 ± 0.4 (0.5–1.6) |
| Body weight at sampling, kg | 1.7 ± 0.4 (0.9–1.7) |
| Feed volume, mL | 14 ± 11 (4 ± 40) |
| Meal frequency | 8 times per day (3 hours intervals) |
| Infant sex | 10 females, 1 male |
| Infant delivery mode | 5 vaginal, 6 caesarian |
| Mother’s age, years | 35 ± 3 (32–39) |
All values are mean ± SD (range), n = 11 for number of paired milk and gastric contents after 1, 2 and 3 h postprandial from preterm infants. GA, gestational age.
Total IgA Concentration
Total IgA (SIgA/IgA) concentration decreased 76% from human milk to the gastric contents at 3 h postprandial in preterm infants (p = 0.037, fig. 3A) but did not significantly decrease in the gastric contents at 1 and 2 h. Total gastric IgA concentration 3 h postprandial was 62% and 46% lower than that at 1 (p = 0.010) and 2 h (p = 0.037), respectively. Total IgA concentration in milk and gastric contents at 1 h decreased with increasing PMA and BWs (p < 0.05) but did not change at 2 or 3 h postprandial. Total IgA concentration in milk and gastric contents at 1 and 2 h decreased with increasing postnatal age (p < 0.05) but was not changed at 3 h postprandial. Total IgA concentration did not change across GA, BWb or feed volume in the gastric contents at 1, 2 or 3 h postprandial.
Fig. 3.
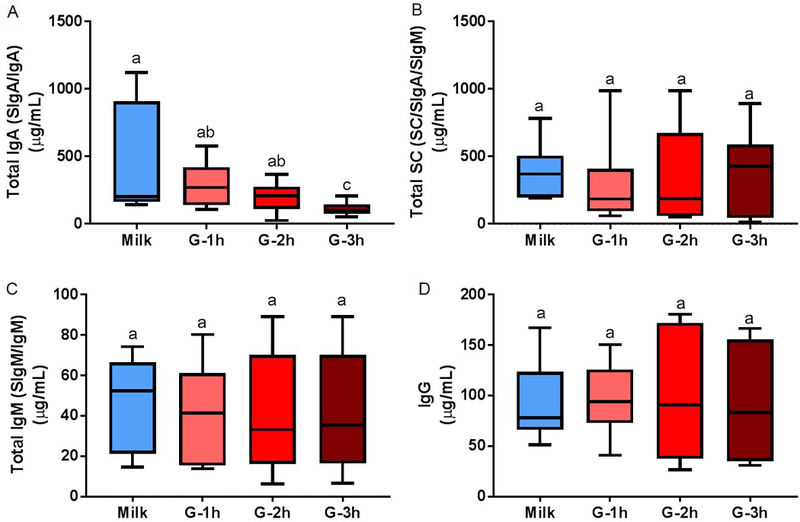
Concentration of immunoglobulin (Ig) in human milk and preterm infant gastric samples at 1 (G-1h), 2 (G-2h) and 3 h (G-3h) after the beginning of feeding. Concentrations of (A) total IgA (secretory IgA (SIgA)/IgA), (B) total secretory component (SC/SIgA/SIgM), (D) total IgM (secretory IgM (SIgM)/IgM) and IgG were determined by ELISA. Paired milk and gastric samples were collected in preterm infants (23–32 wk of gestational age (GA) and 7–98 days of postnatal age). Letters a, b and c show statistically significant differences between groups (p < 0.05) using one-way ANOVA with repeated measures followed by Tukey’s multiple comparison tests. Values are min, median and max, n = 11.
Total SC Concentration
Human milk total SC (SC/SIgA/SIgM) concentration was stable in the gastric contents at 1, 2 and 3 h post-feeding (fig. 3B). Gastric samples did not differ in total SC concentration from 1 to 3 h. Total SC concentration did not change across GA, PMA, postnatal age, BWb, BWs or feed volume in the gastric contents at 1, 2 or 3 h postprandial.
Total IgM Concentration
Total IgM (SIgM/IgM) concentration did not decrease from human milk to the gastric contents at 1 to 3 h postprandial (fig. 3C). Gastric samples did not differ in total IgM concentration from 1 to 3 h. Total IgM concentration in the gastric contents at 1 h decreased with increasing PMA and BWs (p < 0.05) but did not change at 2 or 3 h postprandial or in milk. Total IgM concentration did not change with GA, postnatal age, BWb or feed volume in human milk or gastric contents at 1, 2, or 3 h.
Total IgM Concentration
Total IgM (SIgM/IgM) concentration did not decrease from human milk to the gastric contents at 1 to 3 h postprandial (fig. 3C). Gastric samples did not differ in total IgM concentration from 1 to 3 h. Total IgM concentration in the gastric contents at 1 h decreased with increasing PMA and BWs (p < 0.05) but did not change at 2 or 3 h postprandial or in milk. Total IgM concentration did not change with GA, postnatal age, BWb or feed volume in human milk or gastric contents at 1, 2 or 3 h.
IgG Concentration
IgG concentration did not decrease from human milk to the gastric contents at 1 to 3 h post-feeding (fig. 3D). Gastric samples did not differ in IgG concentration from 1 to 3 h. IgG concentration in the gastric contents at 1 and 3 h decreased with increasing PMA and BWs (p < 0.05) but did not differ across these parameters for the 2 h postprandial or milk samples. IgG concentration did not change with GA, postnatal age, BWb or feed volume in human milk or gastric contents at 1, 2 or 3 h.
Human Ig in Fortifier
No IgA, IgM, IgG or SC were detected in the fortifier alone.
Peptidomic Results
No peptides were detected for Ig alpha-chain, Ig gamma-chain, Ig mu-chain or Ig J-chain in human milk but were detected in the gastric contents. Peptides derived from Ig lambda-chain, Ig kappa-chain, total PIgR (f19–764) and SC (f19–603 of PIgR) were detected in milk and gastric samples. Peptide counts of Ig alpha-chain increased 2.2- and 1.7-fold from gastric contents at 1 h postprandial to the gastric contents at 2 and 3 h, respectively (p < 0.05, fig. 4A) but were constant from 2 to 3 h. Peptide abundance of Ig alpha-chain did not differ in the gastric contents across time. Ig gamma-chain counts increased 1.2- and 1.4-fold from gastric contents at 1 h postprandial to the gastric contents at 2 and 3 h, respectively (fig. 4B) but were constant from 2 to 3 h. Peptide counts and abundance for Ig mu-chain and Ig J-chain did not change from human milk to the gastric contents at 1, 2 or 3 h postprandial (fig. 4C and 4D). Peptide counts of Ig lambda increased from human milk to the gastric contents at 2 and 3 h, respectively (p < 0.05, fig. 5A). Peptide counts of Ig kappa-chain increased from human milk or gastric contents at 1 h, respectively, to 3 h (fig. 5B). Total PIgR (f19–764) counts decreased from milk to the gastric contents, but the gastric contents did not change across time (fig. 5C). Peptide counts of SC (f19–603 of PIgR) were stable from human milk to the gastric contents as well as at different times postprandial in the stomach (fig. 5D). Peptide abundance of Ig lambda-chain, Ig kappa-chain, PIgR and SC did not differ in human milk or gastric contents across time postprandial. FcRn was not detected in either milk or gastric contents.
Fig. 4.
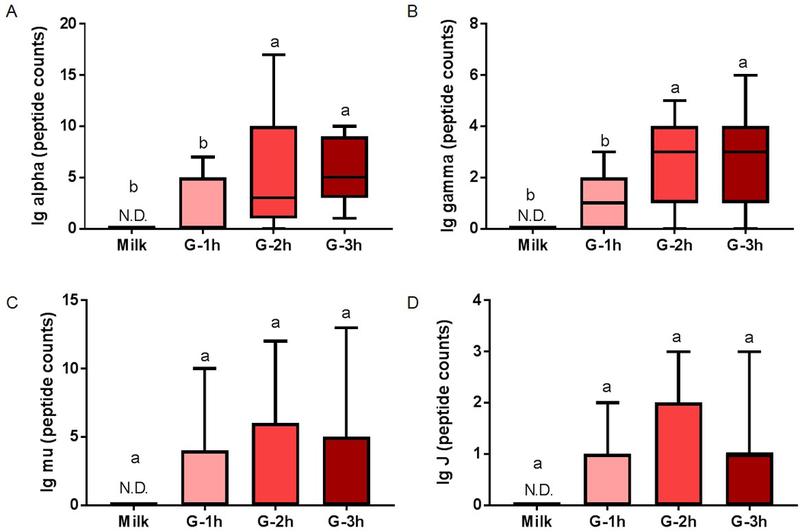
Peptide counts of immunoglobulin (Ig) fragments in human milk and preterm infant gastric samples at 1 (G-1h), 2 (G-2h) and 3 h (G-3h) after the beginning of feeding. Peptide counts of (A) total Ig alpha-chain (from IgA or SIgA), (B) total Ig gamma-chain (from IgG), (C) Ig mu-chain (from IgM or SIgM) and (D) Ig J-chain (from IgA, SIgA, IgM or SIgM). Paired milk and gastric samples were collected in preterm-delivering mother-infant pairs (23–32 wk of gestational age (GA) and 7–98 days of postnatal age). Letters a, b and c show statistically significant differences between groups (p < 0.05) using one-way ANOVA with repeated measures followed by Tukey’s multiple comparison tests. Values are min, median and max, n = 11.
Fig. 5.
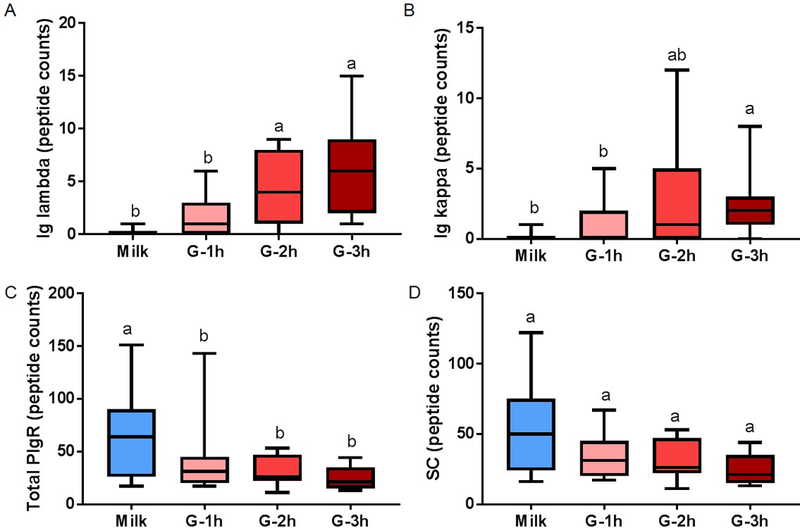
Peptide counts of immunoglobulin (Ig) fragments in human milk (blue boxplot) and preterm infant gastric samples at 1 (G-1h), 2 (G-2h) and 3 h (G-3h) after the beginning of feeding. Peptide counts of (A) Ig lambda (from IgA, SIgA, IgM, SIgM or IgG), (B) Ig kappa (from IgA, SIgA, IgM, SIgM or IgG), (C) total polymeric immunoglobulin receptor (PIgR) (f19–764) and (D) secretory component (SC, f19–603 of PIgR). Paired milk and gastric samples were collected in preterm-delivering mother-infant pairs (23–32 wk of gestational age (GA) and 7–98 days of postnatal age). Letters a, b and c show statistically significant differences between groups (p < 0.05) using one-way ANOVA with repeated measures followed by Tukey’s multiple comparison tests. Values are min, median and max, n = 11.
Individual data points for figures 3, 4 and 5 are shown in supplementary figures 1, 2 and 3.
pH
As expected, the pH values of the preterm infant gastric contents were lower than those of the paired human milk samples (p = 0.002, supplementary fig. 4). Gastric pH decreased significantly across each group from milk to 1 h to 2 h and to 3 h (p < 0.05).
Discussion
This study aimed to determine the survival of human milk Igs—total IgA (SIgA/IgA), total SC (SC/SIgA/SIgM), total IgM (SIgM/IgM) and IgG—in the preterm infant stomach across time postprandial. The range of concentrations for total IgA, total IgM and IgG in human milk from preterm-delivering mothers matched those reported in previous studies [22–26]. Total IgA concentration was 89% and 79% higher than that of total IgM and IgG, respectively (supplemental fig. 5). This proportion matches with the observation reported by Ford et al. [22] that total IgA was 80% higher than total IgM in preterm milk. Similarly, Gross et al. [23] found that total IgA concentration in preterm milk was 95% higher than IgG and total IgM. Total IgM concentration was 2-fold lower than IgG in preterm milk, which is in agreement with the observations reported by Koenig et al. [5] that total IgM was 3.5-fold lower than IgG in preterm milk. On the other hand, Chandra et al. [4] found that total IgM concentration was 1.5-fold higher than IgG in preterm milk. We cannot account for the difference between our results and those of Chandra et al. [4]. Most of the previous studies determined only the total IgA concentration without evaluating the contribution of SIgA alone [24–26]. The concentration of total SC represented 82% of total IgA in human milk, which approximated the observations reported by Goldman et al. [3] that total SC (called “SIgA” by the authors but actually represents total SC, as an anti-SC was used as primary antibody) concentration represented 90% of the total IgA in human milk. As IgA-producing plasma cells are present in human milk [12], we hypothesize that the small proportion of IgA in human milk represents IgA produced by plasma cells inside the alveolar lumen rather than IgA absorbed across the MEC and secreted as SIgA. Tuaillon et al. [12] suggested that, as these plasma cells are at low concentrations in human milk, they likely contribute only a small portion of the Igs present in human milk.
FcRn, which can transport IgG, were identified in normal human MEC [6]. IgG from maternal blood likely binds to FcRn to be transported the membrane of MEC to the alveolar lumen, matching the mechanism reported in the placenta [7]. The mammary gland of the preterm-delivering mother is immaturely developed compared with term-delivering mothers (demonstrated by gene expression in mammary epithelial cells) [27]. Whether this lack of maturation affects milk IgG delivery remains unknown. Two previous studies found similar IgG concentrations between mothers delivering prematurely and at term [5,23], suggesting that the transport of IgG was not affected by birth gestational age.
For the first time, we demonstrated that concentrations of human milk total SC, total IgM and IgG were not significantly decreased in preterm infants across time postprandial and peptides from these Igs (Ig mu-chain, Ig J-chain and SC (f19–603 of PIgR)) did not increase across time postprandial consistent with lack of gastric digestion. The stability of Ig J-chain and SC peptides across time postprandial matched the stable concentration of total SC (SC/SIgA/SIgM) during preterm infant digestion. However, total IgA was partially digested in the preterm infant’s stomach as demonstrated by decreased concentrations at 3 h and increased peptide counts from Ig alpha-chain, Ig gamma-chain, Ig lambda-chain and Ig kappa-chain from milk to gastric contents. This finding is consistent with a previous report of increased Ig alpha-chain (named IGHA1 or Ig alpha1-chain) from human milk (undetectable) to the term infant stomach [28]. The stability of SC/SIgA/SIgM during gastric digestion suggests that SC remains intact and detectable by the anti-SC (regardless of whether it is released from the SIgA complex).
The reduction of total IgA (SIgA/IgA) was likely due to degradation by proteases (pepsin and/or milk proteases) and not acid-induced structural deterioration in the stomach, as incubation of standard IgA in acid conditions did not decrease its concentration (supplemental fig. 6). Previously, SIgA was found to be more resistant than IgA to the in vitro action of pepsin, trypsin and chymotrypsin–due to the presence of the SC, which protects IgA from digestion [13]. Unlike total IgA, total IgM (thought to be mostly SIgM in human milk) and IgG were not degraded in the preterm infant stomach in the present study. Our findings that IgG and total IgM were more resistant to digestion than total IgA agree with those of several previous studies of orally supplemented IgA. For example, a study of preterm infants fed only infant formula or infant formula plus pasteurized pooled human milk supplemented with 600 mg daily of serum-derived human IgA (73%) and IgG (26%) showed that collected stool samples contained 1–10 mg IgG per g of dried feces and no IgA [16]. Moreover, a study [15] of human adults fed a bovine colostrum Ig concentrate (84% IgG, 14% total IgM and 2% total IgA) found that 19% of IgG and total IgM were recovered in the human adult ileum, whereas total IgA (mainly SIgA) was not detected. These studies provided a more complete picture of digestion, by examining stool, which unlike our study, included the effects of intestinal proteases. Yet, they suggested, as did the present study, that IgG and IgM are more resistant to digestion than IgA, even with the attached SC.
Though SIgA/IgA appears to be more digested than the other Igs in the stomach in the present study, some SIgA/IgA has been shown to survive infant digestion. SIgA/IgA was found in higher amounts in the feces and urine of preterm infants fed human milk than those fed a cow milk formula, suggesting that human milk-derived SIgA/IgA could survive intact to the stool and the urine [29,30]. Neither of these studies compared the survival of IgA with IgG and IgM.
A limitation of this study is that we were unable to compare male and female infant digestion, as we had only one male in the study. Future research could examine this question.
This study revealed for the first time that human milk total SC (SC/SIgA/SIgM), total IgM and IgG concentration remained mostly constant through the preterm infant stomach, whereas total IgA concentration decreased, indicating that it was partially digested. Future studies should examine the extent to which milk Igs are digested in the preterm infant intestine, perhaps through studies of feces or ostomy output. The longer milk Igs remain intact within the infant gastrointestinal tract, the longer they can provide passive immune protection to the infant while the overall immune system is immature.
Supplementary Material
Acknowledgements and Statement of Authors’ Contributions to Manuscript
The authors acknowledge the Mass Spectrometry Center at Oregon State University, which is supported in part by the National Institute of Health grant S10OD020111 (CSM). V.D.M. performed the ELISA analyses and conducted the statistical analyses. R.L.B. performed the peptidomic analysis. M.A.U. provided the milk and gastric samples. V.D.M. and D.C.D. designed the study and drafted the manuscript. V.D.M. and D.C.D. had primary responsibility for the final content. This study was supported by the K99/R00 Pathway to Independence Career Award, Eunice Kennedy Shriver Institute of Child Health & Development of the National Institutes of Health (R00HD079561) (D.C.D).
Footnotes
Disclosure Statement
There are no financial relationships or conflicts of interest to disclose.
References
- 1.Oddy WH: Breastfeeding protects against illness and infection in infants and children: a review of the evidence. Breastfeed Rev 2001;9:11. [PubMed] [Google Scholar]
- 2.Van de Perre P: Transfer of antibody via mother’s milk. Vaccine 2003;21:3374–3376. [DOI] [PubMed] [Google Scholar]
- 3.Goldman AS, Garza C, Nichols BL, Goldblum RM: Immunologic factors in human milk during the first year of lactation. J Pediatr 1982;100:563–567. [DOI] [PubMed] [Google Scholar]
- 4.Chandra R: Immunoglobulin and protein levels in breast milk produced by mothers of preterm infants. Nutr Res 1982;2:27–30. [Google Scholar]
- 5.Koenig Á, Diniz EMdA, Barbosa SFC, Vaz FAC: Immunologic factors in human milk: the effects of gestational age and pasteurization. J Hum Lact 2005;21:439–443. [DOI] [PubMed] [Google Scholar]
- 6.Cianga P, Cianga C, Cozma L, Ward ES, Carasevici E: The MHC class I related Fc receptor, FcRn, is expressed in the epithelial cells of the human mammary gland. Hum Immunol 2003;64:1152–1159. [DOI] [PubMed] [Google Scholar]
- 7.Kuo TT, Baker K, Yoshida M, Qiao S- W, Aveson VG, Lencer WI, Blumberg RS: Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol 2010;30:777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson D: Immunological functions of the mammary gland and its secretion-comparative review. Aust J Biol Sci 1980;33:403–422. [DOI] [PubMed] [Google Scholar]
- 9.Bourges D, Meurens F, Berri M, Chevaleyre C, Zanello G, Levast B, Melo S, Gerdts V, Salmon H: New insights into the dual recruitment of IgA+ B cells in the developing mammary gland. Microbiol Immunol 2008;45:3354–3362. [DOI] [PubMed] [Google Scholar]
- 10.Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman J-P, Lamm ME: The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci 1991;88:8796–8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silanikove N: Transcellular route as the most probable explanation for the presence of plasminogen in mammal׳ s milk. J Theor Biol 2016;395:221–226. [DOI] [PubMed] [Google Scholar]
- 12.Tuaillon E, Valea D, Becquart P, Al Tabaa Y, Meda N, Bollore K, Van de Perre P, Vendrell J-P: Human milk-derived B cells: a highly activated switched memory cell population primed to secrete antibodies. J Immunol 2009;182:7155–7162. [DOI] [PubMed] [Google Scholar]
- 13.Lindh E: Increased resistance of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J Immunol 1975;114:284–286. [PubMed] [Google Scholar]
- 14.Brown WR, Newcomb RW, Ishizaka K: Proteolytic degradation of exocrine and serum immunoglobulins. J Clin Invest 1970;49:1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roos N, Mahe S, Benamouzig R, Sick H, Rautureau J, Tome D: 15N-labeled immunoglobulins from bovine colostrum are partially resistant to digestion in human intestine. J Nutr 1995;125:1238–1244. [DOI] [PubMed] [Google Scholar]
- 16.Eibl MM, Wolf HM, Fürnkranz H, Rosenkranz A: Prevention of necrotizing enterocolitis in low-birth-weight infants by IgA–IgG feeding. N Engl J Med 1988;319:1–7. [DOI] [PubMed] [Google Scholar]
- 17.Demers-Mathieu V, Nielsen SD, Underwood MA, Borghese R, Dallas DC: Analysis of milk from mothers who delivered prematurely reveals few changes in proteases and protease inhibitors across gestational age at birth and infant postnatal age. J Nutr 2017;147:1152–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demers-Mathieu V, Nielsen SD, Underwood MA, Borghese R, Dallas DC: Changes in proteases, antiproteases and bioactive proteins from mother’s breast milk to the premature infant stomach. J Pediatr Gastroenterol Nutr 2017 [DOI] [PMC free article] [PubMed]
- 19.Demers-Mathieu V, Qu Y, Underwood MA, Borghese R, Dallas DC: Premature infants have lower gastric digestion capacity for human milk proteins than term infants. J Pediatr Gastroenterol Nutr 2017 [DOI] [PMC free article] [PubMed]
- 20.Dallas DC, Smink CJ, Robinson RC, Tian T, Guerrero A, Parker EA, Smilowitz JT, Hettinga KA, Underwood MA, Lebrilla CB: Endogenous human milk peptide release is greater after preterm birth than term birth. J Nutr 2014;145:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dallas DC, Citerne F, Tian T, Silva VL, Kalanetra KM, Frese SA, Robinson RC, Mills DA, Barile D: Peptidomic analysis reveals proteolytic activity of kefir microorganisms on bovine milk proteins. Food Chem 2016;197:273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford J, Law B, Marshall VM, Reiter B: Influence of the heat treatment of human milk on some of its protective constituents. J Pediatr 1977;90:29–35. [DOI] [PubMed] [Google Scholar]
- 23.Gross SJ, Buckley RH, Wakil SS, McAllister DC, David RJ, Faix RG: Elevated IgA concentration in milk produced by mothers delivered of preterm infants. J Pediatr 1981;99:389–393. [DOI] [PubMed] [Google Scholar]
- 24.Ballabio C, Bertino E, Coscia A, Fabris C, Fuggetta D, Molfino S, Testa T, Sgarrella M, Sabatino G, Restani P: Immunoglobulin-A profile in breast milk from mothers delivering full term and preterm infants. Int J Immunopathol Pharmacol 2007;20:119–128. [DOI] [PubMed] [Google Scholar]
- 25.Hennart PF, Brasseur DJ, Delogne-Desnoeck JB, Dramaix MM, Robyn CE: Lysozyme, lactoferrin, and secretory immunoglobulin A content in breast milk: influence of duration of lactation, nutrition status, prolactin status, and parity of mother. Am Soc Nutrition 1991;53:32–39. [DOI] [PubMed] [Google Scholar]
- 26.Araújo ED, Gonçalves AK, Cornetta MdC, Cunha H, Cardoso ML, Morais SS, Giraldo PC: Evaluation of the secretory immunoglobulin A levels in the colostrum and milk of mothers of term and pre-trerm newborns. Braz J Infect Dis 2005;9:357–362. [PubMed] [Google Scholar]
- 27.Twigger A-J, Hepworth AR, Lai CT, Chetwynd E, Stuebe AM, Blancafort P, Hartmann PE, Geddes DT, Kakulas F: Gene expression in breastmilk cells is associated with maternal and infant characteristics. Sci Rep 2015;5:12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dallas DC, Guerrero A, Khaldi N, Borghese R, Bhandari A, Underwood MA, Lebrilla CB, German JB, Barile D: A peptidomic analysis of human milk digestion in the infant stomach reveals protein-specific degradation patterns. J Nutr 2014;144:815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schanler RJ, Goldblum RM, Garza C, Goldman AS: Enhanced fecal excretion of selected immune factors in very low birth weight infants fed fortified human milk. Pediatr Res 1986;20:711. [DOI] [PubMed] [Google Scholar]
- 30.Goldblum RM, Schanler RJ, Garza C, Goldman AS: Human milk feeding enhances the urinary excretion of immunologic factors in low birth weight infants. Pediatr Res 1989;25:184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


