Abstract
IMPORTANCE
Out-of-hospital cardiac arrest (OHCA) occurs in more than 6000 children each year in the United States, with survival rates of less than 10% and severe neurologic morbidity in many survivors. Post–cardiac arrest hypotension can occur, but its frequency and association with survival have not been well described during targeted temperature management.
OBJECTIVE
To determine whether hypotension is associated with survival to discharge in children and adolescents after resuscitation from OHCA.
DESIGN, SETTING, AND PARTICIPANTS
This post hoc secondary analysis of the Therapeutic Hypothermia After Pediatric Cardiac Arrest (THAPCA) trial included 292 pediatric patients older than 48 hours and younger than 18 years treated in 36 pediatric intensive care units from September 1, 2009, through December 31, 2012. Participants underwent therapeutic hypothermia (33.0°C) vs therapeutic normothermia (36.8°C) for 48 hours. All participants had hourly systolic blood pressure measurements documented during the initial 6 hours of temperature intervention. Hourly blood pressures beginning at the time of temperature intervention (time 0) were normalized for age, sex, and height. Early hypotension was defined as a systolic blood pressure less than the fifth percentile during the first 6 hours after temperature intervention. With use of forward stepwise logistic regression, covariates of interest (age, sex, initial cardiac rhythm, any preexisting condition, estimated duration of cardiopulmonary resuscitation [CPR], primary cause of cardiac arrest, temperature intervention group, night or weekend cardiac arrest, witnessed status, and bystander CPR) were evaluated in the final model. Data were analyzed from February 5, 2016, through June 13, 2017.
EXPOSURES
Hypotension.
MAIN OUTCOMES AND MEASURE
Survival to hospital discharge.
RESULTS
Of 292 children (194 boys [66.4%] and 98 girls [33.6%]; median age, 23.0 months [interquartile range, 5.0–105.0 months]), 78 (26.7%) had at least 1 episode of early hypotension. No difference was observed between the therapeutic hypothermia and therapeutic normothermia groups in the prevalence of hypotension during induction and maintenance (73 of 153 [47.7%] vs 72 of 139 [51.8%]; P = .50) or rewarming (35 of 118 [29.7%] vs 19 of 95 [20.0%]; P = .10) during the first 72 hours. Participants who had early hypotension were less likely to survive to hospital discharge (20 of 78 [25.6%] vs 93 of 214 [43.5%]; adjusted odds ratio, 0.39; 95% CI, 0.20–0.74).
CONCLUSIONS AND RELEVANCE
In this post hoc secondary analysis of the THAPCA trial, 26.7% of participants had hypotension within 6 hours after temperature intervention. Early post–cardiac arrest hypotension was associated with lower odds of discharge survival, even after adjusting for covariates of interest.
Out-of-hospital cardiac arrest (OHCA) occurs in more than 6000 children each year in the United States, with survival rates of less than 10%.1,2 After initial resuscitation from OHCA and admission to a pediatric intensive care unit, many children die in the post–cardiac arrest period before hospital discharge.3 Many survivors of pediatric OHCA sustain short- and long-term neurologic morbidities.3–5
The post–cardiac arrest syndrome is characterized by myocardial dysfunction, a systemic ischemia-reperfusion response, brain injury, and multiorgan dysfunction.6–8 A study of children resuscitated from in-hospital cardiac arrest (IHCA) and OHCA showed that early postresuscitation hypotension (within 6 hours after the return of spontaneous circulation [ROSC]) is common and associated with increased odds of in-hospital mortality and unfavorable neurologic outcome.9 Similarly, adult data suggest that early hypotension after cardiac arrest is associated with higher mortality rates.10–12 In a secondary analysis of a large randomized clinical trial of targeted temperature management (TTM) for adult OHCA, lower mean arterial pressure and use of higher doses of vasopressors were associated with increased mortality.13
The Therapeutic Hypothermia After Pediatric Cardiac Arrest (THAPCA) trial, a recent large randomized clinical trial comparing therapeutic hypothermia (33°C) with therapeutic normothermia (36.8°C) for 48 hours after pediatric OHCA, did not show a difference in 1-year neurobehavioral outcomes or mortality.4 In this post hoc secondary analysis, we aimed to (1) evaluate the association of hypotension within 6 hours (early) and within 72 hours after study intervention with survival to hospital discharge and (2) describe the association of temperature intervention with blood pressure.
Methods
This study is a post hoc secondary analysis of data from the THAPCA trial.4 The trial design, protocol, and results have been described previously.14–16 Enrollment of patients in THAPCA was approved by the institutional review boards of all 36 sites. All patients’ parents or guardians provided written informed consent. This secondary analysis was exempted by the institutional review board of the Children’s Hospital of Philadelphia.
THAPCA Study
The THAPCA trial17 was conducted from September 1, 2009, through December 31, 2012, at 36 pediatric intensive care units in the United States and Canada.4 Children and adolescents older than 48 hours and younger than 18 years who sustained an OHCA, received more than 2 minutes of chest compressions, and were admitted to an intensive care unit while intubated and receiving mechanical ventilation after ROSC were eligible for the THAPCA trial. Major exclusion criteria consisted of the inability to randomize within 6 hours after ROSC and a Glasgow Coma Scale motor response score of less than 5 (range, 1–6, with higher scores indicating a better motor response). The complete list of exclusion criteria is included in the appendix of the original article.4 Participants were randomized 1:1 to therapeutic hypothermia or therapeutic normothermia within 6 hours after ROSC by using permuted blocks stratified by age (<2, 2 to <12, or ≥12 years).
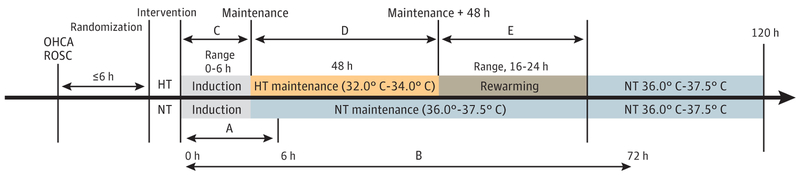
The TTM intervention was initiated as soon as possible after randomization and maintained for 120 hours in the groups undergoing therapeutic hypothermia (33.0°C; range, 32.0°C-34.0°C) and therapeutic normothermia (36.8°C; range, 36.0°C-37.5°C) (Figure 1). Participants were sedated and pharmacologically paralyzed to achieve the target temperature using external cooling blankets (Cincinnati Sub-Zero). Participants undergoing therapeutic hypothermia underwent cooling to 33.0°C, maintenance at 32.0°C to 34.0°C for 48 hours, and rewarming for approximately 16 to 24 hours to 36.8°C, which was actively maintained through hour 120. Participants undergoing therapeutic normothermia had their core temperature actively maintained at 36.8°C (range, 36.0°C-37.5°C) through 120 hours.
Figure 1. Time Frame for Interventions After Out-of-Hospital Cardiac Arrest (OHCA).
A indicates the early (6-hour) blood pressure period from study intervention initiation; B, the 72-hour period from study intervention initiation; C, the period of induction to achieve hypothermia (HT); D, the period of maintenance at goal temperature; and E, the period of rewarming from HT back to normothermia (NT). ROSC indicates return of spontaneous circulation.
Per the THAPCA protocol, blood pressure was monitored by an arterial catheter. Blood pressures were documented hourly during the induction, maintenance, and rewarming phases. No explicit hemodynamic management protocol was used; however, protocol training recommended that the clinical team maintain blood pressure in a normal range for age and diagnosis, with accounting for preload, contractility, and afterload. The primary outcome of the THAPCA trial was survival with a favorable neurologic outcome at 12 months.
Hypotension Study
All participants included in the THAPCA trial were eligible for the hypotension study. Participants were excluded if they did not have the TTM intervention initiated. Hypotension was defined as a systolic blood pressure (SBP) of less than the fifth percentile derived from normative data for age, sex, and height.18 Hourly invasive arterial blood pressure measurements were recorded during the first 72 hours of TTM intervention. Blood pressure measurements from ROSC to initiation of the TTM intervention were not available. If a measured height was not available, the 50th percentile for height and sex was used for normalization. This procedure was followed for 28 participants. Our primary exposure variable was at least 1 episode of hypotension during the first 6 hours after TTM intervention (early hypotension) or from 0 to 72 hours (hypotension in the first 72 hours). Hypotension burden was defined as the percentage of hypotensive measurements during these periods.
Time 0 was defined as the time of initiation of the TTM intervention (Figure 1). Therefore, the 0- to 6-hour blood pressure period occurred during therapeutic hypothermia induction or during therapeutic hypothermia induction and maintenance. The 0- to 72-hour period occurred during therapeutic hypothermia induction, maintenance (48 hours), rewarming (≤24 hours), and part of normothermia. For participants undergoing therapeutic normothermia, the corresponding periods from study intervention (time 0) were used. Because of the variable time to achieve maintenance temperature, we compared hypotension prevalence in the therapeutic normothermia and therapeutic hypothermia groups by the combination of the induction and maintenance phases and by the rewarming phase.
Data collected in the THAPCA study included participant demographics, cardiac arrest characteristics, and cardiac arrest care data after intervention initiation. Data from nights and weekends were previously published.19 The number of vasoactive agents were counted from the participant’s concomitant medication log and included epinephrine, phenylephrine, norepinephrine, dobutamine, and dopamine. Vasopressin, milrinone, and corticosteroids (hydrocortisone or methylprednisolone) were analyzed as yes or no. Indications for medication administration and the timing of initiation, dosing, titration, or discontinuation within a 24-hour period were not documented.
Medications administered were documented by day from randomization (day 0), which concluded at midnight of each day. Day of medication administration was aligned with blood pressure period (0–6 hours or 0–72 hours) for each participant. If the participant’s measurement period crossed 2 days, the higher number of vasoactive agents was used for analysis. Description of vasoactive medications by phase (combined induction-maintenanceandrewarming)couldnotbeperformed because of the variable time to achieve maintenance phase.
Statistical Analysis
Data were analyzed from February 5, 2016, through June 13, 2017. Frequencies and percentages or medians and interquar-tile ranges (IQRs) were used to summarize participant, cardiac arrest, and post–cardiac arrest care characteristics. Univariate associations between characteristics and the presence of hypotension were assessed using the Wilcoxon rank sum test or the χ2 test of no association. A forward stepwise multivariate logistic regression model was used to estimate the asso ciation between survival to hospital discharge and early hypotension. A 2-sided P = .05 was used as the criterion for entering or leaving the model. Other variables entered into the model as potential predictors included age, sex, initial cardiac rhythm, primary cause of cardiac arrest, temperature treatment group, night or weekend cardiac arrest, estimated duration of cardiopulmonary resuscitation (CPR), preexisting condition, witnessed status, and bystander CPR. Estimated duration of CPR was highly collinear with the number of epinephrine doses but had fewer missing data and therefore was entered into the regression model. Lactate levels were not entered into the model because of collinearity with hypotension and high levels of missingness during these periods. The primary outcome was survival to hospital discharge.
Kaplan-Meier estimates of the time to death during the first year after cardiac arrest were generated,and differences between the therapeutic hypothermia and therapeutic normothermia groups were evaluated using the log-rank test. Analyses were completed using SAS software (version 9.4; SAS Institute).
Results
Two hundred ninety-five individuals from the THAPCA trial were eligible. After applying exclusion criteria, 292 participants underwent analysis (194 boys [66.4%] and 98 girls [33.6%]; median age, 23.0 months [IQR, 5.0–105.0 months]); 3 were excluded because they never received the TTM intervention. The median time from ROSC to TTM initiation was 5.8 hours (IQR, 5.1–6.5 hours). A description of the participant, cardiac arrest, and post–cardiac arrest care characteristics by TTM intervention is found in the eTable in the Supplement. We found no significant differences in demographics or cardiac arrest characteristics between groups.
0–6 Hours After TTM Intervention
Seventy-eight of 292 participants (26.7%) had at least 1 episode of hypotension during the first 6 hours of temperature intervention. Participants who had hypotension were more likely to have a preexisting condition, a cardiac cause of arrest, or a higher lactate level and to have received more than 30 minutes of CPR and more than 4 doses of epinephrine (Table 1). We found no difference in sex, race, cardiac arrest time of week, initial rhythm, witnessed status, or time from ROSC to intervention. More participants with hypotension received at least 1 vasoactive agent, milrinone, or vasopressin, although 18 of 78 participants (23.1%) with early hypotension received no vasoactive agents. We found no difference in the prevalence of hypotension by TTM intervention.
Table 1.
Patient and Cardiac Arrest Characteristics by Hypotension Groupa
| Any Hypotension at 0–6 h | ||||
|---|---|---|---|---|
| Characteristic | Overall (N = 292) | No (n = 214) | Yes (n = 78) | P Value |
| Age at randomization, median (IQR), mo | 23.0(5.0–105.0) | 25.5 (7.0–96.0) | 14.0 (3.0–145.0) | .18b |
| Male, No. (%) | 194 (66.4) | 138 (64.5) | 56(71.8) | .24c |
| Race, No. (%) | ||||
| Black or African American | 65 (22.3) | 49 (22.9) | 16 (20.5) | .90c |
| White | 174 (59.6) | 127 (59.3) | 47 (60.3) | |
| Other or unknown | 53 (18.2) | 38 (17.8) | 15 (19.2) | |
| Ethnicity, No. (%) | ||||
| Hispanic or Latino | 65 (22.3) | 48 (22.4) | 17 (21.8) | .27c |
| Not Hispanic or Latino | 214(73.3) | 154 (72.0) | 60 (76.9) | |
| Unknown | 13 (4.5) | 12 (5.6) | 1 (1.3) | |
| Preexisting chronic conditions, No. (%) | ||||
| None | 150 (51.4) | 119 (55.6) | 31 (39.7) | .02c |
| Prenatal | 36 (12.3) | 28(13.1) | 8(10.3) | .52c |
| Lung or airway disease | 66 (22.6) | 49 (22.9) | 17 (21.8) | .84c |
| Congenital heart disease | 35 (12.0) | 24(11.2) | 11(14.1) | .50c |
| Gastrointestinal tract | 41 (14.0) | 32 (15.0) | 9(11.5) | .46c |
| Neurologic | 49 (16.8) | 36 (16.8) | 13 (16.7) | .98c |
| Other | 71 (24.3) | 46 (21.5) | 25 (32.1) | .06c |
| Night or weekend arrest, No. (%) | 141 (48.3) | 100 (46.7) | 41 (52.6) | .38c |
| Initial cardiac arrest rhythm noted by EMS or hospital, No. (%) | ||||
| Asystole | 172 (58.9) | 126 (58.9) | 46 (59.0) | .87c |
| Bradycardia | 19 (6.5) | 13 (6.1) | 6 (7.7) | |
| PEA | 42 (14.4) | 29 (13.6) | 13 (16.7) | |
| Ventricular fibrillation or tachycardia | 23 (7.9) | 18 (8.4) | 5 (6.4) | |
| Unknown | 36 (12.3) | 28(13.1) | 8 (10.3) | |
| Primary cause of cardiac arrest, No. (%) | ||||
| Cardiac | 37(12.7) | 22 (10.3) | 15 (19.2) | .01c |
| Respiratory | 211(72.3) | 163 (76.2) | 48 (61.5) | |
| Other | 10 (3.4) | 4 (1.9) | 6 (7.7) | |
| Unknown | 34(11.6) | 25 (11.7) | 9(11.5) | |
| Cardiac arrest witnessed, No. (%) | 108 (37.0) | 75 (35.0) | 33 (42.3) | .51c |
| Bystander chest compressions, No. (%) | 183 (62.7) | 135 (63.1) | 48 (61.5) | .65c |
| Estimated duration of chest compressions, No. (%) | ||||
| ≤15 min | 66 (22.6) | 48 (22.4) | 18 (23.1) | .03c |
| >15to≤30min | 109 (37.3) | 89 (41.6) | 20 (25.6) | |
| >30min | 106 (36.3) | 68 (31.8) | 38 (48.7) | |
| Unable to determine | 11 (3.8) | 9 (4.2) | 2 (2.6) | |
| Total No. of doses of epinephrine administered by EMS and at hospital, No. (%) | ||||
| 0 | 19 (6.5) | 18 (8.4) | 1 (1–3) | .02c |
| 1 | 35 (12.0) | 28(13.1) | 7 (9.0) | |
| 2 | 51 (17.5) | 40 (18.7) | 11 (14.1) | |
| 3 | 40 (13.7) | 29 (13.6) | 11 (14.1) | |
| 4 | 41 (14.0) | 30 (14.0) | 11 (14.1) | |
| >4 | 76 (26.0) | 45 (21.0) | 31 (39.7) | |
| Missing | 30 (10.3) | 24(11.2) | 6 (7.7) | |
| Time from ROSC to TTM initiation, median (IQR), h [No. of participants] | 5.8 (5.1–6.5) [292] | 5.8(5.1–6.6) [214] | 5.9 (5.1–6.4) [78] | .74b |
| Treatment received, No. (%) | ||||
| Hypothermia | 153 (52.4) | 115 (53.7) | 38 (48.7) | .45c |
| Normothermia | 139 (47.6) | 99 (46.3) | 40 (51.3) | |
| Burden of hypotension, median (IQR), % [No. of participants] | 0.0 (0.0–13.4) [292] | 0.0 (0.0–0.0) [214] | 31.0 (16.7–60.0) [78] | <.001b |
| Maximum measured lactate level, median (IQR), mmol/L [No. of participants] | 4.7 (2.5–7.8) [238] | 3.6(2.0–6.6) [171] | 7.5 (4.2–11.5) [67] | <.001b |
| Minimum measured lactate level, median (IQR), mmol/L [No. of participants] | 3.7 (1.8–6.7) [238] | 3.0 (1.5–5.1) [171] | 5.7 (3.2–8.7) [67] | <.001b |
| No medications, No. (%) | 110 (37.7) | 96 (44.9) | 14(17.9) | <.001c |
| No. of vasoactive agents administered, No. (%) | ||||
| 0 | 129 (44.2) | 111(51.9) | 18(23.1) | <.001c |
| 1 | 80 (27.4) | 60 (28.0) | 20 (25.6) | |
| 2 | 70 (24.0) | 38 (17.8) | 32 (41.0) | |
| 3 | 13 (4.5) | 5 (2.3) | 8 (10.3) | |
| Milrinone administered, No. (%) | 28 (9.6) | 16 (7.5) | 12 (15.4) | .04c |
| Corticosteroids administered. No. (%) | 39 (13.4) | 18 (8.4) | 21 (26.9) | <.001c |
| Vasopressin administered, No. (%) | 42 (14.4) | 25(11.7) | 17 (21.8) | .03c |
Abbreviations: EMS, emergency medical services; IQR, interquartile range; PEA, pulseless electrical activity; ROSC, return of spontaneous circulation; TTM, targeted temperature management.
Indicates hypotension at 0 to 6 hours. Percentages have been rounded and may not total 100.
Calculated using the Wilcoxon rank sum test.
Calculated using the χ2 test of no association.
Among the 78 participants with early hypotension, the median burden of hypotension per participant was 31.0% of measurements (IQR, 16.7%−60.0%). Of participants with a burden of hypotension of at least 16.7% of measurements, 9 of 49 (18.4%) did not receive a vasoactive infusion.
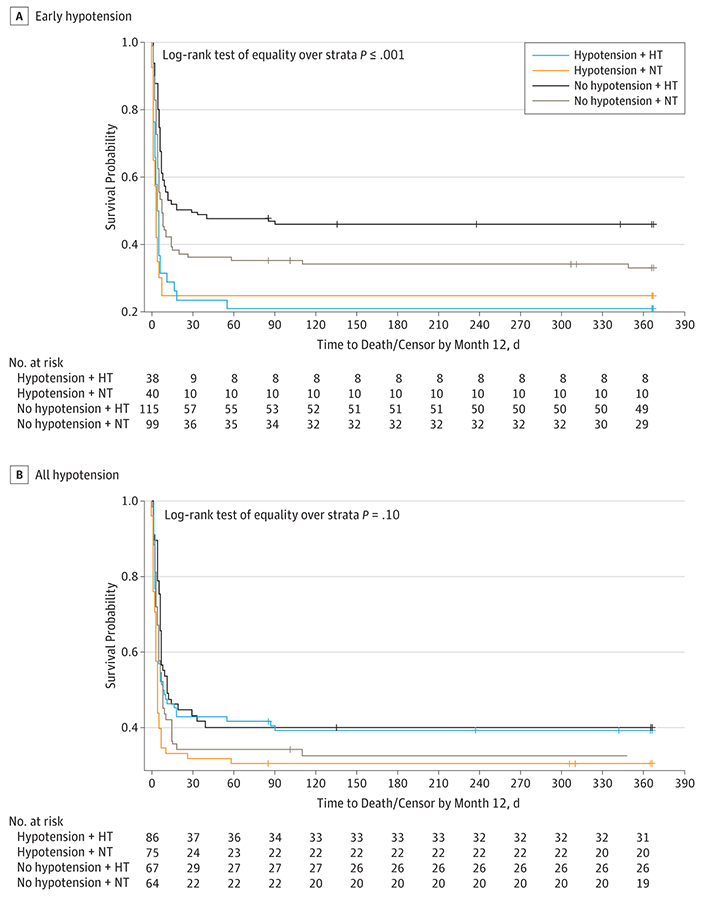
One hundred thirteen participants (38.7%) survived to discharge. Participants who had early hypotension (0–6 hours) had lower rates of survival to hospital discharge (Table 2). After controlling for witnessed status, duration of CPR, and the presence of a preexisting condition, early hypotension was associated with significantly decreased odds of survival to discharge (adjusted odds ratio [AOR], 0.39; 95% CI, 0.20–0.74). Early hypotension burden (0–6 hours) was also associated with significantly decreased odds of survival to discharge. For each 10% increase in the number of hypotension measurements, a 20% decrease occurred in the odds of survival (AOR, 0.80; 95% CI, 0.69–0.93). The probability of survival over 1 year was significantly lower in participants with early hypotension (0–6 hours) regardless of TTM arm (log-rank test of equality over strata, P < .001) (Figure 2A). No interaction was found between TTM and blood pressure (P = .49).
Table 2.
Association With Survival to Hospital Discharge
| Survival to Hospital Dischargea | OR(95%CI) | ||||
|---|---|---|---|---|---|
| Factor | No (n = 179) | Yes (n = 113) | P Value | Unadjusted | Adjusted |
| Any hypotension | |||||
| 0–6 h | 58 (32.4) | 20(17.7) | .006b | 0.45 (0.25–0.80) | 0.39 (0.20–0.74) |
| 0–72 h | 99 (55.3) | 62 (54.9) | .94b | 0.98(0.61–1.58) | NA |
| Age at randomization, median (IQ.R), y | 1.3 (0.3–7.2) | 3.1 (1.0–9.8) | <.001c | 1.05 (1.01–1.09) | NA |
| Sex | |||||
| Male | 112 (62.6) | 82 (72.6) | .08b | 1 [Reference] | NA |
| Female | 67 (37.4) | 31 (27.4) | 0.63 (0.38–1.05) | NA | |
| Cardiac arrest witnessed | |||||
| No | 116 (64.8) | 54 (47.8) | 1 [Reference] | 1 [Reference] | |
| Yes | 52 (29.1) | 56 (49.6) | .001b | 2.31 (1.41–3.80) | 2.04(1.15–3.61) |
| Unable to determine | 11 (6.1) | 3 (2.7) | 0.59 (0.16–2.19) | 0.46 (0.12–1.84) | |
| Chest compressions administered by bystander | |||||
| No | 62 (34.6) | 35 (31.0) | .81b | 1 [Reference] | NA |
| Yes | 110(61.5) | 73 (64.6) | 1.18 (0.71–1.96) | NA | |
| Unable to determine | 7 (3.9) | 5 (4.4) | 1.27 (0.37–4.29) | NA | |
| Initial cardiac arrest rhythm noted by EMS or hospital | |||||
| Asystole | 117 (65.4) | 55 (48.7) | .01b | 1 [Reference] | NA |
| Bradycardia | 9 (5.0) | 10 (8.8) | 2.36 (0.91–6.15) | NA | |
| PEA | 26 (14.5) | 16 (14.2) | 1.31 (0.65–2.64) | NA | |
| Ventricular fibrillation or tachycardia | 8 (4.5) | 15 (13.3) | 3.99 (1.60–9.97) | NA | |
| Unknown | 19 (10.6) | 17(15.0) | 1.90 (0.92–3.94) | NA | |
| Estimated duration of chest compressions | |||||
| ≤15 min | 28 (15.6) | 38 (33.6) | <.001b | 1 [Reference] | 1 [Reference] |
| >15to≤30min | 61 (34.1) | 48 (42.5) | 0.58(0.31–1.08) | 0.57 (0.30–1.10) | |
| >30min | 83 (46.4) | 23 (20.4) | 0.20 (0.10–0.40) | 0.21 (0.10–0.42) | |
| Unable to determine | 7 (3.9) | 4 (3.5) | 0.42 (0.11–1.58) | 0.57(0.14–2.27) | |
| Primary cause of cardiac arrest | |||||
| Cardiac | 15 (8.4) | 22(19.5) | .02b | 1 [Reference] | NA |
| Respiratory | 134 (74.9) | 77 (68.1) | 0.39 (0.19–0.80) | NA | |
| Other | 5(2.8) | 5 (4.4) | 0.68(0.17–2.77) | NA | |
| Unknown | 25 (14.0) | 9 (8.0) | 0.25 (0.09–0.67) | NA | |
| Treatment received | |||||
| Hypothermia | 87 (48.6) | 66 (58.4) | .10b | 1 [Reference] | NA |
| Normothermia | 92 (51.4) | 47 (41.6) | 0.67 (0.42–1.08) | NA | |
| Preexisting condition | 77 (43.0) | 65(57.5) | .02b | 1.79(1.11–2.89) | 1.75 (1.01–3.04) |
| Night or weekend arrest | 91 (50.8) | 50 (44.2) | .27b | 0.77 (0.48–1.23) | NA |
Abbreviations: EMS, emergency medical services; IQR, interquartile range; NA, not applicable; OR, odds ratio; PEA, pulseless electrical activity.
Percentages have been rounded and may not total 100. All variables were considered for entry into logistic regression model using forward stepwise selection.
Calculated using the χ2 test of no association.
Calculated using the Wilcoxon rank sum test.
Figure 2. Kaplan-Meier Survival Curves.
Early hypotension indicates 0 to 6 hours after initiation of target temperature maintenance (TTM) treatment; hypotension, 0 to 72 hours after initiation of TTM treatment. The TTM arms consist of hypothermia (HT) and normothermia (NT), described in detail in the THAPCA Study subsection of the Methods section. Tick marks indicate censorship.
0–72 Hours After TTM Intervention
One hundred sixty-one of 292 participants (55.1%) had hypo-tension during the first 72 hours of temperature intervention (Table 3). Among those with hypotension, the median burden of hypotension per participant was 6.7% (IQR, 2.9%−18.8%) of measurements. Hypotension was more common in participants with no bystander CPR, with preexisting conditions, a cardiac cause of arrest, a witnessed cardiac arrest, and a higher post–cardiac arrest lactate level and in older chil dren. We found no difference in sex, race, cardiac arrest time of week, initial rhythm, duration of CPR, or time from ROSC to intervention. Participants with hypotension were more likely to receive vasoactive infusions (122 of 161 [75.8%] vs 72 of 131 [55.0%]; P < .001) and corticosteroids (41 of 161 [25.5%] vs 16 of 131 [12.2%]; P = .004), although 39 of 161 with hypo-tension (24.2%) did not receive a vasoactive infusion. We found no difference in the prevalence of any hypotension by TTM intervention or in survival to discharge by blood pressure group (Table 2) and no difference in the probability of survival over 1 year by blood pressure group in the first 72 hours regardless of TTM arm (P = .10) (Figure 2B).
Table 3.
Patient and Cardiac Arrest Characteristics by Hypotension Groupa
| Any Hypotension at 0–72 h | ||||
|---|---|---|---|---|
| Characteristic | Overall (N = 292) | No (n = 131) | Yes (n = 161) | P Value |
| Age at randomization, median (IQR), mo | 23.0 (5.0–105.0) | 18.0(5.0–55.0) | 25.0(5.0–146.0) | .049b |
| Male, No. (%) | 194 (66.4) | 85 (64.9) | 109 (67.7) | .61c |
| Race, No. (%) | ||||
| Black or African American | 65 (22.3) | 30 (22.9) | 35 (21.7) | .89c |
| White | 174 (59.6) | 79 (60.3) | 95 (59.0) | |
| Other or unknown | 53 (18.2) | 22 (16.8) | 31 (19.3) | |
| Ethnicity, No. (%) | ||||
| Hispanic or Latino | 65 (22.3) | 26 (19.8) | 39 (24.2) | .15c |
| Not Hispanic or Latino | 214(73.3) | 96 (73.3) | 118 (73.3) | |
| Unknown | 13 (4.5) | 9 (6.9) | 4 (2.5) | |
| Preexisting chronic conditions, No. (%) | ||||
| None | 150 (51.4) | 78 (59.5) | 72 (44.7) | .01c |
| Prenatal | 36 (12.3) | 20 (15.3) | 16 (9.9) | .17c |
| Lung or airway disease | 66 (22.6) | 29(22.1) | 37 (23.0) | .86c |
| Congenital heart disease | 35 (12.0) | 14 (10.7) | 21(13.0) | .54c |
| Gastrointestinal tract | 41 (14.0) | 21 (16.0) | 20 (12.4) | .38c |
| Neurologic | 49 (16.8) | 20 (15.3) | 29 (18.0) | .53c |
| Other | 71 (24.3) | 24 (18.3) | 47 (29.2) | .03c |
| Night or weekend arrest, No. (%) | 141 (48.3) | 60 (45.8) | 81 (50.3) | .44c |
| Initial cardiac arrest rhythm noted by EMS or hospital, No. (%) | ||||
| Asystole | 172 (58.9) | 82 (62.6) | 90 (55.9) | .61c |
| Bradycardia | 19 (6.5) | 8 (6.1) | 11 (6.8) | |
| PEA | 42 (14.4) | 19 (14.5) | 23 (14.3) | |
| Ventricular fibrillation or tachycardia | 23 (7.9) | 7 (5.3) | 16 (9.9) | |
| Unknown | 36 (12.3) | 15 (11.5) | 21 (13.0) | |
| Primary cause of cardiac arrest, No. (%) | ||||
| Cardiac | 37(12.7) | 11 (8.4) | 26 (16.1) | <.001c |
| Respiratory | 211 (72.3) | 109 (83.2) | 102 (63.4) | |
| Other | 10 (3.4) | 0 | 10 (6.2) | |
| Unknown | 34 (11.6) | 11 (8.4) | 23 (14.3) | |
| Cardiac arrest witnessed, No. (%) | 108 (37.0) | 38 (29.0) | 70 (43.5) | .009c |
| Chest compressions administered by bystander, No. (%) | 183 (62.7) | 94 (71.8) | 89 (55.3) | .01c |
| Estimated duration of chest compressions, No. (%) | ||||
| ≤15 min | 66 (22.6) | 24 (18.3) | 42 (26.1) | .053c |
| >15to≤30min | 109 (37.3) | 60 (45.8) | 49 (30.4) | |
| >30min | 106 (36.3) | 42 (32.1) | 64 (39.8) | |
| Unable to be determined | 11 (3.8) | 5 (3.8) | 6 (3.7) | |
| Total No. of doses of epinephrine administered by EMS and at hospital, No. (%) | ||||
| 0 | 19 (6.5) | 9 (6.9) | 10 (6.2) | .57c |
| 1 | 35 (12.0) | 18 (13.7) | 17 (10.6) | |
| 2 | 51 (17.5) | 24 (18.3) | 27 (16.8) | |
| 3 | 40 (13.7) | 21 (16.0) | 19 (11.8) | |
| 4 | 41 (14.0) | 17 (13.0) | 24 (14.9) | |
| >4 | 76 (26.0) | 28 (21.4) | 48 (29.8) | |
| Missing | 30 (10.3) | 14 (10.7) | 16 (9.9) | |
| Time from ROSC to TTM initiation, median (IQR), h [No. of participants] | 5.8 (5.1–6.5) [292] | 5.8(5.2–6.6) [131] | 5.8 (5.0–6.5) [161] | .47b |
| Treatment received, No. (%) | ||||
| Hypothermia | 153 (52.4) | 67 (51.1) | 86 (53.4) | .70c |
| Normothermia | 139 (47.6) | 64 (48.9) | 75 (46.6) | |
| Burden of hypotension, median (IQR), % [No. of participants] | (292)1.4 (0.0–7.8) | 0.0 (0.0–0.0) [131] | 6.7 (2.9–18.8) [161] | <.001b |
| Maximum measured lactate level, median (IQR), mmol/L [No. of participants] | 4.4 (2.3–7.7) [285] | 3.9(1.9–6.1) [128] | 5.3 (2.8–9.0) [157] | <.001b |
| Minimum measured lactate level, median (IQR), mmol/L [No. of participants] | 1.2 (0.8–2.1) [285] | 1.2 (0.8–1.7) [128] | 1.2 (0.8–2.6) [157] | .16c |
| No medications, No. (%) | 78 (26.7) | 50 (38.2) | 28 (17.4) | <.001c |
| No. of vasoactive agents administered, No. (%) | ||||
| 0 | 98 (33.6) | 59 (45.0) | 39 (24.2) | <.001c |
| 1 | 77 (26.4) | 38 (29.0) | 39 (24.2) | |
| 2 | 100 (34.2) | 28 (21.4) | 72 (44.7) | |
| 3 | 17 (5.8) | 6 (4.6) | 11 (6.8) | |
| Milrinone administered, No. (%) | 36 (12.3) | 11 (8.4) | 25 (15.5) | .07c |
| Corticosteroids administered. No. (%) | 57(19.5) | 16(12.2) | 41 (25.5) | .004c |
| Vasopressin administered, No. (%) | 68 (23.3) | 26 (19.8) | 42 (26.1) | .21c |
Abbreviations: EMS, emergency medical services; IQR, interquartile range; PEA, pulseless electrical activity; ROSC, return of spontaneous circulation; TTM, targeted temperature management.
Indicates hypotension at 0 to 72 hours. Percentages have been rounded and may not total 100.
Calculated using the Wilcoxon rank sum test.
Calculated using the χ2 test of no association.
By TTM Intervention Phase
One hundred forty-five of 292 participants (49.7%) had hypotension during the combined induction and maintenance phase (≤48 hours for a median of ±2.8 hours from initiation of TTM intervention), including 73 of 153 participants undergoing therapeutic hypothermia (47.7%) and 72 of 139 undergoing therapeutic normothermia (51.8%) (P = .50). Fifty-four of 213 participants (25.4%) had hypotension during the rewarming period, including 35 of 118 participants undergoing therapeutic hypothermia (29.7%) and 19 of 95 undergoing therapeutic normothermia (20.0%) (P = .10) (eTable in the Supplement).
No difference between therapeutic normothermia and hypothermia groups occurred in the number of participants who received vasoactive infusions or vasopressin during day 0 or 1; however, fewer participants in the therapeutic normothermia group received a vasoactive agent on day 2 (42 of 112 [37.5%] vs 69 of 134 [51.5%]) or 3 (36 of 102 [35.3%] vs 65 of 125 [52.0%]) compared with the therapeutic hypothermia group. A larger percentage of participants undergoing therapeutic normothermia received vasopressin on day 1 only (30 of 131 [22.9%] vs 23 of 152 [15.1%]) and received more corticosteroids on every day (eTable in the Supplement).
Discussion
In this secondary analysis of the THAPCA trial, early hypotension within 6 hours after TTM intervention occurred in approximately one-quarter of participants and was associated with decreased survival to hospital discharge. Furthermore, participants who had a higher hypotension burden were less likely to survive to discharge, a finding supporting a dose-response effect. In contrast, hypotension during the first 72 hours of TTM intervention was not associated with outcome, suggesting less vulnerability to hypotensive episodes during later periods after cardiac arrest in the THAPCA trial.
In the present study, we applied previously published definitions of hypotension (SBP <5th percentile for age and sex) and found the prevalence of hypotension in the first 6 hours after intervention initiation (approximately 6–12 hours after ROSC) to be lower than the 56% described in 0 to 6 hours after ROSC in a pediatric IHCA and OHCA cohort.9 Adult studies10,11 report early hypotension prevalence rates in combined IHCA and OHCA cohorts of 47% to 65%. Hypo-tension is common after cardiac arrest and is caused by a combination of ischemia and reperfusion and myocardial dysfunction.6,20 The lower rate of hypotension in the present cohort suggests that secular improvements in post-cardiac arrest care may have been associated with decreased prevalence of post–cardiac arrest hypotension. Other possible reasons to explain the lower rate in the present cohort may be that (1) research training and monitoring in an intervention trial encouraged hypotension prevention; (2) a Hawthorne effect may have occurred for a consented cohort of a randomized clinical trial; (3) the 0- to 6-hour period in this cohort was approximately 6 to 12 hours after ROSC, not 0 to 6 hours after ROSC; and (4) lack of participants with IHCA may have reduced higher rates of hypotension based on preexisting illnesses.
We also evaluated the hypotension burden during both periods and found a dose-response effect. Similarly, Kilgannon et al12 demonstrated a dose-response effect after adult cardiac arrest by using a different method. Hypotension burden quantifies a “dose” of hypotension per participant accounting for time and severity, not just presence or absence. We selected SBP a priori because of the availability of normative data and previously published pediatric literature.9
In our cohort, fewer participants with early hypotension survived to discharge. Furthermore, we found that as the number of measurements of hypotension increased, the odds of discharge survival decreased. This finding supports previous findings in a combined IHCA and OHCA cohort of 383 children9 that early systolic hypotension was associated with worse outcomes. In that study,9 early systolic hypotension was associated with higher rates of in-hospital mortality and worse hospital discharge neurologic outcomes. Similarly, Lin et al21 found that hypotension within 1 hour from ROSC after pediatric OHCA was associated with lower rates of discharge survival. Despite the consistency of these findings, whether early postresuscitation hypotension causes worse outcomes or is a marker of more severe cardiac arrest, including prolonged CPR requiring more doses of epinephrine and higher post–cardiac arrest lactate levels, remains unclear. Although these significant differences were observed in the early hypotension group, no difference was found between hypotension groups in the 0- to 72-hour analysis despite hypotension being more prevalent. These findings may be attributed to early hypotension being a bio-marker of injury severity, thus leading to death, or early hypotension occurring at an especially vulnerable time for the brain and/or heart, suggesting that improvement in blood pressure may result in more favorable outcomes.
These data support the American Heart Association recommendations22 that parenteral fluids and/or inotropes or vasoactive drugs be used to maintain an SBP greater than the fifth percentile for age. In our study, 163 of 292 participants (55.8%) were treated with at least 1 vasoactive infusion in the first 6 hours after temperature intervention, including 60 of 78 with early hypotension (76.9%) and 103 of 214 without early hypotension (48.1%). In contrast, in the previous study of early post–cardiac arrest hypotension,9 only 41% of participants with hypotension received at least 1 vasopressor compared with 23% without hypotension. This notable increase in the use of vasoactive infusions suggests a change in practice between the 2003–2004 observational cohort in that earlier study9 and the later period when the THAPCA trial was performed. In the THAPCA trial, blood pressure targets and management were not protocolized, but research training included guidelines targeting normal blood pressure for age. Although determining whether the increase in the use of vasoactive infusions has changed outcomes during the past decade is not possible, these data suggest that protocols with criteria for post–cardiac arrest care may drive clinicians’ management and thus could affect outcomes in the longer term.
Hypotension occurred in 49.7% of participants during the combined induction-maintenance phase and 25.4% during the rewarming phase; however, no difference was found between therapeutic hypothermia and therapeutic normothermia groups. Hypotension during rewarming was described in a trial of therapeutic hypothermia for pediatric traumatic brain injury23,24 and was more common during the rewarming period and in patients who received the therapy. Rewarming is a vulnerable time when vasodilation occurs because the post–cardiac arrest syndrome has often not resolved. Compared with the traumatic brain injury trial,23,24 the THAPCA trial had later rewarming at more than 48 hours after acute injury, different definitions of hypotension, and different underlying pathophysiologic features. The THAPCA investigators specifically encouraged hypotension prevention because of the known hypotension during rewarming in the traumatic brain injury trial.23,24
A post hoc analysis from the adult TTM trial comparing 33°C with 36°C for 24 hours after OHCA demonstrated that blood pressures did not differ between treatment arms until rewarming, at which point participants in the therapeutic hypothermia arm had lower mean arterial pressures.13 These findings differed from those of our study, in which during rewarming, 29.7% of participants receiving therapeutic hypothermia had hypotension vs 20.0% of participants receiving therapeutic normothermia. This finding may be attributable to rewarming beginning approximately 24 hours later in the THAPCA trial and thus later after ROSC, with more time for post–cardiac arrest syndrome to resolve.
Limitations
Our study has several limitations. First, this study was a secondary analysis of a randomized clinical trial of TTM of a severely injured pediatric population with OHCA and therefore represents a subset of the population with OHCA and may not be generalizable to all survivors of OHCA who are admitted to a pediatric intensive care unit. Second, blood pressure data were not collected from ROSC to the intervention–approximately the first 6 hours after ROSC–a potentially important time when blood pressure and treatment may affect outcomes. Third, vasoactive medication infusion dosing and timing were not available, and our data are limited to ordered medications rather than medications confirmed to be administered. Fourth, the timing of induction and rewarming were variable, and therefore matching specific periods was challenging. Fifth, echocardiogram data were not available to characterize myocardial function. Sixth, this observational study from a randomized clinical trial cannot determine cause and effect. Finally, we evaluated the association of SBP with discharge mortality but not diastolic or mean blood pressures.
Conclusions
In this secondary analysis of the THAPCA trial, 26.7% of participants had hypotension within 6 hours of the study intervention. Early post–cardiac arrest hypotension and the burden of early hypotension were associated with lower odds of survival to discharge, even after adjusting for covariates of interest.
Supplementary Material
Key Points
Question
Is hypotension after resuscitation from pediatric out-of-hospital cardiac arrest associated with lower rates of survival to discharge in children treated with targeted temperature management?
Findings
In this post hoc secondary analysis of a randomized clinical trial that included 292 children and adolescents treated with therapeutic hypothermia after pediatric cardiac arrest, 78 (26.7%) had at least 1 episode of hypotension in the first 6 hours after temperature intervention. Postarrest hypotension was associated with lower odds of surviving to discharge.
Meaning
Hypotension early after pediatric out-of-hospital cardiac arrest is common and associated with lower odds of survival to discharge.
Footnotes
Conflict of Interest Disclosures:
None reported.
REFERENCES
- 1.Atkins DL, Everson-Stewart S, Sears GK, et al. ; Resuscitation Outcomes Consortium Investigators. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation. 2009;119(11):1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fink EL, Prince DK, Kaltman JR, et al. ; Resuscitation Outcomes Consortium. Unchanged pediatric out-of-hospital cardiac arrest incidence and survival rates with regional variation in North America. Resuscitation. 2016;107:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moler FW, Donaldson AE, Meert K, et al. ; Pediatric Emergency Care Applied Research Network. Multicenter cohort study of out-of-hospital pediatric cardiac arrest. Crit Care Med. 2011;39(1):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moler FW, Silverstein FS, Holubkov R, et al. ; THAPCA Trial Investigators. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372(20):1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Zellem L, Buysse C, Madderom M, et al. Long-term neuropsychological outcomes in children and adolescents after cardiac arrest. Intensive Care Med. 2015;41(6):1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118(23):2452–2483. [DOI] [PubMed] [Google Scholar]
- 7.Kern KB, Hilwig RW, Rhee KH, Berg RA. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol. 1996;28(1):232–240. [DOI] [PubMed] [Google Scholar]
- 8.Kern KB, Hilwig RW, Berg RA, et al. Postresuscitation left ventricular systolic and diastolic dysfunction: treatment with dobutamine. Circulation. 1997;95(12):2610–2613. [DOI] [PubMed] [Google Scholar]
- 9.Topjian AA, French B, Sutton RM, et al. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med. 2014;42(6):1518–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilgannon JH, Roberts BW, Reihl LR, et al. Early arterial hypotension is common in the post-cardiac arrest syndrome and associated with increased in-hospital mortality. Resuscitation. 2008;79(3): 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trzeciak S, Jones AE, Kilgannon JH, et al. Significance of arterial hypotension after resuscitation from cardiac arrest. Crit Care Med. 2009;37(11):2895–2903. [DOI] [PubMed] [Google Scholar]
- 12.Kilgannon JH, Roberts BW, Jones AE, et al. Arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest. Crit Care Med. 2014;42(9):2083–2091. [DOI] [PubMed] [Google Scholar]
- 13.Bro-Jeppesen J, Annborn M, Hassager C, et al. Hemodynamics and vasopressor support during targeted temperature management at 33° C versus 36° C after out-of-hospital cardiac arrest: a post hoc study of the Target Temperature Management Trial. Crit Care Med. 2015;43(2):318–327. [DOI] [PubMed] [Google Scholar]
- 14.Holubkov R, Clark AE, Moler FW, et al. Efficacy outcome selection in the Therapeutic Hypothermia After Pediatric Cardiac Arrest trials. Pediatr Crit Care Med. 2015;16(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pemberton VL, Browning B, Webster A, Dean JM, Moler FW. Therapeutic Hypothermia After Pediatric Cardiac Arrest trials: the vanguard phase experience and implications for other trials. Pediatr Crit Care Med. 2013;14(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moler FW, Silverstein FS, Meert KL, et al. Rationale, timeline, study design, and protocol overview of the Therapeutic Hypothermia After Pediatric Cardiac Arrest trials. Pediatr Crit Care Med. 2013;14(7):e304–e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.clinicaltrials.gov. Therapeutic Hypothermia to Improve Survival After Cardiac Arrest in Pediatric Patients-THAPCA-OH [Out-of-Hospital] Trial. NCT00878644. https://clinicaltrials.gov/ct2/show/NCT00878644. Accessed February 5, 2016.
- 18.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167(6):653–666. [DOI] [PubMed] [Google Scholar]
- 19.Meert KL, Telford R, Holubkov R, et al. ; Therapeutic Hypothermia After Pediatric Cardiac Arrest (THAPCA) Trial Investigators. Pediatric out-of-hospital cardiac arrest characteristics and their association with survival and neurobehavioral outcome. Pediatr Crit Care Med. 2016;17(12): e543–e550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106(5):562–568. [DOI] [PubMed] [Google Scholar]
- 21.Lin YR, Li CJ, Wu TK, et al. Post-resuscitative clinical features in the first hour after achieving sustained ROSC predict the duration of survival in children with non-traumatic out-of-hospital cardiac arrest. Resuscitation. 2010;81(4):410–417. [DOI] [PubMed] [Google Scholar]
- 22.de Caen AR, Berg MD, Chameides L, et al. Part 12: pediatric advanced life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18)(suppl2):S526–S542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutchison JS, Ward RE, Lacroix J, et al. ; Hypothermia Pediatric Head Injury Trial Investigators and the Canadian Critical Care Trials Group. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358(23): 2447–2456. [DOI] [PubMed] [Google Scholar]
- 24.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7)(suppl): S186–S202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.