Abstract
Village chickens are ubiquitous in smallholder farming systems, contributing to household, local and national economies under diverse environmental, economic and cultural settings. However, they are raised in challenging environments where productivity is low while mortality is high. There is much interest in utilizing indigenous genetic resources to produce a chicken resilient to its environment, whilst providing the basis of an economically sustainable enterprise. Globally, however, a wide variety of interventions have so far proved unable to deliver sustainable improvements. Here, we show that regional differences in trait preferences and parasite burden are associated with distinct chicken genepools, likely in response to interacting natural and human-driven (economic and social) selection pressures. Drivers of regional differences include marketing opportunities, cultural preferences, agro-ecologies and parasite populations, and are evident in system adaptations, such as management practices, population dynamics and bird genotypes. Our results provide sound multidisciplinary evidence to support previous observations that sustainable poultry development interventions for smallholder farmers, including breeding programs, should be locally tailored and designed for flexible implementation.
Keywords: local adaptation, management intervention, village chickens, phenotypic diversity, genomic variation, disease resistance
For millions of smallholders in the least developed countries livestock are vital components of their livelihoods, contributing to economic, nutritional and social well-being, and improving families’ resilience as an asset that can be sold in times of need1. Indigenous chickens are of especial value to women and children2 and improving production has become widely accepted as a viable strategy towards poverty alleviation1,3, and increasing household food security4. In this paper, we examine the village production system as a whole and, by comparing two contrasting regions in Ethiopia as cases studies, illustrate how unique adaptations to local circumstances have implications for the success of any widespread intervention program. In doing so, we highlight barriers impeding the transformations required to achieve more sustainable increases in chicken production and, consequently, improvements to local economies, livelihoods and nutrition.
Whilst village chickens are widely accessible and require few inputs, productivity is low and constrained by, among other things, disease, predation and scarcity of feed5. Interventions to improve production include vaccination4,6; bird distribution7; management interventions8; cross-breeding programs9; and combined programs10, but few interventions have been demonstrably sustainable in village chicken production systems8,11. In Ethiopia, where indigenous birds still account for over 97% of egg and meat production12, government-led programs for village poultry development through genetic improvement have included a cockerel exchange program and distribution of high-producing exotic birds13 combined with farmer training on poultry management, based on a commercial model with larger flock sizes and increased inputs14. However, in rural areas, exotic birds adapted poorly to a scavenging environment and were reported by farmers to negatively impact the local birds’ brooding and scavenging abilities15, and may also have introduced very virulent Infectious Bursal Disease virus into indigenous chicken populations16. One alternative to exotic gene introgression is to selectively breed indigenous ecotypes to produce birds that are productive under village conditions while retaining locally-acceptable morphological and adaptive traits17.
Globally, the conventional top-down transfer of technology from researchers to farmers has been criticized for often being inappropriate to the social, physical and economic settings in which farmers operate18. Sustainable agriculture and development implies that systems should avoid the over-exploitation of natural resources, whilst providing for the existing and emerging economic and basic food needs of families and communities19. System changes impact on the future availability of resources, and therefore agro-ecologies need to remain resilient to ensure they can continue to support future generations. Resilience is dependent on adaptability and one approach is to investigate the system using three “pillars” of sustainability, namely environmental, economic and social20.
The aims of our unique, large-scale multidisciplinary study were, therefore, to map the contexts of local chicken production systems, together with investigation of indigenous chicken genetics and health, to identify the challenges facing sustainable productivity increases in this system. Using two Ethiopian indigenous chicken ecotypes for detailed case studies, we employed a systems approach to study the climatic, social, cultural, economic, market, bird productivity and production characteristics, as well as infection prevalence in two distinct geographic woredas (local administrative districts; Horro and Jarso) of Ethiopia (Fig. 1, Table 1, Supplementary Fig.1, Supplementary Table 1) in parallel with genomic studies of adaptive traits in the chickens themselves. We identify key selection pressures that impact on the sustainability and resilience of village chicken production, and suggest how poultry development programs globally could address constraints whilst building on the existing strengths of the local poultry production systems.
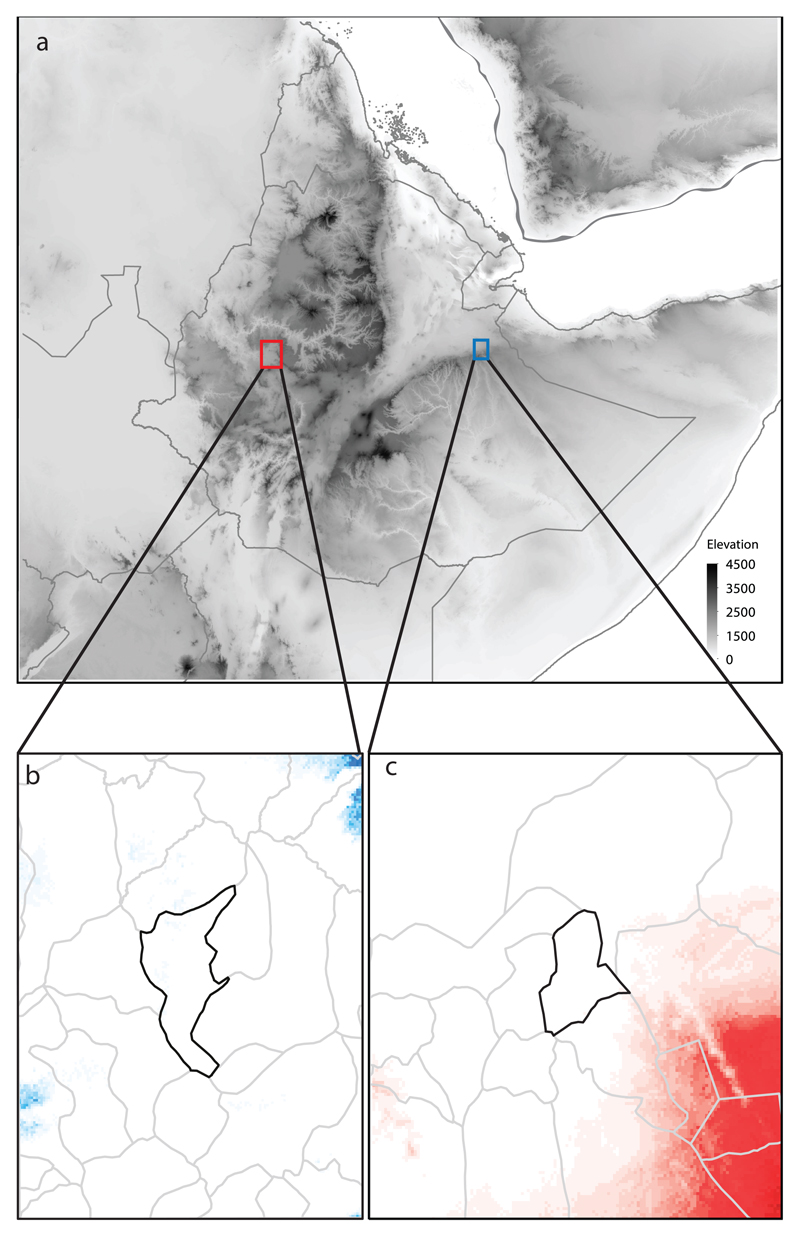
Fig. 1. Location of the study regions (a; red shape indicates Horro, blue shape Jarso) and potential distribution models constructed separately for each breed and projected onto the other.
Models of the potential distribution for each ecotype were constructed separately for each study region based on the bioclimatic variables measured in the other study region. The district map for Horro (b) displays the modelled potential distribution of Jarso birds (indicated by the blue shading) and vice versa for Jarso (c). Darker shading denotes areas of greater climatic similarity. Note that each area has low predicted similarity according to the model built with data from the other population, suggesting climatic dissimilarity. The environmental variables that contributed the most to the model for Horro were Precipitation of Wettest Month (49.2%) and Temperature Seasonality (27.2%), and for Jarso were Temperature Annual Range (38.7%) and Precipitation of Wettest Month (23.2%). Hence, the Horro ecotype is kept in, and may be adapted to, areas with higher precipitation and less temperature seasonality, with higher precipitation in the wettest and driest periods compared to the Jarso ecotype.
Table 1. Household demographics and utilization of chicken assets in the two study regions, Horro and Jarso, Ethiopia.
Data were obtained from 200 randomly selected households per region, collected in May 2011. Quantitative (numerical) variables reported as median [inter-quartile range]. Categorical variables reported as number (percentage: 95% Confidence Interval), unless otherwise indicated.
| Variable | Horro | Jarso | P value | |
|---|---|---|---|---|
| Household head age (years) | 38 [30.0-55.0] | 35 [30.0-47.8] | 0.07* | |
| Female-headed households | 16 (8%: 5-13%) | 10 (5%: 3-9%) | 0.3† | |
| Family size (number of people) | 7.0 [5.0-8.0] | 6.0 [5.0-8.0] | 0.1* | |
| Household head education level§ | None | 37% | 63% | <0.001‡ |
| Primary | 42% | 35% | ||
| Secondary | 13% | 1% | ||
| High school | 7% | 1% | ||
| College | 1% | 0% | ||
| Household land size (Ha) | 2.0 [1.0-3.0] | 0.4 [0.3-0.5] | <0.001* | |
| Chicken ownership¶ (% [95%CI]) | 75% [67-82%] | 71% [63-77%] | 0.5† | |
| TLU# owned | 7.6 [4.3-11.5] | 2.1 [1.2-3.2] | <0.001* | |
| Chicken TLU# | 0.21 [0.10-0.36] | 0.10 [0.06-0.16] | <0.001* | |
| Chicken number | 8.0 [4.0-14.25] | 4 [2-6] | <0.001* | |
| Reported value of flock (ETB) | 173 [98.5-265] | 75 [40-115.8] | <0.001* | |
| Purpose of chicken keeping | Sale | 181 (96%: 93-98%) | 175 (93%: 88-96%) | 0.18† |
| Consumption | 69 (37%: 30-44%) | 35 (19%: 14-27%) | <0.001† | |
| Purpose of egg production | Hatching | 131 (70%: 63-76%) | 60 (30%: 24-37%) | <0.001† |
| Sale | 76 (41%: 34-48%) | 131 (66%: 59-72%) | <0.001† | |
| Consumption | 47 (25%: 19-32%) | 61 (31%: 25-37%) | 0.27† | |
| Sold chickens in last 12 months | 114 (57%: 50-64%) | 76 (38%: 32-45%) | <0.001† | |
| Number of chickens sold‡ | 4 [2 -6] | 2 [1-4] | <0.001* | |
| Sold eggs in last 12 months | 17 (8.5%: 5-13%) | 37 (18.5%: 14-24%) | 0.005† | |
| Income from sale of chickens in past year (ETB) ‖ | 120 [70 -220) | 56 [30 -100] | <0.001* | |
| Percentage of household income from chickens ‖ | 1.68 [0.96 – 2.85} | 0.68 [0.39 – 1.49] | <0.001* | |
| Mean (sd) price per hen (ETB) | 26 (14) | 17 (5) | <0.001* | |
| Mean (sd) price per cock (ETB) | 41 (14) | 27 (12) | <0.001* | |
| Eaten chickens in last 12 month | 132 (68%: 61-74%) | 35 (18%: 13-23%) | <0.001† | |
| Number of chickens consumed∆ | 2 [1-3] | 3 [2-4] | 0.054* | |
| Eaten eggs in last 12 month | 33 (17%: 12-22%) | 45 (23%: 17-29%) | 0.165† | |
| Number of eggs consumed ∆ | 20 [10-40] | 21 [12-50] | 0.33* |
Mann-Whitney U test
Chi-square test
Chi-square test for trend
Primary (grade 1-4), Secondary (grade 5-8), High school (grade 9-12).
estimated from number of households not owning chickens among all households that were visited to identify the required number owning at least 2 adult birds
TLU – Tropical Livestock Units, calculated according to Storck et al (26).
Calculations based on households which sold at least 1 chicken or egg (as appropriate)
Calculations based on households which consumed at least 1 chicken or egg (as appropriate). Among the entire sample, the median number of chickens consumed per household in the last 12 months was 1 in Horro and 0 in Jarso (see also Figure 2), and 0 eggs in both Horro and Jarso.
ETB = Ethiopian Birr. At the time of the study, 1 USD was approximately equal to 17 Ethiopian Birr (ETB)
Results and Discussion
Bird and system adaptations to the cultural and economic environment
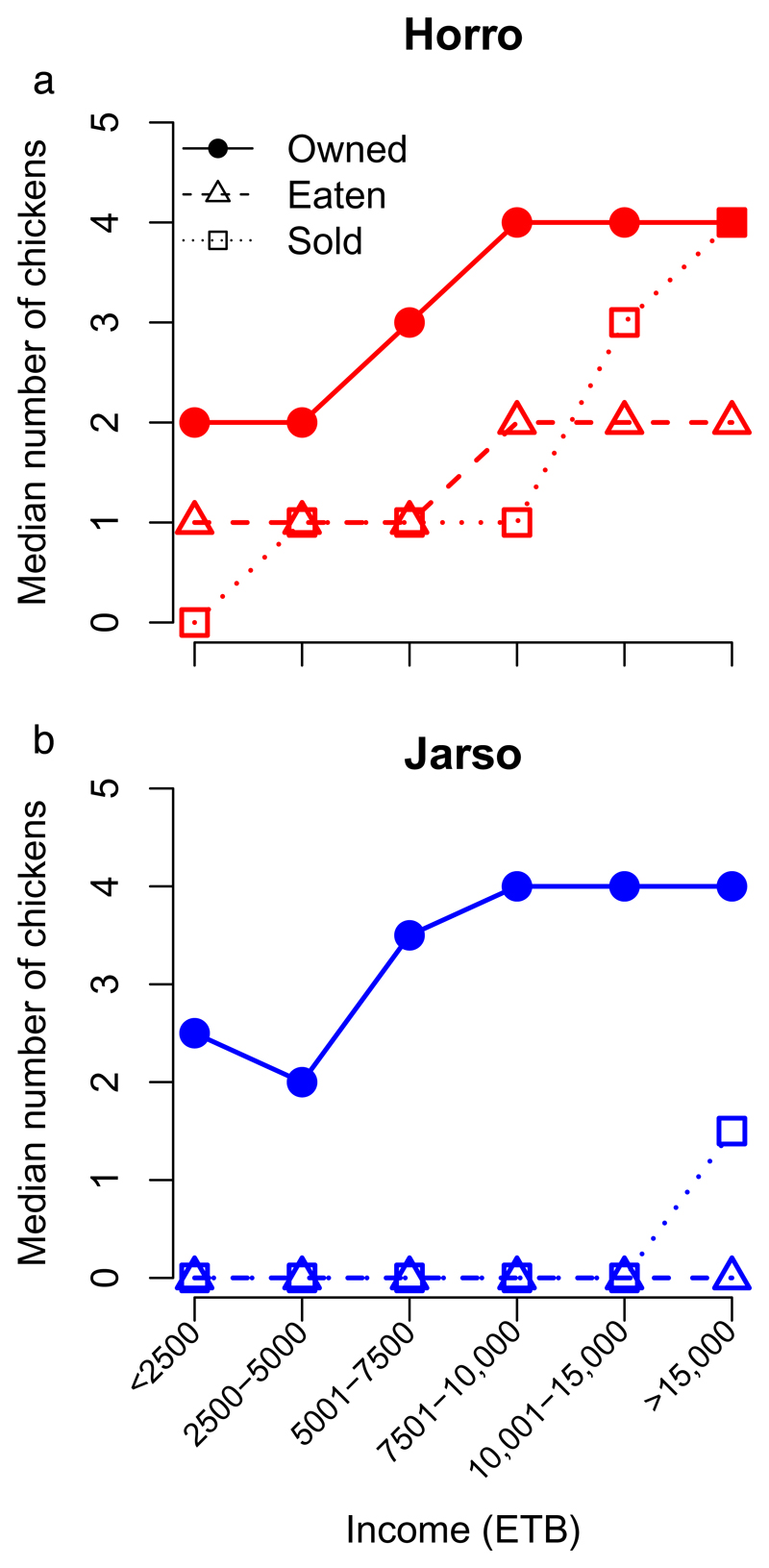
More than 70% of the households in the study regions owned chickens (Table 1), with production, and income from sales, often managed by women. Using the FAO classification system3, households in the study engaged in ‘small scale extensive scavenging’ (1-5 adult chickens, rarely keep other livestock, often landless) or ‘extensive scavenging’ (5-50 adult chickens, usually keep other livestock) chicken production. However, strict classification using this system was problematic; although two-thirds of all participants (66%; 267/400) kept 1-5 adult chickens (Horro 58%, 115/200; Jarso 76%, 152/200; P<0.001), only 9 (3 from Horro, 6 from Jarso) kept no other livestock. Although demographic characteristics, such as family size, proportion of female-headed households and age of household heads were broadly similar between the two woredas, there was significant variation in education, agricultural production and land ownership. Furthermore, chicken flocks were larger and more valuable in Horro (Table 1), mostly due to the greater numbers of young stock. Flock size also increased with household income, as did the number of birds consumed and sold (Fig. 2).
Fig. 2. Ownership and usage of chickens (median value; within previous 12 months) in the two study regions, (a) Horro and (b) Jarso, Ethiopia, for different categories of household income.
Although ownership of chickens is similar in both regions, utilization of chickens was greater in Horro, where there was evidence of increasing utilisation with increasing income. Data from 200 households in Horro and Jarso woreda in Ethiopia (total n = 400). See Supplementary Table 7 for detailed summary data. (ETB Ethiopian Birr).
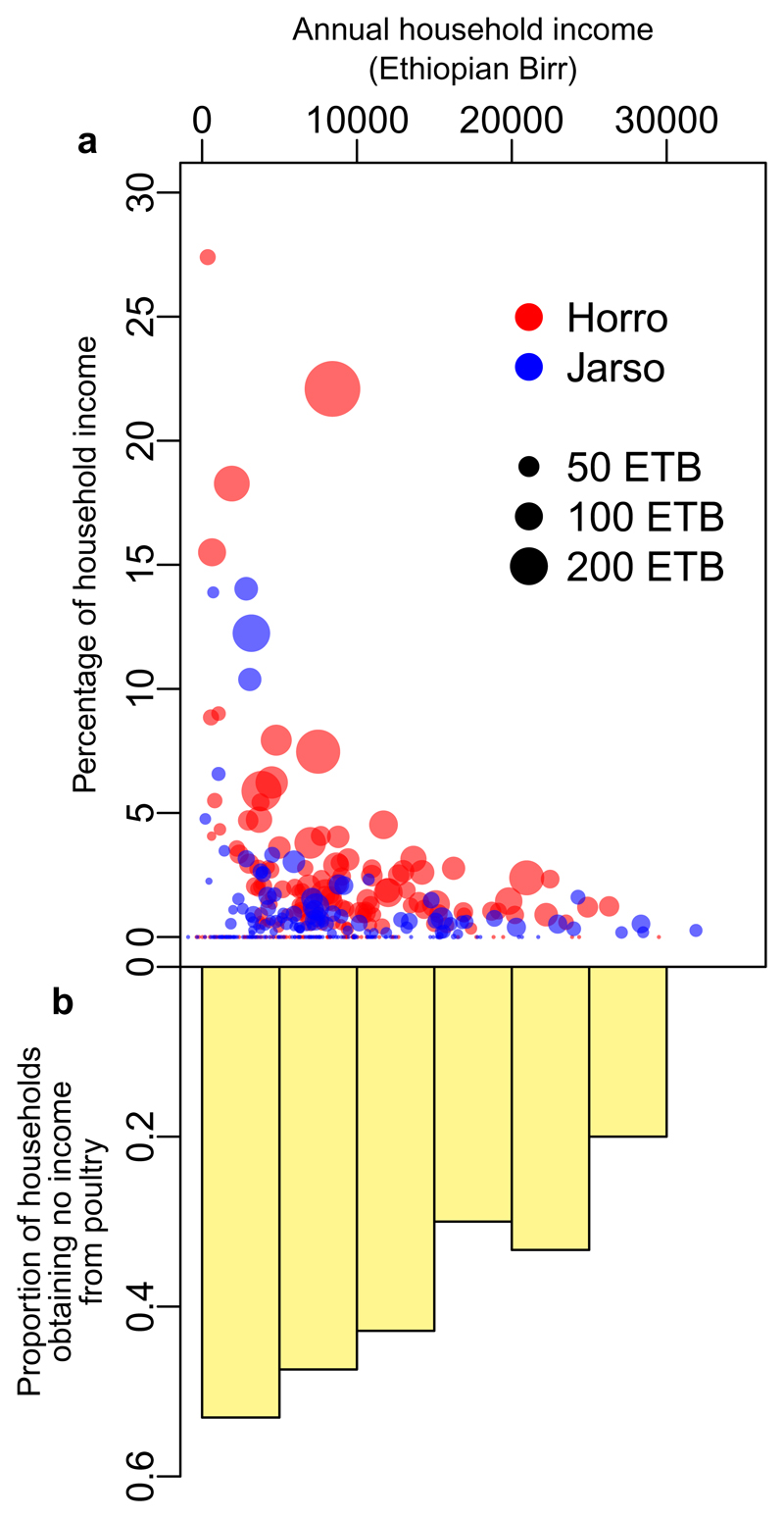
Consumption and sale of chickens and eggs were low, particularly in Jarso where both were significantly lower than in Horro (Table 1), and this is likely to have impacted development of market-oriented food systems, which halts if there is limited demand for farmers’ products21. Furthermore, the roles for chickens differed between the communities; Horro farmers were more likely to sell chickens (around twice as many) where the average prices received for both hens and roosters were significantly higher in Horro (P < 0.001 for both). Neverthless, the median income from sale of chickens was low, despite reportedly being an important source of additional income for incidental expenses in Horro. Chicken production offered even less income in Jarso, where sale of khat provided an important alternative source of incidental or continuous income. Whilst income from chicken production tended to contribute a greater proportion of family income in poorer households, a smaller proportion of the poorest families derived income from their chickens compared to wealthier households (Fig. 3; Chi-square for trend 10.7, P = 0.001).
Fig. 3. Household income from chicken production in two regions of Ethiopia.
Income from chicken production tended to contribute a greater proportion of family income in poorer households in both regions (a). However, a smaller proportion of the poorest families derived income from their chickens compared to wealthier households (a). Points in (a) are scaled relative to the number of breeding female chickens owned. Data from 200 households in Horro and Jarso woreda in Ethiopia (total n = 400).
In Horro, 70% of farmers reported that the purpose of egg production was hatching; just 8.5% reported that they had sold eggs in the past year, compared to 18.5% in Jarso. The greater emphasis on sale and consumption of chickens in Horro may have driven this preference for using eggs for rearing replacement birds. This is consistent with the larger populations of young stock with greater infection-susceptibility and greater fluctuation in chicken population numbers observed in Horro, factors relevant for sustained transmission of infectious organisms.
Markets are also influential in dictating the characteristics of the local chickens17. Village chickens are often assumed by outside researchers to mate in an uncontrolled manner22, but farmers actually select their breeding stock based on morphological traits23. For example, the greater marketing opportunities in Horro, compared to Jarso, may have resulted in greater human-driven selection pressure in favor of the rose comb variant and higher body weight; the rose comb has strong cultural significance and increases the market value of roosters in Ethiopia and was observed in higher frequency in Horro22. Our genome wide association study (GWAS) for rose versus simple comb identified a strong signal of selection in the genomic region previously associated with the rose comb phenotype24 in the Horro chickens (Supplementary Fig. 2C and Supplementary Table 2), confirming results from our previous studies22. Indeed, human selection for rose comb must have been particularly strong given that homozygotes for this mutation have lower sperm mobility24. Our previous studies also identified a stronger signal of selection in the Horro chickens in the genomic regions associated with body weight25, probably a result of the greater importance attached to body size in Horro (reported as important by 43% of respondents in Horro vs 12% in Jarso), and reflected in the greater weight of males and females of the same age in Horro compared to Jarso (P < 0.01; see26). The predicted weight for a 6-month-old male bird in Horro was 1.65 kg; almost 20% greater than for an equivalent bird in Jarso (1.38 kg).
In summary, the role of chickens and demand for products between regions (e.g. sale and consumption) is associated with variation in flock (size, age distribution) and bird phenotypic (weight, rose comb) and genomic characteristics.
Bird and system adaptations to the natural environment
The major constraints to livestock production reported by farmers varied between the woredas, with disease and shortage of land most important in Horro and Jarso, respectively; the median land holding size in Horro was approximately 5 times larger than in Jarso (Table 1). This difference was also reflected in the size of the livestock holdings (in terms of Tropical Livestock Units; TLU27). Scarcity of feed and water, predation and inadequate veterinary and extension services were noted as major chicken production constraints in both woredas.
Our genomic studies show compelling evidence of co-adaptation of these two chicken populations with their parasite populations; parasitic infections were more prevalent and less aggregated in the Horro population compared to Jarso (Supplementary Note 1). Macroparasites tend to cause density-dependent pathology; hence, both the number of chickens infected (prevalence) and the distribution of macroparasites among hosts (aggregation) matters.
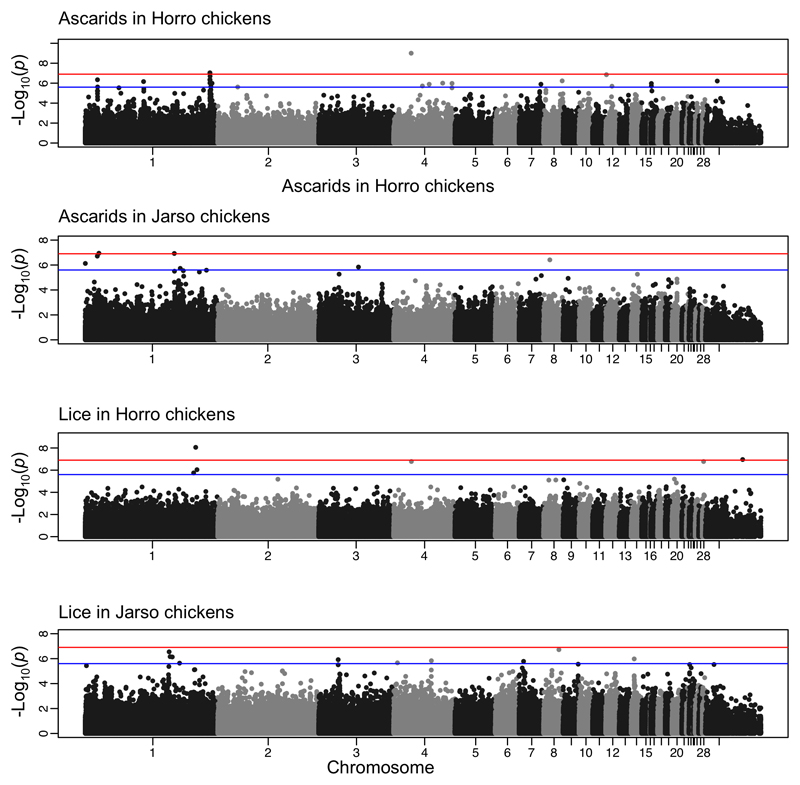
GWAS analyses indicated that genetic resistance to both ascarids and lice parasitism are heritable complex traits. However, distinct genomic regions are associated with these traits in the two populations (Fig. 4, Supplementary Note 2 and Supplementary Table 3). In the Horro chickens, genome-wide significant associations were identified on chromosomes 1 (P = 9.16E-08) and 4 (P = 9.86E-10) for ascarids, and on chromosomes 1 (P = 8.75E-09) and Z (P = 1.10E-07) for lice resistance. In Jarso chickens, two genome-wide significant associations were identified for ascarids on chromosome 1 (P = 1.13E-07 and 1.18E-07), albeit 50 Mb and 160 Mb away from the one identified in Horro, respectively. Several other SNP markers that exceeded the suggestive significance threshold were identified for both ascarids and lice resistance in the two populations (Supplementary Table 3). Collectively, the SNP markers associated with parasitic resistance in Horro chickens accounted for more of the phenotypic variance compared to Jarso chickens. This could have been due to lower selective pressures in Jarso compared to Horro or may have arisen if the two traits have different levels of polygenic complexity in the two populations. Although factors other than host genetic difference may also contribute to variation in parasite measurements28 and thus may reduce the power of a field study compared to controlled challenge experiments, crucially host genetic differences in resistance may still be detected29,30. Hence, while the data presented here cannot dissect the direct role of the parasites driving selection for resistance, we provide, for the first time in indigenous village chicken ecotypes, evidence suggestive of co-adaptation with their parasite populations, as evidenced by our signature of selection and GWAS results.
Fig. 4. Manhattan plots displaying the genome-wide association analysis results for ascarid and lice infection in two regions of Ethiopia (Horro and Jarso).
Genomic location (horizontal axis) is plotted against -log10(P); genome-wide (P < 0.05, after adjusting for multiple testing with Bonferroni correction) and suggestive genome-wide thresholds are shown as red and blue lines, respectively.
Whilst birds may be adapted to meet farmers’ requirements and the local environment, farmers also adapt their management systems accordingly. For example, most Jarso farmers chose to rear chicks during the rainy season, when vegetation affords chicks with protection from predators. In contrast, Horro farmers reported considerably greater numbers of birds lost to disease, prompting farmers to hatch larger numbers of eggs in the lower disease-risk dry season. This lead to regional and temporal/seasonal variation in flock sizes (see Supplementary Table 4). In Horro, flock size was significantly larger in May/June (pre- main rainy season; median = 11 birds), compared to October/November early dry season; (median = 6 birds), whereas in Jarso the pattern was reversed and the seasonal difference in flock size was lower.
The results of our ecological niche modelling, which highlight considerable bioclimatic dissimilarity between the study woredas (Fig. 1 and Supplementary Figure 1) are consistent with these divergent management strategies being, at least in part, a response to distinct climatic conditions, particularly precipitation, which is an important factor in parasite survival and aggregation. Whilst we might expect that reproductive management of birds by the farmers would be the main driver of flock size, it is notable that greater fluctuations were observed in the poultry population with the higher parasite burden, as is observed in wild game bird populations31. The system adaptation to annual population crashes means more chickens can be produced to meet increased demands during the important festivals of Easter and Christmas, but also allows for the regular depopulation in unfavorable conditions, such as in response to, or anticipation of, high epidemic risk during the wet season. However, to allow birds to meet the farmers’ annual breeding requirements, which may not coincide with fluctuations in parasite numbers over longer time periods, chickens, particularly in Horro, may be under increased selection for parasite resistance and/or tolerance due to both the social and physical environments.
Implications and recommendations for breeding programs
A major obstacle for a successful breeding improvement intervention in rural areas is the high level of social and ecological diversity, which makes the identification of suitable breeding goals a difficult task. Our selective sweep analyses, together with our previous findings25, demonstrate that the genomes of the two chicken ecotypes are distinctly selected (Supplementary Fig. 4; see also Supplementary Note 2 and Supplementary Note 3). SweeD analysis, a likelihood based detection method of selective sweeps based on allele frequencies32, mapped a total of 51 genomic regions across the autosomal genome (GGA1–28) in both populations that had been subject to positive selection pressure, of which only 7 (13.7%) were common to both populations (Supplementary Fig. 5A). Moreover, calculation of integrated haplotype score statistics (iHS) at the intra-population level revealed 96 regions under selection pressure in Horro chickens, but only 31 in the Jarso population, with only 2 regions in common (Supplementary Fig. 5B). These results are consistent with the hypothesis that indigenous chickens from different geographic regions have developed distinct adaptive mechanisms responding to local selection pressures. This needs to be considered when designing breeding programs. We previously reported the lack of significant genetic correlations between infections and production traits in these populations, suggesting that selection for enhanced host defense against parasitism and viral and bacterial infection may not compromise productivity and vice versa25. Thus, balanced selection goals towards the enhancement of both productivity and health traits seem to present a valid means for the improvement of indigenous village chickens. However, trade-offs between infection tolerance (i.e. the ability of the host to withstand the pathological consequences of infection) and resistance (i.e. the ability of the host to control pathogen invasion or replication) may complicate the definition of clear goals in breeding programs aiming to improve chicken health. A breeding program aiming to increase tolerance is much easier to implement without the need of specialized phenotyping. However, in spatially segregated populations (as our results demonstrate village chickens to be) tolerance to infection will tend to maintain or increase local pathogen prevalence, whereas resistance will reduce prevalence33. Therefore, determining the relative advantages of tolerance versus resistance as a goal in a localized genetic improvement program may depend on the environment, economics farmers’ preference and attitudes toward investing in supportive measures, such as complementary infection control. However, to fully realize the benefit of a breeding intervention, the complex interactions between host population dynamics, genetic variation and the effects of parasites on host fecundity and mortality need to be well understood within local agro-ecological and socio-cultural environments, in order that these may be predicted and mitigated. For example, potentially adverse consequences of production changes to increase the number of chicks (which tend to be more susceptible to infection) reared at one time may include altered population susceptibility to, and/or transmission dynamics of, infection. As mitigation may entail additional costs and infrastructure (which may not be locally available), the economic and other implications of system perturbations resulting from interventions also need to be considered, as do the effects on farmers who are either unable or unwilling to invest in genetically improved birds, and whose flocks may potentially be put at increased risk by infection-tolerant birds supporting increased pathogen circulation.
Implications and recommendations for development programs utilizing poultry
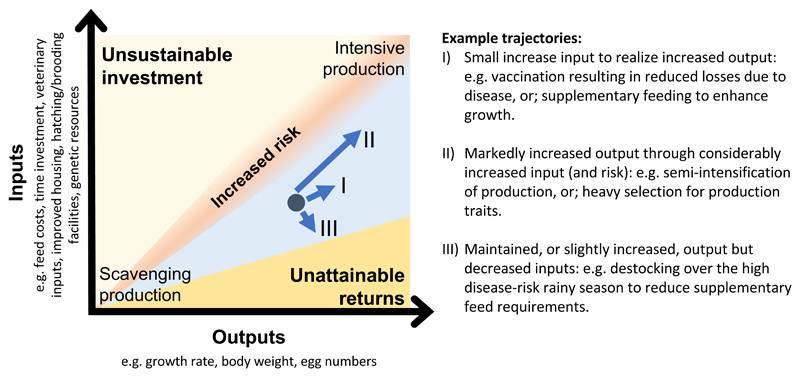
The opportunities and choice to move towards increased chicken production will vary greatly between individual farmers, and will depend on their individual situation and locality. Our results highlight important social and environmental differences between regions that may drive the need for different technologies to support increased production that should be taken into account within intervention planning. As summarise in Fig. 5, sustainable chicken production interventions need to identify an appropriate balance between inputs (such as supplementary feed, veterinary measures to prevent and control disease, improved housing, hatching or brooding facilities, enhanced genetic resource) and outputs (such as increased growth rates, body weights, egg numbers) and recognise that different farmers may prefer different trajectories of change, depending on individual-level factors (e.g. attitudes to risk, desired livelihood role for chicken production) and region-level factors (e.g. access to markets and services, environmental risks, cultural norms).
Fig. 5. Sustainable chicken production intervention framework.
Sustainable chicken production operates within a zone defined by inputs and outputs. The sustainable zone is delineated by regions that are unsustainable (where inputs exceed outputs) or unattainable (where outputs cannot be attained with given inputs). Within the sustainable zone, efficiency of production can be achieved by increasing outputs relative to inputs. Three examples of strategies toward enhanced production efficiency as illustrated.
In our study, Horro farmers expressed considerable interest in vaccines and are likely to be receptive to effective disease control programs, particularly against Newcastle disease, which have already demonstrated economic and social benefits in projects conducted in a number of developing countries4,6. Thus, for a relatively modest investment, Horro farmers may be able to realize the economic benefits of vaccination (Fig. 5: Trajectory I), provided they can access stable markets34. The same intervention programs may be less readily received in Jarso, where chickens have lower cultural and economic importance and where there is poor transport infrastructure, limited local market access and feed shortages. Thus, a prerequisite for successful up-scaling of poultry production in such regions will be the connection of farmers to stable markets, without which any investment may carry more risk than its equivalent in Horro due to the likely lower output for a given level of input. Where markets exist, greater increases in inputs, moving the farmer toward a semi-intensified production system, may enable greater output but may also increase risk (Fig. 5: Trajectory II). Alternatively, a breeding program could make available fertile eggs or young chicks, which farmers can either buy or contract rear, timed for sale during periods of peak demand. This may allow them to depopulate over the rainy season, which would minimize the losses to disease and decrease the supplementary feed requirements, thereby increasing efficiency (Fig. 5: Trajectory III).
However, the low current rate of chicken sale means that interventions to improve production, such as the introduction of improved birds, are unlikely to translate into substantial increase in income for many farmers under the current production systems, especially in Jarso. Furthermore, the observed correlation between wealth, flock size and utilization (both sale and consumption), which has also been noted in other studies35,36, and the relatively small proportion of the poorest people who participated in chicken sales, would suggest that this latter sector may not achieve the same benefit from interventions as more affluent farmers.
The low level of consumption in both woredas, but particularly Jarso, suggests that the potential nutritional benefits of poultry meat and eggs are not being realized. Alimentary habits, which may be subject to social taboos4, have been slow to change following previous successful poultry development projects4. Hence, successful nutritional impact from poultry development schemes will require further work, sensitive to possible variation between regions, to understand how an increase in production of these valuable sources of protein and micronutrients37,38 may be better incorporated into local diets.
Given the low use of chickens for sale and consumption, the primary role of chickens in poorer, more precarious, households is likely to be to provide a buffer from shocks and stresses in the subsistence agriculture system39. Therefore, in the short term, for such farmers it may be more important to reduce wastage and the risk of loss than to increase productivity for this sector, particularly as many smallholders believe additional inputs to chicken production would not be profitable40. Whilst selective breeding for enhanced resistance to specific key viral, bacterial and parasitic disease25 has the potential to bring benefits to farmers willing to adapt their production systems, it will necessarily require the adoption of new technologies, with associated additional costs.
Supporting recommendations from fieldworkers3,5 this study demonstrates that, as well as developing different strains of chickens appropriate to different localities, other flexibilities need to be built in to the program delivery, such as allowing (and assisting) farmers to determine their own capacity to support flocks of varying size, rather than dictating the number of chicks provided, as feed provision is a limiting factor for many farmers. However, the present analysis focused on the household level; given the important role of chicken production to women41 future studies explicitly examining specific gender aspects are warranted.
Conclusions
Using a combination of clinical, socio-economic and genomic studies, data provided by local communities and agricultural officers, and publicly available census, survey and bioclimatic data, we have established a detailed map of the chicken production systems in two diverse regions of Ethiopia. These data identified two distinct production systems and populations, with indigenous poultry adapted to not only the physical but also the economic and cultural environments. Our results reveal that the apparent ubiquity of chickens in village settings globally is obscuring the underlying divergence which is a consequence of distinct local adaptations that underpin sustainable chicken production in ecologically, culturally and economically diverse settings; we find that this diversity matters and should be considered in intervention programs.
Globally, poultry development programs need to consider how they can achieve the required flexibility to deliver interventions sustainably42 and to meet the requirements of “people in places”43. Many previous programs have operated according to prescriptive protocols, such as delivering a fixed number of pullets and cockerels according to an “ideal” ratio and requiring farmers to commit to investing additional time and resources into their poultry. Although this may be efficient in terms of program delivery, and allow these programs to claim success in terms of numbers of smallholders reached, in reality, rigid protocols achieve success only where they can be adapted and redefined to meet the primary goals of all actors in the process44. Drawing on recommendations from fieldworkers3,5, we conclude that enabling farmers to exercise greater flexibility in the development and implementation of future improvement programs in terms of the type of chicken used, how breeding strategies are implemented, the numbers of birds kept and the amount and type of inputs required may promote more widespread and sustainable adoption among those groups in society who are most dependent on this valuable resource.
Materials and methods
The study areas
This study was conducted in two geographically distinct woredas (local administrative districts) within the Oromia region in Ethiopia; Horro and Jarso. Oromia is the largest administrative region in the country and includes 180 woredas, which are further divided into kebeles encompassing several proximate communities or villages. Kebeles may be loosely grouped within one or more market sheds, which describe discrete areas of exchange of products along a network45. Horro woreda is located approximately 310 km west from the capital, Addis Ababa. Jarso woreda lies approximately 560 km east of Addis Ababa. Further details of the geography, climate, demography and agricultural practices of the two woredas are summarized in Supplementary Table 1.
Data collection
Primary data for this study were obtained from two linked studies; a rapid rural appraisal (RRA) conducted in February 2011 and a series of four repeated cross-sectional studies carried out between May 2011 and November 2012. Multistage sampling was used to select villages and participant households. Initially, two market sheds and two kebeles per market shed were selected within district in consultation with local representatives of the Department of Agriculture and local communities. For the RRA, focus group discussions (FGD) were organized comprising up to 50 farmers. We aimed to have an equal balance of male and female participants, but this was only achieved in some kebeles; female participation ranged from 20% to 50%. This may be due to a number of factors, including recruitment bias. Group discussions typically took two hours. Following the FGD, field visits were conducted in each of the kebeles to directly observe village poultry production systems. Discussions with key informants, such as district administrators and livestock extension officers were also undertaken. Data obtained through the RRA included: field notes derived from key informant and focus group interviews and during transect walks; and, quantitative data obtained through discussion and participatory exercises, including seasonal calendars and ranking and scoring of general livelihood activities, poultry production activities and constraints, and preferred bird characteristics.
For the cross-sectional study, systematic random sampling was used to select potential participants in each kebele from list of all household heads obtained from local agricultural development agents. Each kebele was visited four times; in May/June and October/November in each year of the study (2011 and 2012). Visits were timed for before and after the main rainy season (early May or June to September). Different households were selected on each occasion (25 from each kebele in May/June and October/November 2011, and 15 from each kebele in May/June and October/November 2012; total n=640), and visited by Ethiopian staff trained to collect field samples and conduct questionnaires in the local languages. Farmers were interviewed to confirm that no exotic birds or vaccinations had been used in their flock. Moreover, since studies on poultry infectious diseases and genomics were carried out simultaneously, farmers also needed to own two indigenous birds of at least six months of age in order to be included in the study. Farmers responded to questions on management, experience of poultry disease within the previous 12 months, and on important characteristics of poultry (for details, including questionnaires and clinical examination recording sheets, see26).
Two chickens over 6 months of age were randomly selected from each household flock. Where possible, one male and one female were chosen. Bird ages were estimated by the owner, who also provided data on the source of the bird and how long they had owned it. Each bird was weighed, body condition scored on a 0-3 scale46, and examined for a number of morphological characteristics (i.e. comb type, which is a mendelian trait, and plumage colour) and clinical parameters. The birds were scored for lice using a timed count of three areas of the body plus a total count of lice found under one wing and at the base of the tail feathers47. Faecal samples were taken from the basket used to contain the bird or from where the bird was observed to defecate after release. Fecal samples were stored and transported in a refrigerated container to the laboratory, where they were kept at 4 °C until processed. Blood samples were collected from a wing vein into 3.2% sodium citrate, and 0.5 ml was placed on an FTA card (Whatman, GE Healthcare Life Sciences, UK). Fecal and blood samples were examined for a range of pathogens (see48,49). For the present study, we focused on ascarids and lice parasitism for detailed investigation as they are important pathogens of village chickens and there is scarce relevant information regarding genetic resistance. We investigated the two most prevalent gastrointestinal ascarid nematodes; Ascaridia galli and Heterakis gallinarum, the eggs of which were identified using published keys28,50,51.
FTA cards were exported under DEFRA license (TARP/2011/245 and TARP/2012/352) to the University of Nottingham, UK. DNA was extracted from FTA cards as detailed in Smith and Burgoyne 52. A total of 760 birds matching the phenotype described above (384 from Horro and 376 from Jarso) with detailed phenotypes were genotyped using a genome-wide high density (600K) single nucleotide polymorphism (SNP) array (Affymetrix® Axiom® HD)53.
An additional 200 households, 25 in each kebele, were visited during the first round of surveys in May 2011. Selection of these households was identical to that described above, but without the criterion that required them to currently own two adult indigenous chickens. These households only completed the survey relating to the social and economic aspects of chicken keeping.
Data analysis
Data obtained during the farmer questionnaire surveys were analyzed using descriptive statistics and comparisons between woredas were made using chi-squared tests for categorical data and Mann-Whitney tests for continuous data (which was almost universally non-normally distributed). Prior to analysis, data on livestock holdings in each household were converted to a Tropical Livestock Unit (TLU) following Storck et al.27. TLUs adjust for the size and weight of species kept and facilitates comparison between individuals and areas. Parasite aggregations were calculated using the corrected moment estimate, k,54 and the index of discrepancy55. Parasite aggregation was compared between woredas using Kolmogorov-Smirnov tests. Factors associated with bird-level characteristics were identified using mixed effects regression analysis in the lme4 package56. Analyses were performed using R version 3.2.257. Field notes collected during the rapid rural appraisal were analyzed using thematic analysis. Data from the ranking and scoring exercises were tabulated for each woreda.
Climatic conditions between each region were compared using ecological niche modelling58, which applied the climatic conditions of each region to make predictions of the potential distribution of the ecotype found in one region onto other regions. We used the maximum entropy algorithm implemented by Maxent, which estimates the distribution probability of a species using occurrence data, and a set of environmental predictors59 that are commonly used as indicators of annual trends in seasonality, temperature and precipitation60. Initially, we modelled the current potential distribution of each population using 21 environmental variables at 1 km x 1 km resolution as predictors of habitat distribution. As indicators of climatic tolerances, 19 bioclimatic variables and one elevation layer obtained from WorldClim60 were used. As chicken production is linked to smallholder farming2, we used a land cover variable as a proxy to agricultural systems, share of total cultivated land, obtained from the Harmonized World Soil Database v1.261. We used the default settings and additionally selected the minimum training presence threshold and the logistic output format, which generates a probability of occurrence that ranges from 0, low probability of occurrence, to 1, high probability of occurrence, making it easier to interpret. Then, to evaluate if the model for each population would classify as climatically suitable areas where the other ecotype is located, we projected the generated models of each ecotype onto the geographic area of the other. To not restrict our analyses to the woreda administrative boundaries, we extended each area 50 km in all directions. We validated the models using the receiver operating characteristic (ROC) curve and a binomial test of omission (40). The analyses were developed within R version 3.2.257 using the dismo package62
For the selective sweep analysis, 760 chickens (384 from Horro and 376 from Jarso) and 391,384 SNPs that passed the quality control (see Desta63 for details) were used. The R package rehh64 was used to map selective sweeps of genetic markers subjected to moderate selection pressure using iHS statistics, while SweeD32 was used to identify candidate genomic regions that have been subjected to strong selection at intra-population level. The same birds and genotyping data as for the selective sweep analysis were used in GWAS analyses together with relevant phenotypes to detect SNP markers associated with resistance to ascarids and lice parasitism as well as rose versus simple comb. Multidimensional scaling analysis was performed to identify if the two populations were distinct and if further population substructure was present using the GenABEL package of R65. This identified two distinct populations that exactly matched the origin of the birds (in Horro or Jarso; Supplementary Figure 4). GWAS analyses for disease traits were performed using the PLINK v1.966 and GEMMA v0.9467 software and the univariate linear mixed model described previously by Psifidi et al.25; while GenABEL package65 was used to map genomic regions associated with the rose comb phenotype (for details see Desta63). After Bonferroni correction for multiple testing, the significance thresholds were set at P ≤ 1.2 x 10-7 and P ≤ 2.5 x 10-6 for genome-wide (P ≤ 0.05) and suggestive (one false positive per genome scan) levels, respectively. The candidate regions harboring the genes and mutations responsible for resistance to ascarids and lice parasitism, as well as rose comb, were defined as the genomic intervals 100kb upstream and downstream of the significant markers identified in GWAS, based on the average linkage disequilibrium estimated for the two indigenous ecotypes25. The candidate genomic regions for disease traits and rose comb mutation identified in GWAS analyses were compared with targets of signatures of selection in the same data. We used the BioMart data mining tool within the Ensembl genome browser (http://www.ensembl.org/biomart/martview/) and the Galgal5 assembly of the chicken reference genome to identify the genes located in the candidate genomic regions for rose comb, ascarids and lice parasitism resistance, as well as in selective sweep regions.
Data availability
The bioclimatic variables that used in this study are available in “WorldClim” (http://www.worldclim.org/). The land cover variable data is available in “Harmonized World Soil Database” (http://webarchive.iiasa.ac.at/Research/LUC/External-World-soil-database/HTML/index.html?sb=1). All other data that support the findings of this study are available from the corresponding author upon reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
We thank the Chicken Health for Development project team members and the farmers and development agents in the Jarso and Horro districts for their assistance. We also thank David Hume and Georgios Banos for helpful comments on drafts of the manuscript.
We thank Biotechnology and Biological Sciences Research Council (BBSRC), the UK Department for International Development (DFID) and the Scottish Government for providing funding for the ‘Reducing the impact of infectious disease on poultry production in Ethiopia’ project under the Combating Infectious Diseases of Livestock for International Development (CIDLID) program (BB/H009396/1, BB/H009159/1 and BB/H009051/1). JB is supported by CGIAR fund donors http://www.cgiar.org/our-funders/.
Footnotes
Competing Interests
The authors declare not competing interests.
References
- 1.Mack S, Hoffmann D, Otte J. The contribution of poultry to rural development. World Poultry Sci J. 2005;61:7–14. [Google Scholar]
- 2.Alders RG, Pym RAE. Village poultry: still important to millions, eight thousand years after domestication. World Poultry Sci J. 2009;65:181–190. [Google Scholar]
- 3.FAO. Decision tools for family poultry development. Food and Agriculture Organization; 2014. [Google Scholar]
- 4.Bagnol B. The Social Impact of Newcastle Disease Control. In: Alders RG, Spradbrow P, editors. SADC planning workshop on Newcastle Disease control in village chickens. Australian Centre for International Agricultural Research; 2000. [Google Scholar]
- 5.Ahlers C, et al. Improving village chicken production: a manual for fieldworkers and trainers. ACIAR Monograph No. 139. 2009.
- 6.Dwinger RH, Unger H. Improving farmyard poultry production in Africa: Interventions and their economic assessment. International Atomic Energy Agency (IAEA); pp. 1–9. [Google Scholar]
- 7.Pica-Ciamarra U, Dhawan M. A Rapid Rural Appraisal of the Family-Based Poultry Distribution Scheme of West Bengal, India. Pro-Poor Livestock Policy Initiative (PPLPI) Research Report (FAO); 2009. [Google Scholar]
- 8.Sonaiya EB. Constraints to adoption and sustainability of improved practices in scavenging poultry systems. Family Poultry Communications. 2012;21:34–43. [Google Scholar]
- 9.Khobondo JO, et al. Genetic and nutrition development of indigenous chicken in Africa. Livestock Res Rural Dev. 2015;27:122. [Google Scholar]
- 10.Saleque MA, Mustafa S. Landless Women And Poultry: The BRAC Model in Bangladesh. In: Dolberg F, Petersen PH, editors. Integrated Farming in Human Development: Proceedings of a workshop; 1996. pp. 37–55. [Google Scholar]
- 11.FAO. Poultry in the 21st Century: avian influenza and beyond. In: Thieme O, Pilling D, editors. Proceedings of the International Poultry Conference; Rome: FAO Animal Production and Health Proceedings; 2008. No.9. [Google Scholar]
- 12.Report on livestock and livestock characteristics (Private peasant holdings) Central Statistical Agency; 2010/11. [Google Scholar]
- 13.Dessie T, Jobre Y. A review of the importance and control of Newcastle disease in Ethiopia. Ethiop Vet J. 2004;8:71–81. [Google Scholar]
- 14.FAO. Poultry Sector Country Review. Food and Agriculture Organization of the United Nations; Rome: 2008. [Google Scholar]
- 15.Dinka H, Chala R, Dawo F, Bekana E, Leta S. Major Constraints and Health Management of Village Poultry Production in Rift Valley of Oromia, Ethiopia. Am Eurasian J Agric Environ Sci. 2010;9:529–533. [Google Scholar]
- 16.Mazengia H, Bekele ST, Negash T. Incidence of infectious bursal disease in village chickens in two districts of Amhara Region, Northwest Ethiopia. Livestock Res Rural Dev. 2009;21 [Google Scholar]
- 17.Dana N, van der Waaij LH, Dessie T, van Arendonk JA. Production objectives and trait preferences of village poultry producers of Ethiopia: implications for designing breeding schemes utilizing indigenous chicken genetic resources. Trop Anim Health Prod. 2010;42:1519–1529. doi: 10.1007/s11250-010-9602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson J, Scoones I. Addressing the dynamics of agri-food systems:an emerging agenda for social science research. Environ Sci Policy. 2009;12:386–397. [Google Scholar]
- 19.Brown BJ, Hanson ME, Liverman DM, Merideth RW. Global sustainability: Toward definition. Environ Manage. 1987;11:713–719. [Google Scholar]
- 20.Gibson RB. Specification of sustainability-based environmental assessment decision criteria and implications for determining “significance” in environmental assessment. Research and Development Program, Canadian Environmental Assessment Agency; 2001. [Google Scholar]
- 21.Fresco LO. Challenges for food system adaptation today and tomorrow. Environ Sci Policy. 2009;12:378–385. [Google Scholar]
- 22.Desta TT, et al. Signature of artificial selection and ecological landscape on morphological structures of Ethiopian village chickens. Anim Genet Resour. 2013;52:17–29. [Google Scholar]
- 23.Mengesha M, Tsega W. Phenotypic and genotypic characteristics of indigenous chickens in Ethiopia: A review. Afr J Agric Res. 2011;6:5398–5404. [Google Scholar]
- 24.Imsland F, et al. The rose-comb mutation in chickens constitutes a structural rearrangement causing both altered comb morphology and defective sperm motility. PLoS Genet. 2012;8:e1002775. doi: 10.1371/journal.pgen.1002775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Psifidi A, et al. Genome-wide association studies of immune, disease and production traits in indigenous chicken ecotypes. Genet Sel Evol. 2016;48:74. doi: 10.1186/s12711-016-0252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettridge J. The epidemiology and ecology of infectious diseases in Ethiopian village chickens and the role of co-infection in infection risk. PhD thesis; University of Liverpool: 2014. [Google Scholar]
- 27.Storck H, Emana B, Adenew B, Borowiecki A, W/Hawariat S. Farming systems and farm management practices of small holders in the Hararghe highlands. Wissenschaftsverlag Vauk; Kiel, Germany: 1991. [Google Scholar]
- 28.Permin A, Hansen JW. Epidemiology, Diagnosis and Disease Control of Poultry Parasites. Food and Agriculture Organization; 1998. [Google Scholar]
- 29.Bishop SC, Wooliams JA. On the genetic interpretation of disease data. PLoS One. 2010;5:e8940. doi: 10.1371/journal.pone.0008940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishop SC, Doeschl-Wilson AB, Woolliams JA. Uses and implications of field disease data for livestock genomic and genetics studies. Front Genet. 2012;3:114. doi: 10.3389/fgene.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson PJ, Dobson AP, Newborn D. Prevention of population cycles by parasite removal. Science. 1998;282:2256–2258. doi: 10.1126/science.282.5397.2256. [DOI] [PubMed] [Google Scholar]
- 32.Pavlidis P, Živković D, Stamatakis A, Alachiotis N. SweeD: Likelihood-based detection of selective sweeps in thousands of genomes. Mol Biol Evol. 2013;30:2224–2234. doi: 10.1093/molbev/mst112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horns F, Hood ME. The evolution of disease resistance and tolerance in spatially structured populations. Ecol Evol. 2012;2:1705–1711. doi: 10.1002/ece3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Queenan K, et al. An appraisal of the indigenous chicken market in Tanzania and Zambia. Are the markets ready for improved outputs from village production systems? Livestock Res Rural Dev. 2016;28:185. [Google Scholar]
- 35.Aklilu HA, Udo HMJ, Almekinders CJM, Van der Sijpp AJ. How resource poor households value and access poultry: Village poultry keeping in Tigray, Ethiopia. Agric Syst. 2008;96:175–183. [Google Scholar]
- 36.Tadelle D, Million T, Alemu Y, Peters KJ. Village chicken production systems in Ethiopia: 2. Use patterns and performance valuation and chicken products and socio-economic functions of chicken. Livestock Res Rural Dev. 2003;15 [Google Scholar]
- 37.Calloway DH, et al. Village Nutrition in Egypt, Kenya and Mexico: Looking Across the CRSP Projects. University of California; 1992. [Google Scholar]
- 38.Marangoni F, et al. Role of poultry meat in a balanced diet aimed at maintaining health and wellbeing: an Italian consensus document. Food Nutr Res. 2015;59:27606. doi: 10.3402/fnr.v59.27606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halderman M. PPLPI Working Paper No. 19. Pro-Poor Livestock Policy Initiative, Food and Agricultural Organization; 2004. The political economy of pro-poor livestock policy-making in Ethiopia. [Google Scholar]
- 40.Wondmeneh E, Van der Waaij EH, Udo HMJ, Tadelle D, Van Arendonk JAM. Village poultry production system: Perception of farmers and simulation of impacts of interventions. Afr J Agric Res. 2016;11:2075–2081. [Google Scholar]
- 41.FAO. Smallholder poultry production – livelihoods, food security and sociocultural significance. FAO Smallholder Poultry Production Paper No. 4. 2010.
- 42.Vaarst M, Steenfeldt S, Horsted K. Sustainable development perspectives of poultry production. World Poultry Sci J. 2015;71:609–620. [Google Scholar]
- 43.Zussman R. People in places. Qual sociol. 2004;27:351–363. [Google Scholar]
- 44.Timmermans S, Berg M. Standardization in Action: Achieving Local Universality through Medical Protocols. Soc Stud Sci. 1997;27:273–305. [Google Scholar]
- 45.FAO. Seeds, Diversity and Development - Key concepts. Food and Agriculture Organization; 2014. [Google Scholar]
- 46.Gregory NG, Robins JK. A body condition scoring system for layer hens. New Zeal J Agr Res. 1998;41:555–559. [Google Scholar]
- 47.Clayton DH, Drown DM. Critical evaluation of five methods for quantifying chewing lice (Insecta: Phthiraptera) J Parasitol. 2001;87:1291–1300. doi: 10.1645/0022-3395(2001)087[1291:CEOFMF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 48.Bettridge JM, et al. Infection-interactions in Ethiopian village chickens. Prev Vet Med. 2014;117:358–366. doi: 10.1016/j.prevetmed.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luu L, et al. Prevalence and molecular characterisation of Eimeria species in Ethiopian village chickens. BMC Vet Res. 2013;9:208. doi: 10.1186/1746-6148-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lapage G. Veterinary Parasitology. Oliver and Boyd; London, England: 1956. [Google Scholar]
- 51.Soulsby EJL. Helminths, Arthropods and Protozoa of Domesticated Animals. 7th edn. Bailliere and Tindall; East Sussex, UK: 1982. [Google Scholar]
- 52.Smith LM, Burgoyne LA. Collecting archiving and processing DNA form wildlife samples using FTA databasing paper. BMC Ecol. 2004;4:4. doi: 10.1186/1472-6785-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kranis A, et al. Development of a high density 600K SNP genotyping array for chicken. BMC Genomics. 2013;14:59. doi: 10.1186/1471-2164-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gregory RD, Woolhouse MEJ. Quantification of parasite aggregation: A simulation study. Acta Trop. 1993;54:131–139. doi: 10.1016/0001-706x(93)90059-k. [DOI] [PubMed] [Google Scholar]
- 55.Poulin R. The disparity between observed and uniform distributions: a new look at parasite aggregation. Int J Parasitol. 1993;23:937–944. doi: 10.1016/0020-7519(93)90060-c. [DOI] [PubMed] [Google Scholar]
- 56.Bates D, Maechler M, Bolker B, Walker S. lme4: Linear Mixed-Effects Models using 'Eigen' and S4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 57.R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2016. Available: http://www.R-project.org. [Google Scholar]
- 58.Peterson AT. Predicting species geographic distributions based on ecological niche modeling. Condor. 2001;103:599–605. [Google Scholar]
- 59.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006;190:231–259. [Google Scholar]
- 60.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 61.FAO/IIASA/ISRIC/ISSCAS/JRC. Harmonized World Soil Database (version 1.2) 2012.
- 62.Species distribution modeling. R package version 1.1-4. 2017.
- 63.Desta TT. Phenomic and genomic landscape of Ethiopian village chicken. PhD thesis; University of Nottingham: 2015. [Google Scholar]
- 64.Gautier M, Vitalis R. rehh: An R package to detect footprints of selection in genomewide SNP data from haplotype structure. Bioinformatics. 2012;28:1176–1177. doi: 10.1093/bioinformatics/bts115. [DOI] [PubMed] [Google Scholar]
- 65.Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23:1294–1296. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
- 66.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou X, S M. Efficient multivariate linear mixed model algorithms for genome-wide association studies. Nat Methods. 2014;11:407–409. doi: 10.1038/nmeth.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The bioclimatic variables that used in this study are available in “WorldClim” (http://www.worldclim.org/). The land cover variable data is available in “Harmonized World Soil Database” (http://webarchive.iiasa.ac.at/Research/LUC/External-World-soil-database/HTML/index.html?sb=1). All other data that support the findings of this study are available from the corresponding author upon reasonable request to the corresponding author.