Abstract
A beta-elimination reaction generally involves the cleavage of a sigma (σ) bond at the position beta (β) to a pair of electrons that departs a molecule via a nucleophilic leaving group, subsequently leading to the formation of a new pi (π) bond. We describe the importance of β-elimination reactions in the mechanisms of action of two classes of chemotherapeutic agents. First, we evaluate the chemical steps resulting in formation of 5-methyl-cytosine and its disassociation from DNA methytransferase (DNMT) by β-elimination reaction. When carbon 5 (C5) of cytosine is substituted with a nitrogen atom (N) in 5-aza-cytosine analogues, the critical β-elimination reaction cannot proceed, which results in the permanent attachment of 5-aza-cytosine to DNMT. The net outcome is entrapment of the DNMT by 5-aza-cytosine analogues and its eventual degradation, leading to DNA hypomethylation. Second, we analyze the critical role of β-elimination reaction in the activation of cyclophosphamide and ifosfamide. The incapability of undergoing β-elimination results in reduction of the cytotoxic activity of these agents. It appears that the conversion of aldehyde group, in aldophosphamide metabolites of cyclophosphamide and ifosfamide, to carboxyl group by aldehyde dehydrogenase makes the protons on the carbon atom attached to carboxyl group not acidic enough that can be removed under physiologic conditions via initiation of the critical β-elimination reaction. This ultimately culminates in selective cytotoxic effect of these agents against lymphocytes but not hematopoietic and other stem cells with high aldehyde dehydrogenase content.
β-Elimination Reactions
β-elimination reactions play an integral role in normal physiologic function and in activation of prodrugs into active compounds. Both of these scenarios represent key steps that several antineoplastic therapeutic agents exploit in order to achieve clinical activity and efficacy. These include, but not limited to, the hypomethylating agents such as azacitidine and decitabine, and the nitrogen-mustard alkylating agents cyclophosphamide and ifosfamide. It is thus important to have a basic understanding of the intricacies of β-elimination reactions and how they participate in the clinical activity of these agents.
In organic chemistry, an elimination reaction generally involves the cleavage of a sigma (σ) bond and departure of a nucleophilic leaving group, subsequently leading to the formation of a new pi (π) bond (Figure 1) [1]. This process is called β-elimination when the σ bond beta (β) to the pair of nucleophilic electrons breaks in order to create the new π bond. The formation of the π bond may occur by a one- or two-step process via bimolecular or unimolecular elimination, respectively. Unimolecular β-elimination (E1) involves only one molecular entity and represents a two-step process in which: (1) a carbocation intermediate is formed after dissociation of carbon-nucleophile bond; and (2) the carbocation intermediate is deprotonated. Bimolecular β-elimination (E2), on the other hand, involves a one-step process with two molecular entities in which a Lewis base with a lone pair of electrons removes one hydrogen (proton) from the alpha carbon (Cα) to form a double bond between Cα and the beta carbon (Cβ) while a nucleophile (e.g. halide) simultaneously leaves Cβ (Figure 1) [1].
Figure 1.
Top: The mechanism of the bimolecular β-elimination reactions (E2). Simultaneously, (1) a lone pair of electrons from a Lewis base removes a proton (H) from Cα, (2) the pair of electrons between Cα and H forms a new π bond between Cα and Cβ, which (3) promotes the loss of a leaving group (Nu*). Bottom: An E2 β-elimination reaction results in formation of a π bond between Cα and Cβ (double bond or alkene) after departure of a sulfide-containing moiety (:S-R). Professional illustration by John Ott.
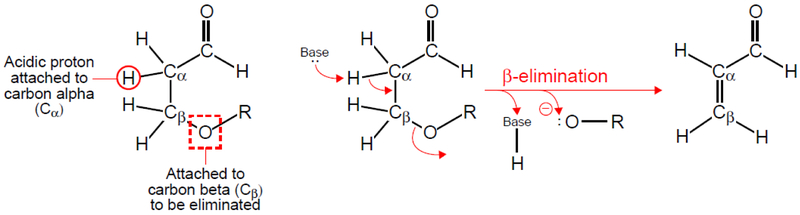
A specific form of β-elimination is called the Elimination Unimolecular Conjugate Base (E1cB) mechanism, which occurs under basic conditions. In an E1cB elimination reaction, there are two steps: (1) an acidic proton on the Cα adjacent to a carbonyl group is removed by a base; and (2) the elimination occurs through formation of a carbanion intermediate that is the conjugate base of the original molecule, in which the negative charge is stabilized by conjugation (i.e. delocalization of electrons) with a carbonyl group (Figure 2) [1].
Figure 2.
An E1cB β-elimination reaction resulting in the formation of acrolein (propenal). Cα is attached to an aldehyde (carbonyl group), and the proton attached to Cα is an acidic proton, which can be removed by a base resulting in formation of a double bond between Cα and Cβ after departure of an oxide-containing moiety (:O-R). Professional illustration by John Ott.
β-Elimination in Azacitidine and Decitabine
Azacitidine and decitabine are DNA methyltransferase inhibitors (DNMTI), which are approved by the United States Food and Drug Administration (FDA) for the treatment of patients with myelodysplastic syndrome (MDS) [2,3]. In the last decade, these agents have been widely used as an alternative to the standard cytarabine and anthracycline-based chemotherapy for treatment of patients with acute myeloid leukemia (AML) who cannot tolerate or have progressed on conventional cytotoxic chemotherapy [4]. A 20–40% overall response rate to DNMTIs in patients with AML has been reported with overall survival rates comparable to those seen with cytotoxic agents [5–7].
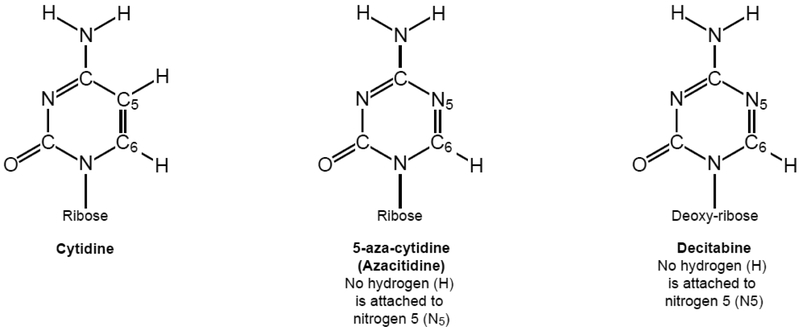
Azacitidine (5-aza-cytidine) and decitabine (5-aza-2’-deoxycytidine) are pyrimidine analogs of cytidine, in which the carbon 5 (C5) of cytosine is substituted with a nitrogen atom (N). Nitrogen 5 (N5) forms three bonds with the two carbons in the cytosine pyrimidine ring and, as opposed to C5, is not attached to a hydrogen (Figure 3).
Figure 3.
The chemical structures of cytidine, azacitidine and decitabine showing their similarities and differences.
The exact mechanism of action of azacitidine and decitabine is not yet fully known. It is believed that the main mechanism through which these agents exert their antineoplastic activity is via the inhibition of DNA methyltransferases culminating in DNA hypomethylation, particularly in the promoter region of tumor suppressor genes [8]. For this reason, these agents are commonly called hypomethylating agents [9].
β-elimination plays an important role in the physiologic process of cytosine methylation mediated by DNA methyltransferase (Figure 4). With DNMTIs, the substitution of N5 in place of C5 prevents the occurrence of the β-elimination reaction, which results in the permanent attachment of azacitidine or decitabine to the DNA methyltransferase enzyme and ultimately to the degradation of the enzyme. After recognition by the DNA methyltransferase, cytosine is positioned in the enzyme by hydrogen bonds. The process of methylation on C5 is initiated by a nucleophilic attack on C6 by a cysteine residue on the DNA methyltransferase forming a covalent bond with the cytosine ring through an addition reaction. Next, the electrons are pushed from the double bond between C6 and C5 to form a double bond between C5 and C4 of the cytosine ring, which in turn results in protonation of N3 [10]. The reverse flow of electrons results in cleavage of the π bond between C5 and C4 and formation of a new σ bond between C5 and a methyl group, which is originated from S-adenosyl-methionine (SAM), a universal methyl group donor. 5-methyl-cytosine dissociates from DNA methyltransferase through a critical β-elimination reaction which results in the simultaneous removal of a proton, formation of a double bond between C6 and C5, and loss of the sulfide-containing leaving group from the DNA methyltransferase [10]. The ultimate product is formation of 5-methylcytosine and availability of DNA methyltransferase for future methylation reactions (Figure 4, Top).
Figure 4.
Top: The chemical reactions result in formation of 5-methylcytosine and its release from DNA methyltransferase mediated by a β-elimination reaction. Bottom: The mechanism by which 5-aza-cytosine analogues are methylated. Due to the absence of the β-elimination reaction they are not released from DNA methyltransferase, resulting in its degradation and unavailability for future methylation reactions, which culminates in therapeutic hypomethylation of DNA. Professional illustration by John Ott.
When 5-aza-cytosine analogues are used, they are incorporated into the DNA methyltransferase enzymes instead of the cytosine. The process of methylation of N5 is very similar to methylation of C5. However, due to the lack of a hydrogen (proton) attached to the N5, the critical β-elimination reaction cannot proceed. The lack of β-elimination results in the permanent attachment of 5-aza-cytosine to DNA methyltransferase via the covalent bond between sulfide and C6. The net outcome is entrapment of the DNA methyltransferase enzyme by 5-aza-cytosine analogues and its eventual degradation, leading to hypomethylation and achievement of the desired antineoplastic effects [11,12].
β-Elimination in Cyclophosphamide and Ifosfamide
Cyclophosphamide and ifosfamide are nitrogen mustard-based alkylating agents that are used for treatment of many solid and hematologic neoplasms (Figure 5) [13,14]. In contrast to DNMTIs, which exert their therapeutic effects via prevention of β-elimination, these agents require β-elimination for their activation. Once activated by β-elimination, cyclophosphamide and ifosfamide can create DNA cross-links, interfering with DNA replication and leading to cell death.
Figure 5.
The chemical structures of cyclophosphamide and ifosfamide.
Cyclophosphamide and ifosfamide are hydroxylated at C4 in the hepatic microsomal cytochrome P450 system and are converted to 4-hydroxycyclophosphamide and 4-hydroxyifosfamide, respectively (Figure 6). Both of these molecules are in equilibrium with their aldehyde tautomers, aldophosphamide. The protons on Cα of aldo tautomers of cyclophosphamide and ifosfomide are acidic enough to promote a base-catalyzed β-elimination reaction via the E1cB mechanism (Figure 2) to form active metabolites, phosphoramide mustards and the leaving group acrolein. Acrolein, or propenal, is a very reactive α-β unsaturated aldehyde responsible for hemorrhagic cystitis after administration of cyclophosphamide or ifosfamide [13]. The phosphoramide mustard forms an unstable reactive aziridinium ring, which readily reacts with different nucleophiles (e.g. N7 of a guanine base in DNA) forming interstrand and intrastrand crosslinks leading to DNA alkylation and apoptosis of the cells [13].
Figure 6.
The mechanism of action activation of cyclophosphamide and ifosfamide underscoring the importance of the E1cB β-elimination reaction. Professional illustration by John Ott.
The role of the β-elimination reaction is critical in the activation of cyclophosphamide and ifosfamide. The inability of undergoing β-elimination results in the lack of cytotoxic activity of these agents. Aldophosphamides can be oxidized to carboxyphosphamides by aldehyde dehydrogenases which are found in high levels within hematopoietic and other stem cells, but not in mature lymphocytes. The protons on Cα attached to carboxyl group are not acidic enough that they can be removed under physiologic conditions via initiation of the critical E1cB-mediated β-elimination reaction necessary for activation of these compounds. The conversion of aldehyde group to carboxyl group by aldehyde dehydrogenase of stem cells culminates in selective cytotoxic effect of cyclophosphamide against lymphocytes but not stem cells. In recent years, administration of high-dose cyclophosphamide after allogeneic blood or bone marrow transplantation has resulted in reducing the rates of graft-versus-host disease (GVHD) by selective removal of allo-reactive T cells, but not donor stem cells, which in turn has expanded the donor pool for many patients by allowing successful use of human leukocyte antigen (HLA)-haploidentical grafts [15].
In conclusion, a basic understanding of β-elimination reactions and the central role they play in the mechanism of action of the 5-aza-pyrimidine analogues discussed here is imperative to optimize these agents’ full potential for clinical efficacy. Further, it is important to appreciate the effect of the fine balance between more acidic and less acidic proton on carbons attached to the carbonyl groups of aldehyde versus carboxyl groups for initiation of the required E1cB β-elimination reaction for activation of cyclophosphamide and ifosfamide.
Footnotes
Authors declare no relevant conflict of interest.
References:
- 1.Clayden J; Greeves N; Warren S Organic chemistry. 2nd Edition ed.; Oxford University Press: New York, 2012. [Google Scholar]
- 2.Kaminskas E; Farrell A; Abraham S; Baird A; Hsieh LS; Lee SL; Leighton JK; Patel H; Rahman A; Sridhara R, et al. Approval summary: Azacitidine for treatment of myelodysplastic syndrome subtypes. Clinical cancer research : an official journal of the American Association for Cancer Research 2005, 11, 3604–3608. [DOI] [PubMed] [Google Scholar]
- 3.Duong VH; Komrokji RS; List AF Update on the pharmacotherapy for myelodysplastic syndromes. Expert opinion on pharmacotherapy 2014, 15, 1811–1825. [DOI] [PubMed] [Google Scholar]
- 4.Tawfik B; Sliesoraitis S; Lyerly S; Klepin HD; Lawrence J; Isom S; Ellis LR; Manuel M; Dralle S; Berenzon D, et al. Efficacy of the hypomethylating agents as frontline, salvage, or consolidation therapy in adults with acute myeloid leukemia (aml). Annals of hematology 2014, 93, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blum W; Garzon R; Klisovic RB; Schwind S; Walker A; Geyer S; Liu S; Havelange V; Becker H; Schaaf L, et al. Clinical response and mir-29b predictive significance in older aml patients treated with a 10-day schedule of decitabine. Proceedings of the National Academy of Sciences of the United States of America 2010, 107, 7473–7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatnagar B; Duong VH; Gourdin TS; Tidwell ML; Chen C; Ning Y; Emadi A; Sausville EA; Baer MR Ten-day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leukemia & lymphoma 2014, 55, 1533–1537. [DOI] [PubMed] [Google Scholar]
- 7.Quintas-Cardama A; Ravandi F; Liu-Dumlao T; Brandt M; Faderl S; Pierce S; Borthakur G; Garcia-Manero G; Cortes J; Kantarjian H Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 2012, 120, 4840–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim TK; Gore SD; Zeidan AM Epigenetic therapy in acute myeloid leukemia: Current and future directions. Seminars in hematology 2015, 52, 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths EA; Gore SD Epigenetic therapies in mds and aml. Advances in experimental medicine and biology 2013, 754, 253–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stresemann C; Lyko F Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. International journal of cancer 2008, 123, 8–13. [DOI] [PubMed] [Google Scholar]
- 11.Baylin SB DNA methylation and gene silencing in cancer. Nature clinical practice. Oncology 2005, 2 Suppl 1, S4–11. [DOI] [PubMed] [Google Scholar]
- 12.Fukushige S; Horii A DNA methylation in cancer: A gene silencing mechanism and the clinical potential of its biomarkers. The Tohoku journal of experimental medicine 2013, 229, 173–185. [DOI] [PubMed] [Google Scholar]
- 13.Emadi A; Jones RJ; Brodsky RA Cyclophosphamide and cancer: Golden anniversary. Nature reviews. Clinical oncology 2009, 6, 638–647. [DOI] [PubMed] [Google Scholar]
- 14.Takimoto CH; Calvo E Principles of oncologic pharmacotherapy. Edition 11th ed.; 2008. [Google Scholar]
- 15.Robinson TM; O’Donnell PV; Fuchs EJ; Luznik L Haploidentical bone marrow and stem cell transplantation: Experience with post-transplantation cyclophosphamide. Seminars in hematology 2016, 53, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]