Abstract
The field of vascular biology has gained enormous insight from the use of Cre and Cre/ERT2 mouse models to temporally and spatially manipulate gene expression within the endothelium. Models are available to constitutively or inducibly modulate gene expression in all, or a specified subset of endothelial cells (ECs). However, caution should be applied to both the selection of allele and the analysis of resultant phenotype: many similarly-named Cre models have divergent activity patterns, while ectopic or inconsistent Cre or Cre/ERT2 expression can dramatically affect results. In an effort to disambiguate previous data and to provide a resource to aid appropriate experimental design, here we summarise what is known about Cre recombinase activity in the most widely used endothelial-specific Cre and Cre/ERT2 mouse models.
Keywords: Tie2-Cre, VE-Cadherin-Cre, Cdh5-Cre/ERT2, Pdgfb-Cre, Sox17-Cre, Nfatc1-Cre, Bmx-Cre/ERT2, Apelin-Cre/ERT2
Subject codes: Angiogenesis, Basic Science Research, Developmental Biology, Vascular Biology, Endothelium/Vascular Type/Nitric Oxide, Genetically Altered and Transgenic Models
Introduction
The Cre-loxP system
The Cre-loxP system is a powerful genetic tool that allows mice with tissue-specific genetic loss or gain of function to be generated with relative ease. Cre recombinase, a protein from the bacteriophage P1, binds a 34bp loxP recognition sequence. Upon binding to two loxP sites in the same orientation, Cre recombinase causes any intervening DNA to be excised from the genome leaving only a single loxP site 1–4. Coding regions of interest can therefore be flanked with loxP sites such that they are removed from the genome upon Cre binding. Restricting the expression of Cre recombinase with specific enhancer/promoter elements therefore allows for spatial control of loxP-mediated excisions. Temporal control of gene excision beyond that which can be regulated by promoter/enhancer activity can be provided by using the modified Cre/ERT2, in which Cre recombinase activity must be actively induced by tamoxifen administration.
Determining expression patterns of Cre and Cre/ERT2 mouse alleles
Successful gene targeting with Cre and Cre/ERT2-loxP is critically determined by the precise expression pattern of Cre recombinase. A single Cre-mediated excision event is passed on during cell division to all subsequent daughter cells. In this way, Cre recombination is able to mark cell lineages. It follows, therefore, that brief expression of Cre in an unreported cell type early in development can have profound consequences on any later phenotype or fate-mapping experiment. So too can lack of Cre expression in a small number of cells within a theoretically Cre-positive population 5. Given the dynamic nature of spatio-temporal gene expression, it can sometimes be challenging to predict or detect these events. Further, copy number variation and site of integration effects can strongly influence the expression patterns of transgenes, such that independently-derived transgenic mouse alleles driving Cre or Cre/ERT2 with identical regulatory sequences may have significantly different Cre expression patterns 6. Consequently, many endothelial-specific Cre and Cre/ERT2 alleles have some degree of unreported activity, inconsistent recombination efficiency and/or parent of origin effects. Generational drift can also strongly influence outcomes, as Cre expression may be lost or altered in mice many generations downstream of those originally characterized. All these variables can lead to results that are difficult to interpret and in some cases seemingly contradictory. A thorough characterisation of Cre expression in each different mouse model is therefore essential in order to generate meaningful data.
Spatial information on Cre recombinase activity can be gained using Cre reporter mice, in which a reporter gene (e.g, GFP, YFP, lacZ) is inserted at a ubiquitously expressed locus but preceded by a loxP-flanked transcriptional termination (tpA) sequence. Therefore, the reporter gene is transcribed only when Cre mediated recombination has excised the termination sequence. The most common of these reporter mice is the R26R-LacZ, where loxP-tpA-loxP precedes the β-gal gene at the ubiquitously expressed ROSA26 locus 7,8. Because unanticipated Cre activity can vary between mice, it is imperative that such analyses extend to testing multiple different mice expressing the Cre transgene, preferably from different breeding pairs 9. However, although Cre reporter mice provide excellent spatial information on Cre activity, their sensitivity is limited by the nature of the reporter protein: it can be challenging to assess small differences in Cre levels, while length of reporter half-life can also be an issue. Precise temporal information can be obtained using qPCR to measure mRNA expression of a floxed gene directly, although this can only reliably detect a difference of 2-fold or more over a control. Improvements on qPCR exist, such as the extension MLPA (eMLPA) assay which also detects the presence of circular excised DNA 10. However, as such assays require tissue homogenization, they do not provide the detailed spatial information afforded by a Cre reporter animal, and are thus best utilized as additional assays rather than as a replacement.
Side-effects of Cre-induced recombination
There have been reports linking Cre-recombinase activity in mammalian cells with growth inhibition, DNA damage and ectopic activation of an anti-viral immune response 11–13. These effects are thought to be independent of legitimate loxP recombination, instead associated with high levels of Cre recombinase, secondary recombination at pseudo-loxP sites and associated DNA damage. While so far poorly studied in mouse models, these potentially confounding responses may be an important consideration for some applications in vascular biology.
Endothelial-specific Cre and Cre/ERT2 Mouse Models
This review provides a summary of the most commonly used endothelial-specific constitutive and inducible Cre models and their Cre recombinase activity, both as a resource to aid experimental design and to facilitate interpretation of previously reported data. Whilst not intended to be an exhaustive list, those discussed here constitute the vast majority of all Cre mouse alleles currently in use to study gene function in the endothelium. To aid reference we have used the official MGI nomenclature throughout: transgenes randomly inserted into the genome are denoted as Tg(Gene locus-Cre)serialnumber labcode, while knock-in models are instead denoted as Genetm#(Cre)labcode, tm# indicating designated targeted mutation number.
Pan-endothelial models
Constitutively active pan-endothelial Cre mouse models
In constitutively active pan-endothelial Cre transgenic mice, Cre recombinase activity is driven by enhancer and/or promoter regions that are generally considered to be expressed in all endothelial cell (EC) populations from early in development. Mouse models using these Cre alleles are therefore frequently referred to simply as endothelial-specific knock out animals. However, important differences exist between different constitutively active pan-endothelial alleles, including wide variations in expression in different vascular beds and onset of enhancer/promoter activity. A detailed understanding of the precise expression pattern of Cre recombinase activity is therefore important when interpreting any phenotypes generated with these models. Here we examine what is known about Cre expression in the most frequently cited pan-endothelial constitutive Cre alleles.
Tek-Cre (Tie2-Cre): The first endothelial-specific Cre mouse models used the regulatory sequences of the Tek gene (also known as Tie2), and remain the most commonly used. Tek/Tie2 encodes an angiopoietin receptor, a member of the receptor tyrosine kinase family, that is expressed in all ECs from very early in development and into adulthood 14–18. In 1997 Schlaeger et al. demonstrated that a combination of the Tek/Tie2 promoter region with an intron 1 enhancer sequence is capable of directing strong EC-specific reporter gene expression 19. Subsequently, by placing Cre cDNA between a 2.1kb promoter region and a 10kb fragment from intron 1, two groups simultaneously created Tek-Cre transgenic mouse alleles: Tg(Tek-cre)1Ywa 20 and Tg(Tek-cre)12Flv 21. Other Tek-Cre alleles made with the same strategy are Tg(Tek-cre)5326Sato, Tg(Tek-cre)1Rwng and Tg(Tek-cre)1Xyfu 22–24. However, these alleles have limited characterisation and are far less widely used than the Tg(Tek-cre)1Ywa and Tg(Tek-cre)12Flv alleles. Despite being made using the same transgenic strategy, important differences in the activity of Cre recombinase exist between the Tg(Tek-cre)1Ywa and Tg(Tek-cre)12Flv mouse alleles, presumably due to copy number variation or position effects from transgene insertion sites.
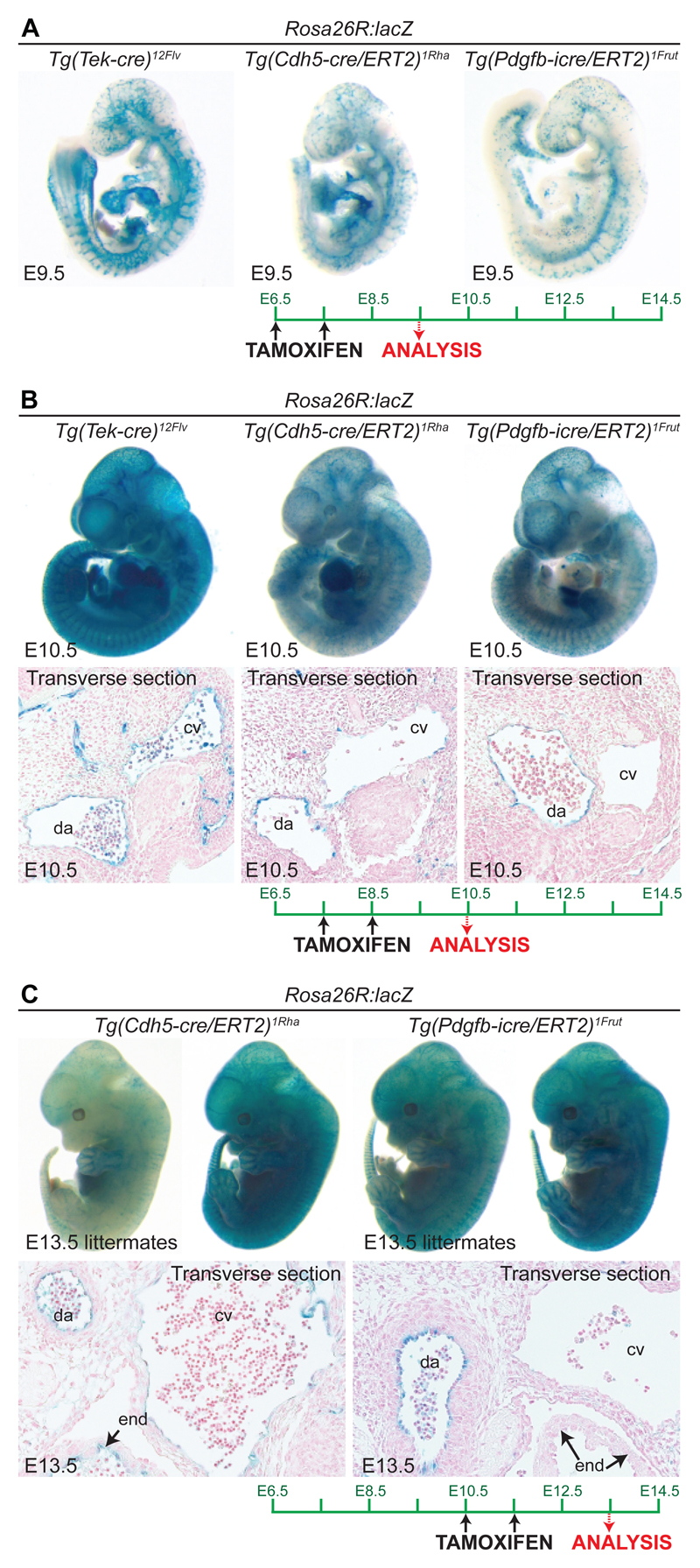
In both Tg(Tek-cre)1Ywa and Tg(Tek-cre)12Flv, Cre activity begins at E7.5 in a subset of cells in the extra embryonic mesodermal yolk sac. At this stage there is no Cre activity observed in the embryo proper. By E8.5, Tg(Tek-cre)1Ywa shows some Cre reporter gene expression within ECs of the embryo, with patchy activity observed in the dorsal aorta and in a small subset of ECs from the common atrial chamber 20. In contrast, Tg(Tek-cre)12Flv is reported to drive strong pan-endothelial Cre recombinase activity in all small blood vessels as well as the dorsal aorta and umbilical vessels at E8.5 25. With both alleles, Cre activity is reported to be pan-EC by E9.5 and remains such throughout development (Fig. 1A,B). In the heart, both alleles show Cre recombinase activity in the endocardium, endocardial cushions and all valves by E13.5, in addition to the coronary vasculature. In the adult, reporter gene activity is observed in all vascular beds 21 and in ECs of the lymphatic vasculature, as is to be expected from their venous endothelial origin 26.
Figure 1. A direct comparison of the embryonic expression pattern and intensity of the Cre reporter R26R:lacZ when crossed with different EC-specific Cre alleles on the C57/Bl6 background.
Embryonic age was established from date of plug in mother, all embryos were fixed, stained and sectioned using identical conditions across each age group as previously described108. For tamoxifen administration, 100μl tamoxifen (10mg/ml in a 10:1 solution of peanut oil and 100% ethanol) was administered orally via gavage on two consecutive days as indicated, and embryos taken 48 hours after last dose. When two embryos for the same allele are shown, they are littermates. E=embryonic day, da=dorsal aorta, cv=cardinal vein, end=endocardial lining of heart
All Tek-Cre mouse models show some degree of Cre recombinase activity in the haematopoietic lineage. With the Tg(Tek-cre)1Ywa allele, a small number of circulating cells are reported to be Cre positive in the adult mouse 20. However, using the Tg(Tek-cre)12Flv allele, Tang et al., report that 82% of blood cells from the embryonic yolk sac and 85% of circulating adult blood cells show Cre reporter gene activity 27. These data suggest that Tie2 is transiently expressed in a precursor cell that gives rise to both blood and endothelial lineages.
The regulatory sequences that drive expression of the Tek-Cre transgene also drive expression in the female germ line. To avoid the generation of a null allele, all Tek-Cre alleles must be transmitted to offspring via the male 21,28. However, even when this is carefully adhered to, null alleles are known to appear within Tie2-Cre colonies 5. This is likely due to variable expression of the Tie2 regulatory sequences very early in embryogenesis and/or their germ cell activation.
Tek-Cre mouse alleles are prone to variable and non-specific Cre recombinase activity 5. Theis et al., report non-specific recombination in 13% of the second generation using the Tg(Tek-cre)5326Sato allele 22. Despite this not being reported in their original characterisations, it is now apparent that other Tek-Cre alelles also suffer from a similar variability. Whilst using the Tg(Tek-cre)12Flv allele, we and others have observed Cre recombinase activity can range from EC-specific to near ubiquitous within a single litter 5.
Cdh5-Cre (VE-Cadherin-Cre): Cadherin 5 (Cdh5, also known as vascular endothelial (VE) cadherin), is localised at the EC junction, where it mediates cell adhesion and plays a crucial role in vessel assembly. Cdh5 null mice die at mid gestation due to severe vascular defects, and abnormal somite and heart development (reviewed 29–31). A region extending 2.2kb from the Cdh5 transcriptional start site is reported to contain both an enhancer element able to independently drive EC expression and a negative regulatory element inhibiting Cdh5 expression in other cell types 32. This 2.2kb regulatory region drives Cre recombinase expression in the Tg(Cdh5-cre)7Mlia mouse, with Cre activity detected in the yolk sac at E7.5, and in the embryo by E8.5. However, Tg(Cdh5-cre)7Mlia is expressed only sporadically within the ECs of the dorsal aorta at E10.5, and Cre activity is also variable between littermates at early stages 33. Cre recombinase reporter gene activity increases during development with expression in all observed blood and lymphatic vasculature by E14.5 and into adulthood 33.
Other constitutive Cdh5-Cre mouse alleles have been independently generated, including Tg(Cdh5-cre)1Nmoc and Tg(Cdh5-cre)1Spe 34,35. These alleles both use the same 2.2kb upstream fragment previously described, but Tg(Cdh5-cre)1Spe has the addition of flanking 2kb insulator sequences from the chicken y-globin gene. Tg(Cdh5-cre)1Spe shows patchy expression at E9.5, with more widespread activity observed at E10.5 but a curious absence in the head vasculature at this time point. The Tg(Cdh5-cre)1Nmoc is not well characterised, but it has been shown that Cre recombinase reporter gene expression can be observed in the dorsal aorta by E10.5 and is pan-EC in the adult 34.
Similar to Tie2-Cre, the Cdh5-Cre alleles show Cre recombinase reporter gene expression in haematopoietic cells. For the Tg(Cdh5-cre)1Spe allele, it has been demonstrated that 85% of foetal blood cells and 96% of CD45+ adult bone marrow-derived cells were positive for Cre recombinase reporter gene activity 35. Away from the haematopoietic system, little non-specific or ubiquitous Cre activity has been noted for these alleles, although they are far less widely used than Tie2/Tek-Cre and therefore variations may not have been reported.
Kdr-Cre (Flk1-Cre, VEGFR2-Cre): Kdr (Flk1/VEGFR2) encodes a receptor tyrosine kinase absolutely required for VEGF-induced EC proliferation, survival and migration 36. Widely expressed in early ECs, Kdr is also robustly expressed in the precursor cells that give rise to endothelial, haematopoietic and muscle cell lineages 37. The Kdr-Cre mouse allele Tg(Kdr-cre)15962Brei was generated using a 969-bp fragment of the Kdr promoter and a previously characterised 510-bp EC-specific enhancer from intron 1 of the Kdr gene 37–39. While the Kdr-intron1 enhancer is active in ECs by E7.8 38, the earliest Cre recombination activity demonstrated in the Tg(Kdr-cre)15962Brei allele is reported at E9.5 exclusively in the head and vessels of the upper trunk, although activity is pan-endothelial by E11.5 39. Tg(Kdr-cre)15962Brei driven reporter gene activity is seen in coronary vessels and the endocardium but is absent from the myocardium 39. Similar to Tek-Cre, Tg(Kdr-cre)15962Brei also shows Cre mediated recombination in most haematopoietic cells and in the bone marrow.
Another Kdr-Cre allele, Kdrtm1(cre)Sato, was generated by knock in of Cre recombinase into the first exon of the Kdr locus, thus creating a null allele. Kdrtm1(cre)Sato is active slightly earlier than Tg(Kdr-cre)15962Brei with Cre recombination observed at E8.5 40. However, the Kdrtm1(cre)Sato allele is also active in haematopoietic cells, skeletal and cardiac muscle, limiting its usefulness as an EC-specific Cre.
Summary: Differences in expression patterns between superficially similar Cre models can have profound consequences for the interpretation of phenotypes driven by these transgenes. Therefore, when using any constitutively active pan-EC Cre model, the origins of the specific Cre allele in use must be considered. Further, the extensive potential for non-specific recombination events, and the inherent variability of many of these Cre alleles, should be taken into account during experimental design. Where possible, Cre activity or target gene expression should be assessed in every animal studied, either by the inclusion of a Cre-reporter allele or by direct analysis of gene expression. Further information on the controls required are also described in detail elsewhere 9.
Inducible pan-endothelial Cre/ERT2 mouse models
Cre/ERT2, an inducible version of Cre recombinase, can be combined with EC-specific enhancer/promoter regions to enable both temporal and spatially controlled activity of Cre recombinase. Cre/ERT2 was created by fusing Cre recombinase cDNA with a mutated version of the estrogen receptor ligand binding domain (ERT2), thus creating a specific receptor for the tamoxifen metabolite OHT. In Cre-ERT2 mouse models, Cre recombinase is still expressed according to its driving regulatory sequences, but remains sequestered in the cytoplasm in complex with Hsp90. After tamoxifen administration, OHT binds to ERT2, preventing interaction with Hsp90 and allowing the Cre recombinase to move to the nucleus where it can recombine loxP sites 41,42. Tamoxifen can therefore be administered to induce Cre recombinase activity at any required developmental stage, with minimal toxicity (reviewed by 43). Cre/ERT2 mouse models are particularly useful in vascular biology, where constitutive gene deletion often causes lethality early in development. Here we detail the most cited pan-endothelial inducible Cre mouse alleles, with an emphasis on recombination specificity, timing and recombination efficiency.
Cdh5-Cre/ERT2 (VE-cadherin-Cre/ERT2): Cdh5-Cre/ERT2 alleles are the most commonly used inducible EC-specific Cre mice. There are three different alleles that use regions of the Cdh5 locus to drive Cre/ERT2 expression: Tg(Cdh5-cre/ERT2)CIVE23Mlia 44, Tg(Cdh5-cre/ERT2)#Ykub 45 and the most widely used Tg(Cdh5-cre/ERT2)1Rha 46. The Tg(Cdh5-cre/ERT2)CIVE23Mlia allele (or VE-cadherin-CreERT2) was developed using the same 2.2kb Cdh5 enhancer/promoter element previously used to drive the constitutively-active VE-cadherin-Cre 32,44. By contrast, the Tg(Cdh5-cre/ERT2)#Ykub was created using a BAC clone containing 200.3kb upstream region of Chd5, 45 while the Tg(Cdh5-cre/ERT2)1Rha allele (commonly referred to as Cdh5-PAC-CreERT2) were created by inserting the Cre/ERT2 cDNA at the start codon of the Cdh5 gene in a Cdh5-carrying PAC, which was then randomly integrated into the genome46,47.
The Tg(Cdh5-cre/ERT2)1Rha allele has been used extensively to study the post-natal vasculature, with tamoxifen administration between P1 and P6 able to induce pan-EC Cre-mediated recombination in both retina and brain endothelium 46 48. Tamoxifen injection at two weeks after birth has also been shown to produce efficient recombination in the endocardium 49. However, as with many inducible Cre alleles, there have been some reports of experimental variability in recombination efficiency, both in relative levels of gene knock-down in different mice given identical tamoxifen doses, and between different floxed allele genes within the same mouse 50. Relatively little information is available about the Cre expression patterns in the post-natal retina of the Tg(Cdh5-cre/ERT2)CIVE23Mlia and Tg(Cdh5-cre/ERT2)#Ykub alleles.
Despite their popularity, the activity of Cdh5-Cre/ERT2 alleles have not been consistently characterised during embryonic development. The Tg(Cdh5-cre/ERT2)CIVE23Mlia allele is best described, with fairly robust Cre activity at most embryonic stages two days after tamoxifen administration, and greater recombination efficiency reported after multiple days of tamoxifen dosing44. Although less characterised, the Tg(Cdh5-cre/ERT2)1Rha allele is also active in embryonic stages, with intraperitoneal (I.P) injection for three consecutive days from E11.5 known to induce reporter gene expression in ECs of the valves, coronary vessels and the endocardium by E15.5 51. When directly compared with Tg(Tek-cre)12Flv for activity during embryonic development, the Tg(Cdh5-cre/ERT2)1Rha allele drove similarly patterned but weaker Rosa26R:lacZ Cre-reporter gene activity at both E9.5 and E10.5 (when induced three and two days earlier), suggesting that genetic deletions using the two different Cre alleles will result in different levels of recombination and therefore potentially affect phenotypes (Fig. 1A,B). Further, section analysis found evidence of mosaic activity in the dorsal aorta similar to that seen with the constitutively active Tg(Cdh5-cre)7Mlia (Fig. 1B,C). As with post-natal mice, considerable intra-litter variability was also detected at E13.5 (Fig. 1C).
Pdgfb-Cre/ERT2: Platelet derived growth factor B (PDGFB) is a high affinity ligand for PDGF receptors PDGFRA and PDGFRB. Pdgfb is expressed in ECs, where it is crucial for the proper arrangements of pericytes and smooth muscle cells in the vessel wall 52. However, Pdgfb is neither exclusively nor ubiquitously expressed in the vasculature: expression is observed in neurons, pericytes, smooth muscle and megakaryocytes 53–55. In E11.5 embryos, Pdgfb is expressed in capillary and arterial ECs but not venous ECs 56, and is down-regulated in the mature vasculature, although expression remains in capillaries into the adult stages 56.
The Tg(Pdgfb-icre/ERT2)1Frut allele was created by insertion of Cre/ERT2 coding cDNA at the start codon of the Pdgfb gene in a PAC, which was then randomly integrated into the genome 57. Like Tg(Cdh5-cre/ERT2)1Rha, this allele has been best characterized in post-natal mice, where it has been used extensively to study angiogenesis in the neonatal retina. When induced at P1, Cre recombination can be observed in all ECs, although Pdgfb itself is only highly expressed at the growing edge of the developing vascular network 57. Mice injected with tamoxifen at P1 to P4 have been shown to have efficient pan-EC recombination at P5 58. In the adult, tamoxifen administration by gavage has been shown to induce Cre recombination in most capillary beds except the liver or the central nervous system after 48 hours, although no Cre expression was seen in larger arteries and veins at this stage 57. Tamoxifen administration in the adult mouse can also result in pan-EC recombination in coronary vessels, with recombination reported to be at ~99% of all coronary vascular ECs but entirely absent in the endocardium 59.
The Tg(Pdgfb-icre/ERT2)1Frut allele is not well characterised in the embryo and may be of limited use for developmental studies due to the highly heterogeneous nature of Pdgfb expression between vascular beds, particularly its absence from venous ECs 56,60. When directly compared to the inducible Tg(Cdh5-cre/ERT2)1Rha and Tg(Tek-cre)12Flv, R26RlacZ reporter gene driven by Tg(Pdgfb-icre/ERT2)1Frut was notably absent in venous ECs at both E10.5 and E13.5 (Fig. 1B-C).
Tek-Cre/ERT2: The Tg(Tek-cre/ERT2)1Arnd allele utilizes a similar approach to the constitutively active Tek-Cre models, placing the Cre/ERT2 cDNA between the promoter and intron 1 enhancer sequences 19,61. The Tg(Tek-cre/ERT2)1Soff allele places Cre/ERT2 cDNA at the coding ATG of the Tie2 gene within a BAC, which was then randomly inserted into the genome 62. Although fairly widely used, Tg(Tek-cre/ERT2)1Arnd requires up to five weeks of tamoxifen induction to induce pan-EC gene recombination in the adult, limiting its applications, while the efficiency and expression profile of the Tg(Tek-cre/ERT2)1Soff allele has not been fully reported.
Summary: Although a powerful tool for vascular researchers, EC Cre/ERT2 mouse models do not come without their problems. In addition to the expression variability and characterization limitations described for standard Cre models, susceptibility of floxed alleles to recombination can differ 63, whilst experimental variations in the method and dose of tamoxifen administration can also affect the efficiency of Cre/ERT2-driven gene deletion 64. Conversely, Cre/ERT2 alleles also have the potential for Cre activity without tamoxifen induction (‘leakiness’), which must be controlled for in experimental design 65. Lastly, there is a time lag between tamoxifen induction and effective excision of loxP sites by Cre recombination that ranges from 6-24 hours 66. Therefore, when using these models, it is essential to accurately determine recombination efficiency in target tissues for each floxed allele and each tamoxifen administration regime, when possible in every experimental animal.
Subtype-specific endothelial models
Endothelial heterogeneity is an essential feature of the vasculature. Differential gene expression in specific EC sub-populations is essential for the creation of a hierarchically branched vascular system, for angiogenesis and lymphangiogenesis, and for organ specialization in response to local signals 67 (and reviewed 68–70). While some “pan-EC” Cre models may inadvertently omit some EC subtypes, a number of Cre alleles have also been generated to deliberately target specific types of ECs. These include arterial and lymphatic ECs, sprouting angiogenic ECs and the endocardium. At the time of writing, there are no reported venous or capillary EC-specific Cre alleles, although both would clearly be valuable additional resources.
Arterial-specific Cre and Cre/ERT2 mouse models
Sox17-Cre: The SOX17 transcription factor is expressed in arterial but not venous ECs from early in development, in addition to the definitive endoderm and multiple haematopoietic lineages. An arterial EC-specific Cre allele, Sox17tm1(iCre)Heli, was generated by knock-in of the codon-improved Cre recombinase 71 into exon 1 of the Sox17 locus 72. This allele should not be confused with Sox17tm2.1(iCre)Heli, which is constitutively active in all Sox17-expressing tissues 73, or Sox17tm4.1(iCre/ERT2)Heli, which is expressed in arterial and venous ECs, pancreas and haematopoietic cells 74. Although Sox17 is first expressed in the early definitive endoderm from E6.0 75, the earliest activity of the constitutive Sox17tm1(iCre)Heli allele is at E8.5 and exclusive to the developing dorsal aorta. This specificity was attributed to a second promoter sequence between exons 3 and 4 of the Sox17 locus, which directs expression of a shorter Sox17 mRNA transcript that functions earlier in the endoderm but does not influence the Cre transcript. At E9.5 and E10.5, Sox17tm1(iCre)Heli-mediated recombination was detected in the dorsal aorta and was absent from the cardinal vein, although it was seen in the umbilical vein. From E10.5 all labelled vessels could be traced directly to the dorsal aorta, and later in development Cre activity was seen in arteries throughout all visceral organs examined. However, reporter activity was also seen in non-ECs within the thymus, spleen, pancreas and liver at E16.5-E18.5. Further, although embryos homozygous for the Sox17tm1(iCre)Heli allele were indistinguishable from wild-type littermates, Sox17 plays a crucial role in acquisition and maintenance of arterial identity 76. Disruption to the Sox17 locus could therefore have a synergistic effect with other genes deleted using this allele, confounding interpretation of any resulting phenotype.
Bmx-Cre/ERT2: Bmx, a member of the Tec tyrosine kinase family, is expressed in arterial ECs of both fetal and adult tissues 77. The Tg(Bmx-cre/ERT2)1Rha allele is an inducible transgenic arterial EC-specific Cre mouse originally developed to investigate the role of Notch signalling in postnatal arteries 78. cDNA encoding Cre/ERT2 was introduced downstream of the promoter for the Bmx gene in a PAC4 vector, which was then randomly inserted in the mouse genome 79. Daily I.P injections of tamoxifen from P10 to P13 induced Cre recombinase activity specifically within arterial ECs in the P28 retina and adult intestine, ovary and uterus. Cre recombination was also observed specifically in arteries of various organs at E18.5, although details of the tamoxifen induction strategy are unclear. In a later study, Kidoya et al induced Tg(Bmx-cre/ERT2)1Rha activity from E10.5 onward, with reporter expression seen in SMA-positive arteries at E14.5 80.
It is unlikely that the Tg(Bmx-cre/ERT2)1Rha allele can be used for studying the earliest events in arterio-venous specification in the embryo, as formation of the dorsal aorta and cardinal vein has occurred before Bmx is first detected at E10.5 81,82. Additionally, BmxlacZ expression was not observed in smaller arterioles, potentially indicating incomplete Tg(Bmx-cre/ERT2)1Rha activity in these beds. Variable Tg(Bmx-cre/ERT2)1Rha activity at the distal ends of arterioles has also been noted 83. It is not reported whether the Tg(Bmx-cre/ERT2)1Rha allele is active in any non-EC tissues following tamoxifen induction, although Bmx expression has been detected in the embryonic endocardium, thymus and tongue of the adult mouse 81.
Summary: The limited characterization of the above arterial specific Cre alleles indicates that testing specificity and activity of Cre at the time point and tissue of interest prior to breeding compound mouse lines would be highly advisable.
Angiogenic-specific Cre mouse models
Sprouting angiogenesis is the best studied form of angiogenesis, in which new blood vessels form from existing vessels. During sprouting angiogenesis, environmental cues induce quiescent ECs to develop motile behaviour and migrate towards a pro-angiogenic signal. These leading ECs, termed ‘tip cells’, are followed by proliferative ‘stalk cells’ that maintain the connection to the pre-existing vasculature 84.
Esm1-CreERT2: The inducible Tg(Esm1-cre/ERT2)1Rha allele (also denoted as Esm1(BAC)-iCreERT2) targets tip cells in the postnatal retina, a commonly used model for studying angiogenesis 85. It was made using a BAC transgenic strategy, in which an iCreERT2-containing cassette was inserted immediately downstream of the Esm1 promoter, deleting exon 1 67. Cre activity of Tg(Esm1-cre/ERT2)1Rha mice was mostly restricted to sprouting ECs in the P5 retina following tamoxifen induction at P4, consistent with the tip cell-specific expression patterns of both endogenous Esm1 mRNA 86,87 and Esm1 protein 67. Retinas analysed at P6, following single tamoxifen pulses between 6-96 hours prior to analysis, demonstrated consistent Cre activity at the angiogenic front. Induction 24-96 hours before analysis also labelled an increasing number of cells in the arteries, but not the veins, a pattern attributed to the migration of angiogenic tip cells into the forming arteries 88,89. The only other tissue in which the expression of this Cre allele is reported is in Lewis Lung carcinoma tumours, where both Esm1 protein and Tg(Esm1-cre/ERT2)1Rha activity is seen not just in tip cells, but throughout the tumour vasculature. Embryonic activity of Tg(Esm1-cre/ERT2)1Rha has not yet been described.
Apelin-Cre/ERT2: Apelin (Apln) is a pro-angiogenic ligand for the Apln receptor APLNR/APJ. The knock-in Aplntm1.1(cre/ERT2)Bz allele was generated by inserting CreERT2 cDNA at the Apln locus, deleting exon 1 90. This inducible allele is able to distinguish sprouting vessels from mature, quiescent vessels: adult mice show significantly fewer ECs labelled one week after tamoxifen induction, compared with induction in the embryo. Further, Aplntm1.1(cre/ERT2)Bz activity is reactivated in various tissue injury models that stimulate sprouting angiogenesis, including myocardial infarction (MI), hindlimb ischaemia-reperfusion, and numerous tumour models. In all cases, robust EC-specific Aplntm1.1(cre/ERT2)Bz activity is seen in newly-formed vessels, a subset of which are actively proliferating, whilst remaining at low levels in regions/tissue remote from the injury 91. The Aplntm1.1(cre/ERT2)Bz allele also targets angiogenic ECs in the postnatal retina: Aplntm1.1(cre/ERT2)Bz -driven reporter activity is more localised to the peripheral angiogenic front of the P6 retina compared with Tg(Cdh5-cre/ERT2)1Rha, and labels more ECs when induced at P2-P4 compared with P5-P7, consistent with decreasing angiogenic sprouting in the more developed retinas 92. Tamoxifen administration in adult mice resulted in no Aplntm1.1(cre/ERT2)Bz activity in the retina, again demonstrating lack of activity in mature, quiescent ECs.
In the embryo, the Aplntm1.1(cre/ERT2)Bz allele drives recombination in the heart specifically within the coronary endothelium, while no recombination is seen in the endocardium, smooth muscle cells or lymphatic ECs after tamoxifen administration at E10.5 90,91. Aplntm1.1(cre/ERT2)Bz is also active in ECs throughout other developing organs in which angiogenesis is occurring, and analysis of Cre-expressing APLN-positive ECs at E10.5 demonstrates they are proliferative 91. Interestingly, Aplntm1.1(cre/ERT2)Bz Cre activity is absent from the dorsal aorta.
There have been some reports of ‘leakiness’ with the Aplntm1.1(cre/ERT2)Bz allele, resulting in Cre/ERT2 recombinase activity in the absence of tamoxifen induction 91. This was observed at levels significantly lower than those seen following tamoxifen treatment. Nevertheless, for accurate interpretation of experimental data it is important to include an un-induced negative control.
Summary: Although both these Cre/ERT2 alleles are described as labelling sprouting angiogenic ECs, there are considerable differences in reporter gene expression driven by Apln-Cre/ERT2 compared to Esm1-Cre/ERT2, indicating that they label different subsets of angiogenic ECs. This should be considered when selecting a model.
Cre models specific to different compartments of the coronary vasculature
In addition to the arterial, venous, lymphatic and microvascular heterogeneity seen in the systemic vasculature, coronary ECs are also developmentally heterogeneous, differentially originating from the sinus venosus (SV), endocardium and proepicardium 93. There has been much debate over the relative contributions of these different sources of ECs, in which the interpretation of various constitutive or inducible Cre alleles has underpinned much of the discrepancies between different fate-mapping studies 94. This final section will give a brief overview of the EC-specific Cre alleles used to address the origins of the coronary endothelium.
Aplnr-Cre/ERT2
The SV is a transient developmental structure at the venous pole of the heart. The apelin receptor APLNR (also known as APJ) is highly expressed in the SV endothelium, but not at appreciable levels in the endocardium or epicardium at E10.5-12.5. The Tg(Aplnr-cre/ERT2)#Krh allele was generated using BAC recombineering technology: Cre/ERT2 cDNA was inserted at the Aplnr/Apj start site, which was then randomly integrated into the genome 95. When induced by tamoxifen at E9.5, robust reporter expression was detected by E10.5 in the SV and in many, but not all, coronary vessels in the developing heart, predominantly on the dorsal and lateral sides. In the embryo, Tg(Aplnr-cre/ERT2)#Krh was reported to mimic that of Aplnr/Apj, with activity seen throughout the microvasculature, in the cardinal vein and intersomitic vessels. In the adult mouse, the Tg(Aplnr-cre/ERT2)#Krh allele has also been used to label EC-specific Aplnr/Apj expression in non-muscularised vessels of <~50µm diameter in skeletal muscle, white adipose tissue and brown adipose tissue 96.
Fabp4-Cre and Fabp4-Cre/ERT2
Fatty acid binding protein 4 (FABP4), often referred to as an adipocyte and macrophage-specific protein AP2, is also expressed in ECs. This includes coronary vessel ECs, although no expression is detected in endocardial ECs 97,98. As expected, the Tg(Fabp4-cre)1Rev allele, generated by driving Cre cDNA expression with a 5.4kb Fabp4/Ap2 promoter/enhancer fragment and then randomly inserting into the genome 99, directs Cre activity in coronary but not endocardial ECs 99. The inducible Tg(Fabp4-cre/ERT2)1Ipc 100 allele was created using the same transgenic promoter/enhancer approach as Tg(Fabp4-cre)1Rev, and was also able to selectively label coronary, but not endocardial, ECs 98.
Nfatc1-Cre and Nfatc1-Cre/ERT2
The endocardium is a specialised EC layer that lines the inner surface of the heart, from which cells can delaminate and migrate to the developing cardiac cushions, as well as contributing to coronary vessel endothelium and mural cell populations 101. Nfatc1, a calcium/calcineurin-dependent transcription factor, is highly expressed in the endocardium whilst relatively absent in most other blood ECs. The Nfatc1 locus has therefore been used to develop a number of Cre alleles primarily utilised to study the endocardium.
A constitutively active Nfatc1tm1.1(cre)Bz allele (generated by Bin Zhou at Albert Einstein College of Medicine and sometimes referred to as Nfatc1-Cre) was created by knock-in of Cre-IRES cDNA downstream of the translational stop codon of the mouse Nfatc1 locus 102. The resulting allele was reported to drive Cre mRNA expression and Cre-driven recombination specifically within endocardial ECs at both E9.5 and E10.5, with no expression reported in SV ECs, proepicardium, epicardium or myocardium. This Nfatc1tm1.1(cre)Bz allele was used to propose a model by which endocardial cells form some coronary vessels via angiogenesis 102. However, other independently generated Nfatc1-Cre alleles have given contradictory results. Confusingly, these were generated by a group led by a different scientist also named Bin Zhou, based at the Shanghai Institutes for Biological Sciences of the Chinese Academy of Sciences. Until recently, mice generated by these two scientists were both labelled using a single lab code (Bz) on MGI. Although this has now been corrected, researchers should be aware of possible confusion in papers published prior to 2018, and check references carefully to ensure they are using their desired mouse model. Bin Zhou (Shanghai, now denoted Bzsh) generated a series of Nfatc1-Cre and Dre alleles with slightly different expression patterns compared to Nfatc1tm1.1(cre)Bz, the crucial difference being activity in SV ECs. For example, their constitutively active Nfatc1tm2(cre)Bzsh allele, which was generated using the identical targeting strategy detailed for Nfatc1tm1.1(cre)Bz, showed Cre recombinase activity in SV ECs at E9.5 and E11.5, in addition to endocardial activity 49. Together, these results suggest that constitutive Nfatc1-Cre activity may not be entirely restricted to the endocardium in the developing heart, and so alone may be unsuitable for definitive studies of coronary vessel origins.
To overcome specificity issues, inducible Nfatc1-Cre/ERT2 models have also been developed. The Nfatc1tm1.1(cre/ERT2)Bzsh allele was generated by homologous recombination to replace exon 1 of the Nfatc1 locus with Cre/ERT2 cDNA 103. Immunostaining for estrogen receptor (ESR) showed at E9.5 Nfatc1tm1.1(cre/ERT2)Bzsh is expressed in atrial and ventricular endocardial ECs, but is absent in the SV. Tamoxifen injection at E8.0-E8.5 could therefore activate Cre activity specifically in the endocardium before the coronary vasculature forms, but presumably after the early transient expression of Nfactc1 in the SV. Consistent with this, no reporter labelling was observed in the SV at E11.5 103, indicating that Nfatc1-CreER is a more reliable tool for labelling endocardial cells when induced in the specified developmental window. A second inducible knock-in allele, Nfatc1em1(cre/ERT2)Bzsh, also showed similar endocardial-specific immunostaining of early Cre/ERT2 expression 104.
Npr3-Cre/ERT2
Nrp3 encodes natriuretic peptide receptor 3 and was found to be specifically expressed in endocardial ECs 49,104. The inducible Npr3tm1.1(cre/ERT2)Bzsh allele was generated using homologous recombination to insert CreERT2 cDNA in frame with the translational start codon at the Npr3 locus 104. Immunostaining for ESR showed Npr3-Cre/ERT2 expression in the atrial and ventricular endocardium, but not SV, between E8.5 to E11.5. Following tamoxifen induction at E8.5, Cre recombination was seen in approximately 73% of ventricular endocardial cells at E10.5, 78% at E11.5 and 80% at E12.5, whilst the SV remained unlabelled. Although Npr3tm1.1(cre/ERT2)Bzsh is not exclusive to the endocardium (expression is also seen in dorsal aorta EC, a subset of non-EC mesenchymal cells, and a subset of TBX18-positive epicardial cells 49), it is active in the adult 105. This is different to Nfatc1tm2(cre)Bzsh, which cannot be activated by tamoxifen injection after embryonic stages. Tamoxifen induction of Npr3tm1.1(cre/ERT2)Bzsh in the adult heart labels over 90% of endocardial cells after 48 hours. As in the embryonic heart, labelling was also seen in a subset of epicardial cells, but not vascular ECs.
Summary
Due to the ongoing controversy regarding the exact origins of different types of vessels within the heart, a detailed knowledge of the expression pattern of a chosen Cre and/or Cre/ERT2 model is crucial for any research in this area. A careful literature check and a detailed genealogy for any chosen mouse model would also be recommended, as many of these alleles (and the labs of origin) have similar or identical names.
Conclusion and Future Outlook
EC-specific Cre mouse models have greatly expanded our knowledge of vascular biology. However many are not thoroughly characterised and have recombination in cell populations other than the intended cell type, confounding the interpretation of resulting data. Constitutively active pan-EC Cre alleles can display variable Cre recombinase activity even between littermates, and all show recombination in cells of the haematopoietic lineage. Inducible Cre mouse alleles will also have inherent problems with Cre leakiness and variation in recombination efficiency. Therefore, to generate reliable and reproducible data using EC-specific Cre mouse models it is clear that Cre allele activity must be adequately monitored for each specific mouse model, target tissue and time point of interest.
As our understanding of the genome expands, so do the options for the manipulation of tissue-specific gene expression. For example, it is now possible to combine Cre-lox with the Dre-rox system, in which Dre recombinase catalyzes recombination between rox sites 106, thus enabling knockout specificity to be controlled by two regulatory sequences 107. To this end, a Dre-switchable CrexER allele has been generated that has two rox sites flanking the ESR, so the Cre-ESR fusion protein is restricted to the cytoplasm until Dre-driven excision of the ESR coding sequence occurs 107. For EC-specific Dre expression, Pu et al. have already generated a novel Tek-Dre allele (provisionally named Tek-Dretm1(dre)Bzsh) by knocking in a Dre cassette in-frame with the ATG of endogenous Tek 107. By combining this Tek-Dre allele with organ- or EC subtype-specific expression of CrexER, recombination of a floxed target gene can be restricted to increasingly precise cell populations.
With our increased understanding of EC heterogeneity, the combination of EC-subtype-specific enhancers with new recombinase technology looks set to provide a powerful next-generation toolbox with which to interrogate the complex systems governing EC development and behaviour.
Highlights.
Summary of Cre recombinase activity from embryo to adulthood in the most widely used vascular specific Cre mouse lines.
Table of vascular specific Cre mouse lines including timing of Cre recombinase onset and non-endothelial Cre expression.
Comparison between Cre recombinase activity, time of onset and expression patterns in the early embryo between mouse lines commonly used to create vascular specific knock outs.
Table 1. Summary of the most widely-used EC-specific Cre and Cre/ERT2 alleles.
CapEC= capillary endothelial cells, AEC= Arterial endothelial cells, SVEC=sinus venosus endothelial cells, CEC= coronary endothelial cells, VEC=vein endothelial cells, EndEC=endocardial endothelial cells. MGI ID number can be used at http://www.informatics.jax.org/ to obtain further information about origin, recombinase activity, mouse availability and a complete list of papers referencing each allele. Note that ID numbers must include “MGI:” when added into search box
| Gene | Mouse Line | Regulation of Cre Activity | Expression | Non-EC Expression | Earliest Reported Activity | MGI ID | Ref |
|---|---|---|---|---|---|---|---|
| Tek (Tie2) | Tg(Tek-cre)1Ywa | 10kb enhancer and 2.1kb promoter | Pan-EC | Heart valves, HCs | E7.5 | MGI:2450311 | 20 |
| Tg(Tek-cre)12Flv | 10kb enhancer and 2.1kb promoter | Pan-EC | Heart valves, HCs | E7.5 | MGI:2136412 | 21 | |
| Tg(Tek-cre)5326Sato | 10kb enhancer and 2.1kb promoter | Pan-EC | HCs, some ectopic expression | E9.5 | MGI:2445474 | 22 | |
| Tg(Tek-cre)1Rwng | 10kb enhancer and 2.1kb promoter | Pan-EC | HCs | E7.5 | MGI:3608912 | 23 | |
| Tg(Tek-cre)1Xyfu | 10kb enhancer and 2.1kb promoter | Pan-EC | HCs | Unknown | MGI:2683222 | 24 | |
| Tg(Tek-cre/ERT2)1Soff | Endogenous locus, Cre/ERT2 inserted at start codon of gene within a BAC | Pan-EC | None reported | Unknown | MGI:3837451 | 62 | |
| Tg(Tek-cre/ERT2)1Arnd | 10kb enhancer and 2.1kb promoter | Pan-EC | None reported | Unknown | MGI:2450312 | 61 | |
|
Cdh5 (VE-cad) |
Tg(Cdh5-cre)7Mlia | 2.2kb promoter | Pan-EC | HCs | E7.5 | MGI:3620560 | 33 |
| Tg(Cdh5-cre)1Nmoc | 2.2kb promoter | Pan-EC | HCs | E10.5 | MGI:3842516 | 34 | |
| Tg(Cdh5-cre)1Spe | 2.2kb promoter flanked by 2kb insulator | Pan-EC | HCs | E9.5 | MGI:3836418 | 35 | |
| Tg(Cdh5-cre/ERT2)CIVE23Mlia | 2.2kb promoter | Pan-EC | HCs | E9.5 | MGI:3700149 | 44 | |
| Tg(Cdh5-cre/ERT2)#Ykub | Endogenous locus, Cre/ERT2 inserted at start codon within BAC | Pan-EC | None reported | Not reported | MGI:5705396 | 45 | |
| Tg(Cdh5-cre/ERT2)1Rha | Endogenous locus, Cre/ERT2 inserted at start codon within PAC | Pan-EC | None reported | E9.5 | MGI:3848982 | 46 | |
| Kdr (Flk1) | Tg(Kdr-cre)15962Brei | 2.3kb enhancer and 939bp promoter | Pan-EC | HCs | E9.5 | MGI:3038968 | 39 |
| Kdrtm1(cre)Sato | Endogenous locus, targeted knock-in of CreERT2 cDNA into exon 1 | Pan-EC | HCs skeletal and cardiac muscle | E8.5 | MGI:2655253 | 40 | |
| Pdgfb | Tg(Pdgfb-icre/ERT2)1Frut | Endogenous locus, Cre/ERT2 inserted at start codon in PAC, | CapEC, AEC | Keratinocytes, megakaryocytes | E9.5 | MGI:3793852 | 57 |
| Sox17 | Sox17tm1(iCre)Heli | Endogenous locus, targeted knock-in of CreERT2 cDNA deleting exon 1 | Some AECs | Thymus, spleen, pancreas and liver | E8.5 | MGI:3852645 | 72 |
| Bmx | Tg(Bmx-cre/ERT2)1Rha | Endogenous locus, Cre/ERT2 inserted at start codon within PAC | Some AECs | None reported | E10.5 | MGI:5513853 | 78 |
| Esm1 | Tg(Esm1-cre/ERT2)1Rha | Endogenous locus, Cre/ERT2 inserted at start codon within BAC | Tip ECs | None reported | P4 | - | 67 |
| Apln | Aplntm1.1(cre/ERT2)Bz | Endogenous locus, targeted knock-in of CreERT2 cDNA into gene deleting exon 1 | Sprouting ECs | None reported | E10.5 | MGI:5637737 | 90 |
| Aplnr (Apj) | Tg(Aplnr-cre/ERT2)#Krh | Endogenous locus, Cre/ERT2 inserted at start codon of BAC | SV and derived CECs, capECs, VECs | None reported | E10.5 | MGI:5637737 | 95 |
| Fabp4 (Ap2) | Tg(Fabp4-cre)1Rev | 5.4kb promoter fragment | CEC | Adipocytes and macrophages | E16.5 | MGI:2386686 | 99 |
| Tg(Fabp4-cre/ERT2)1Ipc | 5.4kb promoter fragment | CEC | Adipocytes and macrophages | E16.5 | MGI:2387425 | 100 | |
| Nfatc1 | Nfatc1tm1.1(cre)Bz | Endogenous locus, targeted knock-in of Cre cDNA into translational stop codon | EndEC | Hair follicle stem cells, osteoblasts, macrophages | E9.0 | MGI:5471107 | 102 |
| Nfatc1tm2(cre)Bzsh | Endogenous locus, targeted knock-in of Cre cDNA into translational stop codon | EndEC, SVEC | None reported | E9.5 | MGI:6163733 | 49 | |
| Nfatc1tm1.1(cre/ERT2)Bzsh | Endogenous locus, targeted knock-in of CreERT2 cDNA into gene deleting exon 1 | EndEC, SVEC | None reported | E8.5 | MGI:5637438 | 103 | |
| Nfatc1em1(cre/ERT2)Bzsh | Endogenous locus, targeted knock-in of Cre/ERT2 cDNA into 3'UTR | EndEC, SVEC | None reported | E7.75 | MGI:5804178 | 104 | |
| Npr3 | Npr3tm1.1(cre/ERT2)Bzsh | Endogenous locus, targeted knock-in of Cre/ERT2 cDNA into translational start codon | EndEC | Some epicardial cells | E8.5 | MGI:5804184 | 104 |
Acknowledgments
Sources of funding
This work was supported by the BHF Centre of Research Excellence, Oxford (RE/08/004) (S.D., A.N.) and the BHF (PG/16/34/32135) (S.P).
Abbreviations
- EC
endothelial cell
- GFP
green fluorescent protein
- YFP
yellow fluorescent protein
- tpA
transcriptional termination
- β-gal
beta-D-galactosidase
- MLPA
multiplex ligation-dependent probe amplification
- MGI
mouse genome informatics
- TG
transgene
- tm
targeted mutation
- bp
base pair
- kb
kilo base pair
- PAC
P1-derived artificial chromosome
- BAC
bacterial artificial chromosome
- E
embryonic day
- P
postnatal day
- DA
dorsal aorta
- CV
cardinal vein
- END
endocardial lining of heart
Footnotes
Disclosures,
Nothing to disclose
References
- 1.Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. Journal of Molecular Biology. 1981;150:467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- 2.Hoess RH, Ziese M, Sternberg N. P1 site-specific recombination: nucleotide sequence of the recombining sites. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:3398–3402. doi: 10.1073/pnas.79.11.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abremski K, Wierzbicki A, Frommer B, Hoess RH. Bacteriophage P1 Cre-loxP site-specific recombination. Site-specific DNA topoisomerase activity of the Cre recombination protein. The Journal of Biological Chemistry. 1986;261:391–396. [PubMed] [Google Scholar]
- 4.Orban PC, Chui D, Marth JD. Tissue- and site-specific DNA recombination in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6861–6865. doi: 10.1073/pnas.89.15.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heffner CS, Herbert Pratt C, Babiuk RP, et al. Supporting conditional mouse mutagenesis with a comprehensive cre characterization resource. Nature Communications. 2012;3 doi: 10.1038/ncomms2186. 1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandler KJ, Chandler RL, Broeckelmann EM, Hou Y, Southard-Smith EM, Mortlock DP. Relevance of BAC transgene copy number in mice: transgene copy number variation across multiple transgenic lines and correlations with transgene integrity and expression. Mammalian genome. 2007;18:693–708. doi: 10.1007/s00335-007-9056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 8.Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song AJ, Palmiter RD. Detecting and Avoiding Problems When Using the Cre-lox System. Trends in genetics : TIG. 2018;34:333–340. doi: 10.1016/j.tig.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leonhard WN, Roelfsema JH, Lantinga-van Leeuwen IS, Breuning MH, Peters DJ. Quantification of Cre-mediated recombination by a novel strategy reveals a stable extra-chromosomal deletion-circle in mice. BMC Biotechnology. 2008;8:18. doi: 10.1186/1472-6750-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loonstra A, Vooijs M, Beverloo HB, et al. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9209–9214. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepin G, Ferrand J, Honing K, et al. Cre-dependent DNA recombination activates a STING-dependent innate immune response. Nucleic acids research. 2016;44:5356–5364. doi: 10.1093/nar/gkw405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janbandhu VC, Moik D, Fassler R. Cre recombinase induces DNA damage and tetraploidy in the absence of loxP sites. Cell cycle. 2014;13:462–470. doi: 10.4161/cc.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumont DJ, Gradwohl G, Fong GH, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes & Development. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 15.Dumont DJ, Gradwohl GJ, Fong GH, Auerbach R, Breitman ML. The endothelial-specific receptor tyrosine kinase, tek, is a member of a new subfamily of receptors. Oncogene. 1993;8:1293–1301. [PubMed] [Google Scholar]
- 16.Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML. tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene. 1992;7:1471–1480. [PubMed] [Google Scholar]
- 17.Wong AL, Haroon ZA, Werner S, Dewhirst MW, Greenberg CS, Peters KG. Tie2 Expression and Phosphorylation in Angiogenic and Quiescent Adult Tissues. Circulation Research. 1997;81:567–574. doi: 10.1161/01.res.81.4.567. [DOI] [PubMed] [Google Scholar]
- 18.Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 19.Schlaeger TM, Bartunkova S, Lawitts JA, et al. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3058–3063. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kisanuki YY, Hammer RE, Miyazaki J-i, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre Transgenic Mice: A New Model for Endothelial Cell-Lineage Analysis in Vivo. Developmental Biology. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 21.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. The Journal of Experimental Medicine. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theis M, de Wit C, Schlaeger TM, et al. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. genesis. 2001;29:1–13. doi: 10.1002/1526-968x(200101)29:1<1::aid-gene1000>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Braren R, Hu H, Kim YH, Beggs HE, Reichardt LF, Wang R. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol. 2006;172:151–162. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kano A, Wolfgang MJ, Gao Q, et al. Endothelial cells require STAT3 for protection against endotoxin-induced inflammation. The Journal of Experimental Medicine. 2003;198:1517–1525. doi: 10.1084/jem.20030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002;129:1397–1410. doi: 10.1242/dev.129.6.1397. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan RS, Dillard ME, Lagutin OV, et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes & Development. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Y, Harrington A, Yang X, Friesel RE, Liaw L. The contribution of the Tie2+ lineage to primitive and definitive hematopoietic cells. Genesis. 2010;48:563–567. doi: 10.1002/dvg.20654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Lange WJ, Halabi CM, Beyer AM, Sigmund CD. Germ line activation of the Tie2 and SMMHC promoters causes noncell-specific deletion of floxed alleles. Physiological Genomics. 2008;35:1–4. doi: 10.1152/physiolgenomics.90284.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vestweber D. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:223–232. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- 30.Gavard J. Endothelial permeability and VE-cadherin. Cell Adhesion & Migration. 2014;8:158–164. doi: 10.4161/cam.29026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris ES, Nelson WJ. VE-Cadherin: At the Front, Center, and Sides of Endothelial Cell Organization and Function. Current opinion in cell biology. 2010;22:651–658. doi: 10.1016/j.ceb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gory S, Vernet M, Laurent M, Dejana E, Dalmon J, Huber P. The vascular endothelial-cadherin promoter directs endothelial-specific expression in transgenic mice. Blood. 1999;93:184–192. [PubMed] [Google Scholar]
- 33.Alva JA, Zovein AC, Monvoisin A, et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Developmental Dynamics. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 34.Kogata N, Arai Y, Pearson JT, et al. Cardiac Ischemia Activates Vascular Endothelial Cadherin Promoter in Both Preexisting Vascular Cells and Bone Marrow Cells Involved in Neovascularization. Circulation Research. 2006;98:897–904. doi: 10.1161/01.RES.0000218193.51136.ad. [DOI] [PubMed] [Google Scholar]
- 35.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsson A-K, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling ? in control of vascular function. Nature Reviews Molecular Cell Biology. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 37.Motoike T, Loughna S, Perens E, et al. Universal GFP reporter for the study of vascular development. Genesis. 2000;28:75–81. doi: 10.1002/1526-968x(200010)28:2<75::aid-gene50>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 38.Kappel A, Rönicke V, Damert A, Flamme I, Risau W, Breier G. Identification of Vascular Endothelial Growth Factor (VEGF) Receptor-2 (Flk-1) Promoter/Enhancer Sequences Sufficient for Angioblast and Endothelial Cell-Specific Transcription in Transgenic Mice. Blood. 1999;93:4284–4292. [PubMed] [Google Scholar]
- 39.Licht AH, Raab S, Hofmann U, Breier G. Endothelium-specific Cre recombinase activity in flk-1-Cre transgenic mice. Developmental Dynamics. 2004;229:312–318. doi: 10.1002/dvdy.10416. [DOI] [PubMed] [Google Scholar]
- 40.Lugus JJ, Park C, Ma YD, Choi K. Both primitive and definitive blood cells are derived from Flk-1+ mesoderm. Blood. 2009;113:563–566. doi: 10.1182/blood-2008-06-162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proceedings of the National Academy of Sciences. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochemical and biophysical research communications. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 43.Feil S, Valtcheva N, Feil R. Humana Press; 2009. Inducible Cre Mice; pp. 343–363. [DOI] [PubMed] [Google Scholar]
- 44.Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML. VE-cadherin-CreERT2 transgenic mouse: A model for inducible recombination in the endothelium. Developmental Dynamics. 2006;235:3413–3422. doi: 10.1002/dvdy.20982. [DOI] [PubMed] [Google Scholar]
- 45.Okabe K, Kobayashi S, Yamada T, et al. Neurons limit angiogenesis by titrating VEGF in retina. Cell. 2014 Oct 23;159:584–596. doi: 10.1016/j.cell.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Nakayama M, Pitulescu ME, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 47.Sörensen I, Adams RH, Gossler A. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood. 2009;113:5680–5688. doi: 10.1182/blood-2008-08-174508. [DOI] [PubMed] [Google Scholar]
- 48.Yanagida K, Liu CH, Faraco G, et al. Size-selective opening of the blood-brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:4531–4536. doi: 10.1073/pnas.1618659114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Pu W, Li G, et al. Endocardium Minimally Contributes to Coronary Endothelium in the Embryonic Ventricular Free Walls. Circulation research. 2016;118:1880–1893. doi: 10.1161/CIRCRESAHA.116.308749. [DOI] [PubMed] [Google Scholar]
- 50.Zarkada G, Heinolainen K, Makinen T, Kubota Y, Alitalo K. VEGFR3 does not sustain retinal angiogenesis without VEGFR2. Proceedings of the National Academy of Sciences. 2015;112:761–766. doi: 10.1073/pnas.1423278112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robson A, Allinson KR, Anderson RH, Henderson DJ, Arthur HM. The TGFβ type II receptor plays a critical role in the endothelial cells during cardiac development. Developmental Dynamics. 2010;239:2435–2442. doi: 10.1002/dvdy.22376. [DOI] [PubMed] [Google Scholar]
- 52.Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 53.Yeh H-J, Ruit KG, Wang Y-X, Parks WC, Snider WD, Deuel TF. PDGF A-chain gene is expressed by mammalian neurons during development and in maturity. Cell. 1991;64:209–216. doi: 10.1016/0092-8674(91)90222-k. [DOI] [PubMed] [Google Scholar]
- 54.Gladwin AM, Carrier MJ, Beesley JE, Lelchuk R, Hancock V, Martin JF. Identification of mRNA for PDGF B-chain in human megakaryocytes isolated using a novel immunomagnetic separation method. British Journal of Haematology. 1990;76:333–339. doi: 10.1111/j.1365-2141.1990.tb06364.x. [DOI] [PubMed] [Google Scholar]
- 55.Wilson E, Mai Q, Sudhir K, Weiss RH, Ives HE. Mechanical strain induces growth of vascular smooth muscle cells via autocrine action of PDGF. The Journal of Cell Biology. 1993;123:741–747. doi: 10.1083/jcb.123.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 57.Claxton S, Kostourou V, Jadeja S, Chambon P, Hodivala-Dilke K, Fruttiger M. Efficient, inducible Cre-recombinase activation in vascular endothelium. Genesis. 2008;46:74–80. doi: 10.1002/dvg.20367. [DOI] [PubMed] [Google Scholar]
- 58.Wilhelm K, Happel K, Eelen G, et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. 2016;529:216–220. doi: 10.1038/nature16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dubé KN, Thomas TM, Munshaw S, Rohling M, Riley PR, Smart N. Recapitulation of developmental mechanisms to revascularize the ischemic heart. JCI Insight. 2017;2:e96800. doi: 10.1172/jci.insight.96800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stanczuk L, Martinez-Corral I, Ulvmar MH, et al. cKit Lineage Hemogenic Endothelium-Derived Cells Contribute to Mesenteric Lymphatic Vessels. Cell Reports. 2015;10:1708–1721. doi: 10.1016/j.celrep.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 61.Forde A, Constien R, Grone HJ, Hammerling G, Arnold B. Temporal Cre-mediated recombination exclusively in endothelial cells using Tie2 regulatory elements. Genesis. 2002;33:191–197. doi: 10.1002/gene.10117. [DOI] [PubMed] [Google Scholar]
- 62.Korhonen H, Fisslthaler B, Moers A, et al. Anaphylactic shock depends on endothelial Gq/G11. The Journal of Experimental Medicine. 2009;206:411–420. doi: 10.1084/jem.20082150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu J, Willet SG, Bankaitis ED, Xu Y, Wright CV, Gu G. Non-parallel recombination limits Cre-LoxP-based reporters as precise indicators of conditional genetic manipulation. Genesis. 2013;51:436–442. doi: 10.1002/dvg.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park EJ, Sun X, Nichol P, Saijoh Y, Martin JF, Moon AM. System for tamoxifen-inducible expression of cre-recombinase from the Foxa2 locus in mice. Developmental Dynamics. 2008;237:447–453. doi: 10.1002/dvdy.21415. [DOI] [PubMed] [Google Scholar]
- 65.Kristianto J, Johnson MG, Zastrow RK, Radcliff AB, Blank RD. Spontaneous recombinase activity of Cre-ERT2 in vivo. Transgenic Research. 2017;26:411–417. doi: 10.1007/s11248-017-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hayashi S, McMahon AP. Efficient Recombination in Diverse Tissues by a Tamoxifen-Inducible Form of Cre: A Tool for Temporally Regulated Gene Activation/Inactivation in the Mouse. Developmental Biology. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 67.Rocha SF, Schiller M, Jing D, et al. Esm1 modulates endothelial tip cell behavior and vascular permeability by enhancing VEGF bioavailability. Circulation research. 2014;115:581–590. doi: 10.1161/CIRCRESAHA.115.304718. [DOI] [PubMed] [Google Scholar]
- 68.dela Paz NG, D'Amore PA. Arterial versus venous endothelial cells. Cell and Tissue Research. 2009;335:5–16. doi: 10.1007/s00441-008-0706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 70.Ulvmar MH, Mäkinen T. Heterogeneity in the lymphatic vascular system and its origin. Cardiovascular Research. 2016;111:310–321. doi: 10.1093/cvr/cvw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimshek DR, Kim J, Hubner MR, et al. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis. 2002;32:19–26. doi: 10.1002/gene.10023. [DOI] [PubMed] [Google Scholar]
- 72.Liao WP, Uetzmann L, Burtscher I, Lickert H. Generation of a mouse line expressing Sox17-driven Cre recombinase with specific activity in arteries. Genesis. 2009;47:476–483. doi: 10.1002/dvg.20520. [DOI] [PubMed] [Google Scholar]
- 73.Engert S, Liao WP, Burtscher I, Lickert H. Sox17-2A-iCre: a knock-in mouse line expressing Cre recombinase in endoderm and vascular endothelial cells. Genesis. 2009;47:603–610. doi: 10.1002/dvg.20540. [DOI] [PubMed] [Google Scholar]
- 74.Engert S, Burtscher I, Liao WP, Dulev S, Schotta G, Lickert H. Wnt/β-catenin signalling regulates Sox17 expression and is essential for organizer and endoderm formation in the mouse. Development. 2013;140:3128–3138. doi: 10.1242/dev.088765. [DOI] [PubMed] [Google Scholar]
- 75.Choi E, Kraus MR, Lemaire LA, et al. Dual lineage-specific expression of Sox17 during mouse embryogenesis. Stem cells. 2012;30:2297–2308. doi: 10.1002/stem.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corada M, Orsenigo F, Morini MF, et al. Sox17 is indispensable for acquisition and maintenance of arterial identity. Nature communications. 2013;4 doi: 10.1038/ncomms3609. 2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rajantie I, Ekman N, Iljin K, et al. Bmx tyrosine kinase has a redundant function downstream of angiopoietin and vascular endothelial growth factor receptors in arterial endothelium. Molecular and Cellular Biology. 2001;21:4647–4655. doi: 10.1128/MCB.21.14.4647-4655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ehling M, Adams S, Benedito R, Adams RH. Notch controls retinal blood vessel maturation and quiescence. Development. 2013;140:3051–3061. doi: 10.1242/dev.093351. [DOI] [PubMed] [Google Scholar]
- 79.Osoegawa K, Tateno M, Woon PY, et al. Bacterial artificial chromosome libraries for mouse sequencing and functional analysis. Genome research. 2000;10:116–128. [PMC free article] [PubMed] [Google Scholar]
- 80.Kidoya H, Naito H, Muramatsu F, et al. APJ Regulates Parallel Alignment of Arteries and Veins in the Skin. Developmental cell. 2015;33:247–259. doi: 10.1016/j.devcel.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 81.Rajantie I, Ekman N, Iljin K, et al. Bmx tyrosine kinase has a redundant function downstream of angiopoietin and vascular endothelial growth factor receptors in arterial endothelium. Molecular and cellular biology. 2001;21:4647–4655. doi: 10.1128/MCB.21.14.4647-4655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fish JE, Wythe JD. The molecular regulation of arteriovenous specification and maintenance. Developmental dynamics. 2015;244:391–409. doi: 10.1002/dvdy.24252. [DOI] [PubMed] [Google Scholar]
- 83.Murphy PA, Kim TN, Huang L, et al. Constitutively active Notch4 receptor elicits brain arteriovenous malformations through enlargement of capillary-like vessels. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:18007–18012. doi: 10.1073/pnas.1415316111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nature reviews. Molecular cell biology. 2011 Aug 23;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stahl A, Connor KM, Sapieha P, et al. The mouse retina as an angiogenesis model. Investigative ophthalmology & visual science. 2010 Jun;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strasser GA, Kaminker JS, Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood. 2010;115:5102–5110. doi: 10.1182/blood-2009-07-230284. [DOI] [PubMed] [Google Scholar]
- 87.del Toro R, Prahst C, Mathivet T, et al. Identification and functional analysis of endothelial tip cell-enriched genes. Blood. 2010;116:4025–4033. doi: 10.1182/blood-2010-02-270819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pitulescu ME, Schmidt I, Giaimo BD, et al. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nature cell biology. 2017;19:915–927. doi: 10.1038/ncb3555. [DOI] [PubMed] [Google Scholar]
- 89.Xu C, Hasan SS, Schmidt I, et al. Arteries are formed by vein-derived endothelial tip cells. Nature communications. 2014;5 doi: 10.1038/ncomms6758. 5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tian X, Hu T, Zhang H, et al. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell research. 2013 Sep;23:1075–1090. doi: 10.1038/cr.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Q, Hu T, He L, et al. Genetic targeting of sprouting angiogenesis using Apln-CreER. Nature communications. 2015;6 doi: 10.1038/ncomms7020. 6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pi J, Cheng Y, Sun H, et al. Apln-CreERT:mT/mG reporter mice as a tool for sprouting angiogenesis study. BMC ophthalmology. 2017;17:163. doi: 10.1186/s12886-017-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharma B, Chang A, Red-Horse K. Coronary Artery Development: Progenitor Cells and Differentiation Pathways. Annual review of physiology. 2017;79:1–19. doi: 10.1146/annurev-physiol-022516-033953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tian X, Pu WT, Zhou B. Cellular origin and developmental program of coronary angiogenesis. Circulation research. 2015;116:515–530. doi: 10.1161/CIRCRESAHA.116.305097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen HI, Sharma B, Akerberg BN, et al. The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Development. 2014 Dec;141:4500–4512. doi: 10.1242/dev.113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hwangbo C, Wu J, Papangeli I, et al. Endothelial APLNR regulates tissue fatty acid uptake and is essential for apelin's glucose-lowering effects. Science translational medicine. 2017;9 doi: 10.1126/scitranslmed.aad4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elmasri H, Karaaslan C, Teper Y, et al. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB journal. 2009;23:3865–3873. doi: 10.1096/fj.09-134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He L, Tian X, Zhang H, Wythe JD, Zhou B. Fabp4-CreER lineage tracing revealstwo distinctive coronary vascular populations. Journal of Cellular and Molecular Medicine. 2014;18:2152–2156. doi: 10.1111/jcmm.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proceedings of the National Academy of Sciences. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Imai T, Jiang M, Chambon P, Metzger D. Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid X receptor alpha mediated by a tamoxifen-inducible chimeric Cre recombinase (Cre-ERT2) in adipocytes. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:224–228. doi: 10.1073/pnas.011528898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang H, Lui KO, Zhou B. Endocardial Cell Plasticity in Cardiac Development, Diseases and Regeneration. Circulation research. 2018;122:774–789. doi: 10.1161/CIRCRESAHA.117.312136. [DOI] [PubMed] [Google Scholar]
- 102.Wu B, Zhang Z, Lui W, et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012;151:1083–1096. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tian X, Hu T, Zhang H, et al. Vessel formation. De novo formation of a distinct coronary vascular population in neonatal heart. Science. 2014;345:90–94. doi: 10.1126/science.1251487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang H, Pu W, Tian X, et al. Genetic lineage tracing identifies endocardial origin of liver vasculature. Nature genetics. 2016;48:537–543. doi: 10.1038/ng.3536. [DOI] [PubMed] [Google Scholar]
- 105.Tang J, Zhang H, He L, et al. Genetic Fate Mapping Defines the Vascular Potential of Endocardial Cells in the Adult Heart. Circulation research. 2018;122:984–993. doi: 10.1161/CIRCRESAHA.117.312354. [DOI] [PubMed] [Google Scholar]
- 106.Sauer B, McDermott J. DNA recombination with a heterospecific Cre homolog identified from comparison of the pac-c1 regions of P1-related phages. Nucleic Acids Research. 2004;32:6086–6095. doi: 10.1093/nar/gkh941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pu W, He L, Han X, et al. Genetic Targeting of Organ-Specific Blood Vessels. Circulation Research. 2018;123:86–99. doi: 10.1161/CIRCRESAHA.118.312981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Val S, Anderson JP, Heidt AB, Khiem D, Xu SM, Black BL. Mef2c is activated directly by Ets transcription factors through an evolutionarily conserved endothelial cell-specific enhancer. Developmental biology. 2004;275:424–434. doi: 10.1016/j.ydbio.2004.08.016. [DOI] [PubMed] [Google Scholar]