Abstract
Developments in breast cancer treatment have resulted in reduction in breast cancer mortality in the developed world. However incidence continues to rise and greater use of preventive interventions including the use of therapeutic agents is needed to control this burden. High quality evidence from 9 major trials involving more than 83000 participants shows that selective oestrogen receptor modulators (SERMs) reduce breast cancer incidence by 38%. Combined results from 2 large trials with 8424 participants show that aromatase inhibitors (AIs) reduce breast cancer incidence by 53%. These benefits are restricted to prevention of ER positive breast cancers. Restricting preventive therapy to high-risk women improves the benefit-harm balance and many guidelines now encourage healthcare professionals to discuss preventive therapy in these women. Further research is needed to improve our risk-prediction models for the identification of high risk women for preventive therapy with greater accuracy and to develop surrogate biomarkers of response. Long-term follow-up of the IBIS-I trial has provided valuable insights into the durability of benefits from preventive therapy, and underscores the need for such follow up to fully evaluate other agents. Full utilisation of preventive therapy also requires greater knowledge and awareness among both doctors and patients about benefits, harms and risk factors. Healthcare professionals should routinely discuss preventive therapy with women at high-risk of breast cancer.
Keywords: breast cancer, breast cancer risk, prevention, tamoxifen, aromatase inhibitors, SERMs
Introduction
Breast cancer is by far the commonest cancer in women today and is a major cause of death. Its incidence continues to rise with an estimated 1.6 million cases occurring worldwide each year [1]. We have, through significant improvements in cancer treatments over past few decades, have reduced breast cancer mortality in the well-developed regions of the world. In comparison, our cancer prevention efforts have been very modest. With incidence still rising, breast cancer is a prime candidate to focus our prevention efforts on, particularly given the availability of agents with well-proven efficacy backed by a large body of evidence [2–5].
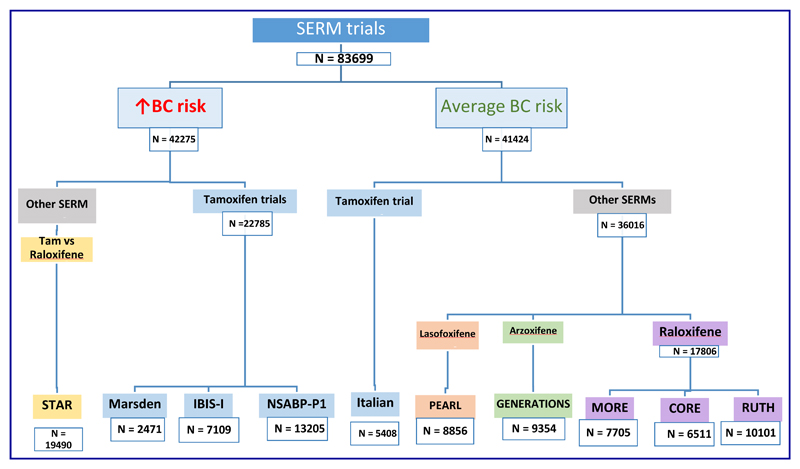
In 1985, Cuzick and Baum first reported a reduction in contralateral breast cancers in women taking tamoxifen [6]. This paved the way for evaluation of tamoxifen as a preventive therapy [7]. The original observation was also confirmed later in the EBCTCG overview of adjuvant tamoxifen trials [8]. A similar observation of a larger reduction in contralateral breast cancers in women receiving anastrozole as compared with tamoxifen in the ATAC trial [9] led to evaluation of aromatase inhibitors (AIs) as preventive therapy [3, 5]. Results from 9 large phase III trials (Figure 1) have demonstrated that preventive therapy with selective oestrogen receptor modulators (SERMs) reduces breast cancer incidence by about 38% [2], whereas 2 large phase III trials have shown that preventive therapy with AIs reduces breast cancer incidence by at least 50% [3, 5].
Figure 1. Overview of trials of Selective Oestrogen Receptor Modulators (SERMs).
The effectiveness of prophylactic therapy for breast cancer prevention as a public health strategy requires that it is used in a large proportion of women who are at an increased risk of breast cancer. Women with a significant family history of breast cancer, mammographically dense breasts, certain precursor lesions such as atypical ductal hyperplasia (ADH), lobular carcinoma in situ (LCIS) or ductal carcinoma in situ (DCIS) are obvious candidates. Several risk-prediction models, particularly the Breast Cancer Risk Assessment Tool (also known as the Gail Model) [10] and IBIS Breast Cancer Risk Evaluation Tool (also known as the Tyrer-Cuzick Model) [11], assist stratification of women into different risk categories to facilitate appropriate use of preventive therapy. In this article, we review the current evidence for prevention of invasive breast cancer using endocrine therapy, discuss barriers to wider use of preventive therapy and identify research priorities to move the field forward.
SERM trials
Selective oestrogen receptor modulators (SERMs) are a class of drugs that compete with endogenous oestrogen to bind to oestrogen receptor and after binding modulate (either inhibit or potentiate) the ligand-receptor action in a tissue-specific manner [12]. The value of four SERMs for breast cancer prevention has been evaluated in large randomised trials (Figure 1); these are tamoxifen, raloxifene, lasofoxifene and arzoxifene. SERMs have been evaluated not only in women at an increased risk of breast cancer, but also in those with an average risk of breast cancer. An individual patient data meta-analysis for 83399 women with 306617 years of follow-up from 9 randomised trials showed a 38% reduction (hazard ratio [HR] 0·62, 95% confidence interval [CI] 0·56–0·69) in breast cancer incidence (including DCIS) and the number needed to treat to prevent one invasive ER positive breast cancer in 10 years was 53 [2]. This meta-analysis included data from three trials of tamoxifen in women at an increased risk of breast cancer, viz. the Royal Marsden trial [7, 13], the IBIS-I trial [14–16] and the NSABP-P1 trial [17, 18] as well as one trial in those at average-risk women, viz. the Italian Tamoxifen Prevention Study [19, 20]. Data from the STAR trial [21, 22] comparing tamoxifen versus raloxifene in women at an increased risk of breast cancer, as well as data from trials of raloxifene in average-risk women (those conducted in fracture and cardiovascular prevention settings) viz. MORE [23]/CORE [24] and RUTH [25] were included. Data from trials of two other SERMs, viz. the PEARL trial [26, 27] evaluating lasofoxifene and the GENERATIONS trial [28, 29] evaluating arzoxifene in average-risk women with osteoporosis were also included, thus covering a range of drugs in a broad population mix.
Royal Marsden trial [7, 13]
The Royal Marsden trial was the first randomised prevention trial of tamoxifen. Between 1986 and 1996, it recruited 2494 (2471 eligible, 1238 in the tamoxifen arm and 1233 in the placebo arm) healthy women aged 30 to 70 years, with a family history of breast cancer to take tamoxifen or placebo for 5-8 years. Although after a median follow-up of 13 years and 2 months, the trial did not observe a statistically significant reduction in all invasive breast cancers (82 on tamoxifen and 104 on placebo; HR = 0.78, 95% CI 0.58-1.04; P = 0.1), the number of ER positive invasive breast cancers was significantly lower in the tamoxifen arm (53 versus 86; HR = 0.61, 95% CI 0.43-0.86; 0.005). Importantly, this benefit mainly accrued in the post-treatment period (HR = 0.48, 95% CI 0.29-0.79; P = 0.004).
IBIS-I [14–16]
The first International Breast Intervention Study (IBIS-I) trial recruited 7154 women at increased risk of breast cancer between April 1992 and March 2001. Women were randomly allocated to 5 years of preventive treatment with tamoxifen (n = 3579) or placebo (n = 3575). After a median follow-up of 16 years, the longest median follow-up so far in the breast cancer prevention trials, tamoxifen reduced breast cancer incidence by 29% (HR = 0.71, 95% CI 0.60-0.83, p < 0.0001) as compared with placebo (251 vs 350 cases; 20y risk 6.8% vs 12.3%) The reduction in breast cancer was similar between years 0 to 10 (HR = 0.72, 95% CI 0.59-0.88) and after 10 years (HR = 0.69, 95% CI 0.53-0.91), indicating a long post-treatment benefit carryover period.
NSABP-P1 [17, 18]
The National Surgical Adjuvant Breast and Bowel Project (NSABP) recruited 13388 women (6681 in the tamoxifen arm; 6707 in the placebo arm) at an increased risk of breast cancer in the Breast Cancer Prevention Trial (P1) between June 1992 and September 1997. Treatment period in the trial was 5 years and after median follow-up of 7 years, tamoxifen reduced the risk of invasive breast cancer by 43% (RR = 0.57, 95% CI 0.46-0.70) and that of non-invasive breast cancer by 37% (RR = 0.63, 95% CI 0.45-0.89).
Italian Tamoxifen Prevention Study [19, 20]
The Italian Tamoxifen Prevention Study randomised 5408 women with or without any breast cancer risk factors but who had undergone hysterectomy to tamoxifen or placebo for 5 years; 53% (n = 2876) of participants had undergone bilateral oophorectomy as well which also reduced their breast cancer risk. Recruitment was from October 1992 to July 1997. At the median follow-up of 11 years, 136 women (74 placebo, 62 tamoxifen; 4.2% 10y risk in control group) had developed breast cancer [Relative Risk (RR) = 0.84, 95% CI 0.60-1.17] and tamoxifen was associated with a reduction in breast cancer incidence in the subgroup of high-risk women (n = 702; RR = 0.24, 95% CI 0.10-0.59).
STAR [21, 22]
In 1999, the NSABP launched its second breast cancer prevention trial, the Study of Tamoxifen and Raloxifene (STAR) P-2 trial. Postmenopausal women at an increased risk of breast cancer (n = 19,747) were randomised to either tamoxifen (20 mg/d) or raloxifene (60 mg/d) over 5 years. Contrary to the initial results, which showed similar invasive breast cancer risk in both arms, the updated analysis with an 81-month median follow-up showed that the risk of invasive was significantly higher in the raloxifene arm (RR = 1.24 95% CI 1.05-1.47) and the higher risk of non-invasive breast cancer (RR = 1.22 95% CI 0.95-1.59) remained statistically non-significant as in the first analysis. The risk of serious adverse events like endometrial cancer (RR = 0.55 95% CI 0.36-0.83) and thromboembolic events (RR = 0.75 95% CI 0.60-0.93) was lower in the raloxifene arm.
MORE [23, 30]/CORE [24]
The Multiple Outcomes of Raloxifene Evaluation (MORE) trial [23, 30] was designed to see whether raloxifene reduces the risk of fracture in postmenopausal women with osteoporosis, with breast cancer incidence as a secondary endpoint. It recruited 7705 women at an average-risk of breast cancer who were randomised to 4 years of placebo (n = 2576) or raloxifene 60 mg/day (n = 2557) or raloxifene 120 mg/day (n = 2572). During this 4 years trial period, a 72% reduction in invasive breast cancers was observed in the combined raloxifene arms (RR = 0.28, 95% CI 0.17-0.46). Subsequently, the Continuing Outcomes Relevant to Evista (CORE) trial [24] was conducted to examine the effect of 4 additional years of raloxifene therapy on incidence of invasive breast cancer in women in MORE who agreed to continue in CORE. Women in the raloxifene arms in MORE were assigned to receive raloxifene (60 mg/day) in CORE (n = 3510), and women in the placebo arm in MORE continued on placebo in CORE (n = 1703). During this 4 years trial period of CORE, raloxifene reduced invasive breast cancers by 59% (HR = 0.41; 95% CI 0.24-0.71). Over the 8 years of both trials, the incidence of invasive breast cancer was 66% lower in raloxifene arm/s (HR = 0.34; 95% CI 0.22-0.50) and incidence of total breast cancer was 58% (HR = 0.42; 95% CI 0.29-0.60).
RUTH [25]
The Raloxifene Use for The Heart (RUTH) trial was designed to investigate raloxifene’s effects on both, coronary heart disease (CHD) and breast cancer. Consequently, the trial had 2 primary outcomes viz. coronary events and invasive breast cancer, and participants were not selected by their breast cancer risk factor profile, but by their CHD risk profile. The trial randomised 10101 postmenopausal women with CHD or multiple risk factors for CHD to either placebo or raloxifene (60 mg/day). After median exposure to the study drug of 5.05 years and median follow-up of 5.56 years, raloxifene reduced the risk of invasive breast cancer by 44% (HR = 0.56; 95% CI 0.38-0.83). There was no effect on primary coronary events.
PEARL [26, 27]
The Postmenopausal Evaluation and Risk-Reduction with Lasofoxifene (PEARL) trial recruited women aged 59 to 80 years who had a bone mineral density T-score of -2.5 or less at the femoral neck or spine. Primary endpoints in the trial were vertebral fractures, ER positive breast cancer, and non-vertebral fractures whereas major coronary heart disease events and stroke were secondary endpoints. The trial recruited 8556 women, randomly assigned to receive lasofoxifene, at a dose of either 0.25 mg per day (n = 2852) or 0.5 mg per day (n = 2852), or placebo (n = 2852). Lasofoxifene at a dose of 0.5 mg per day, as compared with placebo, was associated with an 85% reduced risk of invasive breast cancer (HR = 0.15; 95% CI, 0.04-0.50). Lower dose lasofoxifene (0.25mg/d) did not significantly reduce invasive breast cancer risk (HR = 0.79; 95% CI, 0.41-1.52). Participants were not selected by their breast cancer risk, and the benefits of 0.5 mg of lasofoxifene were similar across Gail score groups.
GENERATIONS [28, 29]
The generations trial enrolled 9,354 postmenopausal women aged 60 to 85 years with either osteoporosis (n = 5252), defined as a femoral neck or lumbar spine bone mineral density T-score of -2.5 or less at either femoral neck or lumbar spine or a vertebral fracture, or with osteopenia (n = 4102), defined as a bone density T-score between -1.0 and -2.5 at either site, both skeletal sites being above -2.5. Women were assigned to arzoxifene 20 mg (n = 4676) or placebo (n = 4678) daily. After 4 years of follow-up, arzoxifene reduced the incidence of invasive breast cancer by 56% (HR = 0.44, 95% CI 0.26-0.76, p <0 .001). Participants were not selected by their breast cancer risk factor profile and breast cancer risk reduction was similar across Gail risk groups (P interaction=0.31).
Non-breast cancer events in SERM trials
Two potentially fatal adverse events associated with SERM use are endometrial cancer and venous thromboembolic events. Overall SERM use was associated with an increased risk of endometrial cancer (HR = 1.56, 95% CI 1.13-2.14) and venous thromboembolic events [Odds Ratio (OR) = 1.73, 95% CI 1.47-2.05] [2]. There were some differences between SERMS on these risks – notably no increase in endometrial cancer has been seen with raloxifene. Also overall, the frequency of these events is much lower than the frequency of breast cancer events. For example, as compared with 852 breast cancers in the placebo arms of 8 SERM trials (excluding STAR), endometrial cancer and venous thromboembolic events numbered 63 and 215 respectively. In terms of changes from baseline 265 breast cancers were prevented, compared to increases of 42 and 160 endometrial cancer and venous thromboembolic events. Given that two-thirds of the participants in these 8 placebo-controlled trials were women at an average risk of breast cancer (Figure 1), the ratio of breast cancer cases prevented to causation of either of these adverse events is likely to be substantially higher in women at an increased risk of breast cancer, who are the most appropriate candidates for preventive therapy. Overall, SERMs did not prevent cardiovascular events (OR = 0.99, 95% CI 0.91-1.09) although a reduction has been seen for lasofoxifene nor did they increase the risk of cataracts (OR = 1.01, 95% CI 0.95-1.06) [2].
An additional benefit associated with use of at least some SERMs was prevention of fractures (OR = 0.85, 95% CI 0.80-0.89), through a small reduction in non-vertebral fractures (OR = 0.93, 95% CI 0.87-0.99) and a large reduction in vertebral fractures (OR = 0.66, 95% CI 0.59-0.73). Participants in MORE/CORE, PEARL and GENERATIONS trials (40% of total) were postmenopausal women with osteoporosis or osteopenia, who are at a higher risk of fractures, influencing the total number of fractures. Overall, there were 2848 fractures in the placebo arms in 8 trials and 450 fewer fractures in SERM arms [2]. The implications of this benefit are however likely to be limited since a majority of postmenopausal women are candidates for preventive therapy with AIs (below).
Deaths in SERM trials
None of the trials were designed with either breast cancer or all-cause mortality as an endpoint. There was no difference in breast cancer deaths (HR = 1.03, 95% CI 0.55-1.92) or any deaths ((HR = 0.98, 95% CI 0.90-1.06) [2]. Even after data from trials are pooled together, the numbers of breast cancer deaths are very small (30 in SERM arms and 29 in placebo arms) and therefore these analyses are underpowered even if the numbers were to be updated (51 in SERM arms and 43 in placebo arms) to include 16-year follow-up data from IBIS-I trial [16]. Furthermore, except for the Royal Marsden, IBIS-I and Italian trials, the median follow-up is also short. This results in an even shorter follow-up after an incident breast cancer event to see any effect on breast cancer mortality.
Aromatase Inhibitors
Two third-generation aromatase inhibitors (AIs), anastrozole, a non-steroidal AI and exemestane, a steroidal AI, have been evaluated in breast cancer prevention trials: the IBIS-II trial [3] and the NCIC CTG MAP.3 trial [5] respectively.
IBIS-II [3]
The International Breast Intervention Study II (IBIS-II) trial [3] recruited 3864 postmenopausal women at an increased risk of breast cancer between February 2003 and January 2012. Participants were randomised to either anastrozole 1mg/d (n = 1920) or placebo (n = 1944). After a median follow-up of 5 years, anastrozole reduced the incidence of invasive breast cancers (32 in anastrozole arm vs 64 in placebo arm) by 50% (HR = 0.50, 95% CI 0.32-0.76; p = 0.001). All breast cancers (40 in anastrozole arm vs 85 in placebo arm) were reduced by 53% (HR = 0.47, 95% CI 0.32-0.68; p < 0.0001). Although the adverse events (any grade) were frequent, occurring in more than 80% of participants in both arms, skeletal fractures, cardiovascular events, and thromboembolic events were not significantly different between arms. Cancers other than breast cancer were less frequent (HR = 0.58, 95% CI 0.39-0.85) in anastrozole arm as compared with placebo arm (40 vs 70) whereas musculoskeletal events (p = 0.0001) and vasomotor symptoms (p < 0.0001) were more frequent in the anastrozole arm.
NCIC CTG MAP.3 [5]
The National Cancer Institute of Canada Clinical Trials Group MAP.3 (NCIC CTG MAP.3) trial [5] recruited 4560 postmenopausal women at an increased risk of breast cancer between February 2004 and March 2010. Participants were randomised to either exemestane 25mg/d (n = 2285) or placebo (n = 2275). After a median follow-up of 35 months, exemestane reduced the incidence of invasive breast cancers (11 in exemestane arm vs 32 in placebo arm) by 65% (HR = 0.35, 95% CI 0.18-0.70; p = 0.002). All breast cancers including DCIS were reduced by 53% (HR = 0.47, 95% CI 0.27-0.79; p = 0.004). Although the adverse events (any grade) were similarly frequent, occurring in more than 80% of participants in both arms, skeletal fractures, cardiovascular events, other cancers were not significantly different between arms.
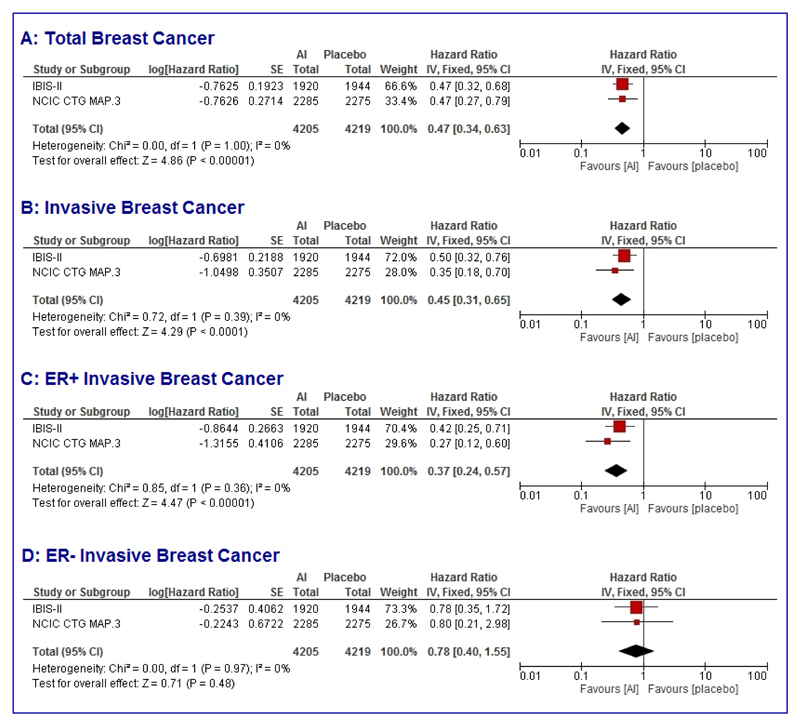
Pooled analyses (Figure 2) of results from these two trials using a fixed effects model show that aromatase inhibitors reduce the risk of breast cancer by 53% (HR = 0.47, 95% CI 0.34-0.63; p < 0.0001); invasive breast cancer by 55% (HR = 0.45, 95% CI 0.31-0.65; p < 0.0001) and ER positive invasive breast cancer by 63% (HR = 0.37, 95% CI 0.24-0.57; p < 0.0001); there was no effect on ER negative invasive breast cancers (HR = 0.78, 95% CI 0.40-1.55; p = 0.97). No heterogeneity been trials was seen for any of these endpoints (heterogeneity quotient I2 = 0% for all analyses).
Figure 2. Forest plots of pooled analyses (fixed effects) of trials evaluating aromatase inhibitors for breast cancer prevention.
Total breast cancer (A); Invasive breast cancer (B); ER positive invasive breast cancer (C); ER negative invasive breast cancer (D).
Contralateral breast cancers in extended aromatase inhibitor trials
A greater reduction in contralateral breast cancers (CBC) with anastrozole as compared with tamoxifen in the ATAC trial [9] formed the basis of breast cancer prevention trials of AIs. The long-term follow-up of the ATAC trial [31] showed a 38% reduction in CBCs (HR = 0.62, 95% CI 0·45–0·85) with anastrozole as compared to tamoxifen in breast cancer patients with hormone receptor positive tumours. Similarly, reductions in CBC have recently been reported from trials of extended AI therapy. The NCIC CTG MA17.R trial [32] evaluated the benefit of 5 years of extended letrozole (n = 959) therapy versus placebo (n = 954) in postmenopausal women who have completed 5 years of letrozole therapy either upfront or after switch from tamoxifen. It reported a 58% reduction in CBC (HR = 0.42, 95% CI 0.22-0.81, p = 0.007) in the extended letrozole arm as compared with the placebo arm. The Investigation on the Duration of Extended Adjuvant Letrozole (IDEAL) trial (n = 1824) of 2.5 vs 5 years of extended letrozole after 5 years of endocrine therapy reported 63% reduction in CBC (HR = 0.37, 95% CI 0.18-0.77, p = 0.008) in the longer extended therapy arm [33]. The NSABP-B42 trial investigating 5 years of letrozole (n = 1959) versus placebo (n = 1964) after 5 years of endocrine treatment reported a 48% reduction in CBC (OR = 0.52, 95% CI 0.33-0.81, p = 0.0041) in the extended letrozole arm as compared with the placebo arm [34].
Other endocrine agents
Goserelin, an LHRH analogue induces menopause through ovarian suppression resulting in low levels of endogenous oestrogen. It has been evaluated in combination with raloxifene (60mg/d) in the Raloxifene and Zoladex Research study (RAZOR) where women aged 30-45 years and at a high genetic risk of developing breast cancer including those with BRCA1 or BRCA2 germ-line mutations in a small feasibility study. Women were randomised to annual screening or goserelin (3.6mg/month) and raloxifene (60mg/d) for 2 years (ClinicalTrials.gov Identifier: NCT00031850). The trial recruited 75 participants and initial results on reductions in breast density, side effects and acceptability have been reported at a meeting [35] but are yet to be published.
Breast cancer prevention guidelines and recommendations
As the breast cancer prevention trials accrue follow-up, the benefit-harm balance has improved since the majority of adverse events are limited to treatment period and net benefit of such preventive interventions has become clearer. This has shaped the more recent updates of guidelines or resulted in new guidelines recommending use of preventive therapy. As compared with the 2009 guidelines [36] of the American Society of Clinical Oncology (ASCO), the 2013 update of these ASCO guidelines [37] is more affirmative. Current ASCO guidelines [37] recommend that for women aged 35 or more and at an increased risk of breast cancer, tamoxifen should be discussed as an option to reduce the risk of ER positive breast cancer, and in postmenopausal women raloxifene and exemestane should also be discussed. The guideline panel also states that “Health care providers are encouraged to discuss the option of chemoprevention among women at increased BC risk.” [36–38].
The UK National Institute for Health and Care Excellence (NICE) reviewed the evidence for preventive therapy in breast cancer and released a new set of guidelines in 2013. These guidelines also recommend tamoxifen or raloxifene as preventive therapy in women with lifetime breast cancer risk above 30% [39]. Recently, a draft of update of these guidelines has been placed for comments. This update draft [40] recommends anastrozole for breast cancer prevention in postmenopausal women at high risk of breast cancer unless they also have severe osteoporosis.
An expert panel consensus statement [41] on behalf of the International Society of Cancer Prevention (ISCaP) now known as International Cancer Prevention Society (ICAPS) also recommended tamoxifen and raloxifene for breast cancer prevention. Recent guidelines [42] from the US National Comprehensive Cancer Network (NCCN) also recommend tamoxifen for breast cancer prevention in women aged 35 or more and at greater than1.7% breast cancer risk over 5 years as estimated from modified Gail model. The panel felt strongly that tamoxifen is a superior choice of risk-reduction agent but raloxifene may be chosen in view of toxicity considerations. The panel also recommended exemestane and anastrozole for breast cancer prevention in postmenopausal women.
Barriers to use of preventive therapy
Therapy to prevent breast cancer remains very much under-utilised [43] and the low uptake of preventive therapy, 8.7% in non-trial settings, is a result of several factors [44]. These include lack of physician and patient awareness, concerns about side effects, lack of demonstrated mortality reduction and other issues such as licensing and indemnity issues [4, 37, 45, 46]. In a qualitative study to investigate the factors affecting preventive therapy use within the UK. Smith and colleagues [46] observed that general practitioners (GPs) were unfamiliar with the concept of preventive therapy. This is particularly important since physician’s recommendation is a key factor in the uptake of preventive therapy [44, 47, 48]. Underestimation of benefits and / or overestimation of harms [48, 49] is another issue not helped by limited general knowledge and use of individual risk-estimation [50, 51]. The majority of these findings are related to use of SERMs and it is possible that AIs with their greater efficacy and better side-effect profile may get more readily accepted. However, AIs are only available to postmenopausal women. Other barriers include low commercial interest as the drugs no longer have patent protection [52, 53] and liability issues, especially for off label use [46, 54]. In the study by Smith and colleagues [46], GPs were reluctant to initiate preventive therapy because it is not licensed, but were willing to continue a prescription if it had been started in secondary or tertiary care. DeCensi and colleagues [4] have discussed these barriers and strategies to overcome barriers in great detail, and we refer reader to this review for a detailed discussion of physician prejudices arising out of lack of demonstrated mortality reduction [4].
Research priorities
The 16-year median follow-up data from IBIS-I trial [16] and the extended follow-up data for the SERM overview [2] have demonstrated the importance of long-term follow-up by not only improving the estimates of benefit-harm balance but also by improving our understanding of the long carryover benefit. The follow-up for trials of arzoxifene and lasofoxifene and AIs has been short, and it is important that participants in these trials continue to be followed-up for at least another 10 years.
Recent improvements in risk prediction models like the Tyrer-Cuzick model [11] through incorporation of SNP panels and mammographic density offer greater accuracy and scope for identifying high risk women [55, 56]. The newest version [57] includes both of these and can now be downloaded from the internet (version 8 [57]; http://www.emstrials.org/riskevaluator/software/v8/winForm/IBIS_RiskEvaluator_v8.zip). Continued research to identify new biomarkers that can further improve risk-prediction models is needed [50] notably for benign breast disease where our understanding is still limited [50, 51]. While the effects of preventive endocrine therapy in reducing ER positive breast cancer are beyond doubt, we lack therapies that can prevent ER negative breast cancer. Predictive biomarkers that can identify women who will respond to specific treatments or differentiate better response to tamoxifen vs. aromatase inhibitors are also lacking.
Reduction in mammographic density has been shown to be a good surrogate marker to evaluate benefit of tamoxifen as preventive therapy [58] and both tamoxifen and aromatase inhibitors in the adjuvant setting [59–62], but is not available until after at least 6 months of treatment, so predictive factors available at baseline are still needed. It may be possible to avoid majority of the side effects of endocrine agents by local delivery to breast and avoiding systemic distribution. Research to develop novel delivery methods like topical gels [63, 64] or intraductal delivery (ClinicalTrials.gov Identifier: NCT02540330) is also an important area.
Conclusions
A large body of evidence from 11 major phase III randomised trials has demonstrated the effect of preventive endocrine therapy in reducing breast cancer, but benefits are limited to ER positive breast cancer. Nine of these trials with over 83000 participants and 306000 women-years of follow-up show that SERMs reduce breast cancer incidence by 38% whereas 2 other trials with more than 8400 participants have shown that AIs reduce breast cancer incidence by 53%. The long-term follow-up of IBIS-I trial has demonstrated that 5 years of tamoxifen treatment has a benefit that lasts at least 15 years; the overall long term reduction of ER positive invasive breast cancers was 34% (HR = 0.66, 95% CI 0.54-0.81, p < 0.0001) with no effect on ER negative invasive breast cancers (HR = 1.05, 95% CI 0.71-1.57, p = 0.8). As a result, many guidelines now recommend use of preventive endocrine therapy in women at an increased risk of breast cancer and encourage healthcare professionals to discuss preventive therapy with their patients. Utilisation of preventive therapy however remains poor largely due to lack of physician and patient awareness, concerns about side effects and particularly overestimation of side effects, as well as other issues such as licensing and indemnity issues. Among the research priorities, improving risk-prediction models, development of surrogate biomarkers of response and novel local drug delivery systems merit attention. The importance of continuation of long-term follow-up of trial participants cannot be overemphasised. In summary, preventive endocrine therapy with its clear and clinically relevant efficacy in preventing ER positive breast cancer merits wider attention including increasing awareness among healthcare professionals. Healthcare professionals should make their patients aware of this important option in breast cancer risk management and discuss it with them.
Acknowledgments
IBIS trials were funded in part by Cancer Research UK (grants C569/A5032; C569/A16891; C8162/A26893). Support was also provided by AstraZeneca and Sanofi-Aventis. AstraZeneca additionally provided anastrozole, tamoxifen, and matching placebos for these trials. Sanofi-Aventis also provided risedronate and matching placebo for the IBIS-II trial.
Funding source:
This work was funded by Cancer Research UK (grant number C569/A16891).
Abbreviations
- AIs
Aromatase Inhibitors
- ATAC
Arimidex, Tamoxifen, Alone or in Combination
- AH
Atypical Hyperplasia
- ADH
Atypical Ductal Hyperplasia
- BCRAT
Breast Cancer Risk Assessment Tool
- DCIS
Ductal Carcinoma in situ
- EBCTCG
Early Breast Cancer Trialists’ Group
- HR
Hazard Ratio
- LCIS
Lobular Carcinoma in situ
- NSABP
National Surgical Adjuvant Breast and Bowel Project
- OR
Odds Ratio
- SERMs
Selective Oestrogen Receptor Modulators
Footnotes
Competing interests:
JC’s institution has received funding for the IBIS trials from Sanofi-Aventis and AstraZeneca.
References
- [1].Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. IARC CancerBase. 2012 ed. Lyon, France: International Agency for Research on Cancer; 2012. [Google Scholar]
- [2].Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827–34. doi: 10.1016/S0140-6736(13)60140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041–8. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- [4].DeCensi A, Thorat MA, Bonanni B, Smith SG, Cuzick J. Barriers to preventive therapy for breast and other major cancers and strategies to improve uptake. Ecancermedicalscience. 2015;9:595. doi: 10.3332/ecancer.2015.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–91. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- [6].Cuzick J, Baum M. TAMOXIFEN AND CONTRALATERAL BREAST CANCER. The Lancet. 1985;326:282. doi: 10.1016/S0140-6736(85)90338-1. [DOI] [PubMed] [Google Scholar]
- [7].Powles T, Eeles R, Ashley S, Easton D, Chang J, Dowsett M, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet. 1998;352:98–101. doi: 10.1016/S0140-6736(98)85012-5. [DOI] [PubMed] [Google Scholar]
- [8].Abe O, Abe R, Enomoto K, Kikuchi K, Koyama H, Nomura Y, et al. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet. 1998;351:1451–67. doi: 10.1016/S0140-6736(97)11423-4. [DOI] [PubMed] [Google Scholar]
- [9].Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–9. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- [10].Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- [11].Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Statistics in medicine. 2004;23:1111–30. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- [12].Williams C, Lin CY. Oestrogen receptors in breast cancer: basic mechanisms and clinical implications. Ecancermedicalscience. 2013;7:370. doi: 10.3332/ecancer.2013.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. Journal of the National Cancer Institute. 2007;99:283–90. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- [14].Cuzick J, Forbes J, Edwards R, Baum M, Cawthorn S, Coates A, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–24. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- [15].Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, et al. Long-term results of tamoxifen prophylaxis for breast cancer-96-month follow-up of the randomized IBIS-I trial. Journal of the National Cancer Institute. 2007;99:272–82. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- [16].Cuzick J, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. The Lancet Oncology. 2015;16:67–75. doi: 10.1016/S1470-2045(14)71171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- [18].Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. Journal of the National Cancer Institute. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- [19].Veronesi U, Maisonneuve P, Costa A, Sacchini V, Maltoni C, Robertson C, et al. Prevention of breast cancer with tamoxifen: Preliminary findings from the Italian randomised trial among hysterectomised women. Lancet. 1998;352:93–7. doi: 10.1016/S0140-6736(98)85011-3. [DOI] [PubMed] [Google Scholar]
- [20].Veronesi U, Maisonneuve P, Rotmensz N, Bonanni B, Boyle P, Viale G, et al. Tamoxifen for the prevention of breast cancer: Late results of the Italian randomized tamoxifen prevention trial among women with hysterectomy. Journal of the National Cancer Institute. 2007;99:727–37. doi: 10.1093/jnci/djk154. [DOI] [PubMed] [Google Scholar]
- [21].Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- [22].Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the national surgical adjuvant breast and bowel project Study of Tamoxifen and Raloxifene (STAR) P-2 trial: Preventing breast cancer. Cancer prevention research. 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cauley JA, Norton L, Lippman ME, Eckert S, Krueger KA, Purdie DW, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-Year results from the MORE trial. Breast cancer research and treatment. 2001;65:125–34. doi: 10.1023/A:1006478317173. [DOI] [PubMed] [Google Scholar]
- [24].Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, et al. Continuing outcomes relevant to Evista: Breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. Journal of the National Cancer Institute. 2004;96:1751–61. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- [25].Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. New England Journal of Medicine. 2006;355:125–37. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- [26].Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, et al. Lasofoxifene in postmenopausal women with osteoporosis. New England Journal of Medicine. 2010;362:686–96. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- [27].Lacroix AZ, Powles T, Osborne CK, Wolter K, Thompson JR, Thompson DD, et al. Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. Journal of the National Cancer Institute. 2010;102:1706–15. doi: 10.1093/jnci/djq415. [DOI] [PubMed] [Google Scholar]
- [28].Cummings SR, McClung M, Reginster JY, Cox D, Mitlak B, Stock J, et al. Arzoxifene for prevention of fractures and invasive breast cancer in postmenopausal women. Journal of Bone and Mineral Research. 2011;26:397–404. doi: 10.1002/jbmr.191. [DOI] [PubMed] [Google Scholar]
- [29].Powles TJ, Diem SJ, Fabian CJ, Neven P, Wickerham DL, Cox DA, et al. Breast cancer incidence in postmenopausal women with osteoporosis or low bone mass using arzoxifene. Breast cancer research and treatment. 2012;134:299–306. doi: 10.1007/s10549-012-2041-5. [DOI] [PubMed] [Google Scholar]
- [30].Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–97. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- [31].Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. The Lancet Oncology. 2010;11:1135–41. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- [32].Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med. 2016;375:209–19. doi: 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Blok E, van de Velde C, Meershoek-Klein Kranenbarg E, Putter H, van den Bosch J, Maartense E, et al. Abstract S1-04: Optimal duration of extended letrozole treatment after 5 years of adjuvant endocrine therapy; results of the randomized phase III IDEAL trial (BOOG 2006-05) Cancer Research. 2017;77 doi: 10.1158/1538-7445.sabcs16-s1-04. S1-04-S1- [DOI] [Google Scholar]
- [34].Mamounas E, Bandos H, Lembersky B, Geyer C, Fehrenbacher L, Graham M, et al. Abstract S1-05: A randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy (tx) with letrozole (L) in postmenopausal women with hormone-receptor (+) breast cancer (BC) who have completed previous adjuvant tx with an aromatase inhibitor (AI): Results from NRG Oncology/NSABP B-42. Cancer Research. 2017;77 doi: 10.1158/1538-7445.sabcs16-s1-05. S1-05-S1- [DOI] [Google Scholar]
- [35].Motion J, Ashcroft L, Dowsett M, Cuzick J, Hickman J, Evans G, et al. Abstract P1-09-05: The RAZOR trial: a phase II prevention trial of screening plus goserilin and raloxifene versus screening alone in pre-menopausal women at increased risk of breast cancer. Cancer Research. 2012;72 doi: 10.1158/0008-5472.sabcs12-p1-09-05. P1-09-5-P1--5. [DOI] [Google Scholar]
- [36].Visvanathan K, Chlebowski RT, Hurley P, Col NF, Ropka M, Collyar D, et al. American society of clinical oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3235–58. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2942–62. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- [38].Chlebowski RT, Col N, Winer EP, Collyar DE, Cummings SR, Vogel VG, 3rd, et al. American Society of Clinical Oncology technology assessment of pharmacologic interventions for breast cancer risk reduction including tamoxifen, raloxifene, and aromatase inhibition. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:3328–43. doi: 10.1200/JCO.2002.06.029. [DOI] [PubMed] [Google Scholar]
- [39].National Institute for Health and Clinical Excellence. Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer. CG164. National Institute for Health and Clinical Excellence; 2013. [Google Scholar]
- [40].National Institute for Health and Clinical Excellence. Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer. CG164. National Institute for Health and Clinical Excellence; 2016. [Google Scholar]
- [41].Cuzick J, DeCensi A, Arun B, Brown PH, Castiglione M, Dunn B, et al. Preventive therapy for breast cancer: a consensus statement. The Lancet Oncology. 2011;12:496–503. doi: 10.1016/S1470-2045(11)70030-4. [DOI] [PubMed] [Google Scholar]
- [42].National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology - Breast Cancer Risk Reduction. National Comprehensive Cancer Network; 2017. [Google Scholar]
- [43].Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526–32. doi: 10.1093/jnci/95.7.526. [DOI] [PubMed] [Google Scholar]
- [44].Smith SG, Sestak I, Forster A, Partridge A, Side L, Wolf MS, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2016;27:575–90. doi: 10.1093/annonc/mdv590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3090–5. doi: 10.1200/JCO.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Smith SG, Side L, Meisel SF, Horne R, Cuzick J, Wardle J. Clinician-Reported Barriers to Implementing Breast Cancer Chemoprevention in the UK: A Qualitative Investigation. Public Health Genomics. 2016;19:239–49. doi: 10.1159/000447552. [DOI] [PubMed] [Google Scholar]
- [47].Kinney AY, Richards C, Vernon SW, Vogel VG. The effect of physician recommendation on enrollment in the Breast Cancer Chemoprevention Trial. Preventive medicine. 1998;27:713–9. doi: 10.1006/pmed.1998.0349. [DOI] [PubMed] [Google Scholar]
- [48].Taylor R, Taguchi K. Tamoxifen for breast cancer chemoprevention: low uptake by high-risk women after evaluation of a breast lump. Annals of family medicine. 2005;3:242–7. doi: 10.1370/afm.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Annals of surgical oncology. 2001;8:580–5. doi: 10.1007/s10434-001-0580-9. [DOI] [PubMed] [Google Scholar]
- [50].Cuzick J, Sestak I, Thorat MA. Impact of preventive therapy on the risk of breast cancer among women with benign breast disease. Breast. 2015 doi: 10.1016/j.breast.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN. Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev. 2010;19:443–6. doi: 10.1158/1055-9965.EPI-09-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Herberman RB, Pearce HL, Lippman SM, Pyenson BS, Alberts DS. Cancer chemoprevention and cancer preventive vaccines--a call to action: leaders of diverse stakeholder groups present strategies for overcoming multiple barriers to meet an urgent need. Cancer Res. 2006;66:11540–9. doi: 10.1158/0008-5472.CAN-06-4122. [DOI] [PubMed] [Google Scholar]
- [53].Veronesi U, Maisonneuve P, Decensi A. Tamoxifen: an enduring star. J Natl Cancer Inst. 2007;99:258–60. doi: 10.1093/jnci/djk072. [DOI] [PubMed] [Google Scholar]
- [54].Tanne JH. Court awards claimant $13.5m in rofecoxib lawsuit. 2006 doi: 10.1136/bmj.332.7547.927-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Evans D, Astley S, Brentnall A, Howell A, Cuzick J. Abstract PD1-07: Mammographic density and SNPs add to Tyrer-Cuzick and Gail model breast cancer risk in a UK screening cohort. Cancer Research. 2016;76 doi: 10.1158/1538-7445.sabcs15-pd1-07. PD1-07-PD1- [DOI] [Google Scholar]
- [56].Evans DG, Astley S, Stavrinos P, Harkness E, Donnelly LS, Dawe S, et al. Improvement in risk prediction, early detection and prevention of breast cancer in the NHS Breast Screening Programme and family history clinics: a dual cohort study; Programme Grants for Applied Research; Southampton (UK). 2016. [PubMed] [Google Scholar]
- [57].IBIS Breast Cancer Risk Evaluation Tool. v8 ed. 2017 [Google Scholar]
- [58].Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744–52. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- [59].Cuzick J. Breast density predicts endocrine treatment outcome in the adjuvant setting. Breast Cancer Res. 2012;14:109. doi: 10.1186/bcr3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ko KL, Shin IS, You JY, Jung SY, Ro J, Lee ES. Adjuvant tamoxifen-induced mammographic breast density reduction as a predictor for recurrence in estrogen receptor-positive premenopausal breast cancer patients. Breast cancer research and treatment. 2013;142:559–67. doi: 10.1007/s10549-013-2726-4. [DOI] [PubMed] [Google Scholar]
- [61].Li J, Humphreys K, Eriksson L, Edgren G, Czene K, Hall P. Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy in postmenopausal patients with breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2249–56. doi: 10.1200/JCO.2012.44.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Engmann NJ, Scott C, Jensen MR, Ma L, Brandt KR, Mahmoudzadeh A, et al. Longitudinal changes in volumetric breast density with tamoxifen and aromatase inhibitors. Cancer Epidemiol Biomarkers Prev. 2017 doi: 10.1158/1055-9965.EPI-16-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lee O, Khan SA. Novel routes for administering chemoprevention: local transdermal therapy to the breasts. Semin Oncol. 2016;43:107–15. doi: 10.1053/j.seminoncol.2015.09.003. [DOI] [PubMed] [Google Scholar]
- [64].Lee O, Ivancic D, Allu S, Shidfar A, Kenney K, Helenowski I, et al. Local transdermal therapy to the breast for breast cancer prevention and DCIS therapy: preclinical and clinical evaluation. Cancer Chemother Pharmacol. 2015;76:1235–46. doi: 10.1007/s00280-015-2848-y. [DOI] [PubMed] [Google Scholar]