Abstract
We report new findings of non-indigenous Indo-Pacific molluscs from shallow water habitats off Israel, Greece and Egypt, eastern Mediterranean Sea. The bivalves Pillucina vietnamica Zorina, 1978 and Alveinus miliaceus (Issel, 1869) were collected from sandy bottoms off Israel, whereas Gregariella cf. ehrenbergi (Issel, 1869) was recovered from a buoy originating from Port Said, Egypt, and stranded on the Israeli coast. The three species are first records for the Mediterranean Sea. Additionally, we report range extensions for several gastropods: Varicopeza pauxilla (A. Adams, 1855) is recorded from Israel, Phidiana militaris (Alder and Hancock, 1864) from southern Israel (Ashqelon), and Viriola cf. bayani Jousseaume, 1884 from Israel and Crete. Shells and valves of an unidentified lucinid bivalve morphologically distinct from any known Mediterranean species were found along the Israeli Mediterranean shore.
Keywords: Lessepsian invasion, Israel, Greece, Egypt, Suez Canal, first records, range extensions
Introduction
Biological invasions rank among the most serious threats to the world’s biodiversity and constitute a major and pervasive element of global change (Galil 2007; Molnar et al. 2008; Occhipinti-Ambrogi and Galil 2010). The Mediterranean Sea, a hotspot of species richness and endemism (Bianchi and Morri 2000; Coll et al. 2010), is affected by the largest marine biological invasion—the so-called “Lessepsian invasion”—which followed the opening of the Suez Canal in 1869. The Suez Canal, a shallow artificial waterway connecting the Mediterranean and Indo-Pacific biogeographic provinces, is the most significant vector for introductions of non-indigenous species (NIS) to the basin, followed by shipping (Galil 2008; Galil et al. 2016; Zenetos 2017). Due to the geographic proximity and prevailing surface currents, the Levantine Sea in the southeastern Mediterranean is the region most affected by Lessepsian NIS (Galil 2008, 2017; Tzomos et al. 2012; Katsanevakis et al. 2014; Galil et al. 2016). Biological invasions have the potential to fundamentally alter the structure and functioning of recipient communities (Molnar et al. 2008; Ehrenfeld 2010; Fanelli et al. 2015); therefore, they not only affect local biodiversity, but may cause major ecological, economic and social damage (Galil 2007; Pyšek and Richardson 2010; Simberloff et al. 2013), particularly if NIS affect the provision of ecosystem services (Wallentinus and Nyberg 2007) or adversely affect human health (Mazza et al. 2014). Considering this and the rapid range expansions observed for many NIS originally restricted to the Levantine Basin (Galil 2009; Tzomos et al. 2012; Galil et al. 2016), the timely reporting of new findings is essential for better understanding the dynamics of the invasion and to recognize potential invasive species at an early stage, when mitigation measures are most effective (Crooks 2005; Simberloff et al. 2013).
As of 2016, 613 established and further 208 casual non-indigenous species representing most marine phyla have been recorded in the Mediterranean, with molluscs being the most diverse taxon (Galil 2009; Zenetos et al. 2017). Herein, we report new records and range extensions of non-indigenous molluscs of Indo-Pacific origin, further increasing the list of Lessepsian species in the Mediterranean Sea.
Methods
The molluscs were collected along the Mediterranean coast of Israel, in southern Crete, Greece, and from a buoy originally moored at the entrance of the Suez Canal in Port Said, Egypt, but detached and stranded in 2014 near Shefayim, Israel, and subsequently transported to the Herzliya marina nearby (Captain M. Solomon and A. Tzindr, pers. comm.) (Figure 1).
Figure 1.
Map of the eastern Mediterranean showing the sampling localities (see Table 1 for details).
In Israel, soft substrates were sampled in September 2016 and April 2017 off Ashqelon and Atlit (Figure 1, Table 1). Molluscan individuals and shells were recovered from bulk sediment samples taken at 10–41 m depth with a van Veen grab (36.5 × 31.8 cm) or boxcorer (25 × 25 cm) aboard RV “Mediterranean Explorer”. The sediment was sieved through a 0.5 mm mesh and the retained material fixed in ethanol. Additional molluscs were collected in spring 2018 from offshore reefs off Ashqelon, southern Israel, with a diver-operated airlift sampler equipped with a 0.5 mm mesh bag, and off Palmachim, central Israel, by snorkeling and overturning rocks (Figure 1).
Table 1. List of stations (coordinates according to datum WGS84).
| Sample | Locality | Latitude | Longitude | Depth [m] | Substrate |
|---|---|---|---|---|---|
| Rh10 | Greece, Crete, Plakias | 35.1793 | 24.3956 | 10 | Posidonia oceanica rhizomes |
| Rh20 | Greece, Crete, Plakias | 35.1793 | 24.3956 | 20 | Posidonia oceanica rhizomes |
| NG10 | Israel, N of Atlit | 32.7820 | 34.9466 | 10 | sand |
| NG30 | Israel, N of Atlit | 32.7422 | 34.9181 | 30 | sand |
| S12 | Israel, Ashqelon | 31.6868 | 34.5516 | 12 | offshore rocky reef |
| SG10 | Israel, Ashqelon | 31.6953 | 34.5588 | 11 | sand |
| SG20 | Israel, Ashqelon | 31.7002 | 34.5498 | 21 | sand |
| SG30 | Israel, Ashqelon | 31.7100 | 34.5406 | 30 | muddy sand |
| SG40 | Israel, Ashqelon | 31.7487 | 34.4960 | 41 | mud |
| – | Israel, Palmachim | 31.9272 | 34.6964 | 3 | rocks |
| – | originally Egypt, Port Said (but buoy detached and stranded near Shefayim, central Israel) | 31.28 | 32.37 | 0–5.5 | buoy |
In Crete, sampling took place off Plakias on the southern coast of the island in the framework of a survey of molluscan assemblages in Posidonia oceanica meadows. A diver-operated airlift sampler was used to collect on three replicate 1 m2-quadrats of Posidonia rhizomes at depths of 5, 10, 15 and 20 m, using 0.5 mm mesh bags.
The dry remains of fouling assemblages on the 13.5 m-long buoy in the Herzliya marina were scraped from 0.1 m2 quadrats approximately every meter from the original water level to its lowermost end (originally 5.5 m water depth), plus a further quadrat in the hollow internal part of the buoy at ca. 5.5 m depth. Samples were sieved with a 0.5 mm mesh.
Molluscs from bulk samples were sorted and identified to the lowest possible taxonomic level. Detailed data for all sampling stations which yielded new records of non-indigenous species and georeferenced record data are provided in Table 1 and Supplementary material Table S1, respectively. Photographs were taken using a Zeiss SteREO Discovery.V20 stereomicroscope and stacked with Helicon Focus 6 (Helicon Soft Ltd., Roseau Valley, Dominica). Scanning electron microscopy (SEM) images were taken with a Fei Inspect S50 scanning electron microscope without coating. The illustrated specimens of the newly recorded non-indigenous species have been deposited as vouchers in the Naturhistorisches Museum Wien (NHMW). A subset of the specimens will be deposited at the Steinhardt Museum of Natural History (SMNH), Tel Aviv University.
Abbreviations
H: height; L: length (for bivalves); LV/s: left valve/s; RV/s: right valve/s; sh/s: empty shell/s (gastropod or complete bivalve); spcm/s: live collected specimen/s; v/s: valve/s; W: width (for gastropods).
Results
Class Gastropoda Cuvier, 1795
Subclass Caenogastropoda Cox, 1960
Order unassigned
Family Cerithiidae J. Fleming, 1822
Varicopeza pauxilla (A. Adams, 1855)
(Figure 2)
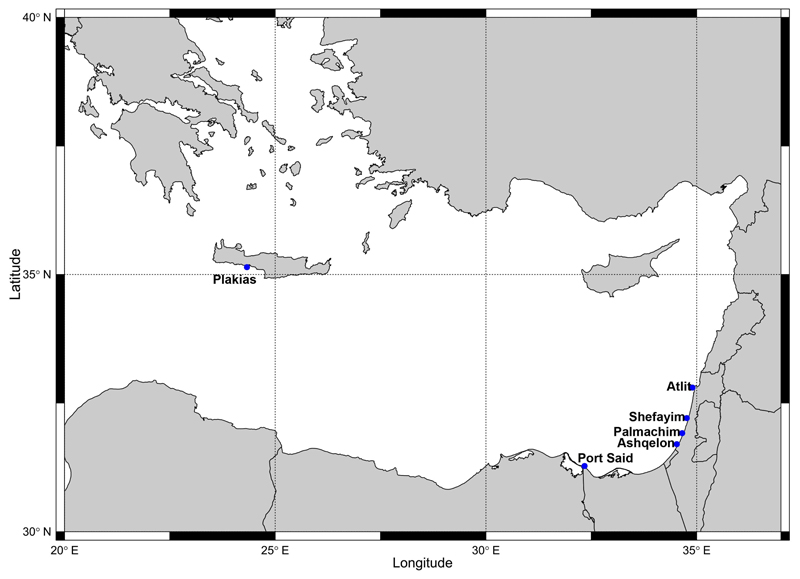
Figure 2.
Varicopeza pauxilla (A. Adams, 1855). A–C, H. NHMW-MO-112644, NG30, N of Atlit, northern Israel, sand, −30 m, 20/09/2016, front (A), side (B) and back (C) view, side view of aperture (H). D. NHMW-MO-112645, locality as previous, protoconch. E–G, I. NHMW-MO-112646, SG40, off Ashqelon, southern Israel, mud, −41 m, 27/04/2017, front (E), side (F) and back (G) view, side view of aperture (I). Scale bars: 1 mm (A–C and E–G), 0.2 mm (D), 0.5 mm (H–I). Photo credit: J. Steger.
Material examined: NG10 (21/09/2016, 3 shs); NG30 (20/09/2016, 37 spcms, 4 shs); SG20 (18/09/2016, 7 spcms; 27/04/2017, 2 spcms); SG30 (27/04/2017, 3 spcms); SG40 (18/09/2016, 88 spcms, 1 sh; 27/04/2017, 1 sh).
Voucher specimens: NHMW-MO-112644: NG30, 20/09/2016, 1 spcm, H 5.9 mm, W 2.3 mm (Figure 2A–C, H); NHMW-MO-112645: locality as previous, 1 juvenile sh, H 1.6 mm, W 0.9 mm (Figure 2D); NHMW-MO-112646: SG40, 27/04/2017, 1 sh, H 8.6 mm, W 2.9 mm (Figure 2E–G, I).
Remarks: Varicopeza pauxilla has a slender, rather straight-sided, turriform shell with a strong sculpture consisting of spiral cords and slightly opisthocline axial ribs, forming tubercles at the intersections. The colour is variable, ranging from yellowish-white to brown (Figure 2), sometimes with a darker spiral band. A strong varix is present opposite the outer lip in adult specimens (Figure 2A, C, E and G; see also Houbrick 1980). In the Mediterranean, V. pauxilla may be confused with the invasive Lessepsian cerithiid Rhinoclavis kochi, particularly when dealing with juvenile specimens. The latter, however, lacks the wide sinus/posterior exhalant channel in the outer lip of adult Varicopeza pauxilla (Figure 2H–I) as well as the prominent notch at the protoconch/teleoconch transition (Figure 2D). Further, the protoconch of V. pauxilla is smooth with only a faint spiral keel visible at high magnification, while two keels are present in R. kochi.
Varicopeza pauxilla has an Indo-West Pacific distribution, inhabiting soft substrates at continental shelf and upper slope depths (Houbrick 1980). In the northern Red Sea, it has been reported from the Gulf of Aqaba from 40–402 m depth, including locations off Elat (Houbrick 1980; Edelman-Furstenberg and Faershtein 2010), and from the Bay of Safaga, at the latter location mainly from mud at 39 m depth (Janssen et al. 2011). In August 2016, specimens were found along the Turkish Levantine coast at depths of 18–55 m, comprising the first record for the Mediterranean Sea (Öztürk et al. 2017). Herein, we extend its known distribution to the Israeli coast, where it was common on soft substrates at 30–40 m depth. The species was particularly abundant in samples collected in September 2016 at 41 m depth off Ashqelon, contributing 73.3% of living molluscan individuals. According to Houbrick (1980), V. pauxilla is a microphageous detritivore and perhaps also a filter feeder. It has a planktonic developmental strategy (Houbrick 1993). The numerous specimens in various growth stages and already wide distribution in the Levantine Sea suggest it is a well-established species in the eastern Mediterranean.
Family Triphoridae Gray, 1847
Viriola cf. bayani Jousseaume, 1884
(Figure 3)
Figure 3.
Viriola cf. bayani Jousseaume, 1884. NHMW-MO-112647, Rh10, Plakias, Crete, Greece, among rhizomes of Posidonia oceanica, -10 m, 17/09/2017, front (A) and side (B) view. Scale bar: 2 mm. Photo credit: P.G. Albano.
Material examined: Rh10 (17/09/2017, 1 spcm); Rh20 (14/09/2017, 1 spcm); Palmachim, Israel, under rocks, −3 m (26/04/2018, 3 spcms); MNHN IM-2000-1388, New Caledonia (syntype, 1 sh).
Voucher specimens: NHMW-MO-112647: Rh10, 17/09/2017, 1 spcm, H 14.7 mm, W 2.8 mm (Figure 3).
Remarks: This striking shell cannot be misidentified with any native Mediterranean triphorid. It belongs to a taxonomically difficult group of Viriola species whose validity is still uncertain, such as V. corrugata (Hinds, 1843), V. senafirensis (Sturany, 1903) and V. tricincta (Dunker, 1882) (Albano and Bakker 2016; Albano et al. 2017). The Mediterranean specimens are closely similar to V. bayani in the colour pattern of white fletches on brown background and in the sculpture of obsolete orthocline axial riblets between the main spiral cords, a conclusion also shared by Angelidis and Polyzoulis (2018). Viriola corrugata and V. tricincta have usually stronger and prosocline axial ribs. V. senafirensis can be distinguished by its compressed pyriform shape.
Empty shells have been previously recorded as V. corrugata from Karpathos, Greece (Micali et al. 2017). Living individuals were recorded as Viriola sp. [cf. corrugata] from Iztuzu, western Turkey (Stamouli et al. 2017) but they clearly belong to this species. Very recently, V. cf. bayani has been reported also from Astypalaia, Greece, where several fresh dead specimens (one with operculum) were trawled in August 2017 at 35–50 m depth and two shells were recovered from bioclastic sand sampled at 6–8 m depth in August 2016 (Angelidis and Polyzoulis 2018). This is the first record of living individuals for Greek and Israeli waters and confirms the species establishment in the eastern Mediterranean Sea and the Suez Canal as likely introduction vector. Due to the poor taxonomic knowledge of the genus, the native range is not known precisely but it is probably a widespread tropical Indo-Pacific species living in the shallow subtidal.
Subclass Heterobranchia Burmeister, 1837
Order Nudibranchia Cuvier, 1817
Family Facelinidae Bergh, 1889
Phidiana militaris (Alder and Hancock, 1864)
(Figure 4)
Figure 4.
Phidiana militaris (Alder and Hancock, 1864). S12, off Ashqelon, Israel, offshore rocky reef, -12 m, 30/04/2018. Photo credit: P.G. Albano.
Material examined: S12 (30/04/2018, 1 spcm, length ca. 10 mm).
Remarks: This nudibranch was found for the first time in the Mediterranean Sea in October 2016 in Haifa Bay, Israel (Rothman et al. 2017; see this paper also for diagnostic features of this species). This finding off Ashqelon extends its known range to southern Israel, suggesting that the Suez Canal may be the vector of introduction. Although this species has not yet been reported from the Red Sea, several nudibranch species were described from the Red Sea many decades after they had been described elsewhere in the Indo-West Pacific (Rothman et al. 2017).
Class Bivalvia Linnaeus, 1758
Subclass Pteriomorphia Beurlen, 1944
Order Mytilida Férussac, 1822
Family Mytilidae Rafinesque, 1815
Gregariella cf. ehrenbergi (Issel, 1869)
(Figure 5)
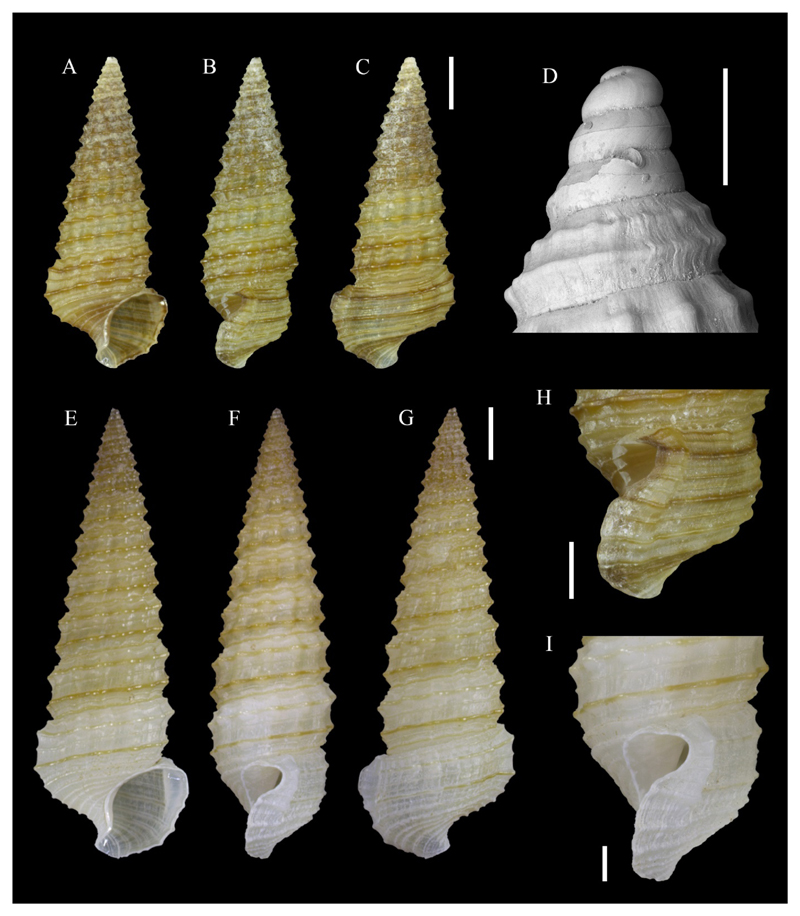
Figure 5.
A–B. Gregariella cf. ehrenbergi (Issel, 1869), NHMW-MO-112648, buoy stranded in Shefayim, Israel (see text), water level, sampled on 28/09/2016, exterior RV (A), interior LV (B). C–D. Gregariella semigranata (Reeve, 1858). C. RV, PGA private coll. 1888, Wied-iz-Zurrieq, Malta, in sediment, -35 m, 03/07/1994, exterior. D. LV, as previous, interior. E–F. Gregariella cf. ehrenbergi (Issel, 1869), elongated form, NHMW-MO-112649, locality as A–B, but -2 m, exterior RV (E), interior LV (F). Scale bars: 1 mm. Photo credit: D. Di Franco, A. Ivkić and P.G. Albano.
Material examined: Port Said, Egypt, on a detached buoy stranded in 2014 in Shefayim, central coast of Israel, originally 0 to −5.5 m (sampled on 28/09/2016, 62 spcms, 1 sh, 32 vs).
Voucher specimens: NHMW-MO-112648: buoy stranded in Shefayim, Israel (see above), water level, 28/09/2016, 1 spcm collected with dried soft body, L 5.6 mm, H 2.9 mm (Figure 5A–B); NHMW-MO-112649: locality as previous, but −2 m, 1 spcm collected with dried soft body, L 8.8 mm, H 3.2 mm (Figure 5E–F).
Material illustrated for comparison: Gregriella semigranata (Reeve, 1858): PGA private coll. 1888, Wied-iz-Zurrieq, Malta, in sediment, -35 m, 03/07/1994, 1 RV (Figure 5C) and 1 LV (Figure 5D).
Remarks: This Gregariella was most abundant on the buoy at the water level, where it probably exploited the barnacle mat to settle, at 5.5 m depth on the ballast at the base of the buoy, where a mat of oysters formed another suitable substrate, and inside the buoy. Indeed, Gregariella often nest in crevices, empty date mussel burrows and similar microhabitats (Morton 1995).
This species differs from the native G. semigranata (Reeve, 1858) (Figure 5C–D) in its generally larger size, more inflated shell, more regularly oval profile, less acute anterior and posterior profiles, crenulations on the posterior margin that extend much deeper into the shell, and darker colour (Figure 5A–B, E–F). It can be readily distinguished from the native G. petagnae (Scacchi, 1832) by the branching periostracal bristles and its smaller size.
The taxonomy of genus Gregariella in the Indo-Pacific is unsettled (Oliver 1992) and we consider the attribution tentative until a revision is undertaken. Our specimens resemble material from Kuwait identified as G. ehrenbergi (G. Oliver, pers. comm.). The type specimen of G. ehrenbergi is stored in the Museo Civico di Storia Naturale “G. Doria” in Genoa, Italy. Two valves are present (likely belonging to different specimens) but unfortunately, they are badly affected by the Byne’s disease and most diagnostic characters cannot be observed any more. Moreover, these specimens are very small (the best-preserved one is just 3 mm long) and likely juvenile, thus the drawing by Issel (1869, Plate I, Figure 12) is of little assistance. Our specimens resemble G. simplicifilis Barnard, 1964 as illustrated by Bosch et al. (1995), though the latter lacks the branching periostracal bristles.
Gregariella ehrenbergi has not been recorded from the Mediterranean Sea but is known from the Suez Canal (Hoenselaar and Dekker 1998). The material is stored in Naturalis in Leiden, the Netherlands, but was unfortunately inaccessible during the preparation of this manuscript due to the ongoing renovation of the museum.
Subclass Heterodonta Neumayr, 1884
Order Lucinida Gray, 1854
Family Lucinidae J. Fleming, 1828
Lucinidae sp.
(Figure 6)
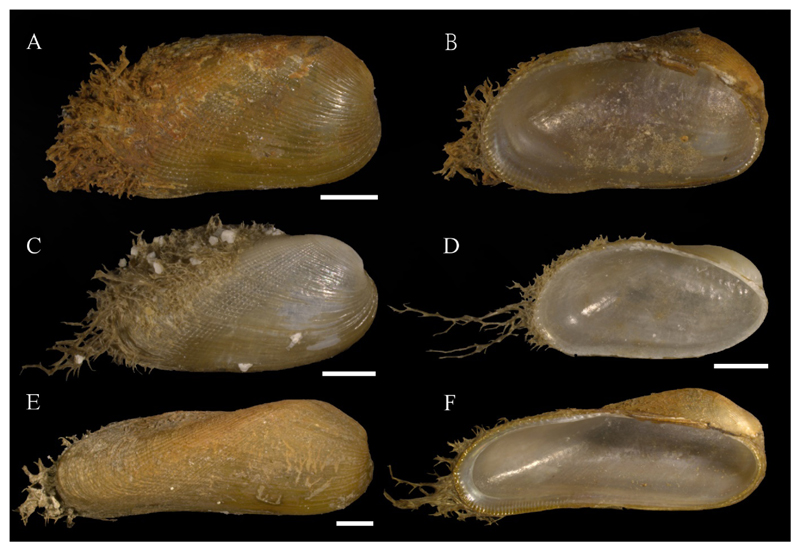
Figure 6.
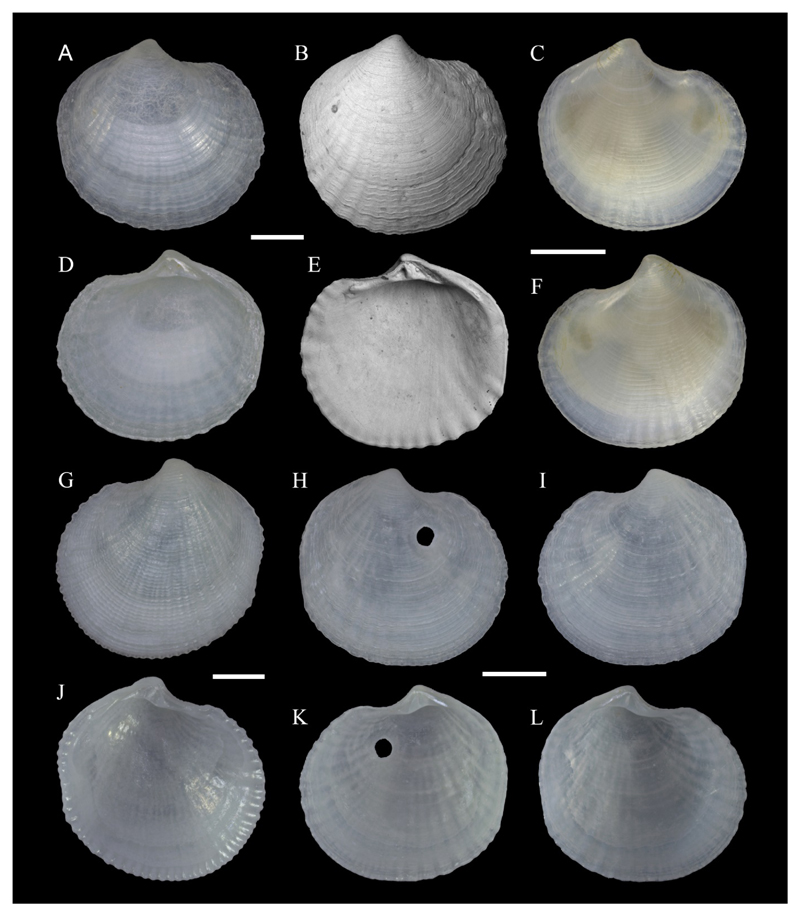
A–I. Lucinidae sp. A, D, G, I. LV, NHMW-MO-112650, SG10, off Ashqelon, southern Israel, sand, -11 m, 27/04/2017, exterior (A), interior (D), hinge (G) and detail of ventral interior shell margin (I). B, E, H. RV, NHMW-MO-112651, locality as previous, but 19/09/2016, exterior (B), interior (E) and hinge (H). C, F. RV, as previous, but different valve, exterior (C) and interior (F). J–N. Loripes orbiculatus Poli, 1791. J, L. RV, University of Vienna, Dept. of Palaeontology collection, Staranzano, northeast Italy, outer tidal flat, −0.3–0.35 m, 10/1999, exterior (J) and interior (L). K, M–N. RV, PGA private coll. 1682, St. Julian’s Bay, Malta, sand, −15 m, 29/06/1994, exterior (K), interior (M) and detail of ventral interior shell margin (irregular linear markings are traces of microbioerosion). Scale bars: 1 mm (A–H, J–M), 0.2 mm (I, N). Photo credit: J. Steger.
Material examined: NG10 (21/09/2016, 2 shs, 19 vs); SG10 (19/09/2016, 19 vs; 27/04/2017, 1 v).
Voucher specimens: NHMW-MO-112650: SG10, 27/04/2017, 1 LV, L 4.6 mm, H 4.4 mm (Figure 6A, D, G, I); NHMW-MO-112651: SG10, 19/09/2016, 2 RVs, L 4.9 mm, H 4.7 mm (Figure 6B, E, H) and L 8.2 mm, H 7.9 mm (Figure 6C, F).
Material illustrated for comparison: Loripes orbiculatus Poli, 1791: University of Vienna, Dept. of Palaeontology collection: Staranzano, northeast Italy, outer tidal flat, −0.3–0.35 m, 10/1999, 1 RV (Figure 6J, L); PGA private coll. 1682: St. Julian’s Bay, Malta, sand, −15 m, 29/06/1994, 1 sh (RV illustrated) (Figure 6K, M–N).
Remarks: We were unable to confidently assign this taxon to any species, but its shell morphology distinguishes it from all known Mediterranean Lucinidae. The alien status of this taxon could therefore not be clarified. The species is characterized by solid, rather tumid, subcircular valves, semi-translucent and waxy white in color (Figure 6). The outer surface is sculptured by several weakly-defined radial ribs which are also part of the internal shell structure and often more obsolete in larger specimens, as well as much finer, very densely spaced commarginal striae, most prominent in the dorsal part of the shell. Irregularly spaced growth marks are visible on the shell surface. The ligament is internal and rather short. Right valve with a single cardinal tooth (Figure 6H), left valve with two cardinal teeth (Figure 6G). The lateral teeth are weak in both valves, the inner shell margin is denticulate (Figure 6I). The characters of our lucinid are similar to Chavania erythraea (Issel, 1869), a species present also in the Gulf of Suez, however, the concentric sculpture of our shells is weaker than in illustrations of C. erythraea, and a posterior sulcus is not evident (e.g. Glover and Taylor 2001). Our unidentified lucinid resembles the Mediterranean Loripes orbiculatus Poli, 1791 (Figure 6J–N, specimens of similar size as the illustrated unidentified Lucinidae), particularly larger specimens with less obvious radial sculpture (Figure 6C, F). L. orbiculatus, however, lacks radial sculpture (although very fine radial striae might be present (Glover and Taylor 2001)) and the distinct marginal denticulation (Figure 6I – Lucinidae sp. – vs. Figure 6N – Loripes orbiculatus).
Genus Pillucina Pilsbry, 1921
Pillucina vietnamica Zorina, 1978
(Figure 7)
Figure 7.
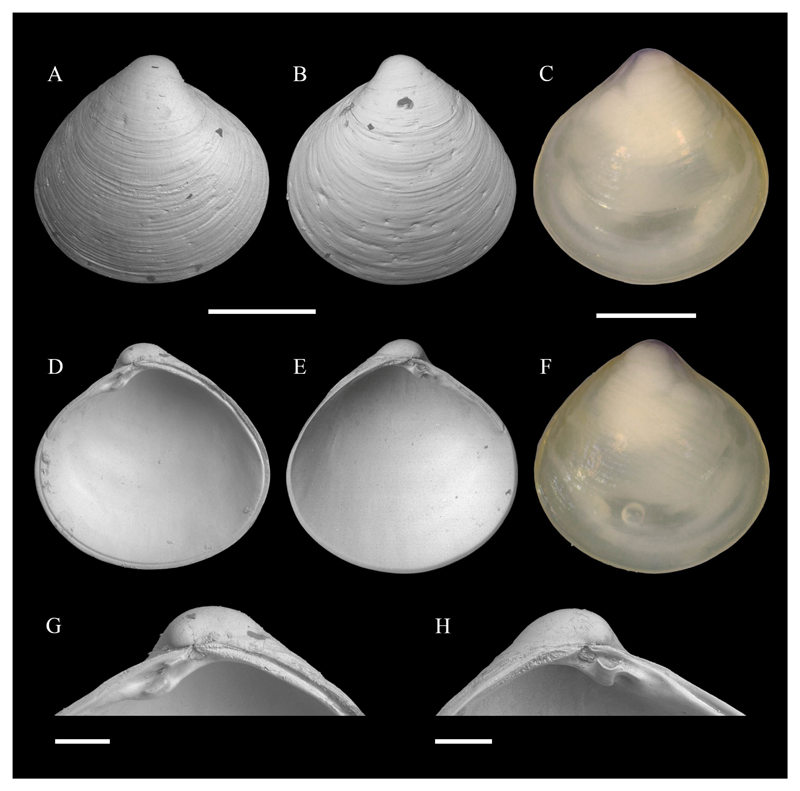
Pillucina vietnamica Zorina, 1978. A–B, D–E. RV, NHMW-MO-112653, SG10, off Ashqelon, southern Israel, sand, −11 m, 19/09/2016, exterior (A, B) and interior (D, E); the slight anterior-posterior compression of images B and E compared to A and D is an artifact of very low magnification SEM-imaging. C, F. NHMW-MO-112652, locality as previous, exterior RV (C) and LV (F). G, J. LV, University of Vienna, Dept. of Palaeontology collection, Abu Dhabi, United Arab Emirates, fine sediment on cap rock, seagrass (Halophila ovalis), −11 m, 09/04/1999, exterior (G) and interior (J). H–I, K–L. As previous, but sh, exterior RV (H) and LV (I), interior RV (K) and LV (L). Scale bars: 1 mm. Photo credit: J. Steger.
Material examined: SG10 (19/09/2016: 1 spcm, 1 v); Abu Dhabi, United Arab Emirates, fine sediment on cap rock, seagrass (Halophila ovalis), −11 m (09/04/1999, 1 sh, 1 v)
Voucher specimens: NHMW-MO-112653: SG10, 19/09/2016, 1 RV, L 4.0 mm, H 3.7 mm (Figure 7A–B, D–E); NHMW-MO-112652: locality as previous, 1 spcm, L 2.8 mm, H 2.6 mm (Figure 7C, F).
Material illustrated for comparison: Pillucina vietnamica Zorina, 1978: University of Vienna, Dept. of Palaeontology collection: Abu Dhabi, United Arab Emirates, fine sediment on cap rock, seagrass (H. ovalis), −11 m, 09/04/1999, 1 LV (Figure 7G, J) and 1 sh (Figure 7H–I, K–L).
Remarks: The surface sculpture consisting of radial ribs most prominent in the anterior and posterior part of the shell, crossed by commarginal ribs, as well as the scalloped shell margins (Figure 7) distinguish this species at first glance from any Mediterranean lucinid. P. vietnamica has a wide geographic distribution, ranging from the Red Sea (here often referred to as P. fischeriana (Issel, 1869), an unavailable name) to China and southern Queensland, Australia (Glover and Taylor 2001). It is reported to be common in the Gulf of Suez (Rusmore-Villaume 2008) and also occurs in the Great Bitter Lake (Hoenselaar and Dekker 1998). Here, we report the first Mediterranean record.
Order Venerida Gray, 1854
Family Kelliellidae P. Fischer, 1887
Alveinus miliaceus (Issel, 1869)
(Figure 8)
Figure 8.
Alveinus miliaceus (Issel, 1869). A, D, G. RV, NHMW-MO-112655, SG20, off Ashqelon, southern Israel, sand, −21 m, 18/09/2016, exterior (A), interior (D) and hinge (G). B, E, H. LV, as previous, exterior (B), interior (E) and hinge (H). C, F. NHMW-MO-112654, locality as previous, but 27/04/2017, exterior LV (C) and RV (F). Scale bars: 0.4 mm (A–F), 0.1 mm (G–H). Photo credit: J. Steger and P.G. Albano.
Material examined: NG30 (20/09/2016, 1 sh, 1 v); SG10 (19/09/2016, 1 sh, 4 vs); SG20 (18/09/2016: 6 spcms, 5 shs, 14 vs; 27/04/2017, 1 spcm); SG30 (18/09/2016, 2 vs).
Voucher specimens: NHMW-MO-112655: SG20, 18/09/2016, 1 RV, L 0.9 mm, H 0.8 mm (Figure 8A, D, G) and 1 LV, L 0.9 mm, H 0.9 mm (Figure 8B, E, H); NHMW-MO-112654: SG20, 27/04/2017, 1 spcm, L 0.9 mm, H 1.0 mm (Figure 8C, F).
Remarks: This minute bivalve reaches a maximum shell size of only 2 mm (Oliver and Zuschin 2000), but may even be much smaller (e.g. specimens in Figure 8). It is a poorly known species, originally described from the Gulf of Suez (Issel 1869), and also known from the Bay of Safaga (Egypt) in the northern Red Sea and Oman (Oliver and Zuschin 2000). Here, we report the first record of Alveinus miliaceus for the Mediterranean Sea. The hinge (Figure 8G, H) is characteristic and allows a distinction from juveniles of other species, particularly the s-shaped cardinal complex of the left valve (Oliver and Zuschin 2000, Figures 3c, 4b, 7b; Figure 8H this study) makes it readily recognizable.
Discussion
Since the opening of the Suez Canal, hundreds of Indo-Pacific species have successfully established populations in the Mediterranean Sea, and several new NIS are reported each year (Galil 2009; Zenetos et al. 2012; Galil et al. 2016; Zenetos 2017). Our findings of Pillucina vietnamica and Alveinus miliaceus in the Mediterranean meet the criteria for “records of confirmed alien species” as proposed by Marchini et al. (2015): i) the species must be unambiguously identified, ii) the native distribution is known, iii) the species has been introduced outside its natural distribution range by direct or indirect human agency, and iv) specimens were found in environments open to the sea. While P. vietnamica currently seems to be very rare, several living individuals of A. miliaceus from Ashqelon and the presence of shells also in sediments from northern Israel (Atlit) suggest that the latter species might have already established viable populations along a large stretch of the Israeli coast. A. miliaceus may represent a recently introduced NIS; however, a significant detection time lag (see e.g. Crooks 2005; Albano et al. 2018) could also be involved: due to its minute size, the species may have been missed in many benthic surveys using mesh sizes too large to retain it, or has been confused with juveniles or post-larval stages of other bivalves. Since the native ranges of P. vietnamica and A. miliaceus both include the Gulf of Suez (and, for P. vietnamica, even the Great Bitter Lake (Hoenselaar and Dekker 1998)), it is likely that these species have entered the Levantine Sea via the Suez Canal, representing “Lessepsian migrants” in the strict sense (Por 1978; Zenetos et al. 2012). The Canal also seems the most likely vector of introduction for Varicopeza pauxilla, Viriola cf. bayani, Phidiana militaris and Gregariella cf. ehrenbergi considering that the new records here reported fill a geographical gap along the expected trajectory of spread from the Suez Canal into the eastern Mediterranean, following the prevalent counter clock-wise currents.
Once introduced outside their natural range, the fate of NIS may differ markedly between species, ranging from extinction to establishing viable populations and range expansion (e.g. Blackburn et al. 2011). The gastropods Varicopeza pauxilla and Viriola cf. bayani belong to the latter group: both species have first been collected in the Mediterranean in 2016, but our findings suggest that the species are widely distributed in its eastern basin. Both species are relatively large (to ≥ 1 cm) and have no Mediterranean congeners; considering the recent benthic surveys conducted in the Levantine Sea (e.g. Bakir et al. 2012; Çinar et al. 2012; Öztürk et al. 2015; Guarnieri et al. 2017) it seems unlikely, at least for the soft-bottom dwelling V. pauxilla, that they have been overlooked for a significant amount of time.
In the framework of our surveys in Israel, we found numerous valves of the unidentified lucinid bivalve in shelly death assemblages from sands at approximately 10 m depth. Due to the lack of species- and genus-level identifications, we were unable to assess the status of this species. However, considering the commonness of shells in the environment, their rather large (up to approx. 1 cm) size, and occurrence in very shallow water, we conjecture that the shells may be evidence for a newly introduced NIS rather than an undescribed native lucinid. We believe that publishing such records is nonetheless important pending the full identification of the species in order to raise awareness on their presence, especially when, as in this case, it is very similar to a native species.
Undoubtedly, the Suez Canal is the most significant vector of NIS introductions to the Mediterranean (Galil 2009), and major concerns were raised about the ecological consequences of the recent doubling of the Canal (Galil et al. 2015). While several studies highlighted the fast pace of NIS introductions to the eastern Mediterranean (Galil 2008; Zenetos et al. 2012; Guarnieri et al. 2017), it has been suggested that the introduction rate of new Lessepsian NIS has recently been on the decline (Zenetos et al. 2017). Our findings suggest caution in this respect as the invasion is evidently ongoing and detection time lags may conceal the actual introduction rates.
Supplementary Material
Acknowledgements
Sampling in Israel was conducted in the framework of the project “Historical ecology of Lessepsian migration” (PI: PGA) funded by the Austrian Science Fund (FWF) P28983-B29. MS’s fieldwork was supported by the Kurzfristige wissenschaftliche Auslandsstipendien of the University of Vienna, the non-profit organization Mare Mundi and the diving school Dive2gether for her master thesis “Assessment of the relevance of Lessepsian alien species in Posidonia oceanica fields along a depth transect in Crete”. AI was supported by an ERASMUS+ traineeship. We are indebted to Martin Zuschin for his support all along the planning and running of the project. We thank Itay Katzman and the crew of the “Mediterranean Explorer” vessel; Captain Michael Solomon, Senior Marine Surveyor, Shipping and Ports Administration, and Amir Tzindr, Harbour Master, Herzliya Marina, for information on the origin and location of the stranded buoy; Jonathan Belmaker, Shahar Malamud, Karolina Czechowska, Justina Givens, Nadja Loferer, Denny Morchner for assistance in the field, and Davide Di Franco for photographing specimens of Gregariella cf. ehrenbergi. Anna Hinterplattner helped in the lab and Jan Päßler classified the sediments of the grab samples. Henk Mienis offered advice on various topics, John Taylor and Emily Glover helped with the identification of lucinids, Graham Oliver offered useful suggestions on the identification of Gregariella. Philippe Bouchet and Virginie Héros kindly sent on loan to PGA the syntypes of Viriola bayani. Helmut Sattmann and the Naturhistorisches Museum Wien supported Anna Hinterplattner for lab work on the Israeli samples. Maria Tavano sent photographs of the type specimens of Gregariella ehrenbergi stored in the Museo Civico di Storia Naturale “G. Doria” in Genoa. The Israel Nature and Parks Authority kindly granted permit 41928 for field sampling. We furthermore thank two anonymous reviewers for their helpful and constructive comments.
References
- Albano P, Bakker PAJ. Annotated catalogue of the types of Triphoridae (Mollusca, Gastropoda) in the Museum für Naturkunde, Berlin, with lectotype designations. Zoosystematics and Evolution. 2016;92:33–78. doi: 10.3897/zse.92.5936. [DOI] [Google Scholar]
- Albano PG, Bakker PAJ, Janssen R, Eschner A. An illustrated catalogue of Rudolf Sturany’s type specimens in the Naturhistorisches Museum Wien, Austria (NHMW): Red Sea gastropods. Zoosystematics and Evolution. 2017;93:45–94. doi: 10.3897/zse.93.10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano PG, Gallmetzer I, Haselmair A, Tomašových A, Stachowitsch M, Zuschin M. Historical ecology of a biological invasion: the interplay of eutrophication and pollution determines time lags in establishment and detection. Biological Invasions. 2018;20:1417–1430. doi: 10.1007/s10530-017-1634-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelidis A, Polyzoulis G. New distributional records of four Indo-Pacific species from Astypalaia Island, south Aegean Sea, Greece. Xenophora Taxonomy. 2018;21:3–10. [Google Scholar]
- Bakir BB, Öztürk B, Doğan A, Önen M. Mollusc fauna of Iskenderun Bay with a checklist of the region. Turkish Journal of Fisheries and Aquatic Sciences. 2012;12:171–184. [Google Scholar]
- Bianchi CN, Morri C. Marine biodiversity of the Mediterranean Sea: situation, problems and prospects for future research. Marine Pollution Bulletin. 2000;40:367–376. doi: 10.1016/S0025-326X(00)00027-8. [DOI] [Google Scholar]
- Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarošík V, Wilson JRU, Richardson DM. A proposed unified framework for biological invasions. Trends in Ecology & Evolution. 2011;26:333–339. doi: 10.1016/j.tree.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Bosch DT, Dance SP, Moolenbeek RG, Oliver PG. Seashells of Eastern Arabia. Motivate Publishing; Dubai, Abu Dhabi, London: 1995. p. 296. [Google Scholar]
- Çinar ME, Katagan T, Öztürk B, Dagli E, Açik S, Bitlis B, Bakir K, Dogan A. Spatio-temporal distributions of zoobenthos in Mersin Bay (Levantine Sea, eastern Mediterranean) and the importance of alien species in benthic communities. Marine Biology Research. 2012;8:954–968. doi: 10.1080/17451000.2012.706305. [DOI] [Google Scholar]
- Coll M, Piroddi C, Steenbeek J, Kaschner K, Ben Rais Lasram F, Aguzzi J, Ballesteros E, Bianchi CN, Corbera J, Dailianis T, Danovaro R, et al. The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0011842. e11842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks JA. Lag times and exotic species: The ecology and management of biological invasions in slow-motion. Ecoscience. 2005;12:316–329. doi: 10.2980/i1195-6860-12-3-316.1. [DOI] [Google Scholar]
- Edelman-Furstenberg Y, Faershtein G. Geological Survey of Israel Report GSI/15/10. 2010. Molluscan fauna of the Gulf of Elat: indicators of ecological change; p. 132. [Google Scholar]
- Ehrenfeld JG. Ecosystem consequences of biological invasions. Annual Review of Ecology, Evolution, and Systematics. 2010;41:59–80. doi: 10.1146/annurev-ecolsys-102209-144650. [DOI] [Google Scholar]
- Fanelli E, Azzurro E, Bariche M, Cartes JE, Maynou F. Depicting the novel eastern Mediterranean food web: a stable isotopes study following Lessepsian fish invasion. Biological Invasions. 2015;17:2163–2178. doi: 10.1007/s10530-015-0868-5. [DOI] [Google Scholar]
- Galil BS. Loss or gain? Invasive aliens and biodiversity in the Mediterranean Sea. Marine Pollution Bulletin. 2007;55:314–322. doi: 10.1016/j.marpolbul.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Galil BS. Alien species in the Mediterranean Sea – which, when, where, why? Hydrobiologia. 2008;606:105–116. doi: 10.1007/s10750-008-9342-z. [DOI] [Google Scholar]
- Galil BS. Taking stock: inventory of alien species in the Mediterranean Sea. Biological Invasions. 2009;11:359–372. doi: 10.1007/s10530-008-9253-y. [DOI] [Google Scholar]
- Galil BS. Eyes wide shut: managing bio-invasions in Mediterranean marine protected areas. In: Goriup PD, editor. Management of Marine Protected Areas: A Network Perspective. Wiley-Blackwell; West Sussex, Weinheim: 2017. pp. 187–206. [DOI] [Google Scholar]
- Galil BS, Boero F, Campbell ML, Carlton JT, Cook E, Fraschetti S, Gollasch S, Hewitt CL, Jelmert A, Macpherson E, Marchini A, et al. ‘Double trouble’: the expansion of the Suez Canal and marine bioinvasions in the Mediterranean Sea. Biological Invasions. 2015;17:973–976. doi: 10.1007/s10530-014-0778-y. [DOI] [Google Scholar]
- Galil BS, Marchini A, Occhipinti-Ambrogi A. East is east and west is west? Management of marine bioinvasions in the Mediterranean Sea. Estuarine, Coastal and Shelf Science. 2016;201:7–16. doi: 10.1016/j.ecss.2015.12.021. [DOI] [Google Scholar]
- Glover EA, Taylor JD. Systematic revision of Australian and Indo-Pacific Lucinidae (Mollusca: Bivalvia): Pillucina, Wallucina and descriptions of two new genera and four new species. Records of the Australian Museum. 2001;53:263–292. doi: 10.3853/j.0067-1975.53.2001.1349. [DOI] [Google Scholar]
- Guarnieri G, Fraschetti S, Bogi C, Galil BS. A hazardous place to live: spatial and temporal patterns of species introduction in a hot spot of biological invasions. Biological Invasions. 2017;19:2277–2290. doi: 10.1007/s10530-017-1441-1. [DOI] [Google Scholar]
- Hoenselaar HJ, Dekker H. Molluscs of the Great Bitter Lake, Suez Canal, Egypt, collected by C. Beets in 1950. Basteria. 1998;62:197–214. [Google Scholar]
- Houbrick RS. Reappraisal of the gastropod genus Varicopeza Gründel (Cerithiidae: Prosobranchia) Proceedings of the Biological Society of Washington. 1980;93(3):525–535. [Google Scholar]
- Houbrick RS. Phylogenetic relationships and generic review of the Bittiinae (Prosobranchia: Cerithioidea) Malacologia. 1993;35(2):261–313. [Google Scholar]
- Issel A. Malacologia del Mar Rosso. Ricerche Zoologiche e Paleontologiche. Memoria letta al congresso dei Naturalisti Italiani in Vicenza nel 1868. Biblioteca Malacologica; Pisa: 1869. xi + 387 pp, pls 1–5. [Google Scholar]
- Janssen R, Zuschin M, Baal C. Gastropods and their habitats from the northern Red Sea (Egypt: Safaga) Part 2: Caenogastropoda: Sorbeoconcha and Littorinimorpha. Annalen des Naturhistorischen Museums in Wien, Serie A. 2011;113:373–509. [Google Scholar]
- Katsanevakis S, Coll M, Piroddi C, Steenbeek J, Ben Rais Lasram F, Zenetos A, Cardoso AC. Invading the Mediterranean Sea: biodiversity patterns shaped by human activities. Frontiers in Marine Science. 2014;1:32. doi: 10.3389/fmars.2014.00032. [DOI] [Google Scholar]
- Marchini A, Galil BS, Occhipinti-Ambrogi A. Recommendations on standardizing lists of marine alien species: lessons from the Mediterranean Sea. Marine Pollution Bulletin. 2015;101:267–273. doi: 10.1016/j.marpolbul.2015.09.054. [DOI] [PubMed] [Google Scholar]
- Mazza G, Tricarico E, Genovesi P, Gherardi F. Biological invaders are threats to human health: an overview. Ethology Ecology & Evolution. 2014;26:112–129. doi: 10.1080/03949370.2013.863225. [DOI] [Google Scholar]
- Micali P, Siragusa F, Agamennone F, Germanà A, Sbrana C. Karpathos Island (Greece) and its Indo-Pacific alien species. Part 1. Bollettino Malacologico. 2017;53(1):40–49. [Google Scholar]
- Molnar JL, Gamboa RL, Revenga C, Spalding MD. Assessing the global threat of invasive species to marine biodiversity. Frontiers in Ecology and the Environment. 2008;6:485–492. doi: 10.1890/070064. [DOI] [Google Scholar]
- Morton B. The biology and functional morphology of Trichomusculus semigranatus (Bivalvia: Mytiloidea) from the Azores. Açoreana. 1995;(Supplement):279–295. [Google Scholar]
- Occhipinti-Ambrogi A, Galil B. Marine alien species as an aspect of global change. Advances in Oceanography and Limnology. 2010;1:199–218. doi: 10.1080/19475721003743876. [DOI] [Google Scholar]
- Oliver PG. Bivalved Seashells of the Red Sea. Christa Hemmen and National Museum of Wales; Wiesbaden, Cardiff: 1992. p. 330. [Google Scholar]
- Oliver PG, Zuschin M. Minute Veneridae and Kelliellidae from the Red and Arabian Seas with a redescription of Kellia miliacea Issel, 1869. Journal of Conchology. 2000;37(2):213–230. [Google Scholar]
- Öztürk B, Bitlis B, Doğan A, Türkçü N. Alien marine molluscs along the Turkish coast, with a new record of Varicopeza pauxilla (A. Adams, 1855) (Mollusca: Gastropoda) from the Mediterranean Sea. Acta Zoologica Bulgarica. 2017;(Supplement 9):83–92. [Google Scholar]
- Öztürk B, Recevik M, Geyran K. New alien molluscs in the Mediterranean Sea. Cahiers de Biologie Marine. 2015;56(3):205–212. [Google Scholar]
- Por FD. Lessepsian Migration - The Influx of Red Sea Biota into the Mediterranean by Way of the Suez Canal. Springer Verlag; Berlin, Heidelberg, New York: 1978. p. 228. [Google Scholar]
- Pyšek P, Richardson DM. Invasive species, environmental change and management, and health. Annual Review of Environment and Resources. 2010;35:25–55. doi: 10.1146/annurev-environ-033009-095548. [DOI] [Google Scholar]
- Rothman SB-S, Mienis HK, Galil BS. Alien facelinid nudibranchs in the eastern Mediterranean: first report of Phidiana militaris (Alder and Hancock, 1864) and report of Caloria indica (Bergh, 1896) 30 years after its previous sighting. BioInvasions Records. 2017;6:125–128. doi: 10.3391/bir.2017.6.2.06. [DOI] [Google Scholar]
- Rusmore-Villaume ML. Seashells of the Egyptian Red Sea. The Illustrated Handbook. American University in Cairo Press; Cairo: 2008. p. 307. [Google Scholar]
- Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, García-Berthou E, Pascal M, Pyšek P, et al. Impacts of biological invasions: what’s what and the way forward. Trends in Ecology & Evolution. 2013;28:58–66. doi: 10.1016/j.tree.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Stamouli C, Akel EHK, Azzurro E, Bakiu R, Bas AA, Bitar G, Boyaci Y, Cakalli M, Corsini-Foka M, Crocetta F, Dragičević B, et al. New Mediterranean biodiversity records (December 2017) Mediterranean Marine Science. 2017;18:534–556. doi: 10.12681/mms.15823. [DOI] [Google Scholar]
- Tzomos T, Kitsos MS, Koutsoubas D, Koukouras A. Evolution of the entrance rate and of the spatio-temporal distribution of Lessepsian Mollusca in the Mediterranean Sea. Journal of Biological Research-Thessaloniki. 2012;17:81–96. [Google Scholar]
- Wallentinus I, Nyberg CD. Introduced marine organisms as habitat modifiers. Marine Pollution Bulletin. 2007;55:323–332. doi: 10.1016/j.marpolbul.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Zenetos A. Progress in Mediterranean bioinvasions two years after the Suez Canal enlargement. Acta Adriatica. 2017;58(2):347–358. [Google Scholar]
- Zenetos A, Gofas S, Morri C, Rosso A, Violanti D, García Raso J, Cinar ME, Almogi-Labin A, Ates AS, Azzurro E, Ballesteros E, et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterranean Marine Science. 2012;13:328–352. doi: 10.12681/mms.327. [DOI] [Google Scholar]
- Zenetos A, Çinar ME, Crocetta F, Golani D, Rosso A, Servello G, Shenkar N, Turon X, Verlaque M. Uncertainties and validation of alien species catalogues: the Mediterranean as an example. Estuarine, Coastal and Shelf Science. 2017;191:171–187. doi: 10.1016/j.ecss.2017.03.031. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.