Abstract
Background
Although hypoalbuminemia is a known risk factor for acute kidney injury (AKI) following surgery, little is known about its effects following aneurysm clipping surgery. We aimed to investigate the predictors of AKI and overall mortality and assessed the relationship between preoperative albumin and postoperative outcomes after aneurysm clipping surgery.
Methods
This study included 2,339 patients who underwent aneurysm clipping surgery. According to the criteria updated by the Kidney Disease: Improving Global Outcomes (KDIGO), patients were classified into AKI and no AKI group. Independent AKI predictors were analyzed by multivariate methods, and the influence of AKI on the outcome variables was assessed with by propensity score matching analysis. Survival in relation to AKI was analyzed using the Kaplan–Meier method.
Results
The total proportion of patients who developed AKI was 1.9%. The cutoff value of preoperative albumin for predicting AKI was 3.9 g/dL. Multivariate analyses showed that preoperative albumin≤ 3.9 g/dL, aneurysmal subarachnoid hemorrhage, male sex, phenylephrine use, and hemoglobin were associated with postoperative AKI development. In multivariate analysis, mortality was increased in AKI patients (p< 0.01). After propensity score matching, preoperative albumin≤ 3.9 g/dL was significantly related to AKI and overall mortality.
Conclusion
Preoperative albumin≤ 3.9 g/dL is associated with postoperative AKI and mortality.
Introduction
Perioperative acute kidney injury (AKI) is a common perioperative complication associated with increased morbidity and mortality [1, 2]. Recently, the Kidney Disease: Improving Global Outcomes (KDIGO) updated the diagnosing criteria for AKI [3]. Various studies have shown that AKI diagnosed based on the KDIGO criteria is associated with increased risk of morbidity and mortality [4, 5]. Because there is no curative treatment for AKI, it would be valuable to determine the modifiable risk factors for patients at increased risk of AKI [6].
One of the modifiable risk factors linked to increased risk of AKI is low albumin level [7, 8]. Albumin is not only responsible for plasma oncotic pressure, but is also a potential natural antioxidant that acts as a core extravascular source of reduced sulfhydryl groups [9]. These sulfhydryl groups, so-called thiols, scavenge reactive oxygen and nitrogen species [10], thereby causing postoperative AKI [11].
Although AKI has been reported as an independent predictor of outcomes after aneurysmal subarachnoid hemorrhage [12, 13], the influence of serum albumin on postoperative AKI development following cerebral artery aneurysm clipping has not been studied. We thus investigated whether preoperative albumin level is associated with postoperative AKI in patients after cerebral artery aneurysm clipping surgery.
Methods
We retrospectively reviewed the electronic medical records and laboratory findings of 2,462 patients who underwent aneurysm-clipping surgery at Asan Medical Center between January 2008 and December 2014. Of these patients, 123 cases were excluded due to chronic kidney disease (n = 34) or incomplete laboratory results (n = 89). Thus, 2,339 patients were registered in the final study population. Among them, 357 patients had accompanying subarachnoidal hemorrhage (SAH). The institutional review board of Asan Medical Center waived the need for informed consent for this study due to its retrospective design and approved the study protocol (2015–0645).
Clinical data
We retrospectively reviewed the computerized medical records at our center (Asan Medical Center Information System Electronic Medical Records) to obtain the demographic, laboratory, and intraoperative data on all patients and their postoperative outcomes. Demographic data included patient sex, age, comorbidities (hypertension, diabetes mellitus, and ischemic heart disease), body mass index, smoking history, and medications (beta-blocker, aspirin, calcium channel blocker, angiotensin-converting enzyme inhibitor, antiplatelet agent, and 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor). Aneurysmal SAH was assessed through a review of medical records. Hypertension and diabetes mellitus were described as use of any antihypertensive or hypoglycemic agents at admission, and ischemic heart disease as positive coronary angiography or compatible electrocardiographic or perfusion scan findings.
Laboratory data included preoperative hemoglobin, platelets, uric acid, albumin, sodium, potassium, serum creatinine (sCr), chloride, and the estimated glomerular filtration rate (eGFR). eGFR was measured from the preoperative sCr concentration based on the Modification of Diet in Renal Disease study equation for adult patients and adjusted for each 1.73 m2 of body surface area [14]. Intraoperative anesthetic data included the mean blood pressure, amount of administered crystalloid solution and mannitol, proportion of patients transfused (ex; red blood cells), total urine output during the operation, anesthetic time, and proportion of patients requiring furosemide and phenylephrine administration.
Anesthetic management
Radial artery was cannulated for invasive arterial pressure monitoring with the administration of fentanyl 50–100 μg. Anesthesia was induced with a bolus intravenous administration of propofol 1.5–2.5 mg/kg. Propofol and remifentanil were then infused with effect site target-controlled infusion (TCI) using a commercially available 2-channel TCI pump (Orchestra, Fresenius Vial, Brezins, France). Endotracheal intubation was facilitated by a single bolus of rocuronium 0.8–1.0 mg/kg. During the maintenance of anesthesia, the target concentrations of propofol and remifentanil were 2.5 μg/mL and 7–9 ng/mL, respectively, maintaining a bispectral index (BIS VISTA, Aspect Medical System, Norwood, MA, USA) of 40–60. During anesthesia, 0.9% normal saline was administered. Until aneurysm clipping, fluid administration was restricted to the sum of 2mL/kg/h and hourly urine output. After aneurysm clipping, the infusion rate of normal saline was increased to 4 mL/kg/h and hourly urine output. If hypotension occurred without significant bleeding, phenylephrine was continuously infused to retain a mean blood pressure > 65 mmHg. If plasma hemoglobin level was reduced to less than 8 g/dL due to bleeding, packed red blood cells were transfused.
Definition of outcomes
Outcome variables included postoperative AKI, hospital and intensive care unit (ICU) stay, and overall mortality. Postoperative AKI was identified on the basis of the KDIGO classification using changes in the sCr on postoperative days 1–7 compared with the baseline sCr, which was the most recent concentration measured prior to operation. In-hospital mortality was determined by reviewing the electrical medical records. To validate the complete follow-up data regarding mortality, information on the date of death was obtained from the National Population Registry of the Korea National Statistical Office by using the unique personal identification number of each patient.
Statistical analysis
Continuous variables are expressed as mean ±standard deviation (SD) or median with interquartile range. All continuous variables were evaluated for normality using the Shapiro-Wilk test; the t-test or Mann-Whitney rank sum test were applied to inspect intergroup differences when appropriate. Categorical variables are expressed as numbers and percentages and were evaluated using the chi-square test or Fisher’s exact test. A receiver operating characteristic (ROC)curve analysis was conducted to determine the cut-off value for anticipating postoperative development of AKI.
Multivariate regression analyses were performed to determine the predictors of AKI. Multivariate analysis was conducted for each variable with p<0.1in the univariate analysis. In addition, a nested case control study comprising 679 patients with hypoalbuminemia (≤3.9 g/dL) and679 matched patients (>3.9g/dL) using propensity score (PS) was conducted to elucidate the influence of hypoalbuminemia on postoperative AKI. PS was calculated for each patient using age; sex; body mass index; diabetes mellitus; hypertension; ischemic heart disease; smoking history; aneurysmal SAH; calcium channel blocker, angiotensin-converting enzyme inhibitor, beta-blocker, aspirin, antiplatelet agent, and statin use; hemoglobin; platelet count; uric acid, sodium, potassium, creatinine, and chloride level; and eGFR. We subsequently used the derived PS to match 679 patients with a serum albumin ≤ 3.9 g/dL with patients with a serum albumin > 3.9g/dL at a ratio of 1:1 using greedy matching algorithms. Patients without corresponding matches were excluded. After all PS matches were performed, we assessed the balance in baseline covariates using standardized mean difference, t tests, and McNemar tests for continuous and categorical variables as appropriate. We analyzed all available data without the imputation of missing values. PS matching was performed with the SAS software package (version 9.1; SAS Institute Inc., Cary, NC, USA).
To assess the adjusted odds ratio (OR) and hazard ratios (HR) of the relationship between a low preoperative albumin level and outcome variables, a weighted logistic regression and multivariate Cox proportional hazard regression analysis was used. The adjusted variables are listed in Table 1. In the PS matching analysis, the association of a lower preoperative albumin level with mortality was evaluated with a weighted logistic regression with generalized estimating equations or Cox proportional hazards regression models with robust standard errors. The PS matching analysis was adjusted with mannitol, crystalloid, and vasopressor use, which were significantly different between the 2 groups. The discrimination of the model was assessed using C statistics (C = 0.750), while calibration was assessed using Hosmer-Lemeshow statistics (χ2 = 9.1944; df = 8; p = .33).
Table 1. Demographic, preoperative, and intraoperative characteristics of the study patients.
| Preoperative albumin level | P value | Preoperative albumin level (Propensity score matched patients) | P value | Standardized difference | |||
|---|---|---|---|---|---|---|---|
| ≤3.9 g/dL (n = 821) |
>3.9 g/dL (n = 1,518) |
≤3.9 g/dL (n = 679) |
>3.9 g/dL (n = 679) |
||||
| Demographic variable | |||||||
| Age, years | 58.1 ± 10.4 | 55.3 ± 9.6 | < .01 | 57.2 ± 10.2 | 57.2 ± 9.5 | .88 | .007 |
| Sex, male | 205 (30.0) | 524 (34.5) | < .01 | 179 (26.4) | 186 (27.4) | .66 | .024 |
| Body mass index, kg/m2 | 24.4 ± 3.4 | 24.5±3.2 | .52 | 24.5 ± 3.4 | 24.6 ± 3.2 | .42 | .042 |
| Diabetes | 71 (8.7) | 142 (9.4) | .57 | 64 (9.43) | 75 (11.1) | .33 | .058 |
| Hypertension | 336 (40.9) | 709 (46.7) | < .01 | 283 (41.7) | 287 (42.3) | .82 | .012 |
| Ischemic heart disease | 46 (5.6) | 52 (3.4) | .01 | 31 (4.6) | 35 (5.2) | .62 | .026 |
| Smoking history | 216 (26.4) | 470 (31.2) | .02 | 180 (26.5) | 197 (29.0) | .30 | .057 |
| aSAH | 171 (20.8) | 186 (12.3) | < .01 | 120 (73.6) | 108 (15.9) | .37 | .044 |
| CCB | 352 (42.9) | 591 (38.9) | .07 | 276 (40.7) | 274 (40.4) | .91 | .006 |
| ACEI | 183 (22.3) | 352 (23.2) | .62 | 153 (22.5) | 155 (22.8) | .89 | .007 |
| Beta-blocker | 134 (16.3) | 172 (11.3) | < .01 | 100 (14.7) | 94 (13.8) | .63 | .024 |
| Aspirin | 79 (9.6) | 155 (10.2) | .65 | 61 (9.0) | 70 (10.3) | .41 | .045 |
| Antiplatelet agent | 105 (12.8) | 169 (11.1) | .24 | 79 (11.6) | 84 (12.4) | .68 | .022 |
| Statin | 133 (16.2) | 315 (20.8) | < .01 | 118 (17.4) | 128 (18.9) | .48 | .04 |
| Laboratory data | |||||||
| Hemoglobin, g/dL | 12.8 ± 1.4 | 13.6 ±1.4 | <.01 | 13.1 ± 1.3 | 13.1 ± 1.3 | .35 | .041 |
| Platelets, ×103/μL | 238.4 ± 63.5 | 243.3 ± 54.4 | .05 | 240.7 ± 63.7 | 239.7 ± 55.4 | .76 | .016 |
| Uric acid, mg/dL | 4.5±1.4 | 4.7± 1.4 | <.01 | 4.6 ± 1.4 | 4.6 ± 1.4 | .91 | .006 |
| Albumin, g/dL | 3.7 ± 0.3 | 4.3±0.2 | <.01 | - | - | - | - |
| Sodium, mmol/L | 140.4 ± 2.9 | 140.7 ± 2.5 | <.01 | 140.5 ± 2.9 | 140.6 ± 2.6 | 0.8 | .013 |
| Potassium, mmol/L | 4.0 ± 0.4 | 4.1 ± 0.3 | <.01 | 4.1 ± 0.4 | 4.1 ± 0.4 | .63 | .025 |
| Creatinine, mg/dL | 0.7 ± 0.2 | 0.8 ± 0.2 | <.01 | 0.7 ± 0.2 | 0.7 ± 0.2 | .70 | .018 |
| Chloride, mmol/L | 105.1 ± 3.0 | 103.7 ± 2.6 | <.01 | 104.6 ± 2.6 | 104.6 ± 2.5 | .87 | .007 |
| GFR, mL/min/1.73m2 | 74.6 ± 14.1 | 72.8 ± 12.8 | <.01 | 74.2 ± 13.7 | 74.6 ± 13.6 | .56 | .031 |
| Intraoperative data | |||||||
| Mean blood pressure | 63.6 ± 5.6 | 63.6 ± 5.7 | .85 | 63.6 ± 5.5 | 63.5 ± 6.0 | .68 | .023 |
| Crystalloid solution, L | 1.9 (1.4–2.2) | 1.9 (1.4–2.2) | .45 | 1.9 ± 1.1 | 2.0 ± 1.0 | .03 | .073 |
| Mannitol, mL | 73.6 (0–100) | 86.0 (50–100) | <.01 | 66.9 ± 76.6 | 87.6 ± 72.0 | <.01 | .268 |
| PRBC transfusion, n (%) | 88 (10.7) | 84 (5.5) | <.01 | 54 (8.0) | 54 (8.0) | 1.0 | .000 |
| FFP transfusion, n (%) | 3 (0.4) | 5 (0.3) | .99 | 2 (0.3) | 5 (0.7) | .26 | .073 |
| Platelet transfusion, n (%) | 5 (0.6) | 5 (0.3) | .34 | 4 (0.6) | 2 (0.29) | .41 | .038 |
| Urine output, L | 0.6 (0.4–1.0) | 0.7 (0.4–1.1) | .06 | 0.8 ± 0.5 | 0.8 ± 0.6 | .24 | .094 |
| Anesthetic time, min | 286.6 ± 80.8 | 285.5 ± 76.6 | .99 | 283.2 ± 78.5 | 287.0 ± 75.5 | .35 | .047 |
| Vasopressor use | 343 (42) | 406 (27) | <.01 | 315 (46.4) | 183 (27.0) | <.01 | .412 |
| Furosemide, n (%) | 14 (1.7) | 20 (1.3) | .45 | 9 (1.3) | 10 (1.5) | .82 | .011 |
Values are expressed as mean ± SD, median (interquartile range), or n (%). aSAH, aneurysmal subarachnoid hemorrhage; CCB, calcium channel blocker; ACEI, angiotensin converting enzyme inhibitor; GFR, glomerular filtration rate; PRBC, packed red blood cells; FFP, fresh-frozen plasma
The cumulative survival rate between groups was analyzed using the Kaplan–Meier method, and alterations between curves were assessed using the log-rank test. All p values < 0.05 were considered statistically significant. Data manipulation and statistical analyses were performed using SAS or R software (version 2.10.1).
Results
The median follow-up duration for the entire patient population was 3.8 years (interquartile range:2.1–5.4years). According to the ROC curve analysis, a cut-off value of preoperative albumin of 3.9 g/dL predicted the development of postoperative AKI (sensitivity 65.5%, specificity 65.9%). Of the 2,339 study patients, 821patients (35.1%) showed preoperative albumin level of ≤3.9 g/dL (3.7±0.3) and 1,518 patients had preoperative albumin level of >3.9 g/dL(4.3±0.2). Patients with lower preoperative albumin levels (≤3.9 g/dL) were older, more likely to be female, and showed higher incidence rates of IHD and aneurysmal SAH. In addition, patients with preoperative albuminlevels≤3.9 g/dL had lower levels of hemoglobin (12.8 ± 1.4 vs 13.6 ± 1.4), uric acid (4.5 ± 1.4 vs 4.7 ± 1.4), and sodium (140.4 ± 2.9 vs 140.7 ± 2.5), and higher level of chloride (105.1 ± 3.0 vs 103.7 ± 2.6)compared with patients with preoperative albumin levels>3.9 g/dL. Baseline eGFR was higher in patients with preoperative albumin levels of ≤3.9 g/dL as well. Intraoperative data showed that patients with preoperative albumin levels of ≤3.9 g/dL received lower amounts of mannitol and more frequently required transfusions and vasopressor infusion. The demographic and intraoperative data of the patients are presented in Table 1.
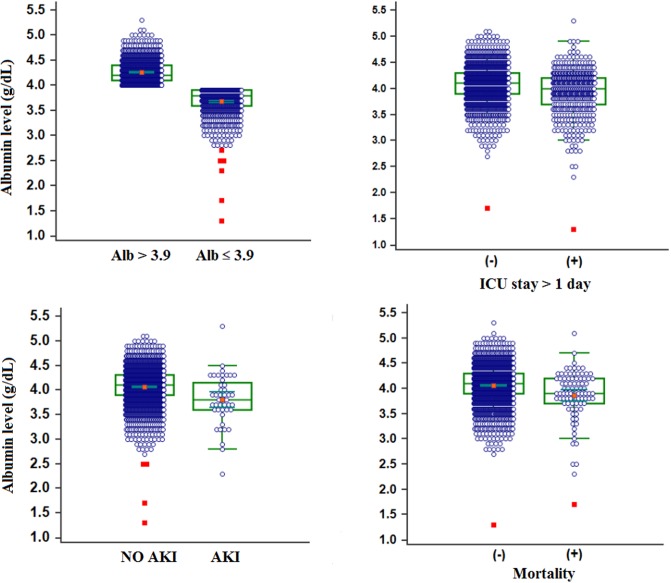
The overall proportion of patient who developed AKI was 1.9%. The rate of AKI development was higher in patients with lower preoperative albumin levels (3.5%; n = 29) compared with patients with higher preoperative albumin levels (1.0%; n = 15). Patients with lower preoperative albumin levels stayed longer in the hospital [7 (6–11) vs 6 (5–8) days; p< 0.001) and in the ICU (p< 0.001). Mortality was also higher in patients with lower preoperative albumin levels [47 (5.7%) vs 40 (2.6%); p< 0.001]. The preoperative albumin level according to the outcome variables is depicted in Fig 1.
Fig 1. Preoperative albumin level according to the outcome variable.
Central box represents the values from the lower to the upper quartile (25th to 75th percentile). The middle line represents the median. A line extends from the minimum to maximum value, excluding outliers. Patients with poorer outcomes such as AKI, ICU stay more than 1 day, and mortality had lower levels of albumin (p < 0.05 for all outcome variables). *; p < 0.05.
Multivariate logistic regression revealed that the predictors of AKI were preoperative albumin level of ≤3.9 g/dL (OR 2.59, CI 1.31–5.13; p < 0.01), aneurysmal SAH (OR 3.91, CI 2.06–7.42; p <0.01), male sex (OR 3.39, CI 1.65–6.97; p<0.01), vasopressor use (OR 0.197, CI 0.057–0.676; p<0.01), and hemoglobin level (OR 0.72, CI 0.59–0.89; p< .01) (Table 2).
Table 2. Univariate and multivariate analysis of AKI predictors using the KDIGO criteria.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |||
| Sex (male) | 1.86 | 1.023 | 3.397 | .04 | 3.729 | 1.797 | 7.740 | <.01 |
| Hemoglobin, g/dL | 0.728 | 0.596 | 0.888 | <.01 | 0.691 | 0.563 | 0.849 | <.01 |
| Creatinine, mg/dL | 0.021 | 0.003 | 0.163 | <.01 | ||||
| Preoperative albumin≤3.9g/dL | 3.669 | 1.955 | 6.884 | <.01 | 2.978 | 1.512 | 5.866 | <.01 |
| Mannitol, ml | 1.007 | 1.004 | 1.011 | <.01 | ||||
| Crystalloid, L | 1.000 | 1.000 | 1.000 | <.01 | ||||
| PRBC transfusion | 3.871 | 1.879 | 7.976 | <.01 | ||||
| Aneurysmal SAH | 5.851 | 3.204 | 10.684 | <.01 | 3.570 | 1.898 | 6.712 | <.01 |
| Urine output, ml | 1.001 | 1.000 | 1.001 | <.01 | ||||
| Vasopressor use | 0.15 | 0.04 | 0.42 | <.01 | 0.197 | 0.057 | 0.676 | <.01 |
| Anesthetic time,min | 1.003 | 1.000 | 1.006 | .03 | ||||
PRBC = packed red blood cells; SAH = subarachnoid hemorrhage
The predictive variables of overall mortality were age (HR1.08, CI 1.05–1.11; p<0.01), medication history of aspirin (HR0.29, CI 0.09–0.93; p = 0.04), aSAH (HR2.48, CI 1.56–3.93; p<0.01), red blood cell transfusion (HR2.00, CI 1.18–3.34; p = 0.01), urine output (HR1.0, CI 1.000–1.001; p<0.01), and AKI (HR2.38, CI 1.10–5.13; p = 0.03) (Table 3). In addition, we performed an analysis between the patients with SAH and without SAH. We summarized these results as a S1 Table.
Table 3. Univariate and multivariate analysis of overall mortality predictors.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Preoperative albumin ≤ 3.9 g/dL | 2.429 | 1.592 | 3.706 | <.01 | ||||
| Age, yr | 1.088 | 1.062 | 1.115 | <.01 | 1.079 | 1.053 | 1.105 | <.01 |
| Mannitol, ml | 1.003 | 1.000 | 1.006 | .02 | ||||
| CCB | 1.578 | 1.036 | 2.403 | .03 | ||||
| Aspirin | 0.314 | 0.099 | 0.992 | <.05 | 0.290 | 0.090 | 0.932 | .03 |
| Aneurysmal SAH | 3.423 | 2.228 | 5.260 | <.01 | 2.480 | 1.564 | 3.933 | <.01 |
| Hemoglobin, g/dL | 0.769 | 0.668 | 0.885 | <.01 | ||||
| Crystalloid, L | 1.000 | 1.000 | 1.000 | <.01 | ||||
| MBP, mmHg | 0.967 | 0.931 | 1.005 | .09 | ||||
| PRBC transfusion | 4.647 | 2.917 | 7.403 | <.01 | 2.003 | 1.184 | 3.387 | .01 |
| Urine output, ml | 1.000 | 1.000 | 1.001 | .02 | 1.000 | 1.000 | 1.001 | <.01 |
| AKI | 5.357 | 2.684 | 10.692 | <.01 | 2.376 | 1.102 | 5.126 | .03 |
CCB = Calcium channel blocker; SAH = subarachnoid hemorrhage; MBP = mean blood pressure; PRBC = packed red blood cells.
The associations between preoperative albumin level ≤3.9 g/dL and clinical outcomes are shown in Table 4. Multivariate analysis revealed that preoperative albumin level ≤3.9 g/dL was related to AKI (OR2.59, CI 1.31–5.13; p <0.01), ICU stay (OR 1.82, CI 1.47–2.25; p<0.01), and hospital stay (OR 1.87, CI 1.56–2.23; p<0.01), but not to overall mortality (HR 1.43, CI 0.91–2.25; p = 0.12). After PS matching, preoperative albumin level ≤ 3.9 g/dL was independently associated with AKI (OR 2.80, CI 1.29–6.06; p < 0.01) and overall mortality (HR 1.90, CI 1.02–3.30, p = 0.04).
Table 4. Outcomes adjusted by preoperative albumin level ≤ 3.9 g/dL.
| Crude | Multivariate | PS matching1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | ||||
| AKI | 3.7 | 2.0 | 6.9 | <.01 | 2.6 | 1.3 | 5.1 | <.01 | 2.8 | 1.3 | 6.1 | <.01 |
| ICU stay > 1day | 1.8 | 1.4 | 2.2 | <.01 | 1.8 | 1.5 | 2.3 | <.01 | 1.1 | 0.9 | 1.5 | .3 |
| Hospital stay >7 days | 1.8 | 1.5 | 2.2 | <.01 | 1.9 | 1.6 | 2.2 | <.01 | 1.2 | 1.0 | 1.5 | .1 |
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | ||||
| Mortality | 2.4 | 1.6 | 3.7 | <.01 | 1.4 | 0.9 | 2.3 | <.12 | 1.9 | 1.02 | 3.3 | .04 |
1Adjusted by mannitol, crystalloid, and vasopressor use.
AKI = acute kidney injury; ICU = intensive care unit
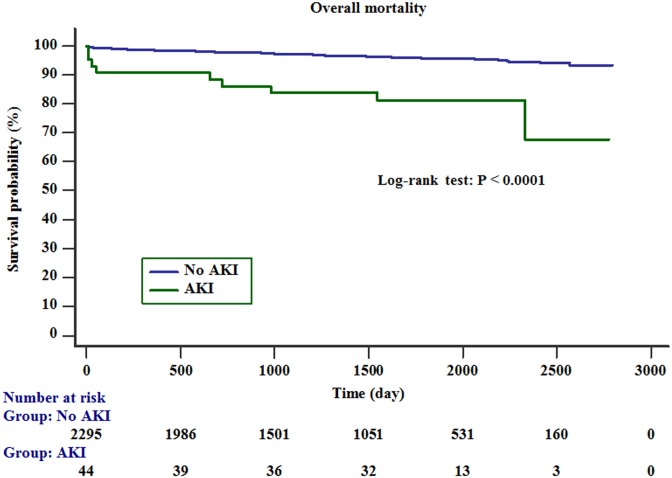
The survival rate of the patients with AKI was significantly lower than that of the patients without AKI (Kaplan–Meier method and log-rank test; p <0.01) (Fig 2).
Fig 2. Kaplan–Meier survival curve.
The survival rate was significantly lower in patients with AKI than in those without AKI (log-rank test; p < 0.001).
Discussion
We investigated whether preoperative albumin levels affected postoperative outcomes, including postoperative AKI, ICU and hospital stay, and overall mortality inpatients who underwent aneurysm clipping surgery. We found that a low preoperative albumin level (≤3.9 g/dL) was associated with postoperative AKI and longer hospital stay, but not with overall mortality. After PS matching analysis, a lower preoperative albumin level was associated with AKI development (p <0.01) and overall mortality (p = 0.04). Multivariate analysis revealed that AKI was an independent predictor of overall mortality (p = 0.03).
Previous studies have reported the risk factors of AKI after hemorrhagic or traumatic brain injury [12, 13]. However, there is little information on the risk factors for postoperative AKI following aneurysm clipping surgery. Our present study revealed that lower preoperative albumin level, male sex, low hemoglobin level, and aneurysmal SAH are associated with AKI following aneurysm clipping surgery. Intraoperative phenylephrine infusion effectively prevented AKI.
Previous studies have reported an association between hypoalbuminemia and increased risk of postoperative AKI [8, 15, 16]. The relationship of hypoalbuminemia to postoperative AKI might be attributable to the renoprotective properties of albumin. Albumin improves renal perfusion and glomerular filtration through prolonged potent renal vasodilation, which is induced by serum albumin reacting with the oxides of nitrogen to form S-nitroso-albumin [17]. Moreover, albumin inhibits apoptosis in renal tubular cells by scavenging reactive oxygen species and carrying protective lysophosphatidic acid [18]. Albumin enhances the proliferation of renal tubular cells through the activation of phosphatidylinositide 3-kinase [19]. In addition, albumin has a ligand-binding capacity and can mitigate the effects of nephrotoxic mediations [20]. Due to these properties, albumin is crucial for maintaining the structural integrity and function of the proximal tubule; thus, a lower level of serum albumin may increase the risk of postoperative AKI. Moreover, recent reports suggest that exogenous albumin administration is beneficial for protecting the kidneys from AKI [21, 22].
Although postoperative AKI develops by a multifactorial mechanism, one of the most common causes is acute tubular necrosis due to hypoxic damage to the nephrons in the medulla of the kidney, which may be induced by hypotension, hypovolemia, and/or dehydration [23]. A decrease in the effective circulating volume leads to the activation of vasoconstriction and salt-retaining neurohumoral systems such as the sympathoadrenal system, and increase in angiotensin, aldosterone, and antidiuretic hormone [23]. The resultant reabsorption of salt and water in the medullary thick ascending limb is associated with an increased demand for oxygen and induces hypoxic injury to the medullary region [24, 25]. Albumin might exert protective effects against acute tubular necrosis, which results from hypoxic injury to the renal medulla. Therefore, administration of exogenous albumin may benefit patients with low preoperative albumin levels. However, exogenous albumin has been reported to be associated with increased mortality in patients with traumatic brain injury [26], which may be due to albumin-induced increases in intracranial pressure [27]. This, along with previous concerns regarding the administration of exogenous albumin, may induce increases in interstitial colloid osmotic pressure and increase intracranial pressure by the extravasation of albumin through the damaged blood-brain barrier [28]. Nevertheless, the status of the blood-brain barrier has not been investigated in patients who undergo aneurysm clipping surgery. Therefore, further prospective studies are needed to identify the clinical effects of exogenous albumin administration to patients with low albumin levels who undergo aneurysm clipping surgery.
In the present study, aneurysmal SAH was also found to be associated with AKI. Dysfunction of non-neurological organs has been correlated with the extent of neurological impairment [29]. Patients with aneurysmal SAH have greater risk of diminished mental capacity, and thus require intubation or surgical or endovascular procedures. As a result, patients with aneurysmal SAH may frequently require radiographic studies and interventions with radiocontrast agents, as well as hyperosmolar therapeutic agents and nephrotoxic antibiotics, which carry greater risks of AKI.
In the present study, an intraoperative phenylephrine infusion was shown to effectively prevent AKI. Although phenylephrine was used more often in hypoalbuminemic patients, it was associated with a low rate of AKI. In fact, there has been concern that vasopressor use is accompanied by renal vasoconstriction and AKI. However, there is also a rationale for vasopressor therapy use in hypotensive states [30]: physiologically, in all regional circulation including renal, splanchnic, cerebral, and coronary beds, blood flow is pressure-dependent outside of the levels of pressure that remain within the autoregulation values for a given regional circulation. This means that if the cardiac output is preserved, organ blood flow is also preserved as long as sufficient blood pressure is maintained. If blood pressure falls below an autoregulatory threshold, organ blood flow also decreases in an almost linear fashion. Especially for the kidneys, the autoregulatory threshold is a mean arterial pressure of 80 mmHg, which is higher than those of the brain or heart, and the risk of ischemic injury is greater. Therefore, it is essential to maintain the mean arterial pressure to preserve renal blood flow. This may be the underlying mechanism of why phenylephrine infusion was helpful for preventing AKI.
Our study had several limitations. First, due to the retrospective design of these analyses, we could not control for all confounding parameters that might have affected our results. Although we performed IPTW analysis to reduce for selection bias, we could not control every residual confounding factor. Second, because we enrolled patients who underwent aneurysm-clipping surgery, our results may not be directly applicable for other types of patients, and data interpretation should thus be performed with care.
In conclusion, a preoperative albumin level of ≤ 3.9 g/dL was associated with postoperative AKI and overall mortality in patients who underwent aneurysm clipping surgery. In addition, postoperative AKI was related to overall mortality following aneurysm clipping surgery.
Supporting information
(DOCX)
(XLSX)
Abbreviations
- AKI
Acute kidney injury
- CCB
Calcium channel blocker
- DM
Diabetes mellitus
- eGFR
Estimated glomerular filtration rate
- HR
Hazard ratio
- HTN
Hypertension
- ICU
Intensive care unit
- IHD
Ischemic heart disease
- KDIGO
Kidney disease improving global outcome
- PS
propensity score
- SAH
subarachnoid hemorrhage
- sCr
Serum creatinine
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009; 249: 851–858. 10.1097/SLA.0b013e3181a40a0b [DOI] [PubMed] [Google Scholar]
- 2.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009; 119: 2444–2453. 10.1161/CIRCULATIONAHA.108.800011 [DOI] [PubMed] [Google Scholar]
- 3.Section 2: AKI Definition. Kidney Int Suppl (2011). 2012; 2: 19–36. 10.1038/kisup.2011.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grams ME, Sang Y, Coresh J, Ballew S, Matsushita K, Molnar MZ, et al. Acute Kidney Injury After Major Surgery: A Retrospective Analysis of Veterans Health Administration Data. Am J Kidney Dis. 2015. 10.1053/j.ajkd.2015.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu HC, Lee LC, Wang WJ. Incidence and mortality of postoperative acute kidney injury in non-dialysis patients: comparison between the AKIN and KDIGO criteria. Ren Fail. 2016; 38: 330–339. 10.3109/0886022X.2015.1128790 [DOI] [PubMed] [Google Scholar]
- 6.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009; 119: 495–502. 10.1161/CIRCULATIONAHA.108.786913 [DOI] [PubMed] [Google Scholar]
- 7.Lee EH, Kim HR, Baek SH, Kim KM, Chin JH, Choi DK, et al. Risk factors of postoperative acute kidney injury in patients undergoing esophageal cancer surgery. J Cardiothorac Vasc Anesth. 2014; 28: 936–942. 10.1053/j.jvca.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 8.Lee EH, Baek SH, Chin JH, Choi DK, Son HJ, Kim WJ, et al. Preoperative hypoalbuminemia is a major risk factor for acute kidney injury following off-pump coronary artery bypass surgery. Intensive Care Med. 2012; 38: 1478–1486. 10.1007/s00134-012-2599-8 [DOI] [PubMed] [Google Scholar]
- 9.Margarson MP, Soni N. Serum albumin: touchstone or totem? Anaesthesia. 1998; 53: 789–803. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B. Albumin—an important extracellular antioxidant? Biochem Pharmacol. 1988; 37: 569–571. [DOI] [PubMed] [Google Scholar]
- 11.Doi K, Suzuki Y, Nakao A, Fujita T, Noiri E. Radical scavenger edaravone developed for clinical use ameliorates ischemia/reperfusion injury in rat kidney. Kidney Int. 2004; 65: 1714–1723. 10.1111/j.1523-1755.2004.00567.x [DOI] [PubMed] [Google Scholar]
- 12.Zacharia BE, Ducruet AF, Hickman ZL, Grobelny BT, Fernandez L, Schmidt JM, et al. Renal dysfunction as an independent predictor of outcome after aneurysmal subarachnoid hemorrhage: a single-center cohort study. Stroke. 2009; 40: 2375–2381. 10.1161/STROKEAHA.108.545210 [DOI] [PubMed] [Google Scholar]
- 13.Moore EM, Bellomo R, Nichol A, Harley N, Macisaac C, Cooper DJ. The incidence of acute kidney injury in patients with traumatic brain injury. Ren Fail. 2010; 32: 1060–1065. 10.3109/0886022X.2010.510234 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 15.Wiedermann CJ, Wiedermann W, Joannidis M. Hypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studies. Intensive Care Med. 2010; 36: 1657–1665. 10.1007/s00134-010-1928-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim CS, Oak CY, Kim HY, Kang YU, Choi JS, Bae EH, et al. Incidence, predictive factors, and clinical outcomes of acute kidney injury after gastric surgery for gastric cancer. PLoS One. 2013; 8: e82289 10.1371/journal.pone.0082289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufmann MA, Castelli I, Pargger H, Drop LJ. Nitric oxide dose-response study in the isolated perfused rat kidney after inhibition of endothelium-derived relaxing factor synthesis: the role of serum albumin. J Pharmacol Exp Ther. 1995; 273: 855–862. [PubMed] [Google Scholar]
- 18.Iglesias J, Abernethy VE, Wang Z, Lieberthal W, Koh JS, Levine JS. Albumin is a major serum survival factor for renal tubular cells and macrophages through scavenging of ROS. Am J Physiol. 1999; 277: F711–722. 10.1152/ajprenal.1999.277.5.F711 [DOI] [PubMed] [Google Scholar]
- 19.Dixon R, Brunskill NJ. Activation of mitogenic pathways by albumin in kidney proximal tubule epithelial cells: implications for the pathophysiology of proteinuric states. J Am Soc Nephrol. 1999; 10: 1487–1497. [DOI] [PubMed] [Google Scholar]
- 20.Contreras AM, Ramirez M, Cueva L, Alvarez S, de Loza R, Gamba G. Low serum albumin and the increased risk of amikacin nephrotoxicity. Rev Invest Clin. 1994; 46: 37–43. [PubMed] [Google Scholar]
- 21.Dubois MJ, Orellana-Jimenez C, Melot C, De Backer D, Berre J, Leeman M, et al. Albumin administration improves organ function in critically ill hypoalbuminemic patients: A prospective, randomized, controlled, pilot study. Crit Care Med. 2006; 34: 2536–2540. 10.1097/01.CCM.0000239119.57544.0C [DOI] [PubMed] [Google Scholar]
- 22.Lee EH, Kim WJ, Kim JY, Chin JH, Choi DK, Sim JY, et al. Effect of Exogenous Albumin on the Incidence of Postoperative Acute Kidney Injury in Patients Undergoing Off-pump Coronary Artery Bypass Surgery with a Preoperative Albumin Level of Less Than 4.0 g/dl. Anesthesiology. 2016. 10.1097/aln.0000000000001051 [DOI] [PubMed] [Google Scholar]
- 23.Sear JW. Kidney dysfunction in the postoperative period. Br J Anaesth. 2005; 95: 20–32. 10.1093/bja/aei018 [DOI] [PubMed] [Google Scholar]
- 24.Brezis M, Rosen S. Hypoxia of the renal medulla—its implications for disease. N Engl J Med. 1995; 332: 647–655. 10.1056/NEJM199503093321006 [DOI] [PubMed] [Google Scholar]
- 25.Heyman SN, Fuchs S, Brezis M. The role of medullary ischemia in acute renal failure. New Horiz. 1995; 3: 597–607. [PubMed] [Google Scholar]
- 26.Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, Bishop N, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007; 357: 874–884. 10.1056/NEJMoa067514 [DOI] [PubMed] [Google Scholar]
- 27.Cooper DJ, Myburgh J, Heritier S, Finfer S, Bellomo R, Billot L, et al. Albumin resuscitation for traumatic brain injury: is intracranial hypertension the cause of increased mortality? J Neurotrauma. 2013; 30: 512–518. 10.1089/neu.2012.2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffcote T, Ho KM. Associations between cerebrospinal fluid protein concentrations, serum albumin concentrations and intracranial pressure in neurotrauma and intracranial haemorrhage. Anaesth Intensive Care. 2010; 38: 274–279. [DOI] [PubMed] [Google Scholar]
- 29.Solenski NJ, Haley EC Jr., Kassell NF, Kongable G, Germanson T, Truskowski L, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Participants of the Multicenter Cooperative Aneurysm Study. Crit Care Med. 1995; 23: 1007–1017. [DOI] [PubMed] [Google Scholar]
- 30.Bellomo R, Wan L, May C. Vasoactive drugs and acute kidney injury. Crit Care Med. 2008; 36: S179–186. 10.1097/CCM.0b013e318169167f [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.