Abstract
Background:
The role of lifestyle factors and sleep for dementia is uncertain.
Objective:
To examine the associations of major lifestyle factors and sleep duration with risk of late-onset dementia.
Methods:
We used data from a population-based cohort of 28,775 Swedish adults who were ≥65 years of age and completed a questionnaire about lifestyle and other modifiable factors in the autumn of 1997. Dementia cases were ascertained by linkage with the Swedish National Patient Register.
Results:
During a mean follow-up of 12.6 years, dementia was diagnosed among 3,755 participants (mean age at diagnosis 83.2±5.1 years). There were no associations of an overall healthy diet (defined by a modified Dietary Approaches to Stop Hypertension Diet score or a Mediterranean diet score), alcohol and coffee consumption, or physical activity with dementia incidence. Compared with never smokers, dementia risk was increased in former and current smokers (hazard ratio [95% confidence interval] = 1.13 [1.04–1.23] and 1.10 [1.00–1.21], respectively). Extended time of sleep (>9 h per night) was associated with an increased risk of dementia. However, this association appeared to be related to a reverse causation effect since the association did not remain after exclusion of cases diagnosed within the first five or ten years of follow-up.
Conclusions:
This study found no evidence that major lifestyle factors, aside from smoking, or sleep duration influence the risk of dementia.
Keywords: Cohort studies, dementia, diet, lifestyle, prospective studies, sleep
INTRODUCTION
Dementia is a syndrome caused by neurodegeneration, with Alzheimer’s disease and vascular, Lewy body, and frontotemporal dementia being the most common underlying pathologies [1]. The number of people living with dementia was about 47 million in 2015, and this number is expected to triple by 2050 [2]. Identification of risk factors for dementia is therefore a public health priority. The chief risk factor for dementia is age, and the age of 65 years is commonly used to classify dementia patients in early- and late-onset groups. Family history and genetic susceptibility genes such as the apolipoprotein ɛ4 allele also play an important role in the development of dementia [3]. However, none of these factors are modifiable. Observational studies have provided some evidence that lifestyle factors, such as a healthy diet, moderate alcohol and coffee consumption, smoking, and regular physical activity, may influence the risk of dementia but results are not consistent [2–15]. The inconsistent findings may be related to residual confounding, reverse causation bias, or small sample sizes, leading to a spurious association or lack of association.
Besides lifestyle factors, sleep quality such as long sleep duration, insomnia, and sleep disturbances have been linked to risk of dementia in some but not all observational studies [16–23]. A potential issue is that a clinical diagnosis of dementia is usually preceded by a long preclinical phase [24], and dementia often leads to sleep disturbances [25]. It remains unclear whether extended time of sleep and disturbed sleep are causally related to an increased risk of dementia or are consequences of the disease.
To clarify the potential role of lifestyle factors for the development of dementia, we used data from a large population-based cohort study of Swedish adults to assess the associations of an overall healthy diet, alcohol and coffee consumption, smoking, and physical activity with incidence of late-onset dementia. Furthermore, we examined whether long sleep duration is associated with risk of dementia. To address the question of potential reverse causality (that is, preclinical dementia leads to extended time of sleep, and not vice versa), we repeated the analyses after exclusion of cases diagnosed during the first five or 10 years of follow-up.
METHODS
Study population
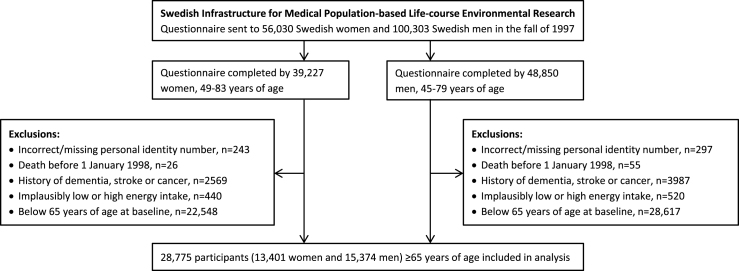
We used data from the National Research Infrastructure SIMPLER (Swedish Infrastructure for Medical Population-based Life-course Environmental Research, previously the Swedish Mammography Cohort and the Cohort of Swedish Men). Eligible for inclusion in the study were all women who were born 1914–1948 and residing in Uppsala and Västmanland counties and all men born 1918–1952 and residing in Västmanland and Örebro counties. Participants completed a questionnaire about diet, beverage consumption, lifestyle factors, and other risk factors for aging-associated diseases in the autumn of 1997. They are well representative of the Swedish population in terms of age distribution, educational attainment, and prevalence of obesity [26]. To form the analytic cohort for the present analysis of potentially modifiable risk factors for late-onset dementia, we excluded those with an erroneous or a missing personal identity number (as they could not be followed up through population-based registers); those with a previous diagnosis of dementia, stroke, or cancer (identified through record linkage with the Swedish National Patient Register); those with implausibly low or high energy intake (i.e., 3 SDs from the loge-transformed mean energy intake in women and men separately), probably reflecting careless completion of the questionnaire; and those below 65 years of age (Fig. 1). This left 28,775 participants (13,401 women and 15,374 men), 65 to 83 years of age, for analysis. The Regional Ethical Review Board at Karolinska Institutet in Stockholm, Sweden, approved the study.
Fig.1.
Flow chart of participants.
Exposure assessment
In the autumn of 1997, participants completed a questionnaire that sought information on diet, coffee and alcohol consumption, smoking, physical activity (walking/bicycling and exercise), body weight and height, sleep duration, educational level, and history of hypertension, hypercholesterolemia, and diabetes. Information on average food consumption over the past year was obtained through a validated 96-item food frequency questionnaire [27].
We created two healthy dietary patterns using modified versions of the Dietary Approaches to Stop Hypertension (DASH) diet [28, 29] and Mediterranean (MED) diet [29]. The modified DASH diet included fruits, vegetables, nuts and legumes, whole grains, and low-fat dairy products as “healthy foods” and meat and sweetened beverages as less healthy foods. The modified MED diet included fruits, vegetables, nuts and legumes, whole grains, and fish as healthy foods, and full-fat dairy foods and red meat and processed meat as less healthy foods. The modified MED diet score also included olive oil use. We did not include alcohol as component of the MED diet because alcohol consumption was analyzed as a separate lifestyle factor. Participants were classified into quintiles according to their consumption of each food. For the “healthy foods” participants were given a score from 1 to 5 from being in the lowest to the highest quintile of consumption. Scores were reversed for the foods considered to be less healthy. The scores were added up to create a DASH diet score that ranged from 7 to 35, and a MED diet score that ranged from 8 to 40. Higher scores indicate higher adherence to the healthy dietary patterns. We categorized participants into sex-specific quartiles according to their DASH and MED diet scores.
Participants reported their average consumption, over the previous year, of coffee (in cups per day; one cup was specified as 150 mL), light beer (alcohol by volume <2.25%), beer (2.8–3.5%), strong beer (4.4–5.6%), wine (12–13.5%), fortified wine (15–22%), and liquor (40%). Alcohol (ethanol) intake was calculated as the sum of consumption of the different beverages, taking into account the frequency and amount consumed at each occasion and the alcohol content. We converted alcohol consumption into drinks per week assuming that one standard drink contains 12 grams of alcohol.
Pack-years of smoking were calculated by multiplying the number of packs smoked per day by the number of years the participant had smoked. The questions on physical activity have been previously validated and described in detail [30, 31]. Briefly, the questionnaire had six predefined categories for walking/cycling (almost never, <20 min/day, 20–40 min/day, 40–60 min/day, 1–1.5 h/day, and >1.5 h/day) and five categories for leisure-time exercise (<1 h/week, 1 h/week, 2–3 h/week, 4–5 h/week, and >5 h/week). In the present study, we combined the two highest categories for each physical activity measure (due to few participants in the highest categories) resulting in five and four exposure categories for walking/bicycling and exercise, respectively.
Case ascertainment and follow-up
Dementia cases were identified by record linkage with the Swedish National Patient Register, which covers in-patient care in Sweden since 1987 and outpatient visits from private and public caregivers since 2001. Information on deaths within the cohort was obtained from the Swedish Cause of Death Register. Participants were followed up from January 1, 1998 to the date of diagnosis of dementia, date of death, or December 31, 2014, whichever occurred first.
Statistical analysis
Hazard ratios (HR) with 95% confidence intervals (CI) were derived using Cox proportional hazards regression models with age as the time variable. In addition to age, all analyses were adjusted for sex (as a stratum variable). The multivariable model further included adjustment for educational attainment (less than high school, high school, or university), body mass index (weight divided by the square of height;<22.5, 22.5–24.9, 25.0–29.9, or ≥30) kg/m2), and history of hypertension (yes/no), hypercholesterolemia (yes/no), and diabetes (yes/no). In the multivariable model, each lifestyle factor was adjusted for the other lifestyle factors and sleep through inclusion in the same model. There was no evidence of significant violation of the proportional hazards assumption, as evaluated by a test based on Schoenfeld residuals.
Analysis stratified by sex was conducted. To evaluate whether reverse causation bias may have influenced the results for sleep duration and dementia risk, we repeated the analyses after exclusion of dementia cases diagnosed within the first five or 10 years of follow-up. All statistical tests were two-sided. The statistical analyses were conducted in SAS (version 9.4, SAS Institute Inc., Cary, NC).
RESULTS
The mean follow-up was 12.6 years (363,357 person-years). During this period, dementia was diagnosed among 3,755 participants (2,022 women and 1,733 men). The mean age at diagnosis was 83.2 (±5.1) years. Baseline characteristics of the study population are shown in Table 1.
Table 1.
Baseline characteristics of the study population
| Characteristic* | All participants (n = 28,775) | Women (n = 13,401) | Men (n = 15,374) |
| Age, y | 71.6 (4.5) | 72.0 (4.7) | 71.2 (4.2) |
| Postsecondary education, % | 8.4 | 7.7 | 8.9 |
| Body mass index, kg/m2 | 25.4 (3.6) | 25.2 (3.9) | 25.6 (3.3) |
| History of hypertension, % | 32.0 | 29.5 | 34.1 |
| History of hypercholesterolemia, % | 14.9 | 10.2 | 18.9 |
| History of diabetes, % | 8.8 | 7.2 | 10.3 |
| DASH diet score† | 21.6 (4.4) | 21.6 (4.4) | 21.6 (4.3) |
| MED diet score† | 22.5 (4.7) | 22.5 (4.7) | 22.6 (4.6) |
| Coffee consumption, cups/day | 3.0 (1.7) | 2.9 (1.6) | 3.1 (1.8) |
| Alcohol consumption, drinks/week‡ | 5.2 (8.9) | 2.8 (4.7) | 7.1 (10.7) |
| Current smoker, % | 19.4 | 15.9 | 22.4 |
| Walking/bicycling ≥ 40 min/day, % | 40.3 | 38.8 | 41.6 |
| Leisure-time exercise ≥2 h/week, % | 66.8 | 62.2 | 70.5 |
| Sleep duration, h/night | 7.2 (1.2) | 7.1 (1.2) | 7.4 (1.1) |
*Values are means (±standard deviation) if not otherwise indicated. †A measure of an overall healthy diet. The DASH (Dietary Approaches to Stop Hypertension) diet score ranges from 7 (minimal adherence) to 35 (maximal adherence). The MED (Mediterranean) diet score ranges from 8 (minimal adherence) to 40 (maximal adherence). ‡Among current drinkers.
Among the lifestyle factors, only smoking was associated with risk of dementia (Table 2). Compared with never smokers, the multivariable HRs (95% CI) of dementia were 1.13 (95% CI 1.04–1.23) for former smokers and 1.10 (95% CI 1.00–1.21) for current smokers. Current smokers of at least 20 pack-years had a 17% increased risk of dementia (HR 1.17; 95% CI 1.02–1.34). Extended time of sleep was associated with an increased risk of dementia (Table 2). The multivariable HR for >9 h of sleep/night compared with 7.1–9 h/night was 1.54 (95% CI 1.27–1.85). However, the association did not remain after exclusion of cases diagnosed during the first five years (HR 1.22; 95% CI 0.97–1.52) or 10 years (HR 1.03; 95% CI 0.76–1.40) of follow-up. The associations of the lifestyle factors and sleep duration with dementia risk were similar in women and men (Table 2).
Table 2.
Associations of lifestyle factors and sleep duration with Alzheimer’s disease among 28,775 Swedish adults ≥65 years of age, 1998–2014
| All participants | Women | Men | ||||||
| Modifiable factor | Casesa | Person-yearsa | Age- and sex-adjusted HR (95% CI) | Multivariable HR (95% CI)b | Casesa | Multivariable HR (95% CI)b | Casesa | Multivariable HR (95% CI)b |
| DASH diet score | ||||||||
| Quartile 1 | 921 | 84,732 | 1.00 (reference) | 1.00 (reference) | 503 | 1.00 (reference) | 418 | 1.00 (reference) |
| Quartile 2 | 916 | 89,802 | 0.96 (0.88–1.05) | 0.96 (0.88–1.06) | 509 | 1.02 (0.90–1.15) | 407 | 0.90 (0.79–1.04) |
| Quartile 3 | 913 | 91,126 | 0.94 (0.86–1.03) | 0.94 (0.85–1.03) | 498 | 0.97 (0.85–1.10) | 415 | 0.90 (0.78–1.03) |
| Quartile 4 | 1005 | 97,696 | 0.98 (0.90–1.08) | 0.96 (0.87–1.05) | 512 | 0.95 (0.84–1.08) | 493 | 0.97 (0.84–1.11) |
| Mediterranean diet score | ||||||||
| Quartile 1 | 1055 | 95,348 | 1.00 (reference) | 1.00 (reference) | 587 | 1.00 (reference) | 468 | 1.00 (reference) |
| Quartile 2 | 877 | 88,237 | 0.93 (0.85–1.01) | 1.03 (0.88–1.21) | 465 | 1.09 (0.89–1.33) | 412 | 0.92 (0.70–1.21) |
| Quartile 3 | 836 | 82,319 | 0.94 (0.86–1.03) | 1.11 (0.95–1.31) | 450 | 1.11 (0.91–1.35) | 386 | 1.10 (0.84–1.43) |
| Quartile 4 | 987 | 97,452 | 0.96 (0.88–1.05) | 1.12 (0.96–1.31) | 520 | 1.13 (0.93–1.38) | 467 | 1.08 (0.84–1.41) |
| Coffee consumption | ||||||||
| <1.0 cups/day | 176 | 17,351 | 1.00 (reference) | 1.00 (reference) | 95 | 1.00 (reference) | 81 | 1.00 (reference) |
| 1.0–2.9 cups/day | 1483 | 143,919 | 0.98 (0.84–1.14) | 0.99 (0.85–1.16) | 807 | 0.94 (0.76–1.17) | 676 | 1.07 (0.85–1.35) |
| 3.0–4.9 cups/day | 1383 | 133,880 | 1.01 (0.86–1.18) | 1.03 (0.88–1.21) | 774 | 1.03 (0.83–1.28) | 609 | 1.03 (0.82–1.31) |
| ≥5.0 cups/day | 486 | 49,254 | 1.07 (0.90–1.27) | 1.07 (0.90–1.28) | 225 | 1.11 (0.87–1.41) | 261 | 1.07 (0.83–1.37) |
| Alcohol consumption | ||||||||
| Lifelong abstainer | 589 | 48,928 | 1.00 (reference) | 1.00 (reference) | 462 | 1.00 (reference) | 127 | 1.00 (reference) |
| Former drinker | 218 | 20,591 | 1.05 (0.89–1.22) | 0.97 (0.83–1.15) | 47 | 0.80 (0.59–1.09) | 171 | 1.12 (0.89–1.42) |
| Current <1 drink/week | 835 | 72,747 | 1.04 (0.94–1.16) | 1.03 (0.92–1.14) | 605 | 0.98 (0.87–1.11) | 230 | 1.18 (0.94–1.47) |
| Current 1–6 drinks/week | 1424 | 145,586 | 0.98 (0.89–1.09) | 0.96 (0.86–1.06) | 703 | 0.97 (0.85–1.09) | 721 | 1.01 (0.83–1.23) |
| Current 7–14 drinks/week | 389 | 44,608 | 0.96 (0.84–1.10) | 0.93 (0.81–1.07) | 87 | 0.85 (0.67–1.08) | 302 | 1.03 (0.83–1.28) |
| Current 14–21 drinks/week | 81 | 9482 | 0.98 (0.77–1.24) | 0.94 (0.74–1.19) | 10 | 0.94 (0.50–1.76) | 71 | 1.01 (0.75–1.36) |
| Current >21 drinks/week | 71 | 8489 | 1.00 (0.78–1.29) | 0.94 (0.73–1.21) | 6 | 0.63 (0.28–1.42) | 65 | 1.06 (0.78–1.44) |
| Smoking | ||||||||
| Never | 2100 | 196,510 | 1.00 (reference) | 1.00 (reference) | 1432 | 1.00 (reference) | 668 | 1.00 (reference) |
| Former (any pack-years) | 1037 | 103,271 | 1.13 (1.04–1.22) | 1.13 (1.04–1.23) | 329 | 1.19 (1.05–1.35) | 708 | 1.10 (0.98–1.23) |
| <20 pack-yearsc | 598 | 59,075 | 1.12 (1.02–1.23) | 1.12 (1.02–1.24) | 232 | 1.14 (0.98–1.31) | 366 | 1.11 (0.97–1.27) |
| ≥20 pack-yearsc | 275 | 31,122 | 1.10 (0.96–1.25) | 1.10 (0.96–1.26) | 62 | 1.37 (1.05–1.77) | 213 | 1.04 (0.88–1.22) |
| Current (any pack-years) | 618 | 63,576 | 1.11 (1.01–1.21) | 1.10 (1.00–1.21) | 261 | 1.05 (0.91–1.20) | 357 | 1.14 (1.00–1.30) |
| <20 pack-yearsc | 281 | 29,691 | 1.02 (0.90–1.15) | 1.01 (0.89–1.15) | 156 | 1.00 (0.85–1.19) | 125 | 1.02 (0.84–1.24) |
| ≥20 pack-yearsc | 248 | 26,931 | 1.18 (1.03–1.34) | 1.17 (1.02–1.34) | 86 | 1.08 (0.86–1.34) | 162 | 1.23 (1.03–1.47) |
| Walking/bicycling | ||||||||
| Almost never | 424 | 38,891 | 1.00 (reference) | 1.00 (reference) | 252 | 1.00 (reference) | 172 | 1.00 (reference) |
| <20 min/day | 559 | 62,860 | 0.88 (0.77–1.00) | 0.89 (0.78–1.01) | 279 | 0.88 (0.74–1.05) | 280 | 0.91 (0.75–1.10) |
| 20–40 min/day | 1017 | 103,747 | 0.91 (0.81–1.02) | 0.91 (0.81–1.03) | 564 | 0.86 (0.74–1.01) | 453 | 0.99 (0.82–1.19) |
| 40–60 min/day | 679 | 64,923 | 0.99 (0.88–1.12) | 0.99 (0.87–1.13) | 374 | 0.98 (0.83–1.16) | 305 | 1.01 (0.83–1.24) |
| ≥1 h/day | 901 | 80,596 | 1.09 (0.97–1.23) | 1.08 (0.95–1.22) | 438 | 1.02 (0.86–1.21) | 463 | 1.16 (0.96–1.40) |
| Leisure-time exercise | ||||||||
| <1 h/week | 475 | 48,792 | 1.00 (reference) | 1.00 (reference) | 280 | 1.00 (reference) | 195 | 1.00 (reference) |
| 1 h/week | 562 | 57,124 | 1.01 (0.89–1.14) | 1.04 (0.91–1.17) | 335 | 1.02 (0.87–1.21) | 227 | 1.05 (0.89–1.14) |
| 2–3 h/week | 1137 | 111,350 | 1.03 (0.92–1.14) | 1.04 (0.93–1.16) | 616 | 1.04 (0.90–1.21) | 521 | 1.01 (0.92–1.14) |
| ≥4 h/week | 1222 | 117,073 | 1.11 (0.99–1.23) | 1.06 (0.95–1.19) | 554 | 1.12 (0.96–1.32) | 668 | 0.99 (0.83–1.17) |
| Sleep duration | ||||||||
| ≤6.0 h/night | 988 | 87,964 | 1.00 (0.92–1.09) | 0.99 (0.92–1.08) | 627 | 0.96 (0.86–1.07) | 361 | 1.05 (0.93–1.19) |
| 6.1–7.0 h/night | 1110 | 111,490 | 1.02 (0.94–1.10) | 1.01 (0.93–1.09) | 599 | 1.02 (0.91–1.14) | 511 | 1.00 (0.89–1.12) |
| 7.1–9.0 h/night | 1454 | 149,828 | 1.00 (reference) | 1.00 (reference) | 692 | 1.00 (reference) | 762 | 1.00 (reference) |
| >9.0 h/night | 119 | 7796 | 1.56 (1.30–1.88) | 1.54 (1.27–1.85) | 55 | 1.63 (1.24–2.14) | 64 | 1.44 (1.11–1.86) |
CI, confidence interval; HR, hazard ratio; DASH, Dietary Approaches to Stop Hypertension diet. aThe number of cases and person-years may not add up to the total number owing to missing data on some of the lifestyle factors. A dummy variable for missing was included in the model. bAdjusted for age, sex, education, body mass index, and history of hypertension, hypercholesterolemia and diabetes, and mutually for the other lifestyle factors (except the Mediterranean diet score due to strong correlation with the DASH diet score) and sleep duration. cThe number of cases included in the analysis of pack-years does not sum up to the number included in the analysis of smoking status owing to missing information on pack-years.
DISCUSSION
Findings from this cohort study indicate that smoking but no other lifestyle factors influence the risk of dementia. Long sleep duration was associated with an increased risk of dementia in the overall analysis but this association did not remain after removing cases diagnosed during the first five or 10 years of follow-up.
We have previously shown that high adherence to healthy dietary patterns, including the modified DASH and MED diets, are inversely associated with risk of cardiovascular disease [28, 32] and cancer [29] as well as with all-cause mortality [33, 34] in this study population. However, the modified DASH and MED diets were not found to be associated with risk of dementia in the present analysis. Previous cohort studies assessing the association of a MED-like diet with risk of dementia were based on small sample sizes (ranging from 923 [8] to 2,258 [4]) and yielded inconsistent results, with an inverse association found in some studies [4, 5, 8] but not in other [6, 7]. Other studies have found that the DASH diet [8], but not a Healthy Diet Indicator or a low carbohydrate and high protein diet score [7], is inversely associated with dementia risk.
Prospective studies of coffee [13, 14] consumption in relation to dementia have produced conflicting results. A meta-analysis of six studies including 31,399 individuals and 2873 cases showed that moderate (1–2 cups/day) but not high (>3 cups/day) coffee consumption was associated with a reduced risk of dementia [13]. In contrast, recent results from a multiethnic cohort study of 185,855 individuals, including 1,404 cases, showed a borderline significant positive association between coffee consumption and risk of Alzheimer’s disease (HR = 1.07 [95% CI 1.00–1.15] per 1 cup/day increase in coffee consumption) [14]. Furthermore, a Mendelian randomization study found that genetic predisposition to consume more coffee was associated with higher risk of Alzheimer’s disease [35].
With regard to alcohol consumption, a meta-analysis of 11 prospective studies with a total of 73,330 participants and 4,586 dementia cases showed a J-shaped relationship between alcohol consumption and risk of dementia [12]. An alcohol intake up to at most 12.5 g/day (about 1 drink/day) was associated with a reduced risk of dementia, with the lowest risk (9% reduction in risk) observed at an intake of about 6 g/day. In our study, including nearly the same number of dementia cases as in the meta-analysis, we found no significant association between alcohol consumption and risk of dementia but we cannot rule out that we may have overlooked a weak association.
The present study did not confirm a beneficial association between physical activity and dementia risk, which was observed in a meta-analysis of observational studies, most of which included small number of cases [11]. A recent genome-wide association study found that a genetic variant (rs429358) in the apolipoprotein E gene was significantly associated with moderate-to-vigorous physical activity [36]. Genetic variants in the apolipoprotein E gene are strongly associated with risk of dementia, in particular Alzheimer’s disease, and therefore we cannot exclude the possibility that the lack of association between physical activity and risk of dementia in the present study was due to confounding by apolipoprotein E genotypes. Further studies evaluating the associations of different types and intensities of physical activity with risk of dementia, adjusted for apolipoprotein E genotypes, are necessary to elucidate the role of physical activity in dementia.
Smoking has been inconsistently associated with risk of dementia in observational studies but a meta-analysis of 17 prospective studies found an overall 30% increased risk of dementia among smokers [37]. Our study confirms a modest increased risk of dementia associated with smoking.
Our overall results showing an association between long sleep duration and increased risk of dementia are consistent with findings from several previous smaller cohort studies of older adults [16, 17, 20–22]. However, sleep duration was not associated with dementia risk among 4835 older Dutch adults who were cognitively intact at baseline [23]. Furthermore, in the Framingham Heart Study long sleep duration in the past was not associated with an increased dementia risk and sleep duration at baseline was only associated with higher risk of dementia in those with mild cognitive impairment [22]. In our study, the association between long sleep duration and dementia risk did not remain after removing cases diagnosed during the first five or 10 years of follow-up, suggesting that the association was explained by a reverse causation effect. Disturbed sleep is often seen in dementia and may involve damage to hypothalamic and brainstem nuclei that control sleep-wake cycles and perturbations in neurotransmitter signaling (e.g., serotonin, norepinephrine, and melatonin) [25]. Collectively, there is evidence that transitioning to being a long sleeper is an early marker of neurodegeneration.
Important strengths of this study include the large sample size, the prospective design, the long follow-up, and the objective ascertainment of dementia through linkage with population-based registers. The large sample size and long follow-up and thus, the large number of cases provided high statistical power to detect weak associations such as the association between smoking and dementia risk.
A shortcoming of this study is that we did not have information on the apolipoprotein E ɛ4 genotype. Another limitation is that some degree of underascertainment of dementia cases was inevitable since data on dementia were retrieved from the National Patient Register. A further limitation is that the lifestyle factors and sleep were self-reported on a questionnaire. Thus, some measurement error in the exposures may have occurred. Nevertheless, in previous studies based on this cohort and the same questionnaire, we have observed associations of healthy dietary patterns, alcohol and coffee consumption, smoking, and physical activity with risk of cardiovascular disease (e.g., [26, 28, 38–44]) and mortality [33], indicating that the questionnaire have adequate validity to capture true associations. Finally, this study could not address whether adopting and maintaining healthy lifestyle behaviors in early adulthood and midlife reduces the risk of developing dementia in late-life.
In conclusion, while adopting and maintaining healthy lifestyle behavior throughout life is important in the prevention of several chronic diseases and premature death, this study found no evidence that major lifestyle factors, aside from smoking, or sleep duration in late life are associated with risk of developing late-onset dementia. The role of specific dietary factors (foods, nutrients, and other bioactive compounds) and other potentially modifiable risk factors for dementia merits study in future studies.
ACKNOWLEDGMENTS
This work was supported by the Swedish Brain Foundation (Hjärnfonden). We acknowledge the Swedish Infrastructure for Medical Population-based Life-course Environmental Research (SIMPLER) for provisioning of facilities and database. SIMPLER receives funding through the Swedish Research Council under the grant no 2017-00644.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0529r3).
REFERENCES
- [1]. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013) The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement 9, 63–75 e62. [DOI] [PubMed] [Google Scholar]
- [2]. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbaek G, Teri L, Mukadam N (2017) Dementia prevention, intervention, and care. Lancet 390, 2673–2734. [DOI] [PubMed] [Google Scholar]
- [3]. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H (2015) Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 11, 718–726. [DOI] [PubMed] [Google Scholar]
- [4]. Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA (2006) Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol 59, 912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y (2009) Physical activity, diet, and risk of Alzheimer disease. JAMA 302, 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Feart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, Barberger-Gateau P (2009) Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 302, 638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Olsson E, Karlstrom B, Kilander L, Byberg L, Cederholm T, Sjogren P (2015) Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J Alzheimers Dis 43, 109–119. [DOI] [PubMed] [Google Scholar]
- [8]. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT (2015) MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 11, 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Strand BH, Langballe EM, Hjellvik V, Handal M, Naess O, Knudsen GP, Refsum H, Tambs K, Nafstad P, Schirmer H, Bergem AL, Selmer R, Engedal K, Magnus P, Bjertness E (2013) Midlife vascular risk factors and their association with dementia deaths: Results from a Norwegian prospective study followed up for 35 years. J Neurol Sci 324, 124–130. [DOI] [PubMed] [Google Scholar]
- [10]. Di Marco LY, Marzo A, Munoz-Ruiz M, Ikram MA, Kivipelto M, Ruefenacht D, Venneri A, Soininen H, Wanke I, Ventikos YA, Frangi AF (2014) Modifiable lifestyle factors in dementia: A systematic review of longitudinal observational cohort studies. J Alzheimers Dis 42, 119–135. [DOI] [PubMed] [Google Scholar]
- [11]. Xu W, Tan L, Wang HF, Jiang T, Tan MS, Tan L, Zhao QF, Li JQ, Wang J, Yu JT (2015) Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry 86, 1299–1306. [DOI] [PubMed] [Google Scholar]
- [12]. Xu W, Wang H, Wan Y, Tan C, Li J, Tan L, Yu JT (2017) Alcohol consumption and dementia risk: A dose-response meta-analysis of prospective studies. Eur J Epidemiol 32, 31–42. [DOI] [PubMed] [Google Scholar]
- [13]. Wu L, Sun D, He Y (2017) Coffee intake and the incident risk of cognitive disorders: A dose-response meta-analysis of nine prospective cohort studies. Clin Nutr 36, 730–736. [DOI] [PubMed] [Google Scholar]
- [14]. Park SY, Freedman ND, Haiman CA, Le Marchand L, Wilkens LR, Setiawan VW (2017) Association of coffee consumption with total and cause-specific mortality among nonwhite populations. Ann Intern Med 167, 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Russ TC, Kivimaki M, Starr JM, Stamatakis E, Batty GD (2014) Height in relation to dementia death: Individual participant meta-analysis of 18 UK prospective cohort studies. Br J Psychiatry 205, 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Bokenberger K, Strom P, Dahl Aslan AK, Johansson AL, Gatz M, Pedersen NL, Akerstedt T (2017) Association between sleep characteristics and incident dementia accounting for baseline cognitive status: A prospective population-based study. J Gerontol A Biol Sci Med Sci 72, 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Sindi S, Kåreholt I, Johansson L, Skoog J, Sjoberg L, Wang HX, Johansson B, Fratiglioni L, Soininen H, Solomon A, Skoog I, Kivipelto M (2018) Sleep disturbances and dementia risk: A multicenter study. Alzheimers Dement 14, 1235–1242. [DOI] [PubMed] [Google Scholar]
- [18]. Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, Shi J, Vitiello MV, Lu L (2018) Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev 40, 4–16. [DOI] [PubMed] [Google Scholar]
- [19]. Pase MP, Himali JJ, Grima NA, Beiser AS, Satizabal CL, Aparicio HJ, Thomas RJ, Gottlieb DJ, Auerbach SH, Seshadri S (2017) Sleep architecture and the risk of incident dementia in the community. Neurology 89, 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Benito-Leon J, Louis ED, Villarejo-Galende A, Romero JP, Bermejo-Pareja F (2014) Long sleep duration in elders without dementia increases risk of dementia mortality (NEDICES). Neurology 83, 1530–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Benito-Leon J, Bermejo-Pareja F, Vega S, Louis ED (2009) Total daily sleep duration and the risk of dementia: A prospective population-based study. Eur J Neurol 16, 990–997. [DOI] [PubMed] [Google Scholar]
- [22]. Westwood AJ, Beiser A, Jain N, Himali JJ, DeCarli C, Auerbach SH, Pase MP, Seshadri S (2017) Prolonged sleep duration as a marker of early neurodegeneration predicting incident dementia. Neurology 88, 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Lysen TS, Wolters FJ, Luik AI, Ikram MK, Tiemeier H, Ikram MA (2018) Subjective sleep quality is not associated with incident dementia: The Rotterdam Study. J Alzheimers Dis 64, 239–247. [DOI] [PubMed] [Google Scholar]
- [24]. Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC (2012) Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Rothman SM, Mattson MP (2012) Sleep disturbances in Alzheimer’s and Parkinson’s diseases. Neuromolecular Med 14, 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Stackelberg O, Bjorck M, Larsson SC, Orsini N, Wolk A (2014) Sex differences in the association between smoking and abdominal aortic aneurysm. Br J Surg 101, 1230–1237. [DOI] [PubMed] [Google Scholar]
- [27]. Messerer M, Johansson SE, Wolk A (2004) The validity of questionnaire-based micronutrient intake estimates is increased by including dietary supplement use in Swedish men. J Nutr 134, 1800–1805. [DOI] [PubMed] [Google Scholar]
- [28]. Larsson SC, Wallin A, Wolk A (2016) Dietary approaches to stop hypertension diet and incidence of stroke: Results from 2 prospective cohorts. Stroke 47, 986–990. [DOI] [PubMed] [Google Scholar]
- [29]. Larsson SC, Hakansson N, Wolk A (2017) Healthy dietary patterns and incidence of biliary tract and gallbladder cancer in a prospective study of women and men. Eur J Cancer 70, 42–47. [DOI] [PubMed] [Google Scholar]
- [30]. Norman A, Bellocco R, Bergstrom A, Wolk A (2001) Validity and reproducibility of self-reported total physical activity–differences by relative weight. Int J Obes Relat Metab Disord 25, 682–688. [DOI] [PubMed] [Google Scholar]
- [31]. Orsini N, Bellocco R, Bottai M, Hagstromer M, Sjostrom M, Pagano M, Wolk A (2008) Validity of self-reported total physical activity questionnaire among older women. Eur J Epidemiol 23, 661–667. [DOI] [PubMed] [Google Scholar]
- [32]. Tektonidis TG, Akesson A, Gigante B, Wolk A, Larsson SC (2015) A Mediterranean diet and risk of myocardial infarction, heart failure and stroke: A population-based cohort study. Atherosclerosis 243, 93–98. [DOI] [PubMed] [Google Scholar]
- [33]. Larsson SC, Kaluza J, Wolk A (2017) Combined impact of healthy lifestyle factors on lifespan: Two prospective cohorts. J Intern Med 282, 209–219. [DOI] [PubMed] [Google Scholar]
- [34]. Bellavia A, Tektonidis TG, Orsini N, Wolk A, Larsson SC (2016) Quantifying the benefits of Mediterranean diet in terms of survival. Eur J Epidemiol 31, 527–530. [DOI] [PubMed] [Google Scholar]
- [35]. Larsson SC, Traylor M, Malik R, Dichgans M, Burgess S, Markus HS (2017) Modifiable pathways in Alzheimer’s disease: Mendelian randomisation analysis. BMJ 359, 5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Klimentidis YC, Raichlen DA, Bea J, Garcia DO, Wineinger NE, Mandarino LJ, Alexander GE, Chen Z, Going SB (2018) Genome-wide association study of habitual physical activity in over 377,000 UK Biobank participants identifies multiple variants including CADM2 and APOE. Int J Obes (Lond) 42, 1161–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y (2015) Smoking is associated with an increased risk of dementia: A meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One 10, e0118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Larsson SC, Drca N, Jensen-Urstad M, Wolk A (2016) Combined impact of healthy lifestyle factors on risk of atrial fibrillation: Prospective study in men and women. Int J Cardiol 203, 46–49. [DOI] [PubMed] [Google Scholar]
- [39]. Larsson SC, Tektonidis TG, Gigante B, Akesson A, Wolk A (2016) Healthy lifestyle and risk of heart failure: Results from 2 prospective cohort studies. Circ Heart Fail 9, e002855. [DOI] [PubMed] [Google Scholar]
- [40]. Larsson SC, Akesson A, Wolk A (2014) Healthy diet and lifestyle and risk of stroke in a prospective cohort of women. Neurology 83, 1699–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Akesson A, Larsson SC, Discacciati A, Wolk A (2014) Low-risk diet and lifestyle habits in the primary prevention of myocardial infarction in men: A population-based prospective cohort study. J Am Coll Cardiol 64, 1299–1306. [DOI] [PubMed] [Google Scholar]
- [42]. Larsson SC, Virtamo J, Wolk A (2011) Coffee consumption and risk of stroke in women. Stroke 42, 908–912. [DOI] [PubMed] [Google Scholar]
- [43]. Larsson SC, Wolk A, Bäck M (2017) Alcohol consumption, cigarette smoking and incidence of aortic valve stenosis. J Intern Med 282, 332–339. [DOI] [PubMed] [Google Scholar]
- [44]. Larsson SC, Wolk A, Håkansson N, Bäck M (2018) Coffee consumption and risk of aortic valve stenosis: A prospective study. Nutr Metab Cardiovasc Dis 28, 803–807. [DOI] [PubMed] [Google Scholar]