Abstract
Background:
Bacillus Calmette-Guerin (BCG) is the most effective intravesical therapy for non-muscle invasive bladder cancer (NMIBC), but patients can fail or supply shortages can develop. For BCG failures, radical cystectomy is recommended. However, in patients who desire bladder preservation or are poor surgical candidates, alternative salvage intravesical therapies should be explored.
Objective:
To determine whether dual sequential intravesical gemcitabine and docetaxel is effective in treating NMIBC.
Methods:
We evaluated our initial experience with 45 patients treated with intravesical gemcitabine and docetaxel between June 2009 and May 2014. Patients were treated with 6 weekly instillations of gemcitabine (1 gram of gemcitabine in 50 ml of sterile water) followed immediately by docetaxel (37.5 mg of docetaxel in 50 mL of saline). Treatment success was defined as no bladder cancer recurrence and no cystectomy. Intention-to-treat analysis was performed using the Kaplan Meier method.
Results:
Forty-five patients received treatment with a median overall follow-up of 15 months. Median follow up for treatment success was 6 months in all patients and 13 months for responders. Five patients were unable to tolerate a full induction course. Treatment success was 66% at first surveillance, 54% at 1 year, and 34% at 2 years after initiating induction. Ten patients received cystectomy (median of 5.6 months from starting induction) with no positive margins or lymph nodes on final pathology.
Conclusions:
Sequential dual intravesical gemcitabine and docetaxel can salvage some patients in a challenging NMIBC cohort.
Keywords: Urinary bladder neoplasms, instillation, bladder, docetaxel, gemcitabine, salvage therapy
INTRODUCTION
Bladder cancer is the sixth most common new cancer diagnosis in the United States with an expected 74,690 new cases in 2014 [1]. Non-muscle invasive bladder cancer (NMIBC) constitutes 75% of new bladder cancer diagnoses [2]. Treatment includes a complete transurethral resection of bladder tumors and repeat resection in select cases, followed by adjuvant intravesical therapy with Bacillus Calmette-Guerin (BCG) for intermediate and high-risk tumors [2]. Intravesical BCG with maintenance has been shown to decrease rates of both recurrence [3, 4] and progression [5, 6].
Despite BCG therapy, treatment failure occurs in approximately 40% of patients followed for 2 years [7]. In the event of BCG failure, the preferred treatment for high-risk patients per EAU and AUA guidelines is to proceed with cystectomy [2, 8]. However, some patients have a strong preference for bladder preservation or are poor surgical candidates, making alternative intravesical salvage therapies essential for this cohort. Bladder preserving therapies including immunotherapy, chemotherapy, device-assisted therapies (i.e. electromotive [9] or hyperthermic [10]) and combination therapy are available. Repeat BCG therapy for patients with prior failure is only successful in approximately one third of patients [11] and combination therapies have been incompletely evaluated. Additionally, given recent shortages of intravesical agents such as mitomycin [12] and BCG [13], there is an unmet need to explore alternative intravesical therapies.
At our institution, intravesical gemcitabine and mitomycin has been offered as a salvage option for the treatment of NMIBC [14]. However, during the mitomycin shortage [12] in 2009, we transitioned to using docetaxel in combination with gemcitabine. We report the results of our pilot experience with sequential gemcitabine and docetaxel (Gem/Doce) as salvage therapy for patients with NMIBC.
MATERIALS AND METHODS
Study population
After Institutional Review Board approval, we retrospectively reviewed all patients treated with sequential intravesical Gem/Doce for NMIBC at the University of Iowa Hospitals and Clinics between June 2009 and May 2014 (n = 45).
Patients were counseled about the recommendation for cystectomy. Initially, only patients unfit for cystectomy were offered this treatment regimen. Over time, the regimen was also offered to cystectomy candidates (n = 30) who desired to pursue alternative salvage options. Cystectomy candidacy was documented by the treating physician in the clinic records.
Patient characteristics are listed in Table 1. The median age at treatment was 72 years (range 50–91). Four of 45 patients were BCG naïve, of whom BCG was not used in 3 due to underlying immunosuppression (two with history of stem cell transplant, one with myelodysplastic syndrome) and one due to advanced age (91 years old). Forty-one of the 45 patients (91%) were previously treated with a BCG-based regimen but had recurrence (n = 37) or could not tolerate BCG due to side effects (n = 4). The median number of prior induction BCG courses was 2 (range 0–4). Of the 17 patients who received 1 prior BCG course, 4 patients had prior BCG intolerance. The remaining 13 patients had disease features which were felt to minimize the chance of success with repeat BCG, including persistent or early recurrent high risk disease (CIS or T1HG) in 8 and 2 patients who recurred while receiving maintenance therapy.
Table 1.
Baseline characteristics of patients that received treatment with intravesical gemcitabine and docetaxel for NMIBC
| Median age (range) | 72 years (50–91) |
| No. of patients | 45 |
| Male | 37 (82%) |
| Race | |
| White | 42 (93%) |
| Other | 3 (7%) |
| Smoking status | |
| Current | 5 (11%) |
| Former | 23 (51%) |
| Never | 17 (38%) |
| Median pack years | 25 |
| Stage | |
| CIS alone | 20 (44%) |
| TaLG | 4 (9%) |
| TaLG + CIS | 0 |
| TaHG | 8 (18%) |
| TaHG + CIS | 5 (11%) |
| T1HG | 4 (9%) |
| T1HG + CIS | 4 (9%) |
| T1LG | 0 |
| T1LG + CIS | 0 |
| Prior treatments | |
| Median induction courses (range) | 2 (0–4) |
| BCG naïve | 4 (9%) |
| 1 prior BCG failure | 17 (38%) |
| ≥2 prior BCG failures | 24 (53%) |
| BCG status | |
| BCG naïve | 4 (9%) |
| BCG refractory | 19 (42 %) |
| BCG relapsing | 18 (40%) |
| BCG intolerant | 4 (9%) |
Patients with prior BCG treatment were divided into failure type subgroups [15], specifically refractory (rapidly recurrent or progressive disease noted at 3 months after diagnosis or persistent disease at 6 months after diagnosis in light of 2 BCG induction courses or induction plus maintenance), relapsing (recurrence after becoming disease free by 6 months) or intolerant (disease recurrence after a less than adequate treatment course due to symptomatic intolerance or a serious adverse event). Patients with BCG refractory disease comprised 42% (19/45) of the cohort.
Prior to initiating therapy, patients with visibletumor burden (n = 25) underwent complete transurethral resection of bladder tumors (TURBT). Patients with CIS alone were diagnosed either by bladder biopsy (n = 11) or high grade urothelial carcinoma cytology (n = 9).
Gemcitabine/docetaxel intravesical treatment
Gemcitabine is a non-vesicant chemotherapeutic agent that acts as a deoxycytidine nucleoside analog, thereby inhibiting deoxyribonucleic acid (DNA) synthesis, resulting in cell apoptosis [16]. Docetaxel is an anti-mitotic chemotherapeutic agent which inhibits tubulin disassembly, thereby stopping cell division [17]. Based on these different mechanisms of action, gemcitabine was administered first as it requires active DNA synthesis for incorporation and effectiveness, a process which might be hampered by the anti-mitotic effects of docetaxel.
Prior to all instillations, patients were treated with 1300 mg oral sodium bicarbonate the evening prior and the morning of treatment to alkalinize their urine. Alkalinization is thought to prevent some of the side effects of the acidic gemcitabine (pH 2.5). Oral ondansetron was also administered prophylactically to patients who reported nausea after their first instillation. After catheter placement and complete bladder drainage, 1 g of gemcitabine in 50 ml of sterile water was instilled. The catheter was plugged, and gemcitabine was retained for 90 minutes. Following bladder drainage, 37.5 mg of docetaxel dissolved in 50 mL of saline was instilled via the catheter, which was then removed. Patients were instructed to not urinate for 120 minutes after catheter removal. This induction regimen was administered weekly for 6 weeks.
Surveillance
Surveillance was initiated 12 to 16 weeks after beginning Gem/Doce and involved either an evaluation under anesthesia (formal restaging) or office cystoscopic follow-up. A restaging procedure was offered to all high-grade cases and included cystoscopy, bladder barbotage cytology, bilateral upper tract barbotage cytologies, bilateral retrograde pyelograms, random bladder biopsies, and prostatic urethral biopsies [18]. If patients refused formal restaging or were considered too high an anesthetic risk, office cystoscopy with bladder cytology was performed with upper tract imaging at least every 2 years. Patients found to be recurrence-free received monthly maintenance instillations for 24 months. Surveillance cystoscopy with bladder cytology was subsequently performed at 3-month intervals for 2 years and then 6-month intervals if disease free beyond 2 years.
Analysis
Data was retrospectively collected and stored using REDCap software, supported by University of Iowa NIH/CTSA program grant 2UL1TR000442-06. Descriptive statistics were calculated. Treatment success was defined as both 1) no evidence of recurrence and 2) bladder preservation (no cystectomy). Analysis was based on intention-to-treat. Recurrence and survival analysis was performed via Kaplan-Meier method. The log-rank test was utilized to compare subgroups (disease stage at Gem/Doce initiation, number of prior BCG failures, BCG failure classification, and surveillance method). Kaplan-Meier creation and statistical analysis were performed using GraphPad Prism version 4.0. P < 0.05 was considered statistically significant.
RESULTS
Treatment tolerance
Five patients were unable to tolerate the full six treatment Gem/Doce induction course. Four of these patients had significant baseline lower urinary tract symptoms. One patient was admitted just prior to treatment for gross hematuria requiring continuous bladder irrigation (CBI) and again soon after instillation with the same issue. In the 5 patients who required cessation of treatment, symptoms included frequency (n = 4), hematuria (n = 4), and dysuria (n = 2).
Twenty-eight patients reported symptoms during treatment (62%), but only 7 patients (16%) had symptoms that ultimately impacted the treatment schedule. Five of these 7 patients were the previously mentioned patients who could not tolerate the full induction course. The remaining 2 patients had treatment delay for a urinary tract infection (1) and significant urinary frequency, dysuria and bladder spasms requiring multiple oral medications and a 1-week treatment delay (1). In all patients, the most common side effects were mild dysuria (33%), mild urinary frequency/urgency (33%), hematuria (11%), and nausea (7%).
Treatment surveillance
Of the 5 patients unable to tolerate treatment, two patients proceeded with immediate cystectomy and 1 patient sought no further surveillance/treatment after being diagnosed with acute lymphocytic leukemia and enrolling in hospice. Two patients refused cystectomy and proceeded with other intravesical therapies. Of the remaining 40 patients who underwent surveillance, evaluation under anesthesia was completed in 33 (full restaging in 29 patients, bladder biopsies alone in 4), while the other 7 patients had office cystoscopy with bladder cytology.
Treatment success
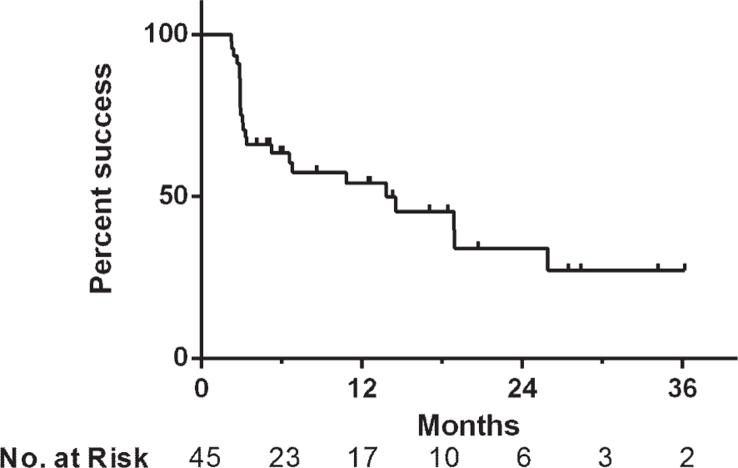
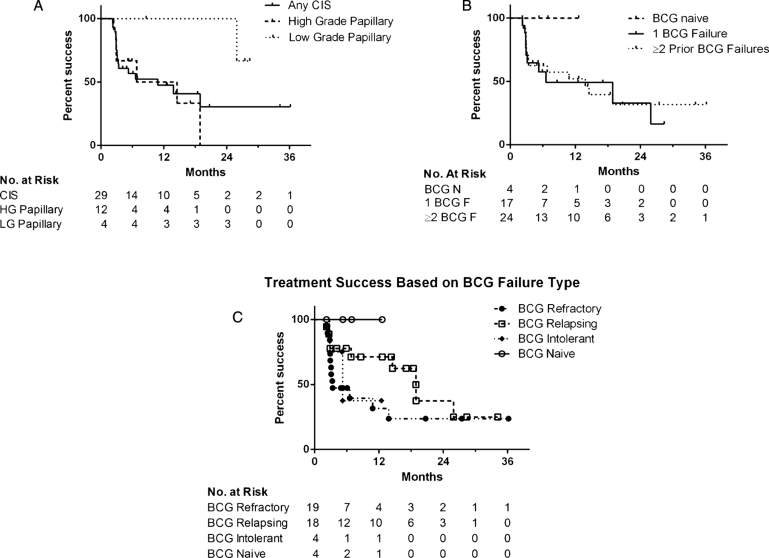
Treatment success was 66% at first surveillance, 54% at 1 year and 34% at 2 years after initiating induction (Fig. 1). Median overall follow-up for treatment success was 5.9 months, while the 30 patients with initial treatment success had a median follow up of 12.5 months (range 2.2–36.2). In those who failed therapy, the median time to failure was 3.1 months (range 2.2–25.9) and 67% of failures occurred within six months. There were no statistically significant differences in treatment success rates when the cohort was stratified based upon disease stage at Gem/Doce initiation (p = 0.36), presence/absence of CIS (p = 0.47), number of prior BCG failures (p = 0.38), classification of BCG failures (p = 0.23), or surveillance method (p = 0.43) (Fig. 2).
Fig.1.
Kaplan-Meier plot of salvage treatment success with intravesical gemcitabine and docetaxel in patients with NMIBC (n = 45).
Fig.2.
Kaplan-Meier plot of treatment success with intravesical gemcitabine and docetaxel depending on (a) disease stage at the time of Gem/Doce initiation, (b) number of prior BCG failures (BCG F = BCG Failure, BCG N = BCG Naïve), and (c) classification of prior BCG failures.
Cystectomy candidates
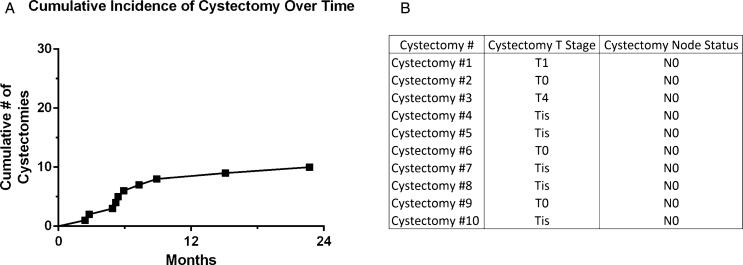
Of the 30 potential cystectomy candidates prior to Gem/Doce, 10 patients underwent cystectomy at a median of 5.6 months (range 2.4–22.7) from the time of first instillation (Fig. 3). Two patients were those unable to tolerate induction and proceeded with immediate cystectomy, while the remaining 8 underwent cystectomy based on recurrence. Cystectomy pathology was 3 patients with T0, 5 with CIS, 1 with T1, and 1 with T4 disease based on prostatic stromal invasion. All surgical margins and lymph nodes were negative.
Fig.3.
Cumulative number of cystectomies in patients who were cystectomy candidates and received intravesical gemcitabine and docetaxel intravesical therapy for NMIBC.
Survival analysis
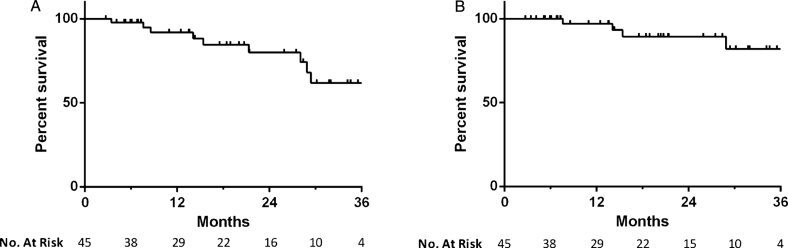
Median overall follow-up for survival determination was 15.4 months. There were a total of 10 deaths during the study period, 4 of which were attributable to bladder cancer. Two bladder cancer deaths were attributable to cardiac arrests during post-cystectomy admission, while the other two occurred in patients who were not cystectomy candidates. One went on to receive radiation therapy and ultimately elected to pursue palliative care, while the other received chemotherapy alone. The all-cause mortality rate was 8% at 1 year and 20% at 2 years, while the bladder cancer specific mortality rate was 3% and 11% , respectively (Fig. 4).
Fig.4.
Kaplan-Meier plot of (a) all-cause and (b) bladder cancer specific mortality in patients treated with intravesical gemcitabine and docetaxel.
DISCUSSION
Patients with NMIBC who fail BCG therapy remain a challenging cohort to treat. The EAU and AUA recommendations for BCG failure patients remains cystectomy; however, cystectomy is a procedure with significant morbidity and mortality rates [19], as well as dramatic lifestyle changes. As a result, some patients refuse to accept cystectomy without first exhausting all bladder preserving treatments. Other patients are poor surgical candidates, thus cystectomy is not a viable option. To our knowledge, this is the first study to report on the use of sequential intravesical Gem/Doce for NMIBC. The treatment was generally well tolerated, as most local and systemic side effects were mild and self-resolving. Salvage treatment demonstrated reasonable efficacy in a population at high-risk for recurrence, as treatment was successful in 54% of patients at 1 year.
In treating such a high-risk cohort, the oncologic safety of delaying cystectomy is a major concern, specifically the possibility of disease progression during intravesical treatment. Millan-Rodriquez and colleagues estimated that in high-risk NMIBC patients, progression occurred in 8% at 1 year with a mortality rate of 1% [20]. Similarly, Herr and Sogani found that patients with recurrent T1 disease who failed BCG and underwent cystectomy within 2 years of initial BCG had significantly higher disease-specific survival as compared to those who underwent surgery beyond 2 years (92% vs 56%) [21].
Given the risk of progression and need to balance the oncologic safety of cystectomy with bladder preservation, treatment choice remains a personal decision best made through shared decision making. However, it appears that there may be a window of opportunity to explore second line salvage intravesical therapy. In select BCG failure patients who are appropriately counseled about the potential for adverse outcomes, we feel a trial of salvage intravesical chemotherapy (Gem/Doce) prior to radical surgery is reasonable and can allow some patients to achieve a disease-free status. However this approach with Gem/Doce will require further validation and follow-up.
In order to avoid delays in identifying treatment failures, we pursued repeat evaluation under anesthesia to pathologically confirm treatment response, rather than depending on office evaluation. This approach avoids the ambiguous cystoscopic and cytologic findings that can occur in the early post-inflammatory period after intravesical therapy and identifies failures earlier, both intravesically and extravesically [18]. While our cohort of Gem/Doce failing patients who proceeded to cystectomy was small, the findings of favorable pathology (no positive surgical margins or lymph node positive disease) and low progression rate (1 of 10 with T4 disease based upon prostatic stromal invasion) suggest that treatment delay to allow a trial of Gem/Doce is oncologically safe.
There are a host of intravesical options that have been reported for patients with BCG failures which appear to be similar or less effective than our response rates with Gem/Doce. For example, re-induction with BCG monotherapy provided a durable response (>2 years) in only 35% of patients after a single BCG failure [15]. While the addition of interferon to BCG has not shown an improvement in BCG naïve patients [7], those with a prior BCG failure may have a mild improvement in treatment efficacy (45% at 2 years) [22]. However, patients in this study were likely not at as high a risk of recurrence as our cohort.
Mitomycin-C (MMC) monotherapy after a single BCG failure was found to be an effective short-term therapy with 1 year recurrence-free survival (RFS) of 65% but was not durable (19% RFS at 3 years) [23]. Valrubicin has been used in the treatment of carcinoma in situ (CIS) in patients with prior BCG failures, however 6 and 30 months RFS was 18–21% and 8% , respectively [24, 25]. Induction with single-agent intravesical docetaxel provided 1 and 3 year RFS rates of 40% and 25% in a BCG failure cohort without maintenance [26, 27]. The docetaxel study cohort received a variable medication dose and some patients underwent additional TURBT but were considered successes as no further intravesical therapy was needed, thus making direct comparison to our cohort not possible. A SWOG study evaluated single-agent intravesical gemcitabine and found RFS rates of 28% at 1 year and 21% at 2 years post-therapy [28]. Notably, their study was conducted in patients with 2 previous BCG failures and utilized a 6 week induction course, followed by monthly maintenance for 12 months, a similar regimen to our study.
Combination intravesical therapies have also been examined. Sequential instillation of Mitomycin C followed by BCG was not superior to BCG monotherapy in the treatment of CIS [29]. Gemcitabine plus MMC has also been investigated with an initial report of 50% disease free rate at 18 months follow-up [30]. A larger, multi-institutional series demonstrated an initial complete response in 68% with recurrence-free-survival rates of 48% at 1 year and 38% at 2 years post treatment [14].
Currently, there is no evidence-based management algorithm for salvage intravesical therapy in NMIBC patients who fail BCG therapy. The literature above has brought to light many promising treatment regimens but the variable methodology (different patient populations, treatment administration schedules, and varying use of maintenance therapy) makes direct comparisons difficult. As such, a multi-arm randomized control trial to directly compare these treatment modalities is crucial to elucidate optimal therapies. Intravesical Gem/Doce provides another treatment option in the armamentarium of the urologist. Given the current BCG shortage in the United States, Gem/Doce may potentially provide an alternative to BCG during this time of need if BCG becomes even lessavailable.
This study is limited by its retrospective nature, moderate sized cohort, and no direct comparator arm. Further, we acknowledge that the follow-up period in our overall cohort was moderate, but comparable to prior reports. While not statistically different, equivalent effectiveness in our subgroups could also not be proven given the cohort size. Analysis of mitomycin utilization post-TURBT was unable to be performed as many patients were referred for therapy and their records did not specifically indicate usage. Given the current national BCG shortage in the United States, however, we felt it was justified to report our experience with this Gem/Doce alternative intravesical therapy.
In conclusion, sequential use of intravesical Gem/Doce in the treatment of NMIBC in patients with prior BCG failures demonstrated reasonable efficacy and tolerability in a difficult to treat population. Further longitudinal evaluation and randomized clinical trials are needed to determine the optimal treatment regimen for BCG failures.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
None.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics. CA: A Cancer Journal for Clinicians. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update European Urology. 2013;64(4):639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: A formal meta-analysis of comparative studies on recurrence and toxicity. The Journal of Urology. 2003;169(1):90–95. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 4.Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized Southwest Oncology Group Study. The Journal of Urology. 2000;163(4):1124–1129. [PubMed] [Google Scholar]
- 5.Bohle A, Bock PR. Intravesical bacille Calmette-Guerin versus mitomycin C in superficial bladder cancer: Formal meta-analysis of comparative studies on tumor progression. Urology. 2004;63(4):682–686. doi: 10.1016/j.urology.2003.11.049. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 6.Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. The Journal of Urology. 2002;168(5):1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 7.Nepple KG, Lightfoot AJ, Rosevear HM, O’Donnell MA, Lamm DL, Bladder Cancer Genitourinary Oncology Study G Bacillus Calmette-Guerin with or without interferon alpha-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. The Journal of Urology. 2010;184(5):1915–1919. doi: 10.1016/j.juro.2010.06.147. [DOI] [PubMed] [Google Scholar]
- 8.Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): update. The Journal of Urology. 2007;178(6):2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Di Stasi SM, Giannantoni A, Stephen RL, Capelli G, Navarra P, Massoud R, et al. Intravesical electromotive mitomycin C versus passive transport mitomycin C for high risk superficial bladder cancer: A prospective randomized study. The Journal of Urology. 2003;170(3):777–782. doi: 10.1097/01.ju.0000080568.91703.18. [DOI] [PubMed] [Google Scholar]
- 10.Gofrit ON, Shapiro A, Pode D, Sidi A, Nativ O, Leib Z, et al. Combined local bladder hyperthermia and intravesical chemotherapy for the treatment of high-grade superficial bladder cancer. Urology. 2004;63(3):466–471. doi: 10.1016/j.urology.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 11.Catalona WJ, Hudson MA, Gillen DP, Andriole GL, Ratliff TL. Risks and benefits of repeated courses of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer. The Journal of Urology. 1987;137(2):220–224. doi: 10.1016/s0022-5347(17)43959-0. [DOI] [PubMed] [Google Scholar]
- 12.Holzbeierlein JM, Waldsachs Isett W. Supply and demand: Dealing with drug shortages. Global Connections. 2013. pp. 4–8.
- 13. American Urological Association. Policy Blog: Important Updates on BCG Availability 2014 [cited 2014 September 9th]. Available from: http://www.auanet.org/advocacy/policy-blog.cfm
- 14.Lightfoot AJ, Breyer BN, Rosevear HM, Erickson BA, Konety BR, O’Donnell MA. Multi-institutional analysis of sequential intravesical gemcitabine and mitomycin C chemotherapy for non-muscle invasive bladder cancer. Urologic Oncology. 2014;32(1):35 e15–35 e19. doi: 10.1016/j.urolonc.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donnell MA, Boehle A. Treatment options for BCG failures. World J Urol. 2006;24(5):481–487. doi: 10.1007/s00345-006-0112-0. [DOI] [PubMed] [Google Scholar]
- 16. Eli Lilly and Company. Gemzar (gemcitabine for injection, USP) [Package Insert]. Indianapolis, IN: Eli Lilly and Company; 2014 [cited 2014 September 18, 2014]. Available from: http://www.gemzar.com
- 17. Sanofi-Aventis U.S. LLC. Taxotere [Package Insert]. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2013 [cited 2014 September 18, 2014]. Available from: http://products.sanofi.us/Taxotere/taxotere.html
- 18.Lightfoot AJ, Rosevear HM, Nepple KG, O’Donnell MA. Role of routine transurethral biopsy and isolated upper tract cytology after intravesical treatment of high-grade non-muscle invasive bladder cancer. International Journal of Urology: Official Journal of the Japanese Urological Association. 2012;19(11):988–993. doi: 10.1111/j.1442-2042.2012.03089.x. [DOI] [PubMed] [Google Scholar]
- 19.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2001;19(3):666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 20.Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Algaba F, Vicente-Rodriguez J. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. The Journal of Urology. 2000;164(3 Pt 1):680–684. doi: 10.1016/s0022-5347(05)67280-1. [DOI] [PubMed] [Google Scholar]
- 21.Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? The Journal of Urology. 2001;166(4):1296–1299. [PubMed] [Google Scholar]
- 22.Joudi FN, Smith BJ, O’Donnell MA, National BCGIPIG. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urologic Oncology. 2006;24(4):344–348. doi: 10.1016/j.urolonc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 23.Malmstrom PU, Wijkstrom H, Lundholm C, Wester K, Busch C, Norlen BJ. 5-year followup of a randomized prospective study comparing mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinoma. Swedish-Norwegian Bladder Cancer Study GrouThe Journal of Urology. 1999;161(4):1124–1127. [PubMed] [Google Scholar]
- 24.Dinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guerin. Urologic Oncology. 2013;31(8):1635–1642. doi: 10.1016/j.urolonc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg G, Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M. Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study GrouThe Journal of Urology. 2000;163(3):761–767. [PubMed] [Google Scholar]
- 26.McKiernan JM, Masson P, Murphy AM, Goetzl M, Olsson CA, Petrylak DP, et al. Phase I trial of intravesical docetaxel in the management of superficial bladder cancer refractory to standard intravesical therapy. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2006;24(19):3075–3080. doi: 10.1200/JCO.2005.03.1161. [DOI] [PubMed] [Google Scholar]
- 27.Barlow LJ, McKiernan JM, Benson MC. Long-term survival outcomes with intravesical docetaxel for recurrent nonmuscle invasive bladder cancer after previous bacillus Calmette-Guerin therapy. The Journal of Urology. 2013;189(3):834–839. doi: 10.1016/j.juro.2012.10.068. [DOI] [PubMed] [Google Scholar]
- 28.Skinner EC, Goldman B, Sakr WA, Petrylak DP, Lenz HJ, Lee CT, et al. SWOG SPhase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guerin. The Journal of Urology. 2013;190(4):1200–1204. doi: 10.1016/j.juro.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oosterlinck W, Kirkali Z, Sylvester R, da Silva FC, Busch C, Algaba F, et al. Sequential intravesical chemoimmunotherapy with mitomycin C and bacillus Calmette-Guerin and with bacillus Calmette-Guerin alone in patients with carcinoma in situ of the urinary bladder: Results of an EORTC genito-urinary group randomized phase 2 trial 3) European Urology. 2011;59(3):438–446. doi: 10.1016/j.eururo.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 30.Breyer BN, Whitson JM, Carroll PR, Konety BR. Sequential intravesical gemcitabine and mitomycin C chemotherapy regimen in patients with non-muscle invasive bladder cancer. Urologic Oncology. 2010;28(5):510–514. doi: 10.1016/j.urolonc.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]