Abstract
Recently, immunotherapy with checkpoint inhibitors has been showing promise in clinical trials for stage IV bladder cancer. Herein, we review the literature regarding the role for radiation therapy, the role for immunotherapy, and the potential synergy of these treatments combined in bladder cancer. There is ample pre-clinical data in a number of different tumor models, coupled with a growing body of clinical evidence in melanoma and other malignancies to suggest combining radiation and immunotherapy could lead to substantial advances in treatment outcomes for bladder cancer. Yet, these data for bladder cancer remain at the pre-clinical stage, and further study is needed.
Keywords: Immunotherapy, radiation therapy, bladder cancer, checkpoint inhibitor, BCG, bladder-sparing therapy
ROLE OF RADIATION IN BLADDER CANCER TREATMENT
The standard of care for localized muscle-invasive bladder cancer (MIBC) is a consideration of neoadjuvant chemotherapy followed by radical cystectomy [1, 2]. The potential morbidity of this approach is well described [3, 4], and increases with age [5]. The effectiveness of trimodality therapy (transurethral resection of bladder tumor (TURBT) followed by chemoradiation) as a viable alternative to upfront cystectomy for selected patients with MIBC and for those either unwilling or unable to undergo surgery has also been evaluated [6–9]. Despite the lack of completed studies randomizing patients to either trimodality therapy or cystectomy, the available data suggests that outcomes can be similar between the two treatment approaches at comparable stages [10, 11]. A recent RTOG pooled analysis with a median follow-up of 7.8 years among survivors demonstrated a complete response (CR) rate to trimodality therapy of 72% [12], and a Massachusetts General Hospital study looking at long-term outcomes for trimodality therapy in MIBC showed a CR rate in 72% (78% in cT2) [13]. A small study also demonstrated that trimodality therapy for T2 recurrence after bacillus Calmette-Guérin (BCG) failure is viable with actuarial disease-specific survival (DSS) of 77% at 5 years and 70% at 7 years [14]. Furthermore, according to another RTOG pooled analysis, the reported rates of late pelvic toxicity following bladder-sparing therapy are low [15], and a quality of life (QOL) study based on patient reported data demonstrated high QOL among long-term survivors [16]. Indeed, the data seem encouraging that chemoradiation could play an important role in bladder cancer therapy for appropriately selected patients [6], and given these data it would seem only natural to explore new combinations with novel emerging immunotherapeutics.
The current recommendation for non-muscle invasive bladder cancer (NMIBC) including Ta, T1, and carcinoma in-situ (CIS) is a TURBT followed by intravesicular chemotherapy or BCG with BCG recommended as the first line for CIS and a favored option for high-grade Ta and T1 disease [17, 18]. Data from the Surveillance, Epidemiology and End Results (SEER)-Medicare database has shown that BCG reduces bladder cancer deaths by 23% for patients with high-grade tumors [19]. In the absence of adjuvant therapy, the risk of progression to T2 or greater disease at 3 years approaches 50% [20, 21].
Despite the well-established benefit of BCG therapy, a substantial proportion of patients who receive it will still develop BCG refractory disease [21–23]. In these cases the standard recommendation has been cystectomy. As a potential alternative, Weiss et al. demonstrated an 88% complete response rate with radiotherapy following TURBT for high risk T1 disease [24], and currently RTOG 0926 is investigating the effectiveness of trimodality therapy in recurrent BCG refractory T1 disease. In fact, prior to the widespread use of BCG, radical radiotherapy was used to treat T1 disease, but this fell out of favor following a phase III comparison between radiotherapy alone versus conservative measures (including BCG) that showed no difference in overall or progression free survival [25, 26]. NCCN guidelines currently dictate that radiation therapy can be considered for poor surgical candidates with recurrent T1 disease [1]. As will be described later, radiation in combination with other immunomodulatory drugs, in fact, may be the ideal treatment for restimulating an immune system that is no longer functionally responsive to BCG.
IMMUNOTHERAPY FOR BLADDER CANCER
An association between febrile illness and cancer regression has been known for centuries [27]. However, it was not until the 19th century that surgical oncologist William Coley demonstrated, using a systematic approach of injecting a mixture of heat-killed Streptococcus pyogenes and Serratia marcescens into 210 patients with soft-tissue sarcomas, that the immune system’s impact could be more accurately quantified. A response was observed in 60 patients, but it would take time before cancer immunotherapy gained broader mainstream acceptance.
Tumor growth requires modulation and suppression of the immune system. It is known that the incidence of malignancies increases in immunosuppressed individuals [28, 29]. In addition, the immunosurveillance hypothesis, in which organisms are under constant thymic-dependent surveillance for neoplastic growth originally postulated by F. MacFarlane Burnett, has gained added traction with later supporting studies [29–32]. This idea was further modified by Robert Schreiber who described a process of “immunoediting”. According to Schreiber, a malignancy undergoes three phases before becoming clinically relevant: 1) the elimination phase or the classical concept of immunosurveillance; 2) the equilibrium phase in which a metastable state is reached between tumor and immune system; and 3) the escape phase in which the tumor has developed immuno-evasive and suppressive mechanisms under the harsh evolutionary pressure of the immune system, thereby allowing it to grow unencumbered [33, 34]. To effectively harness the immune system against cancer these latter evolved barriers must be overcome.
Overcoming the high threshold of tumor immune tolerance is the goal of immunotherapy. There are a number of approaches which range from improving endogenous tumor antigen presentation and lymphocyte activation to ex-vivo manipulation of lymphocytes and re-infusion [35, 36]. Improved antigen presentation occurs with increased tumor antigen liberation and the endogenous production or exogenous addition of adjuvants necessary to fully mature/activate dendritic cells. BCG, a robust adjuvant and a product not dissimilar to the original Coley’s toxin, is the forerunner to all other immunotherapy being trialed for NMIBC. Its effective application is already an indication that influencing T-cell responses can impact bladder cancer’s risk of recurrence. In fact, in one study it was shown that a febrile response to BCG was a good prognostic factor and correlated with longer recurrence free survival [37]. Urinary IL-2, a potent T-cell mitogen, and IL-2 production by peripheral blood lymphocytes (PBL) also were prognosticators of a good response to BCG. Thalmann et al. demonstrated that patients with elevated urinary IL-18, a molecule critical for effective TH1 responses, have significantly longer disease free survival [38]. Using a mouse model, Biot et al. suggested that T-cell memory is likely involved in robust anti-tumor activity following intravesicular BCG administration as prior exposure to BCG enhances the anti-tumor immune response. The authors show that the recruitment of inflammatory monocytes into the bladder was abrogated by CD4 and CD8 T-cell depletion prior to intravesicular BCG instillation. Additionally, repeated BCG instillations also increased the numbers of T-cells infiltrating the bladder. Finally, the authors show in a clinical series of 55 patients, who were stratified by PPD positivity before intravesicular BCG therapy, that prior exposure to mycobacterium confers asignificantly better recurrence-free survival. This again indicates that a likely anamnestic response is at work [39]. All these observations are consistent with Sharma et al. who previously demonstrated that higher numbers of CD8 + tumor-infiltrating lymphocytes in muscle invasive disease correlates with superior disease-free and overall survival [40]. Alternatively, other studies have shown that certain genetic signatures can also be predictive of a BCG response [41, 42], specifically polymorphisms in oxidative stress genes [43]. Despite BCG being an established adjuvant for bladder cancer, BCG failure remains a concern.
BCG failure can occur due to patient intolerance to the side effects or tumor resistance to or recurrence following the treatment. BCG intolerance is relatively common, occurring in 20% of patients during maintenance therapy. These patients then require intravesicular therapy with an alternate agent [44, 45]. In contrast, patients who were able to tolerate BCG and had an initial response were reported to have a 38.6% recurrence rate after a median follow-up of 26 months [46]. BCG unresponsiveness is defined as persistent high grade tumor at 6 months or a recurrence within 6 months or less after achieving a disease-free state [47, 48]. Herr and Dalbagni showed that at 6 months 20% of BCG treated patients had persistent or recurrent tumors. In addition to the cytokines described earlier that correlate with a good response to BCG, there is evidence to suggest an aberrant TH2 response instead of a robust TH1 response is associated with failure[49, 50].
Alternative approaches to cystectomy have been examined following BCG failure. Interferon-α2b (IFN-α2b) in combination with BCG was studied in a multicenter Phase II trial including 231 patients with BCG failure. These patients were treated with a 6-week induction course of BCG and IFN-α, followed by 3 additional treatments. With a median follow-up of 2 years, 57.3% of BCG naïve patients and 42% of the patients with prior BCG failure remained tumor free [51]. However, one randomized trial examining BCG combined with IFN-α2b did not show superiority compared to BCG alone for BCG-naïve patients [52, 53]. Nevertheless, IFN-α2b may still be effective in BCG failure patients because it has been shown in-vitro to inhibit production of IL-10, an immunosuppressive cytokine, allowing potentiation of the TH1 response [54]. IL-10 has been shown to impair the antitumor activity of intravesicular BCG therapy with IL-10 knockout mice demonstrating enhanced delayed-type hypersensitivity and an enhancement of the antitumor response [55]. Finally combined BCG and IL-12, in an effort to skew the immune response towards a dominant TH1 phenotype, appears to have some potential efficacy in animal models [56].
Bladder cancer along with melanoma and non-small cell lung cancer represent good candidates for cell-mediated immunotherapy due to their relatively high somatic mutation rates [57]. This generates many novel tumor antigens [57, 58] that are critical for an effective immune response because it creates a variety of epitopes that may differ from native peptides and to which T-cells have not been negatively selected. For effective T-cell activation, T-cells require three signals: 1) effective TCR ligation by its cognate MHC-antigen complex, 2) co-stimulation through CD28 on the T-cell membrane, and 3) a cytokine milieu that determines the T-cells’ phenotype. There are also negative regulators of T-cell activation including CTLA-4 and PD-1 which increase the T-cell activation threshold or impair its effector function. Current immunotherapy has focused on disrupting these negative regulators, termed checkpoint blockade, thereby increasing the chance for a functional adaptive immune response. Another group of molecules which include OX40 (CD134) and CD40 are activating receptors on T-cells and antigen presenting cells (APC) respectively. These are also being targeted with agonistic antibodies to promote an anti-tumor response. These biologics are an exciting addition to bladder cancer given the lack of effective therapeutic options for advanced disease [59, 60]. Initial results are encouraging and are examined in more detail here [61].
The newer checkpoint inhibitors improve treatment outcomes for localized as well as metastatic disease. Checkpoint blockade of CTLA-4 using ipilimumab, first approved by the FDA in 2011, has demonstrated encouraging results for unresectable and metastatic melanoma [62, 63]. This drug acts at the initial point of T-cell activation, recruiting T-cells to the immune response that would not normally have received the threshold trigger for activation. Ipilimumab has also been shown to impact regulatory T-cell number and function in a few pre-clinical studies [64, 65]. Carthon et al. at MD Anderson showed in a small dose escalation study of ipilimumab for localized urothelial carcinoma of the bladder that there was limited toxicity. They also noted an increased frequency of CD4 + ICOShigh (activated T-cells) in the systemic circulation of these patients [66]. There is currently an on-going phase II clinical trial (NCT01524991) examining the combination of gemcitabine, cisplatin and ipilimumab for metastatic urothelial carcinoma with a primary outcome measurement of one-year overall survival.
The standard of care for stage IV bladder cancer remains platinum-based chemotherapy regimens- methotrexate, vinblastine, doxorubicin, cisplatin (MVAC), high dose MVAC and methotrexate, cisplatin and vinblastin (MCV) -which have demonstrated a survival benefit. The 5-year overall survival for MVAC is 15.3% [67]. Additionally, MVAC is associated with a substantial toxic death rate and toxicities that can reach 4% [68]. Any addition to our armamentarium has the potential for lasting consequences, and disruption of the PD-1/PD-L1 axis appears likely to be one of the most promising recent developments. PD-1, another negative regulator of T-cell activation, can encounter its ligand, PD-L1, in multiple places including tumor cells and tumor-infiltrating immune cells. A phase I trial for lambrolizumab (anti-PD-1) in advanced melanoma enrolled 135 patients, 48 of whom had received prior treatment with ipilimumab. 13% of patients reported grade 3 or 4 toxicity, and the overall response rate (ORR) was 44% which did not vary with prior exposure to ipilimumab. Regressing lesions which were biopsied showed densely infiltrated CD8 + T-cells [69]. Data from Tumeh et al., suggests that pre-existing intratumoral CD8 + T-cells are responsible for tumor regression in advanced melanoma following PD-1 blockade [70]. The authors were able to develop a predictive model for response to PD-1 inhibition based on CD8 + T-cell density at the tumor invasive margin. This was validated in an independent 15 patient cohort. These studies of PD-1/PD-L1 disruption have been extended to bladder cancer.
Recent data from Powles et al. demonstrated in an expanded phase I trial with 67 metastatic bladder cancer patients treated with MPDL3280A (anti-PD-L1) that there was an objective response rate of 11% for patients with PD-L1 negative/low and 43% for PD-L1 intermediate/high expressing tumor-infiltrating immune cells. Interestingly, in this study the response rate was dependent on PD-L1 expression by the tumor infiltrating immune cells (likely myeloid-derived suppressor cells) rather than the tumor cells themselves [61, 71]. This has also been seen in non-small cell lung cancer and renal cell carcinoma [72]. An abstract presented at the European Society for Medical Oncology in 2014 showed encouraging results for pembrolizumab (anti-PD-1) for patients with advanced urothelial tumors [73]. Another recently published study from Harvard, showed that expression of PD-L1 by tumor-infiltrating mononuclear cells (TIMCs) correlated with improved overall survival even in patients treated only with platinum-based chemotherapy. This is consistent with the notion that PD-L1 expression by TIMCs is induced following intratumoral T-cell activation and cytokine production [74]. Of note, it has also been reported that PD-L1 expression is dynamic and PD-L1 upregulation may be an effective surrogate marker for T-cell activation and lytic potential [75]. There are a number of clinical trials (NCT02302807, NCT02108652) still recruiting which are testing the role of MPDL3280A in locally advanced and metastatic bladder carcinoma, in addition to trials testing anti-PD-1 inhibitors (NCT02324582).
Another approach being examined for a variety of metastatic malignancies including bladder cancer is the use of a fusion protein consisting of IL-2 and a humanized soluble T-cell receptor directed against p53 derived epitopes (ALT-801). A number of malignancies, including bladder cancer, overexpress p53 and therefore this approach confers a level of specificity [76, 77]. A phase I trial with a heterogeneous collection of advanced malignancies demonstrated reasonable tolerability and encouraging efficacy [78]. Currently there are trials (NCT01625260) examining ALT-801 in BCG refractory NMIBC or an engineered IL-15 super-agonist (ALT-803) in combination with BCG (NCT02138734) also for NMIBC. Another interesting approach involves an engineered adenovirus, CG0070, which preferentially replicates in RB protein-defective cells and carries a granulocyte macrophage colony-stimulating factor (GM-CSF) gene [79, 80]. This virus is postulated to have direct oncolytic activity in addition to the GM-CSF mediated immunostimulatory effects. In a preliminary phase I trial, patients with BCG failure received single or multiple intravesicular instillations of the virus. An 81.8% response rate was observed in those patients with borderline or high RB phosphorylation who received a multidose regimen. The complete response rate across all cohorts was 48.6% [81]. A phase II/III trial (NCT01438112) evaluating the virus in patients with BCG failure is ongoing. Given these exciting results, it seems reasonable to combine these immunotherapeutics with other immunomodulatory modalities like radiation.
IMMUNOLOGIC EFFECTS OF RADIATION THERAPY IN BLADDER CANCER
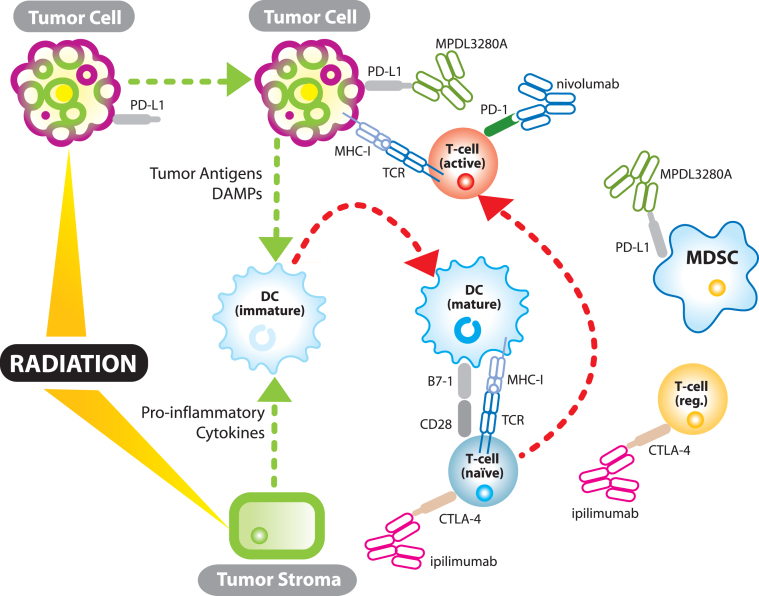
Much has been written recently about the immunostimulatory effects of radiation [82–84] (see Fig. 1). Although still somewhat controversial, there is a growing body of evidence that immunogenic cell death induced by radiation liberates tumor antigens along with endogenous adjuvants promoting APC maturation and effective cytotoxic T-cell priming [85]. Radiation induced immunogenic cell death is characterized, in part, by activation of the endoplasmic reticulum (ER) stress pathway and tumor cell surface expression of calreticulin (CRT), a pro-phagocytic molecule, facilitating APC uptake of tumor antigens [86]. In addition, passive secretion of HMGB1, an evolutionarily conserved nuclear protein, by tumor cells undergoing late apoptosis or necrosis can bind toll-like receptor 4 (TLR-4), the lipopolysaccharide (LPS) receptor, on dendritic cells (DC) and lead to DC maturation and effective T-cell priming [87, 88]. Radiation has also been shown to promote effector T-cell recruitment into the tumor through chemokine induction and upregulation of tumor cell MHC-I expression. Prior to RT, many tumors secrete chemokines which recruit immunosuppressive cells including regulatory T-cells [89, 90]. However, radiation in a 4T1 mouse breast cancer model induces CXCL16 production, a chemokine which recruits effector T-cells and whose expression correlates with increased numbers of tumor-infiltrating lymphocytes and improved survival [91, 92]. Radiation also induces a bystander effect influencing tumor cell survival through its effects on stromal components. The bystander effect is largely mediated by stromal cell-cell communication through gap junctions or production of soluble mediators including tumor necrosis factor-α which may help mature APCs for effective antigen presentation [93, 94]. Finally, and perhaps most importantly, there is data that demonstrates radiation and checkpoint inhibitors activate the immune system through two non-redundant mechanisms [65]. Twyman Saint-Victor et al. show that radiation enhances the diversity of the T-cell receptor repertoire intratumorally while anti-CTLA4 promotes expansion of effector T-cells, and anti-PD-L1 reverses T-cell exhaustion. It is these non-overlapping mechanisms that make clinical synergy so exciting.
Fig.1.
The synergistic relationship between immunotherapy and radiation. TCR – T-cell receptor; MHC-I – major histocompatability complex 1; DC – dendritic cell; MDSC – myeloid derived suppressor cell; DAMPs- damage associated molecular patterns (endogenous adjuvants which mature dendritic cells); T-cell (reg.) – regulatory T-cell.
In the specific context of urinary bladder carcinoma, O’Toole et al. used an interesting technique to evaluate the effect of tumor irradiation on PBL cytotoxicity. In this study, patients with T1-T4 bladder cancer received radiation using a three-field technique (two anterior wedge fields and an open posterior field). Prior to, during, and following radiation, patient PBL were collected and cultured with target cells to assess cytotoxicity, and it was shown that two of three patients with T3 cancer who were negative for PBL cytotoxic activity prior to radiation were positive afterwards [95]. In a follow-up study, the authors demonstrated that the patients who were clinically tumor free 5 years after treatment all had a more rapid post-radiotherapy increase in lymphocyte numbers than the patients who recurred again suggesting that adaptive immunity plays a role in bladder cancer control [96].
There is a subsequent report that irradiation of bladder carcinoma cell lines may enhance their susceptibility to NK cell mediated killing. Mizutani et al. showed that T24, a known NK cell resistant bladder cancer cell line, was increasingly sensitive to lysis following as little as 1 Gy of radiation. This effect is likely not antigen specific, but further suggests the immuno-enhancing effects of ionizing radiation in bladder cancer [97]. More recently, Demaria et al. demonstrated in a pre-clinical model that if a DC growth factor was administered to mice bearing a syngeneic mammary carcinoma, there was regression of tumor cells outside of the radiation field (abscopal effect) [98]. In a follow-up “proof-of principle” clinical trial, 14 patients with advanced metastatic disease, including bladder cancer, were treated with GM-CSF and received radiation to one lesion at 3.5 Gy×10 fractions over 2 weeks. The overall results were encouraging with 30% achieving an abscopal response, while others showed a decreased SUV of non-irradiated lesions [99].
Admittedly, at this time most studies examining this exciting synergy between radiation and immunotherapy are not focused on urinary bladder cancer (Fig. 1). In patients with metastatic cutaneous melonoma there have been a number of case studies reporting encouraging results after receiving a CTLA-4 antagonist and radiation [100, 101]. A report out of Memorial Sloan Kettering followed a 39 year old woman with diffuse disease who received palliative radiation to a paraspinal metastasis while also receiving ipilimumab. Four months following treatment, imaging showed regression of both the irradiated lesion and unirradiated distant metastases [101]. NCT01689974 is a phase II trial evaluating ipilimumab alone versus ipilimumab and radiation in metastatic melanoma. A number of clinical trials are investigating this interaction in a variety of other malignancies including non-small cell lung cancer (NCT02221739) and head and neck cancer (NCT01935921). NCT00861614 is a completed phase 3 trial evaluating radiation and ipilimumab for castration-resistant prostate cancer. The primary outcome measure was overall survival and it compared ipilimumab plus radiation to radiation alone for patients that had received prior treatment with docetaxel. Results published in 2014 demonstrated no statistically significant (p-value = 0.053) difference between the ipilimumab and placebo groups, however, the authors do state the overall survival hazard ratio decreased over time and ipilimumab at later time points correlated with improved survival relative to placebo [102]. Additionally, a subgroup analysis of patients with more favorable prognostic features including an alkaline phosphatase less than 1.5 times the upper limit of normal, a hemoglobin concentration of 110 g/l or higher and no visceral metastases showed a median overall survival with ipilimumab of 22.7 months compared to 15.8 months with placebo and a p-value of 0.0038.
The PD-1 axis inhibitors and radiation are being evaluated in pre-clinical models with data suggesting improved local tumor control [103] and this interaction is also being investigated in some upcoming clinical trials (Table 1). As a parallel, there have also have been a number of trials demonstrating an improved response rate when multiple immunomodulatory drugs are combined in renal cell carcinoma and melanoma- perhaps physiologically analogous to a combined radiation/immunotherapeutic approach [104, 105]. Results from clinical trials evaluating radiation therapy and PD-1/PD-L1 blockade are still outstanding.
Table 1.
A sampling of combination trials
| Clinical trials. gov identifier |
Sponsor | Immunotherapy | Radiotherapy | Treatment timing | Phase | Condition |
| NCT01436968 | Advantagene, Inc | ProstAtak (AdV-tk) injected into prostate | Standard of care | Radiation 0–3 days after second AdV-tk injection | 3 | Prostate Cancer |
| NCT00751270 | Advantagene, Inc | AdV-tk injected into tumor/ tumor bed | Standard of care | Radiation 3–7 days following AdV-tk injection | 1b | Malignant Glioma |
| NCT00589875 | Advantagene, Inc | AdV-tk injected into tumor bed | Standard of care | Radiation 3–7 days following AdV-tk injection | 2a | Malignant Glioma |
| NCT00634231 | Advantagene, Inc | AdV-tk injected into tumor/ tumor bed | Standard of care | Radiation 3–7 days following AdV-tk injection | 1 | Pediatric Brain Tumors |
| NCT00589875 | Advantagene. Inc | AdV-tk injected into tumor bed | Standard of care | Radiation 3–7 days following AdV-tk injection | 2 | Malignant Glioma |
| NCT01836432 | NewLink Genetics Corporation | Algenpantucel-L (HAPa1, HAPa2) | 50.4 Gy in 28 fractions | Radiation and immunotherapy on day 1. | 3 | Pancreatic Cancer |
| NCT01896271 | University of Texas Southwestern Medical Center | High dose IL-2 | Stereotactic ablative radiation therapy (SART) 8–20 Gy in 1–3 fractions | IL-2 administered immediately following radiation | 2 | Metastatic Renal Cancer |
| NCT01497808 | Abramson Cancer Center of the University of Pennsylvania | Ipilimumab | Dose escalation for Stereotactic body radiation therapy (SBRT) | Not described | 1/2 | Metastatic Melanoma |
| NCT02303990 | Abramson Cancer Center of the University of Pennsylvania | Pembrolizumab (anti-PD-1) | Hypofractionated radiation | Not described | 1 | Metastatic Cancers |
| NCT02086721 | Maastricht Radiation Oncology | L19-IL-2 | Patients receive a schedule of 1×30 Gy, 3×15–20 Gy; 5×12 Gy; 8×7.5 Gy | L19-IL-2 given one week after completion of radiation | 1 | Oligometastatic Solid Tumors |
| NCT01935921 | National Cancer Institute | Ipilimumab | Standard of care | Ipilimumab started at beginning of week 4 of cetuximab course, given 3 courses total | 1 | Stage III-IVB Head and Neck Cancer |
| NCT02298946 | National Cancer Institute | AMP-224 (PD-1 inhibitor) | Stereotactic body radiation therapy | Radiation day 0, AMP-224 on day 1 then q14 days | 1 | Metastatic Colorectal Cancer |
| NCT02239900 | M.D. Anderson Cancer Center | Ipilimumab | Depends on group- SBRT 50 Gy in 4 fractions (group 1) | Depends on group - Radiation days 1–4 of cycle 1 of ipilimumab (group 1) | 1/2 | Advanced Solid Tumors |
| NCT01689974 | New York University School of Medicine | Ipilimumab | 6 Gy×5 IMRT | Ipilimumab first given day 4 after radiation | 2 | Metastatic Melanoma |
| NCT02221739 | New York University School of Medicine | Ipilimumab | 6 Gy×5 IMRT | Ipilimumab first given within 24 hours of starting radiation | 2 | Metastatic Non-Small Cell Lung Cancer |
| NCT01276730 | James Graham Brown Cancer Center | Interferon- α2b | 40–45 Gy in conventional fx to whole pelvis | Interferon- α starts first day of radiation | 2 | Advanced Cervical Cancer |
| NCT01347034 | H. Lee Moffit Cancer and Research Institute | Intratumoral injection of autologous dendritic cells | Conventional radiation with boost | Radiation begins prior to DC injection | 2 | Soft Tissue Sarcoma |
| NCT01449279 | Stanford University | Ipilimumab | Palliative radiation | Ipilimumab given up to two days before radiation | 1 | Melanoma |
| NCT02254772 | Stanford University | TLR-9 agonist (SD-101), ipilimumab | Not specified | SD-101 given intratumoral given starting day 1 and ipilimumab given on day 1, radiation on days 1,2 | 1/2 | Low-Grade Recurrent B-Cell Lymphoma |
| NCT01565837 | Wolfram Samlowski | Ipilimumab | Stereotactic ablative radiation | Radiation to 1–5 lesions after initial dose of ipilimumab | 2 | Melanoma |
| NCT01758458 | Fred Hutchison Cancer Research Center | MCPyV TAg-specific polyclonal autologous CD8-positive T-cell vaccine | Unspecified | Radiation is given day 2 to 4 before treatment | 1 | Merkel Cell Carcinoma |
| NCT02303366 | Peter MacCallum Cancer Center, Australia | MK-3475 (anti-PD-1) | Stereotactic ablative radiation | Radiation followed by 8 cycles of MK-3475 | 1 | Oligometastatic Breast Neoplasia |
| NCT02318771 | Thomas Jefferson University | MK-3475 | 8 Gy×1 | Radiation day 1, MK-3475 3–17 days later | 1 | Many Advanced Cancers |
| NCT01703507 | Thomas Jefferson University | Ipilimumab | Whole brain or stereotactic radiation | Receive ipilimumab 4× over 10 weeks. WBRT done for weeks 1-2. | 1 | Melanoma with Brain Metastases |
There is no overriding consensus about the timing of radiation relative to immunotherapy although initial data seems to support concurrent administration. In a pre-clinical trial, commencing anti-PD-L1 administration on either the first or the last day of radiation had equivalent overall survival, but both were superior to commencing PD-L1 blockade 7 days after radiation completion [106]. Administering radiation following immunotherapy could potentially blunt an already active response [84]. There is also limited data with regards to optimal dose and fractionation of the radiation with immunotherapy. For induction of an abscopal effect, preclinical data suggests a fractionated regimen is superior to a single dose: 24 Gy in 3 is superior to 30 Gy in 5 which is superior to 20 Gy in 1 fraction [107, 108]. Clinical data has demonstrated abscopal effects for a variety of different fractionation regimens including 8 Gy in one and 30 Gy in five fractions [107, 109, 110]. Although specific dosing information has yet to be fully established, data again from pre-clinical studies also suggests that increasing dose leads to increased interferon-γ production and improved antigen presentation, the caveat being that at higher doses there appears to be a concurrent rise in regulatory T-cells. Currently, when evaluating the data in totality, it is difficult to make a specific recommendation, but a dose of 8 Gy in one fraction up to 24 Gy in three fractions given with a concurrent checkpoint inhibitor appears to be the most well supported treatment regimen based on the above preclinical data and the encouraging results from NCT00861614 (ipilimumab with radiation for castration-resistant prostate cancer) which gave radiation within 2 days of initiating ipilimumab.
Melanoma has been at the forefront of the radio-immunotherapy clinical trials but it is now time to incorporate urinary bladder cancer. It was one the first malignancies in which an effective immunotherapy was utilized, and it behooves us to examine potential synergy with radiation. There are numerous contexts in which this combination therapy could be applied including BCG refractory NMIBC. A new phase of RTOG 0926 could include a checkpoint inhibitor and an immune activating dose of radiation (such as 8Gy x 1). Alternatively, further investigation may yield a novel hypofractionated regimen that in combination with immunotherapy optimizes radiation antigenicity. Immunotherapy could also be added as an upfront component of trimodality therapy for MIBC, or for use in salvage following trimodality therapy failure in MIBC or in non-muscle invasive disease. Finally, immunotherapy may even have an application in combination with radiation for metastatic disease to evaluate the likelihood of an abscopal response.
CONCLUSION
In conclusion, both radiation and immunotherapy are playing an increasingly important role in a number of malignancies including bladder cancer. It is evident that immunotherapy has great potential to improve survival for patients with both localized and advanced disease. This potential may be improved even further if these novel immunotherapeutic modalities are combined with radiation. Using melanoma as the model, there seems to be reason for great excitement for the future of bladder cancer therapy.
REFERENCES
- 1.National Comprehensive Cancer Network [NCCN.org]. Bladder Cancer; 2015 [cited 2015 Feb 9]. Available from: http://www.nccn.org/professionals/physician gls/pdf/bladder.pdf
- 2.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP, Jr, Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 3.Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, Raj G, Bochner BH, Dalbagni G, Herr HW, Donat SM. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55(1):164–174. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Bochner BH, Dalbagni G, Sjoberg DD, Silberstein J, Keren Paz GE, Donat SM, Coleman JA, Mathew S, Vickers A, Schnorr GC, Feuerstein MA, Rapkin B, Parra RO, Herr HW, Laudone VP. Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: A Randomized Clinical Trial. Eur Urol. 2014. Dec 8 [cited Feb 10] [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 5.Froehner M, Brausi MA, Herr HW, Muto G, Studer UE. Complications following radical cystectomy for bladder cancer in the elderly. Eur Urol. 2009;56(3):443–454. doi: 10.1016/j.eururo.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Gakis G, Efstathiou JA, Lerner SP, Cookson MS, Keegan KA, Guru KA, Shipley WU, Heidenreich A, Schoenberg MP, Sagaloswky AI, Soloway MS, Stenzl A, International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder C ICUD-EAU International Consultation on Bladder Cancer 2012: Radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63(1):45–57. doi: 10.1016/j.eururo.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman DS, Shipley WU, Griffin PP, Heney NM, Althausen AF, Efird JT. Selective bladder preservation by combination treatment of invasive bladder cancer. N Engl J Med. 1993;329(19):1377–1382. doi: 10.1056/NEJM199311043291903. [DOI] [PubMed] [Google Scholar]
- 8.Ploussard G, Daneshmand S, Efstathiou JA, Herr HW, James ND, Rodel CM, Shariat SF, Shipley WU, Sternberg CN, Thalmann GN, Kassouf W. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: A systematic review. Eur Urol. 2014;66(1):120–137. doi: 10.1016/j.eururo.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 9.James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, Crundwell M, Sizer B, Sreenivasan T, Hendron C, Lewis R, Waters R, Huddart RA, Investigators BC. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366(16):1477–1488. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 10.Chen RC, Shipley WU, Efstathiou JA, Zietman AL. Trimodality bladder preservation therapy for muscle-invasive bladder cancer. J Natl Compr Canc Netw. 2013;11(8):952–960. doi: 10.6004/jnccn.2013.0116. [DOI] [PubMed] [Google Scholar]
- 11.Huddart RA, Hall E, Lewis R, Birtle A, Group STM. Life and death of spare (selective bladder preservation against radical excision): Reflections on why the spare trial closed. BJU Int. 2010;106(6):753–755. doi: 10.1111/j.1464-410X.2010.09537.x. [DOI] [PubMed] [Google Scholar]
- 12.Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, Kaufman DS, Heney NM, Zietman AL. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: A pooled analysis of Radiation Therapy Oncology Group protocols and J Clin Oncol. 2014;32(34):3801–3809. doi: 10.1200/JCO.2014.57.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efstathiou JA, Spiegel DY, Shipley WU, Heney NM, Kaufman DS, Niemierko A, Coen JJ, Skowronski RY, Paly JJ, McGovern FJ, Zietman AL. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: The MGH experience. Eur Urol. 2012;61(4):705–711. doi: 10.1016/j.eururo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Wo JY, Shipley WU, Dahl DM, Coen JJ, Heney NM, Kaufman DS, Zietman AL. The results of concurrent chemo-radiotherapy for recurrence after treatment with bacillus Calmette-Guerin for non-muscle-invasive bladder cancer: Is immediate cystectomy always necessary? BJU Int. 2009;104(2):179–83. doi: 10.1111/j.1464-410X.2008.08299.x. [DOI] [PubMed] [Google Scholar]
- 15.Efstathiou JA, Bae K, Shipley WU, Kaufman DS, Hagan MP, Heney NM, Sandler HM. Late pelvic toxicity after bladder-sparing therapy in patients with invasive bladder cancer: RTOG 89-03, 95-06, 97-06, 99-06. J Clin Oncol. 2009;27(25):4055–4061. doi: 10.1200/JCO.2008.19.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mak KS, Smith A, Eidelmann A, Clayman RH, Cheng J-S, Matthews J, Niemierko A, Nielsen ME, Feldman AS, Lee RJ, Zietman AL, Shipley WU, Chen RC, Milowsky MI, Efstathiou JA. Quality of life in long-term survivors of muscle-invasive bladder cancer. ASCO Meeting Abstracts. 2015;33(7_suppl):319. [Google Scholar]
- 17.Burger M, Oosterlinck W, Konety B, Chang S, Gudjonsson S, Pruthi R, Soloway M, Solsona E, Sved P, Babjuk M, Brausi MA, Cheng C, Comperat E, Dinney C, Otto W, Shah J, Thurof J, Witjes JA, International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder C ICUD-EAU International Consultation on Bladder Cancer Non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63(1):36–44. doi: 10.1016/j.eururo.2012.08.061. [DOI] [PubMed] [Google Scholar]
- 18.Kamat AM, Flaig T, Grossman B, Konety B, Lamm D, O’Donnell M, Uchio E, Efstathiou JA, Taylor JA. Consensus statement on best practice management regarding the use of intravesicular immunotherapy with BCG for bladder cancer. Nature Reviews Urology. 2015;12(4):225–235. doi: 10.1038/nrurol.2015.58. [DOI] [PubMed] [Google Scholar]
- 19.Spencer BA, McBride RB, Hershman DL, Buono D, Herr HW, Benson MC, Gupta-Mohile S, Neugut AI. Adjuvant intravesical bacillus calmette-guerin therapy and survival among elderly patients with non-muscle-invasive bladder cancer. J Oncol Pract. 2013;9(2):92–98. doi: 10.1200/JOP.2011.000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heney NM, Ahmed S, Flanagan MJ, Frable W, Corder MP, Hafermann MD, Hawkins IR. Superficial bladder cancer: Progression and recurrence. J Urol. 1983;130(6):1083–1086. doi: 10.1016/s0022-5347(17)51695-x. [DOI] [PubMed] [Google Scholar]
- 21.Gray PJ, Shipley WU, Efstathiou JA, Zietman AL. Recent advances and the emerging role for chemoradiation in nonmuscle invasive bladder cancer. Curr Opin Urol. 2013;23(5):429–434. doi: 10.1097/MOU.0b013e328363de04. [DOI] [PubMed] [Google Scholar]
- 22.Martin FM, Kamat AM. Definition and management of patients with bladder cancer who fail BCG therapy. Expert Rev Anticancer Ther. 2009;9(6):815–820. doi: 10.1586/era.09.35. [DOI] [PubMed] [Google Scholar]
- 23.Shelley MD, Court JB, Kynaston H, Wilt TJ, Fish RG, Mason M. Intravesical Bacillus Calmette-Guerin in Ta and T1 Bladder Cancer. Cochrane Database Syst RevCD. 2000. p. 001986. [DOI] [PMC free article] [PubMed]
- 24.Weiss C, Wolze C, Engehausen DG, Ott OJ, Krause FS, Schrott KM, Dunst J, Sauer R, Rodel C. Radiochemotherapy after transurethral resection for high-risk T1 bladder cancer: An alternative to intravesical therapy or early cystectomy? J Clin Oncol. 2006;24(15):2318–2324. doi: 10.1200/JCO.2006.05.8149. [DOI] [PubMed] [Google Scholar]
- 25.Harland SJ, Kynaston H, Grigor K, Wallace DM, Beacock C, Kockelbergh R, Clawson S, Barlow T, Parmar MK, Griffiths GO, National Cancer Research Institute Bladder Clinical Studies G A randomized trial of radical radiotherapy for the management of pT1G3 NXM0 transitional cell carcinoma of the bladder. J Urol. 2007;178(3 Pt 1):807–813. doi: 10.1016/j.juro.2007.05.024. discussion 13. [DOI] [PubMed] [Google Scholar]
- 26.Gray PJ, Shipley WU, Efstathiou JA. T1 high-grade bladder cancer recurring after BCG therapy: A curative alternative to radical cystectomy exists. Con Oncology (Williston Park) 2013;27(9):873. 921. [PubMed] [Google Scholar]
- 27.Hobohm U. Fever and cancer in perspective. Cancer Immunol Immunother. 2001;50(8):391–396. doi: 10.1007/s002620100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4(6):905–913. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 29.Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB, Horowitz MM, Witherspoon RP, Hoover RN, Sobocinski KA, Fraumeni JF, Jr, Boice JD., Jr Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336(13):897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 30.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 31.Burnet FM. Immunological Factors in the Process of Carcinogenesis. Br Med Bull. 1964;20:154–158. doi: 10.1093/oxfordjournals.bmb.a070310. [DOI] [PubMed] [Google Scholar]
- 32.Burnet FM. Immunological surveillance in neoplasia. Transplant Rev. 1971;7:3–25. doi: 10.1111/j.1600-065x.1971.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 33.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 34.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saint F, Salomon L, Quintela R, Cicco A, Hoznek A, Abbou CC, Chopin DK. Do prognostic parameters of remission versus relapse after Bacillus Calmette-Guerin (BCG) immunotherapy exist? analysis of a quarter century of literature. Eur Urol. 2003;43(4):351–360. doi: 10.1016/s0302-2838(03)00048-4. discussion 60-1. [DOI] [PubMed] [Google Scholar]
- 38.Thalmann GN, Sermier A, Rentsch C, Mohrle K, Cecchini MG, Studer UE. Urinary Interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette-Guerin. J Urol. 2000;164(6):2129–2133. [PubMed] [Google Scholar]
- 39.Biot C, Rentsch CA, Gsponer JR, Birkhauser FD, Jusforgues-Saklani H, Lemaitre F, Auriau C, Bachmann A, Bousso P, Demangel C, Peduto L, Thalmann GN, Albert ML. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med. 2012;4(137):137ra72. doi: 10.1126/scitranslmed.3003586. [DOI] [PubMed] [Google Scholar]
- 40.Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ, Sato E. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007;104(10):3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lima L, Oliveira D, Ferreira JA, Tavares A, Cruz R, Medeiros R, Santos L. The role of functional polymorphisms in immune response genes as biomarkers of BCG Immunotherapy outcome in bladder cancer: Establishment of a predictive profile in a Southern Europe population. BJU Int. 2014. Jun 16 [cited 2015 Feb 15]; [Epub ahead of print] [DOI] [PubMed]
- 42.Kim YJ, Ha YS, Kim SK, Yoon HY, Lym MS, Kim MJ, Moon SK, Choi YH, Kim WJ. Gene signatures for the prediction of response to Bacillus Calmette-Guerin immunotherapy in primary pT1 bladder cancers. Clin Cancer Res. 2010;16(7):2131–2137. doi: 10.1158/1078-0432.CCR-09-3323. [DOI] [PubMed] [Google Scholar]
- 43.Wei H, Kamat A, Chen M, Ke HL, Chang DW, Yin J, Grossman HB, Dinney CP, Wu X. Association of polymorphisms in oxidative stress genes with clinical outcomes for bladder cancer treated with Bacillus Calmette-Guerin. PLoS One. 2012;7(6):e38533. doi: 10.1371/journal.pone.0038533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Meijden AP, Sylvester RJ, Oosterlinck W, Hoeltl W, Bono AV, Group EG-UTC Maintenance BacillusCalmette-Guerin for Ta T1 bladder tumors is not associated with increased toxicity: Results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase III Trial. Eur Urol. 2003;44(4):429–434. doi: 10.1016/s0302-2838(03)00357-9. [DOI] [PubMed] [Google Scholar]
- 45.Witjes JA. Management of BCG failures in superficial bladder cancer: A review. Eur Urol. 2006;49(5):790–797. doi: 10.1016/j.eururo.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: A formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169(1):90–95. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 47.Sylvester RJ, van der Meijden AP, Witjes JA, Kurth K. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: A meta-analysis of the published results of randomized clinical trials. J Urol. 2005;174(1):86–91. doi: 10.1097/01.ju.0000162059.64886.1c. discussion 91-2. [DOI] [PubMed] [Google Scholar]
- 48.Herr HW, Dalbagni G. Defining bacillus Calmette-Guerin refractory superficial bladder tumors. J Urol. 2003;169(5):1706–1708. doi: 10.1097/01.ju.0000062605.92268.c6. [DOI] [PubMed] [Google Scholar]
- 49.Zlotta AR, Fleshner NE, Jewett MA. The management of BCG failure in non-muscle-invasive bladder cancer: An update. Can Urol Assoc J. 2009;3(6 Suppl 4):S199–S205. doi: 10.5489/cuaj.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zlotta AR, Drowart A, Huygen K, De Bruyn J, Shekarsarai H, Decock M, Pirson M, Jurion F, Palfliet K, Denis O, Mascart F, Simon J, Schulman CC, Van Vooren JP. Humoral response against heat shock proteins and other mycobacterial antigens after intravesical treatment with bacille Calmette-Guerin (BCG) in patients with superficial bladder cancer. Clin Exp Immunol. 1997;109(1):157–165. doi: 10.1046/j.1365-2249.1997.4141313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Donnell MA, Lilli K, Leopold C, National Bacillus Calmette-Guerin/Interferon Phase 2 Investigator G Interim results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alfa-2b for superficial bladder cancer. J Urol. 2004;172(3):888–893. doi: 10.1097/01.ju.0000136446.37840.0a. [DOI] [PubMed] [Google Scholar]
- 52.Nepple KG, Lightfoot AJ, Rosevear HM, O’Donnell MA, Lamm DL, Bladder Cancer Genitourinary Oncology Study G Bacillus Calmette-Guerin with or without interferon alpha-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. J Urol. 2010;184(5):1915–1919. doi: 10.1016/j.juro.2010.06.147. [DOI] [PubMed] [Google Scholar]
- 53.Lamm D, Brausi M, O’Donnell MA, Witjes JA. Interferon alfa in the treatment paradigm for non-muscle-invasive bladder cancer. Urol Oncol. 2014;32(1):35 e21–35 e30. doi: 10.1016/j.urolonc.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Luo Y, Chen X, Downs TM, DeWolf WC, O’Donnell MA. IFN-alpha 2B enhances Th1 cytokine responses in bladder cancer patients receiving Mycobacterium bovis bacillus Calmette-Guerin immunotherapy. J Immunol . 1999;162(4):2399–2405. [PubMed] [Google Scholar]
- 55.Nadler R, Luo Y, Zhao W, Ritchey JK, Austin JC, Cohen MB, O’Donnell MA, Ratliff TL. Interleukin 10 induced augmentation of delayed-type hypersensitivity (DTH) enhances Mycobacterium bovis bacillus Calmette-Guerin (BCG) mediated antitumour activity. Clin Exp Immunol . 2003;131(2):206–216. doi: 10.1046/j.1365-2249.2003.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Donnell MA, Luo Y, Chen X, Szilvasi A, Hunter SE, Clinton SK. Role of IL-12 in the induction and potentiation of IFN-gamma in response to bacillus Calmette-Guerin. J Immunol. 1999;163(8):4246–4252. [PubMed] [Google Scholar]
- 57.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortes ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Lee RS, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CW, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, MD Leiserson, Miller CA, Welch JS, Walter MJ, Wendl MC, Ley TJ, Wilson RK, Raphael BJ, Ding L. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choueiri TK, Ross RW, Jacobus S, Vaishampayan U, Yu EY, Quinn DI, Hahn NM, Hutson TE, Sonpavde G, SC Morrissey, Buckle GC, Kim WY, Petrylak DP, Ryan CW, Eisenberger MA, Mortazavi A, Bubley GJ, Taplin ME, Rosenberg JE, Kantoff PW. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol. 2012;30(5):507–512. doi: 10.1200/JCO.2011.37.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bellmunt J, Theodore C, Demkov T, Komyakov B, Sengelov L, Daugaard G, Caty A, Carles J, Jagiello-Gruszfeld A, Karyakin O, Delgado FM, Hurteloup P, Winquist E, Morsli N, Salhi Y, Culine S, von der Maase H. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454–4461. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- 61.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDLA (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 62.Di Giacomo AM, Danielli R, Guidoboni M, Calabro L, Carlucci D, Miracco C, Volterrani L, Mazzei MA, Biagioli M, Altomonte M, Maio M. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: Clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58(8):1297–1306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lipson EJ, Drake CG. Ipilimumab: An anti-CTLA-4antibody for metastatic melanoma. Clin Cancer Res. 2011;17(22):6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107(9):4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Victor CT, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, Ku G, Troncoso P, Logothetis CJ, Allison JP, Sharma P. Preoperative CTLA-4 blockade: Tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16(10):2861–2871. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 68.Saxman SB, Propert KJ, Einhorn LH, Crawford ED, Tannock I, Raghavan D, Loehrer PJ, Sr, Trump D. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J Clin Oncol. 1997;15(7):2564–2569. doi: 10.1200/JCO.1997.15.7.2564. [DOI] [PubMed] [Google Scholar]
- 69.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buchwald ZS, Efstathiou JA. Words of wisdom. Re: MPDLA (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Eur Urol. 2015;67(5):975. doi: 10.1016/j.eururo.2014.12.065. [DOI] [PubMed] [Google Scholar]
- 72.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDLA in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Plimack ER, Gupta S, Bellmunt J, Berger R, Montgomery B, Gonzalez EJ, Pulini J, Dolled-Filhart M, Emancipator K, Pathiraja K, Gause C, Perini R, CHeng JD, O’Donnell PH. LBA23 - A phase 1b study of pembrolizumab (Pembro; MK-in patients (Pts) with advanced urothelial tract cancer. ESMO Meeting Abstracts. 2014;25(5):1–41. [Google Scholar]
- 74.Bellmunt J, Mullane SA, Werner L, Fay AP, Callea M, Leow J, Choueiri TK, Hodi FS, Freeman GJ, Signoretti S. Association of PD-L1 Expression on Tumor Infiltrating Mononuclear Cells and Overall Survival in Patients with Urothelial Carcinoma. Ann Oncol. 2015;26(4):812–817. doi: 10.1093/annonc/mdv009. [DOI] [PubMed] [Google Scholar]
- 75.Powderly JD, Koeppen H, Hodi FS, Sosman JA, Gettinger SN, Desai R, Tabernero J, Soria J-C, Hamid O, Fine GD, Xiao Y, Mokatrin A, Wu J, Anderson M, Irving BA, Chen DS, Kowanetz M. Biomarkers and associations with the clinical activity of PD-L1 blockade in a MPDLA study. ASCO Meeting Abstracts. 2013;31(15_suppl):3001. [Google Scholar]
- 76.Hoffmann TK, Nakano K, Elder EM, Dworacki G, Finkelstein SD, Appella E, Whiteside TL, DeLeo AB. Generation of T cells specific for the wild-type sequence p53 (264-272) peptide in cancer patients: Implications for immunoselection of epitope loss variants. J Immunol. 2000;165(10):5938–5944. doi: 10.4049/jimmunol.165.10.5938. [DOI] [PubMed] [Google Scholar]
- 77.Vousden KH, Prives C. P53 and prognosis: New insights and further complexity. Cell. 2005;120(1):7–10. doi: 10.1016/j.cell.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 78.Fishman MN, Thompson JA, Pennock GK, Gonzalez R, Diez LM, Daud AI, Weber JS, Huang BY, Tang S, Rhode PR, Wong HC. Phase I trial of ALT-801, an interleukin-2/T-cell receptor fusion protein targeting p53 (aa264-272)/HLA-A*complex, in patients with advanced malignancies. Clin Cancer Res. 2011;17(24):7765–7775. doi: 10.1158/1078-0432.CCR-11-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramesh N, Ge Y, Ennist DL, Zhu M, Mina M, Ganesh S, Reddy PS, Yu DC. CGa conditionally replicating granulocyte macrophage colony-stimulating factor–armed oncolytic adenovirus for the treatment of bladder cancer. Clin Cancer Res. 2006;12(1):305–313. doi: 10.1158/1078-0432.CCR-05-1059. [DOI] [PubMed] [Google Scholar]
- 80.Weintraub MD, Li QQ, Agarwal PK. Advances in intravesical therapy for the treatment of non-muscle invasive bladder cancer (Review) Mol Clin Oncol. 2014;2(5):656–660. doi: 10.3892/mco.2014.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burke JM, Lamm DL, Meng MV, Nemunaitis JJ, Stephenson JJ, Arseneau JC, Aimi J, Lerner S, Yeung AW, Kazarian T, Maslyar DJ, McKiernan JM. A first in human phase 1 study of CGa GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol. 2012;188(6):2391–2397. doi: 10.1016/j.juro.2012.07.097. [DOI] [PubMed] [Google Scholar]
- 82.Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol. 2014;4:325. doi: 10.3389/fonc.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Golden EB, Formenti SC. Radiation therapy and immun-otherapy: Growing pains. Int J Radiat Oncol Biol Phys. 2015;91(2):252–254. doi: 10.1016/j.ijrobp.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 84.Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: A synergistic combination. J Clin Invest. 2013;123(7):2756–2763. doi: 10.1172/JCI69219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol . 2012;2:88. doi: 10.3389/fonc.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, Yuan J, Zitvogel L, Madeo F, Williams DB, Kroemer G. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28(5):578–590. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5(4):331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 88.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 89.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 90.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 91.Hojo S, Koizumi K, Tsuneyama K, Arita Y, Cui Z, Shinohara K, Minami T, Hashimoto I, Nakayama T, Sakurai H, Takano Y, Yoshie O, Tsukada K, Saiki I. High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res. 2007;67(10):4725–4731. doi: 10.1158/0008-5472.CAN-06-3424. [DOI] [PubMed] [Google Scholar]
- 92.Gutwein P, Schramme A, Sinke N, Abdel-Bakky MS, Voss B, Obermuller N, Doberstein K, Koziolek M, Fritzsche F, Johannsen M, Jung K, Schaider H, Altevogt P, Ludwig A, Pfeilschifter J, Kristiansen G. Tumoural CXCL16 expression is a novel prognostic marker of longer survival times in renal cell cancer patients. Eur J Cancer. 2009;45(3):478–489. doi: 10.1016/j.ejca.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 93.Prise KM, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9(5):351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chargari C, Clemenson C, Martins I, Perfettini JL, Deutsch E. Understanding the functions of tumor stroma in resistance to ionizing radiation: Emerging targets for pharmacological modulation. Drug Resist Updat. 2013;16(1-2):10–21. doi: 10.1016/j.drup.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 95.O’Toole C, Perlmann P, Unsgaard B, Almgard LE, Johansson B, Moberger G, Edsmyr F. Cellular immunity to human urinary bladder carcinoma. II. Effect of surgery and preoperative irradiation. Int J Cancer. 1972;10(1):92–98. doi: 10.1002/ijc.2910100112. [DOI] [PubMed] [Google Scholar]
- 96.O’Toole C, Unsgaard B. Clinical status and rate of recovery of blood lymphocyte levels after radiotherapy for bladder cancer. Cancer Res. 1979;39(3):840–843. [PubMed] [Google Scholar]
- 97.Mizutani Y, Uchida A, Fujimoto T, Ikenaga M, Yoshida O. Enhancement by X-ray irradiation of target cell susceptibility to natural killer cells. Immunol Lett. 1989;22(3):247–251. doi: 10.1016/0165-2478(89)90199-5. [DOI] [PubMed] [Google Scholar]
- 98.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3):862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 99.Formenti SC, Friedman K, Chao K, Adams S, Fenton-Kerimian M, Donach ME, Demaria S. Abscopal Response in Irradiated Patients: Results of a Proof of Principle Trial. Int J Radiat Oncol Biol Phys. 2008;72(1) [Google Scholar]
- 100.Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, Swetter SM, Knox SJ. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol. 2012;5(6):404–407. doi: 10.1593/tlo.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: A review of clinical outcomes. Int J Radiat Oncol Biol Phys. 2014;88(5):986–997. doi: 10.1016/j.ijrobp.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengelov L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR, Investigators CA. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liang H, Deng L, Chmura S, Burnette B, Liadis N, Darga T, Beckett MA, Lingen MW, Witt M, Weichselbaum RR, Fu YX. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol. 2013;190(11):5874–5881. doi: 10.4049/jimmunol.1202612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hammers HJ, Plimack ER, Infante JR, Ernstoff MS, Rini BI, McDermott DF, Razak ARA, Pal SK, Voss MH, Sharma P, Kollmannsberger CK, Heng DYC, Spratlin JL, Shen Y, Kurland JF, Gagnier P, Amin A. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC) ASCO Meeting Abstracts. 2014;32(15_suppl):4504. [Google Scholar]
- 106.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, Jones H, Wilkinson RW, Honeychurch J, Illidge TM. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 107.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dovedi SJ, Melis MH, Wilkinson RW, Adlard AL, Stratford IJ, Honeychurch J, Illidge TM. Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma. Blood. 2013;121(2):251–259. doi: 10.1182/blood-2012-05-432393. [DOI] [PubMed] [Google Scholar]
- 109.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1(6):365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman RA, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83(4):1306–1310. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180(5):3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]