Abstract
Percutaneous, image-guided musculoskeletal biopsy, due to its minimal invasive nature, when compared with open surgical biopsy, is a safe and effective technique which is widely used in many institutions as the primary method to acquire tissue and bone samples. Indications include histopathologic and molecular assessment of a musculoskeletal lesion, exclusion of malignancy in a bone/vertebral fracture, examination of bone marrow, and infection investigation. Preprocedural workup should include both imaging (for lesion assessment and staging) and laboratory (including coagulation tests and platelet count) studies. In selected cases, antibiotic prophylaxis should be administered before the biopsy. Core needle biopsy of musculoskeletal lesions has a diagnostic accuracy that ranges from 66 to 98% with higher diagnostic yield for lytic, large-size, malignant lesions and when multiple and long specimens are obtained. Reported complication rates range between 0 and 10% and usually do not exceed 5%, with a suggested threshold of 2%. The purpose of this review article is to illustrate the technical aspects, the indications, and the methodology of percutaneous image-guided bone biopsy that will assist the interventional radiologist to perform these minimal invasive techniques.
Keywords: musculoskeletal lesion, percutaneous biopsy, Imaging guidance, interventional radiology
Objectives : Upon completion of this article, the reader will be able to describe indications, contraindications, and the methodology for accessing bone lesions for needle biopsy via imaging guidance.
Accreditation : This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit : Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Musculoskeletal lesions are frequently encountered in clinical practice presenting as painless masses, pain sources, or as incidental findings in imaging studies. Axial imaging can be used in the diagnosis of primary and secondary musculoskeletal lesions; however, the histopathologic verification is lacking. Percutaneous, image-guided musculoskeletal biopsy, due to its minimal invasive nature when compared with open surgical biopsy, is a safe and effective technique which is widely used in many institutions as the primary method to acquire tissue and bone samples for histopathological and molecular analysis of a lesion, for recurrence prediction in curative cases (which can assist in the treatment stratification, identification, and validation of new targets), or for culturing and antibiogram testing (in cases of infection). 1 2 3 4 5 Percutaneous biopsies obviate the risk of destabilizing an already diseased spinal or peripheral skeleton segment (which can occur with open biopsies), while imaging guidance additionally provides immediate confirmation of the correct needle location inside the target area/lesion.
The purpose of this article is to illustrate the technical aspects, the indications, and the methodology of percutaneous image-guided bone biopsy that will assist the interventional radiologist to perform these minimal invasive techniques.
Indications and Contraindications
Indications for image-guided percutaneous biopsy of a bone lesion include assessment of the nature of a bone lesion with nonspecific or aggressive imaging findings, confirmation of bone metastasis in a patient with known primary tumor, exclusion of malignancy in a bone/vertebral fracture, examination of bone marrow for hematologic disease diagnosis, and investigation of infection obtaining sample for microbiological analysis and antibiogram test ( Figs. 1 2 3 ). 1 2 3 4 Additionally, molecular analysis of malignant tumors is very important for personalized cancer treatment. 5 Absolute contraindications of image-guided percutaneous bone biopsies are rare and include incomplete imaging of the lesion prior to biopsy, inaccessible sites, lack of a safe biopsy path, soft-tissue infection that poses the underlying bone to a high risk of contamination, incomplete information regarding definite surgical excision route, uncooperative or unwilling/unable to provide consent patient, and uncorrected bleeding diathesis. 1 6 However, bleeding complications from musculoskeletal biopsies are reported to be low, even when coagulation assessment is omitted prior to biopsy. 7
Fig. 1.
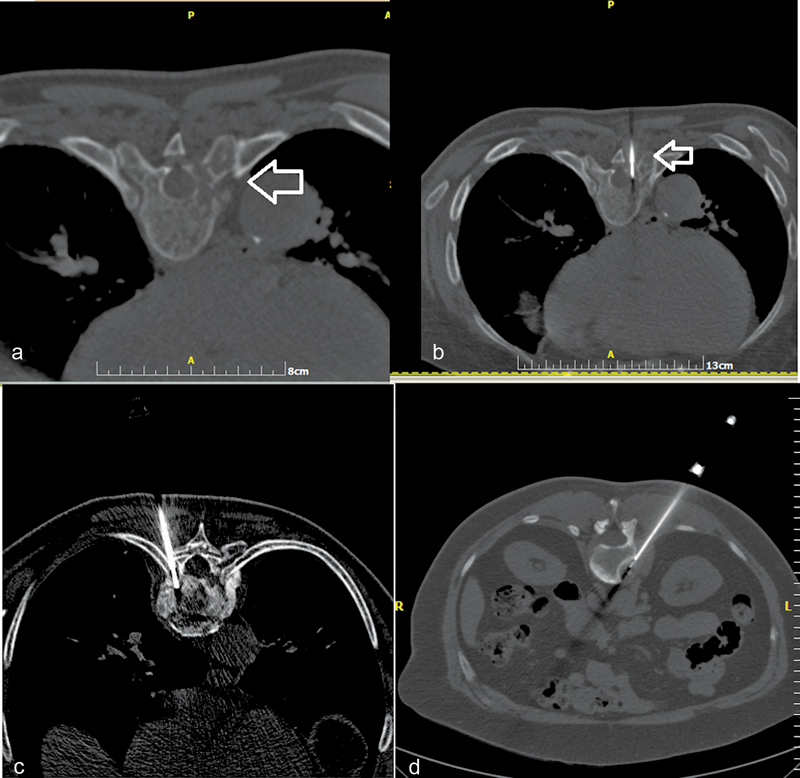
( a ) Computed tomography (CT) axial scan illustrating a lytic lesion in the right pedicle of T8 vertebral body (arrow). ( b ) CT axial scan (same patient)—biopsy needle is placed inside the lesion (transpedicular approach) (arrow). ( c ) CT axial scan (different patient from a, b )—biopsy needle is placed inside T9 vertebral body (costovertebral approach). ( d ) CT axial scan (different patient from a, b , c )—biopsy needle is placed inside the L1 lesion (posterolateral approach).
Fig. 2.
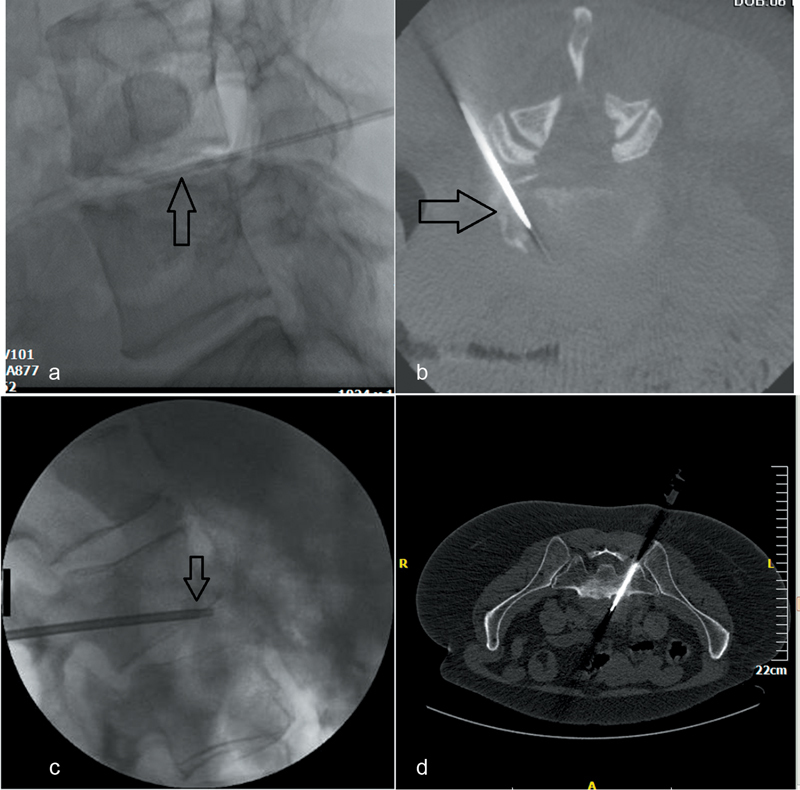
Percutaneous biopsy for spondylodiscitis. ( a ) Lateral fluoroscopic view—needle is inside L4–L5 intervertebral disc (arrow)—biopsy is performed for culture in a patient with suspected spondylodiscitis. ( b ) Cone beam CT axial reconstruction (same patient)—needle is inside L4–L5 intervertebral disc (posterolateral approach). ( c ) Lateral fluoroscopic view (different patient from a, b )—biopsy needle (arrow) is placed inside L2–L3 intervertebral disc trough a transpedicular access crossing the end plate. Sampling is performed both from the end plate and the intervertebral disc. ( d ) Computed tomography axial scan (different patient from a, b, c )—biopsy needle is placed inside the lesion located anterior to the L5–S1 intervertebral disc (transsacral approach).
Fig. 3.
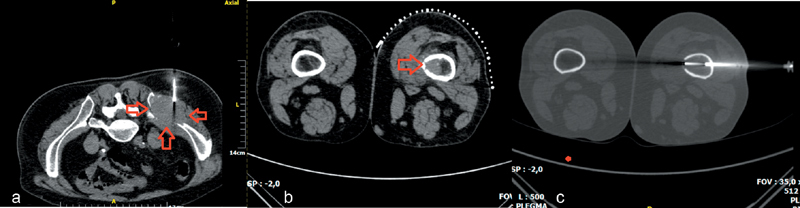
( a ) Computed tomography (CT) axial scan illustrating a large-size soft-tissue mass of the right iliac bone (arrows)—soft-tissue semiautomatic biopsy needle is placed in the periphery of the mass. ( b ) CT axial scan illustrating a hyperdense intraosseous lesion of the femoral bone. ( c ) CT axial scan (same patient with b )—after consulting with the surgeon, a lateral approach was performed; using a coaxial system, needle is located inside the lesion.
Preprocedural Workup
Preprocedural workup consists of the review of patient's clinical data including coagulation tests and platelet count, as well as of all diagnostic and functional tests that should provide a definite regional assessment and also staging of the lesion. 1 2 Depending on the clinical scenario, the imaging studies could include computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography/CT (PET/CT), and bone scans. Information obtained from the aforementioned studies should lead to the selection of the appropriate biopsy route and the imaging guidance modality and also to the selection of the appropriate biopsy needle. Surgical consultation may be required especially in the case of limb-salvage surgery where avoidance of crossing of more than one anatomic compartment is crucial as there is risk of tumor cells seeding across the needle trajectory; needle track staining with methylene blue following biopsy facilitates its subsequent surgical excision. 8 9 10 11
Preprocedurally, there are additional issues that have to be decided, including administration of sedation and/or analgesia, patient positioning, number of samples, and assessment of contraindications and complication risks. 1 Informed consent is mandatory for any interventional procedure following a full explanation of indications and complication risks and has to be obtained directly by the interventional radiologist who will carry out the biopsy. 1 Patients should be on nil per os for 4 to 6 hours prior to the procedure especially when sedation is planned. A peripheral venous access (18–20 gauge) should be placed prior to biopsy and monitoring of vital signs is needed during the procedure. 1 Antibiotic prophylaxis is not routinely used, but, when indicated, should be administered before the biopsy. 1
Technical Factors
Patient positioning is very important for percutaneous biopsy of musculoskeletal lesions; the patient should be placed in a comfortable position depending on the decided access route which will allow optimal access to the biopsy site. 1 2 12 The most common imaging-guided methods used are ultrasound, CT, and fluoroscopy. 13 MRI and PET/CT are used more rarely for biopsy guidance but frequently they reveal lesions that are CT occult. 13 Ultrasound can be used for bone lesions with extraosseous component or for soft-tissue lesions. 2 14 Choice of imaging guidance modality depends on many parameters including availability and operator's preferences but should provide proper visualization of both relevant anatomy and biopsy equipment during the interventional procedure and also assessment of possible complications exposing the patient to as low ionizing radiation as possible. Contemporary equipment allows fusing of images from different imaging modalities facilitating needle tracking. 1 Ultrasound image guidance provides real-time imaging without ionizing radiation exposure, but it is an operator-dependent method with a limited field of view and suboptimal visualization of deep lesions, especially in obesity. 1 14 MRI provides near real-time imaging with a large field of view with multiplanar detailed visualization of the region of interest without ionizing radiation exposure; however, MRI-compatible instruments are required, and the procedure time is longer in comparison to other methods. 1 2 12 Availability issues and high cost are additional disadvantages of MRI that have to be mentioned. 12 CT is the most common method for image guidance, as it provides excellent and rapid visualization of the instruments, the lesion, and the regional compartmental anatomy but at the cost of ionizing radiation exposure. 1 2 12 CT fluoroscopy can be used for real-time imaging and using intermittent fluoroscopy, radiation exposure is greatly reduced. 1 Musculoskeletal biopsies should be core biopsies and a variety of different needles exist; so, the selection of the appropriate needle depends on the location and type of the lesion and the operator's preference. 1 2 A trocar should be used for coaxial biopsy, in the case of an intact cortical bone, allowing for multiple samples to be obtained using the same access site 15 ( Fig. 4 ). In the case of densely sclerotic lesions, increased bone thickness, and excessive periosteal reaction, the use of a drill (manual or electric) may improve diagnostic yield and be technically easier. 16 17 18 19 20
Fig. 4.
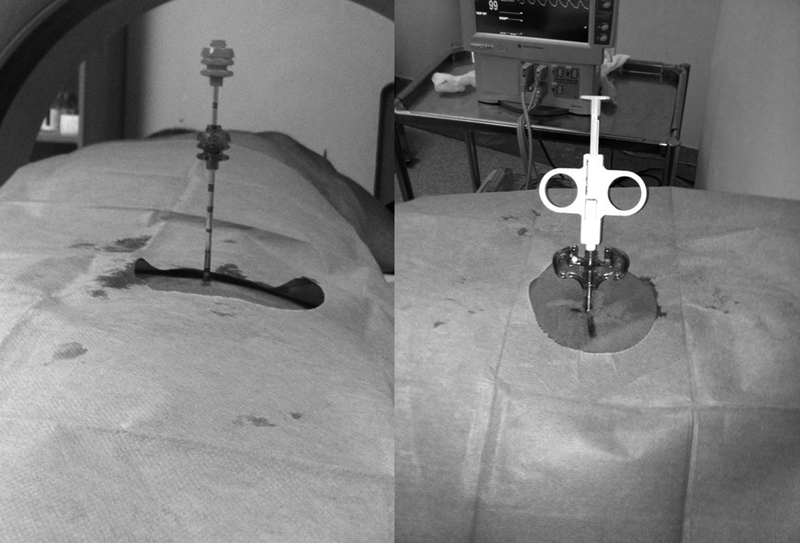
Coaxial approach offers the advantage of multiple bone sampling with a single puncture. Through the initial trocar, either a bone or a soft-tissue biopsy needle can be inserted depending on the lesion.
Sedation and analgesia provide patient comfort and cooperation during and after the biopsy and most bone biopsies are performed under conscious sedation or using local and cutaneous anesthesia. 1 A 22-gauge spinal needle can be used to deliver local anesthetic from the entry site up to the lesion or the periosteum. General anesthesia is usually recommended when patient cooperation is suboptimal (e.g., children) or for patients with high narcotic tolerance levels. 1 12 21 22 Bone biopsy should be performed under strict sterile conditions and on completion of biopsy and removal of the needle, the biopsy site is evaluated with imaging to rule out hemorrhage. Patients are usually kept for evaluation of immediate complications and prior to discharge a full explanation, or more preferably printed advice, about potential complications and appropriate postprocedural care must be provided to the patient. 23
Efficacy and Complications
Core needle biopsy of musculoskeletal lesions has a diagnostic accuracy that ranges from 66 to 98%, 24 25 26 27 28 but it is lower for spinal lesions 29 and infectious disease, 30 as well as in the case of benign lesions or low-grade tumors (in contrast with malignant tumors). 25 31 Diagnostic yield is reported to be higher in lytic lesions, 29 32 in large targets, and when multiple and long specimens are obtained. 32 Sclerotic lesions, such as prostate cancer bone metastases, are more challenging to biopsy and often the sample obtained is inadequate for diagnosis and molecular analysis but drill-assisted systems increase accuracy and improve diagnostic yield, 33 and even blood clots obtained through drilling can be used for next-generation sequencing with favorable results. 33 Concerning acquisition of cellular and molecular material, imaging modality and multiple samples are associated with higher diagnostic yield, which is also positively affected when bone lesions contain radiolucent areas, have ill-defined margins, and exhibit recent interval growth. 4 34 In initially nondiagnostic findings, repeat core biopsy can be useful as an alternative to open biopsy, but it may be underutilized. 35
Mukherjee et al performed spine biopsy in patients with presumed osteoporosis and no prior cancer as well as known cancer patients presumed to be in remission reporting an overall cancer diagnosis rate of 5.5% when combined the results of routine vertebral biopsy in both groups. 36 Image-guided spinal biopsy for spondylodiscitis has a reported sensitivity of 52.2% and a specificity of 99.9%, while the microbiological yield varies from 36 to 91%. 37 According to de Lucas et al, diagnostic rates obtained in patients with previous antibiotic treatment are significantly lower (23 vs. 60%, p = 0.013) than in those with discontinuation of antibiosis prior to biopsy. 38 As far as spinal infection is concerned, percutaneous biopsy can lead to a change in decision management in 35% of the cases. 39
Complication risk for percutaneous bone biopsy is lower than for open biopsy and reported complication rates range between 0 and 10% and usually do not exceed 5%, with a suggested threshold of 2%. 1 12 21 40 Clinically significant complications occur in less than 1% of cases 2 40 and procedure-related mortality rate does not exceed 0.05%. 1 21 Risk factors for delayed minor complications following percutaneous biopsy of musculoskeletal lesions are patient age, female gender, and lesion location. 41 Reported complications include bleeding and hematoma, infection, neural injury, bone fracture, needle tip breakage, surrounding organ damage, and needle tract seeding. 1 42 Significant bleeding is rare, 1 fracture risk is higher in very lytic lesions 42 and needle tract seeding may be affected by needle size, number of needle passes, tumor type, and location of the lesion. 21 Careful planning of the biopsy route is very important. For grading and classification of complications, international systems can be used combining outcome, presence of complication, effect upon hospitalization, and severity of a specific complication and sequelae in patient's everyday life. 43 44
Conclusion
Percutaneous, image-guided musculoskeletal biopsy is a safe (2% suggested threshold of complications rate) and effective (diagnostic accuracy ranging from 66 to 98%) technique widely used for tissue and bone sampling. Percutaneous musculoskeletal biopsies as opposed to open surgical approaches obviate the risk of destabilizing an already diseased spinal or peripheral skeleton segment, while imaging guidance additionally provides immediate confirmation of the correct needle location inside the target area/lesion.
Conflict of Interest Authors have no conflict of interest to disclose.
Note
The reader is referred to a prior article in Seminars in Interventional Radiology that also covers musculoskeletal biopsies. 12
References
- 1.Veltri A, Bargellini I, Giorgi L, Almeida P AMS, Akhan O. CIRSE guidelines on percutaneous needle biopsy (PNB) Cardiovasc Intervent Radiol. 2017;40(10):1501–1513. doi: 10.1007/s00270-017-1658-5. [DOI] [PubMed] [Google Scholar]
- 2.Gogna A, Peh W C, Munk P L.Image-guided musculoskeletal biopsy Radiol Clin North Am 20084603455–473., v [DOI] [PubMed] [Google Scholar]
- 3.Liu B, Limback J, Kendall M et al. Safety of CT-guided bone marrow biopsy in thrombocytopenic patients: a retrospective review. J Vasc Interv Radiol. 2017;28(12):1727–1731. doi: 10.1016/j.jvir.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Holmes M G, Foss E, Joseph G et al. CT-guided bone biopsies in metastatic castration-resistant prostate cancer: factors predictive of maximum tumor yield. J Vasc Interv Radiol. 2017;28(08):1073–10810. doi: 10.1016/j.jvir.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Tam A L, Lim H J, Wistuba I I et al. Image-guided biopsy in the era of personalized cancer care: proceedings from the society of interventional radiology research consensus panel. J Vasc Interv Radiol. 2016;27(01):8–19. doi: 10.1016/j.jvir.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel I J, Davidson J C, Nikolic B et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(06):727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Shif Y, Kung J W, McMahon C J et al. Safety of omitting routine bleeding tests prior to image-guided musculoskeletal core needle biopsy. Skeletal Radiol. 2018;47(02):215–221. doi: 10.1007/s00256-017-2784-5. [DOI] [PubMed] [Google Scholar]
- 8.Liu P T, Valadez S D, Chivers F S, Roberts C C, Beauchamp C P.Anatomically based guidelines for core needle biopsy of bone tumors: implications for limb-sparing surgery Radiographics 20072701189–205., discussion 206 [DOI] [PubMed] [Google Scholar]
- 9.Anderson M W, Temple H T, Dussault R G, Kaplan P A. Compartmental anatomy: relevance to staging and biopsy of musculoskeletal tumors. AJR Am J Roentgenol. 1999;173(06):1663–1671. doi: 10.2214/ajr.173.6.10584817. [DOI] [PubMed] [Google Scholar]
- 10.Toomayan G A, Robertson F, Major N M. Lower extremity compartmental anatomy: clinical relevance to radiologists. Skeletal Radiol. 2005;34(06):307–313. doi: 10.1007/s00256-005-0910-2. [DOI] [PubMed] [Google Scholar]
- 11.Toomayan G A, Robertson F, Major N M, Brigman B E. Upper extremity compartmental anatomy: clinical relevance to radiologists. Skeletal Radiol. 2006;35(04):195–201. doi: 10.1007/s00256-005-0063-3. [DOI] [PubMed] [Google Scholar]
- 12.Le H B, Lee S T, Munk P L. Image-guided musculoskeletal biopsies. Semin Intervent Radiol. 2010;27(02):191–198. doi: 10.1055/s-0030-1253517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillen T J, Talbert R J, Friedman M V et al. Biopsy of CT-occult bone lesions using anatomic landmarks for CT guidance. AJR Am J Roentgenol. 2017;209(01):214–221. doi: 10.2214/AJR.16.17468. [DOI] [PubMed] [Google Scholar]
- 14.Chira R I, Chira A, Manzat-Saplacan R M et al. Ultrasound-guided bone lesions biopsies - a systematic review. Med Ultrason. 2017;19(03):302–309. doi: 10.11152/mu-1118. [DOI] [PubMed] [Google Scholar]
- 15.Filippiadis D K, Tutton S, Kelekis A. Percutaneous bone lesion ablation. Radiol Med (Torino) 2014;119(07):462–469. doi: 10.1007/s11547-014-0418-8. [DOI] [PubMed] [Google Scholar]
- 16.Chang I J, Ilaslan H, Sundaram M, Schils J, Subhas N. CT-guided percutaneous biopsy of sclerotic bone lesions: diagnostic outcomes. Skeletal Radiol. 2018;47(05):661–669. doi: 10.1007/s00256-017-2828-x. [DOI] [PubMed] [Google Scholar]
- 17.Filippiadis D, Gkizas C, Kostantos C et al. Percutaneous biopsy and radiofrequency ablation of osteoid osteoma with excess reactive new bone formation and cortical thickening using a battery-powered drill for access: a technical note. Cardiovasc Intervent Radiol. 2016;39(10):1499–1505. doi: 10.1007/s00270-016-1366-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee R K, Ng A W, Griffith J F. CT-guided bone biopsy with a battery-powered drill system: preliminary results. AJR Am J Roentgenol. 2013;201(05):1093–1095. doi: 10.2214/AJR.12.10521. [DOI] [PubMed] [Google Scholar]
- 19.Cohen M G, McMahon C J, Kung J W, Wu J S. Comparison of battery-powered and manual bone biopsy systems for core needle biopsy of sclerotic bone lesions. AJR Am J Roentgenol. 2016;206(05):W83–W86. doi: 10.2214/AJR.15.15067. [DOI] [PubMed] [Google Scholar]
- 20.Jelinek J S, Murphey M D, Welker J A et al. Diagnosis of primary bone tumors with image-guided percutaneous biopsy: experience with 110 tumors. Radiology. 2002;223(03):731–737. doi: 10.1148/radiol.2233011050. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Wallace M J, Cardella J F, Kundu S, Miller D L, Rose S C; Society of Interventional Radiology Standards of Practice Committee.Quality improvement guidelines for percutaneous needle biopsy J Vasc Interv Radiol 20102107969–975. [DOI] [PubMed] [Google Scholar]
- 22.Mubarak W M, Pastor C, Gnannt R et al. Technique, safety, and yield of bone biopsies for histomorphometry in children. J Vasc Interv Radiol. 2017;28(11):1577–1583. doi: 10.1016/j.jvir.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Lee M J, Fanelli F, Haage P, Hausegger K, Van Lienden K P. Patient safety in interventional radiology: a CIRSE IR checklist. Cardiovasc Intervent Radiol. 2012;35(02):244–246. doi: 10.1007/s00270-011-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hau A, Kim I, Kattapuram S et al. Accuracy of CT-guided biopsies in 359 patients with musculoskeletal lesions. Skeletal Radiol. 2002;31(06):349–353. doi: 10.1007/s00256-002-0474-3. [DOI] [PubMed] [Google Scholar]
- 25.Altuntas A O, Slavin J, Smith P J et al. Accuracy of computed tomography guided core needle biopsy of musculoskeletal tumours. ANZ J Surg. 2005;75(04):187–191. doi: 10.1111/j.1445-2197.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- 26.Datir A, Pechon P, Saifuddin A. Imaging-guided percutaneous biopsy of pathologic fractures: a retrospective analysis of 129 cases. AJR Am J Roentgenol. 2009;193(02):504–508. doi: 10.2214/AJR.08.1823. [DOI] [PubMed] [Google Scholar]
- 27.Rimondi E, Rossi G, Bartalena T et al. Percutaneous CT-guided biopsy of the musculoskeletal system: results of 2027 cases. Eur J Radiol. 2011;77(01):34–42. doi: 10.1016/j.ejrad.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Frassica F J, Fayad L, Clark D P, Weber K L. Analysis of nondiagnostic results after image-guided needle biopsies of musculoskeletal lesions. Clin Orthop Relat Res. 2010;468(11):3103–3111. doi: 10.1007/s11999-010-1337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg V, Kosmas C, Josan E S et al. Computed tomography-guided percutaneous biopsy for vertebral neoplasms: a department's experience and hybrid biopsy technique to improve yield. Neurosurg Focus. 2016;41(02):E17. doi: 10.3171/2016.4.FOCUS1614. [DOI] [PubMed] [Google Scholar]
- 30.Sehn J K, Gilula L A. Percutaneous needle biopsy in diagnosis and identification of causative organisms in cases of suspected vertebral osteomyelitis. Eur J Radiol. 2012;81(05):940–946. doi: 10.1016/j.ejrad.2011.01.125. [DOI] [PubMed] [Google Scholar]
- 31.Omura M C, Motamedi K, UyBico S, Nelson S D, Seeger L L. Revisiting CT-guided percutaneous core needle biopsy of musculoskeletal lesions: contributors to biopsy success. AJR Am J Roentgenol. 2011;197(02):457–461. doi: 10.2214/AJR.10.6145. [DOI] [PubMed] [Google Scholar]
- 32.Wu J S, Goldsmith J D, Horwich P J, Shetty S K, Hochman M G. Bone and soft-tissue lesions: what factors affect diagnostic yield of image-guided core-needle biopsy? Radiology. 2008;248(03):962–970. doi: 10.1148/radiol.2483071742. [DOI] [PubMed] [Google Scholar]
- 33.Sailer V, Schiffman M H, Kossai M et al. Bone biopsy protocol for advanced prostate cancer in the era of precision medicine. Cancer. 2018;124(05):1008–1015. doi: 10.1002/cncr.31173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tacher V, Le Deley M C, Hollebecque A et al. Factors associated with success of image-guided tumour biopsies: results from a prospective molecular triage study (MOSCATO-01) Eur J Cancer. 2016;59:79–89. doi: 10.1016/j.ejca.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Wu J S, McMahon C J, Lozano-Calderon S, Kung J W. JOURNAL CLUB: Utility of repeat core needle biopsy of musculoskeletal lesions with initially nondiagnostic findings. AJR Am J Roentgenol. 2017;208(03):609–616. doi: 10.2214/AJR.16.16220. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee S, Thakur B, Bhagawati D et al. Utility of routine biopsy at vertebroplasty in the management of vertebral compression fractures: a tertiary center experience. J Neurosurg Spine. 2014;21(05):687–697. doi: 10.3171/2014.7.SPINE121015. [DOI] [PubMed] [Google Scholar]
- 37.Pupaibool J, Vasoo S, Erwin P J, Murad M H, Berbari E F. The utility of image-guided percutaneous needle aspiration biopsy for the diagnosis of spontaneous vertebral osteomyelitis: a systematic review and meta-analysis. Spine J. 2015;15(01):122–131. doi: 10.1016/j.spinee.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 38.de Lucas E M, González Mandly A, Gutiérrez A et al. CT-guided fine-needle aspiration in vertebral osteomyelitis: true usefulness of a common practice. Clin Rheumatol. 2009;28(03):315–320. doi: 10.1007/s10067-008-1051-5. [DOI] [PubMed] [Google Scholar]
- 39.Rankine J J, Barron D A, Robinson P, Millner P A, Dickson R A. Therapeutic impact of percutaneous spinal biopsy in spinal infection. Postgrad Med J. 2004;80(948):607–609. doi: 10.1136/pgmj.2003.017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peh W. CT-guided percutaneous biopsy of spinal lesions. Biomed Imaging Interv J. 2006;2(03):e25. doi: 10.2349/biij.2.3.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang A J, Halpern E F, Rosenthal D I. Incidence of delayed complications following percutaneous CT-guided biopsy of bone and soft tissue lesions of the spine and extremities: a 2-year prospective study and analysis of risk factors. Skeletal Radiol. 2013;42(01):61–68. doi: 10.1007/s00256-012-1433-2. [DOI] [PubMed] [Google Scholar]
- 42.Exner G U, Kurrer M O, Mamisch-Saupe N, Cannon S R. The tactics and technique of musculoskeletal biopsy. EFORT Open Rev. 2017;2(02):51–57. doi: 10.1302/2058-5241.2.160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filippiadis D K, Binkert C, Pellerin O, Hoffmann R T, Krajina A, Pereira P L. Cirse Quality Assurance Document and Standards for Classification of Complications: the Cirse classification system. Cardiovasc Intervent Radiol. 2017;40(08):1141–1146. doi: 10.1007/s00270-017-1703-4. [DOI] [PubMed] [Google Scholar]
- 44.Leoni C J, Potter J E, Rosen M P, Brophy D P, Lang E V. Classifying complications of interventional procedures: a survey of practicing radiologists. J Vasc Interv Radiol. 2001;12(01):55–59. doi: 10.1016/s1051-0443(07)61403-1. [DOI] [PubMed] [Google Scholar]


