Abstract
Interventional radiology expertise in image guidance has expanded the treatment options for cancer patients with unstable osseous disease. Percutaneous fixation by internal cemented screw (FICS) describes the technique by which the interventional radiologist stabilizes a fracture or impending fracture with the percutaneous placement of a cannulated screw that is locked in position by polymethyl methacrylate cement. The durable metallic screws provide added resistance to torque and tension stresses that complement the axial compression resistance of cement. Compared with cementoplasty alone, the procedure has been advanced as a more durable and precise technique for stabilization of osseous disease for certain disease presentations in cancer patients. The application of advanced image guidance techniques improves upon existing percutaneous surgical techniques to facilitate approaches that would otherwise prove quite challenging, particularly with stabilization of the pelvic flat bones. This article examines the applications of percutaneous FICS procedures for the treatment of unstable osseous disease in cancer patients. Indications, techniques, and follow-up care are reviewed. Case examples in which FICS can be performed in unstable pathology are detailed.
Keywords: bone fracture, pain palliation, cement consolidation, screw fixation, cementoplasty, interventional radiology
Objectives : Upon completion of this article, the reader will be able to describe the indications, technique, and outcomes of percutaneous fixation by internal cemented screw (FICS) for stabilization of unstable osseous disease in cancer patients.
Accreditation: This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Skeletal events related to unstable osseus disease in cancer patients can have severe consequences that include debilitating pain, limitations in activities of daily living, and significant impairment in quality of life. 1 2 The potential impact on the patient and burden to healthcare systems are substantial given the high prevalence of skeletal metastases. Osseous metastases can directly result in structural bone weakening that increases the risk of pathological fracture. In addition, structural bone weakening and fracture may result from osteoporosis caused by effects on bone metabolism related directly to the cancer, immobility from cancer pain, and various cancer therapies that include certain chemotherapies, hormone therapy, long-term steroid therapy, and external radiotherapy.
Cementoplasty is among the most prominent interventional radiology stabilization techniques. The physical properties of PMMA cement provide resistance to axial compressive forces experienced during weight-bearing activities. Cementoplasty has shown sustained palliative effect for pathologic vertebral body fractures, 3 4 pelvic bone fractures of the sacrum and acetabulum, 5 and proximal femoral fractures. 6 While cementoplasty does confer palliative benefit, stabilization by cementoplasty alone can be suboptimal. A major limitation of PMMA cement is the poor resistance to tension and torques stresses. These nonaxial forces are particularly pertinent for unstable disease in the long and flat bones. Another drawback of cementoplasty is the poor technical control of cement distribution. Multifocal or extensive cortical destruction can channel cement away from the zone of instability and result in incomplete fracture filling or cement leakage outside of the bone that can cause joint or nerve injury. 7
Screw fixation of unstable osseous disease can provide critical pain palliation that overcomes the fallacies of cementoplasty; however, traditional surgical approaches can prove technically challenging in certain situations. Comorbidities in cancer patients with advanced metastatic disease may preclude extensive open surgery. Similarly, certain tumor locations and sizes may render surgical techniques less appealing due to long recovery times and necessity for delays in systemic therapies during surgical healing. Furthermore, large osseous tumors may not be completely resectable, which can predispose surgical hardware to early loosening.
Given these hurdles to open surgical approach, particularly in the pelvis, many surgeons have begun exploring the utility of minimally invasive percutaneous approach in nonpathologic fractures. 8 9 10 11 12 13 Percutaneous fixation techniques can demonstrate more reduced morbidity than open surgical procedures. 8 Despite the surgeon's technical experience, the percutaneous approach can be technically challenging due to the variable shape, size, and contour of the pelvic girdle. In addition, lytic tumor destruction of the bone may render a traditional fluoroscopic approach futile as bony landmarks may be defaced. These considerations have prompted surgical interest in imaging navigation technologies to assist in screw placement. 14 15 16
A recent paradigm shift applies interventional radiology techniques to enable highly accurate and minimally invasive osseous fixation. Percutaneous fixation by internal cemented screw (FICS) describes the percutaneous advancement of cannulated screws across a fracture line or unstable osseous metastasis, with additional cement consolidation. Although the technique and equipment were developed by surgeons, the application of advanced CT or cone beam CT (CBCT) needle guidance software has been recently successfully propagated by interventional radiologists. Needle guidance navigation software available for both fluoroscopy and CT units allows improved precision for screw placement planning and real-time track verification. Several small series have demonstrated the technical successes and short-term outcomes for percutaneous FICS using either fluoroscopic or CT guidance. 17 18 19 20 21 22 23
In this article, the application of percutaneous FICS is examined for the treatment of unstable osseous disease within the cancer population. Clinical indications with pertinent literature are examined. Procedural techniques are reviewed. Several case examples are provided to demonstrate the technique in various disease presentations in the pelvis and femoral neck.
Literature Review
Several small retrospective case series demonstrate that percutaneous FICS performed by interventional radiologists provides reliable technical success and valuable short-term outcome to treat oncologic disease that can prove challenging for surgical methods. The technique is predominantly applied in the pelvic flat bones or femoral neck, although recent studies have suggested a potential role in other locations. 20 24 Applications for both prophylactic treatment and fixation of fractures have been evaluated.
Regarding the preventative approach of impending pathologic fracture, orthopedic surgeons have long employed multifactoral scoring systems to predict risk within long bones and guide treatment indications. The two most common of these systems are the Harrington criteria and Mirels criteria. Harrington's criteria lists more than 50% destruction of diaphyseal cortices, more than 50 to 75% destruction of metaphysis (>2.5 cm), permeative destruction of the subtrochanteric femoral region, and persistent pain following irradiation as factors indicating high risk for impending fracture. 25 Mirels' criteria provides a numeric scoring system based on tumor location, fraction of involved cortex, presence of functional pain, and radiographic appearance regarding lytic and sclerotic composition. 26 An overall Mirels score greater than 8 indicates prophylactic fixation is appropriate due to the high risk of pathological fracture (>33%).
In patients with lytic femoral neck lesions, IR studies have evaluated the role for prophylactic percutaneous FICS to prevent pathologic fractures following a loose application of Mirels' criteria. An initial study published in 2012 reported 12 percutaneous FICS procedures for impeding pathological fracture of the femoral neck. 23 No fracture occurred during the follow-up and symptomatic patients ( n = 8) experienced a significant pain improvement with decreased of the visual analogic score (VAS) from 6.5/10 (range, 2–9) before treatment to 1/10 (range, 0–3) on assessment performed 1 month after treatment. Based on the results of the largest cohort published ( n = 35 patients, Mirels' score: 10.1), it seems that percutaneous FICS of proximal femurs results in an appropriate consolidation of impending pathological fractures, with a fracture rate of 5.7% after a median follow-up of over 6 months. 17 These preliminary results have been confirmed by more recent studies. 27 28
To the authors' best knowledge, no specific indications or criteria are routinely applied to predict fracture risk for flat bones. For the palliative application of percutaneous FICS in the pelvic location, several studies have been demonstrated safe and effective application. 17 20 29 The largest series was published in 2014, with 21 patients suffering from 33 pelvic fractures. Significant pain improvement was reported with mean postprocedure VAS score at 1 month of 2.5/10 compared with 8/10 before the procedure. 17 Similar results have been confirmed by two additional studies. 20 27 Pusceddu et al have demonstrated that the mean VAS score decreased from 7.1 before treatment to 1.6 at 1 month and to 1.4/10 at 6 months, demonstrating a long-term effect. 20 Similarly, a double-center retrospective analysis of percutaneous FICS demonstrated 87.1% rate of pain improvement in a series of cancer patients that included 25 pelvic locations. 27
Outside of the pelvis, reports of percutaneous FICS procedure have explored the potential applications in cancer patients with painful lesions in the scapula and spine. Spine fixation may be appealing for complex vertebral body fractures, pedicle or transverse fractures. 20 24
Clinical Indications and Preprocedural Workup
Clinical presentations will vary based on the variability in osteoporotic disease and osseous tumor biology, size, location, and vascularity. Patients may present with or without symptoms. Asymptomatic patients typically are identified by imaging to have lytic osseous disease in a load bearing bone, which is concerning for impending fracture. Symptomatic patients typically present with musculoskeletal pain that has a mechanical component exacerbated during movement or weight bearing. The symptoms may reflect an underlying pathologic fracture or instability due to bone destruction from metastases that forebodes impending fracture.
Regardless of the clinical presentation, the cross-sectional imaging should be closely evaluated to establish the underlying pathology. A definitive biopsy may be obtained if differentiation between pathologic fracture and osteoporotic fracture cannot be concluded based on imaging. Metastatic disease should be evaluated for the potential locoregional control before FICS. The finding of osteoporotic fractures should prompt medical workup to identify etiology of bone resorption. An attempt to reverse osteoporosis can be pursued with appropriate pharmacologic therapy that may include bisphosphonate or biologic agents. Furthermore, the patient history should be reviewed for any correctable cancer treatment–related factors that might have exacerbated the osteoporosis. A trial of conservative treatment may be pursued, as can palliative cementoplasty.
Clinical evaluation should include focused history and physical exam. Sensory nerve pain may prompt further investigation into the etiology for nerve pain, as this may be related to the osseous disease or conversely represent a separate process. The patient should be counseled on whether or not these symptoms are expected to be affected by the FICS procedure. Any sensory or motor deficits should be noted to provide accurate baseline documentation. The VAS pain score and specifics related to both exacerbating and relieving factors should be recorded. Opioid usage and performance status may be recorded to assess functional postprocedural pain assessment. Laboratory evaluation should exclude coagulopathies, or prompt appropriate corrective measures. In general, the procedures have low blood loss and do not require blood type and screening. Finally, a complete anesthesiology assessment should be performed as the procedures are typically performed under general anesthesia.
Upon confirmation of clinical appropriateness and laboratory parameters, preprocedural workup should include imaging review to identify the most reasonable image guidance modality. Percutaneous FICS performed by the interventional radiologist applies the procedural advantage of needle guidance software via either CT or CBCT acquisition. Modality selection often entails evaluation of lesion location, trajectory approach, patient body habitus, imaging modality availability, and operator comfort. Should locoregional treatments be indicated, this may be performed in the same procedural setting immediately before fixation for the patients comfort and to minimalize anesthesia inductions. This may dictate selection of FICS imaging modality. The additional locoregional control measures by embolization may predispose selection for CBCT guidance, while percutaneous ablation may predispose for CT guidance. The availability of an integrated CT-fluoroscopy unit facilitates this decision process. A Foley catheter may be warranted if procedure is expected to last longer than 3 hours, which is particularly pertinent if additional locoregional measures are applied before screw fixation.
Fixation by Internal Cemented Screw Technique
Percutaneous FICS procedures adhered to the same strict sterility requirements of the conventional surgical operating rooms. A one-time administration of intravenous prophylactic antibiotics to cover skin flora is typical immediately before the procedure. Orthopedic equipment should be preordered based on preprocedural planning. The described technique uses cannulated screws, although noncannulated screws are also available. Screws come in varied diameters and lengths, in titanium and stainless steel construct, and with partially and fully threaded options. The most common variety of screw diameter employed are 6.5 or 8 mm diameter. The screw length is chosen so that the screw tip anchors into at least 1 cm of intact trabecular bone, with the screw head snuggly abutting the intact cortex at the osseous entry point. A metallic washer may be used to improve compressive traction; no washer is indicated if the intent is to bury the screw head into the cortex to minimize soft-tissue discomfort. The number of screws used depends on the size and location of the pathologic fracture or the metastasis. The majority of screws are self-taping, although cannulated drills may be used to facilitate passage through dense bone or hardened cement injected in a previous cementoplasty procedure.
Percutaneous screw advancement begins with a small 1-cm incision. A Kirschner wire is advanced under image guidance to follow the planned trajectory using a mallet or power drill. Alternatively, an 8-gauge bone needle may be advanced along the planned trajectory using a mallet. Once in bone needle has established the trajectory, the Kirschner wire may be coaxially advanced. The 8-gauge needle confers added directional capability due to greater rigidity compared with the Kirschner wire. Once the Kirschner wire is positioned with the tip in the desired location of the future screw tip, measurement of screw length may be obtained with manual measuring tool or based on image acquisition. The appropriate screw is then coaxially advanced over the Kirschner wire into position using a hand or power screwdriver. The Kirschner wire is removed and the incision is closed.
Cement consolidation during the FICS procedure improves consolidative effects and decreases the risk for screw migration. The cementoplasty is performed to anchor the screw more solidly in trabecular bone to prevent screw retraction and migration. The “anchorage technique” can be performed by injecting cement through the positioned 8-gauge needle before a rapid coaxial exchange that advanced the screw into setting cement. In addition, cementoplasty can be performed to provide substantial cementoplasty consolidative effect for screws that bridge large lytic metastases. This technique not only improves the tenacity of the screw but also distributes the pressure load around the screw to provide additive resistance to compressive axial forces with weight bearing. In this technique, additional bone needles are advanced under imaging guidance, and PMMA cement is injected around the screw into the lytic metastasis. Either one or both techniques ( Fig. 1 ) are used for each screw placed depending on the fracture location, size of the lytic metastasis, and character of the adjacent bone.
Fig. 1.
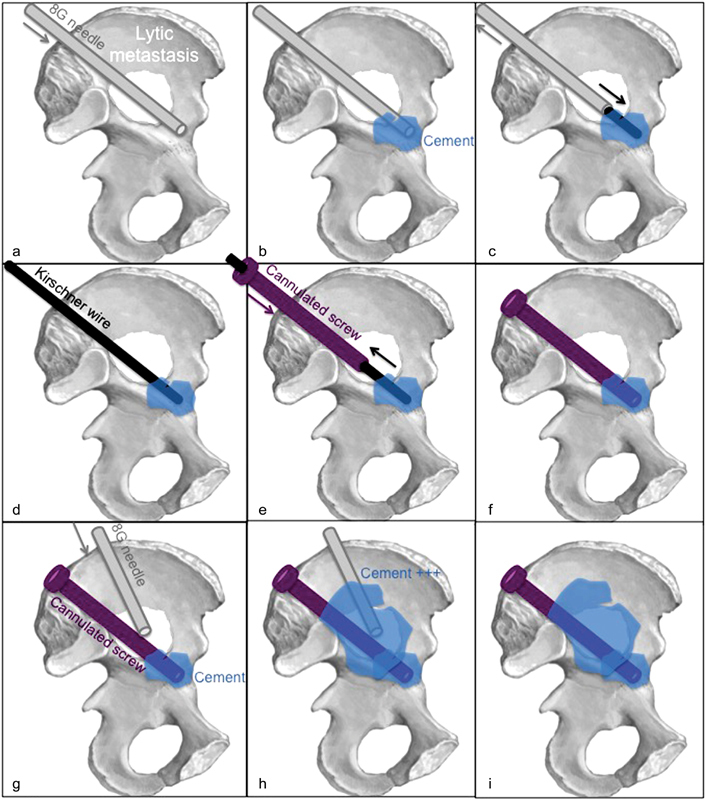
Cementoplasty for better screw anchorage : The lytic metastasis is crossed with the 8-gauge needle ( a ), through which cement is injected ( b ). The 8-gauge needle is coaxially exchanged using a 3.2-mm Kirschner wire ( c and d ) for the 8-mm screw that is advanced into the liquid cement ( e ). The Kirschner wire is removed before cement consolidation ( f ). Cementoplasty for improved consolidation : A second 8- to 11-gauge needle is advanced within the lytic lesion ( g ). Cement is injected around the screw ( h and i ).
The screw insertion track is chosen based on the location and extent of the fracture or lytic osseous metastasis. A 3D acquisition (CBCT or CT scan) of the bone structure is performed to delineate the most appropriate screw trajectory using needle guidance software. For fracture palliation, the screw must perpendicularly cross the fracture line for better stabilization. For preventive consolidation, the screw must bridge the metastasis to act as a lintel beam to reinforce the bone structure.
Pelvic and Femoral Neck Fixation by Internal Cemented Screw
The screw insertion track is chosen based on the location and extent of the fracture or lytic osseous metastasis ( Fig. 2 ). To perform FICS in sacral disease, a lateral sacroiliac track is chosen. A unilateral approach may be pursued for unilateral sacral disease. Optimally two screws are placed to improve rotational stability. If sacral dimensions permit, both screw tips may be anchored within healthy bone in the S1 vertebral body. For smaller sacral dimensions, the first screw tip may be anchored within healthy bone in the S1 vertebra and the second screw tip may be anchored into the S2 vertebra ( Fig. 3 ). If bilateral sacral fractures are present, then this same technique may be mirrored bilaterally. An alternative plan exists for bilateral sacral fixation that may also be appealing for extensive unstable sacral fractures that obscure visualization of the sacral neuroforamina. This alternative plan applies a unilateral approach with a single screw spanning the entire sacrum and bilateral sacroiliac joints ( Fig. 4 ).
Fig. 2.
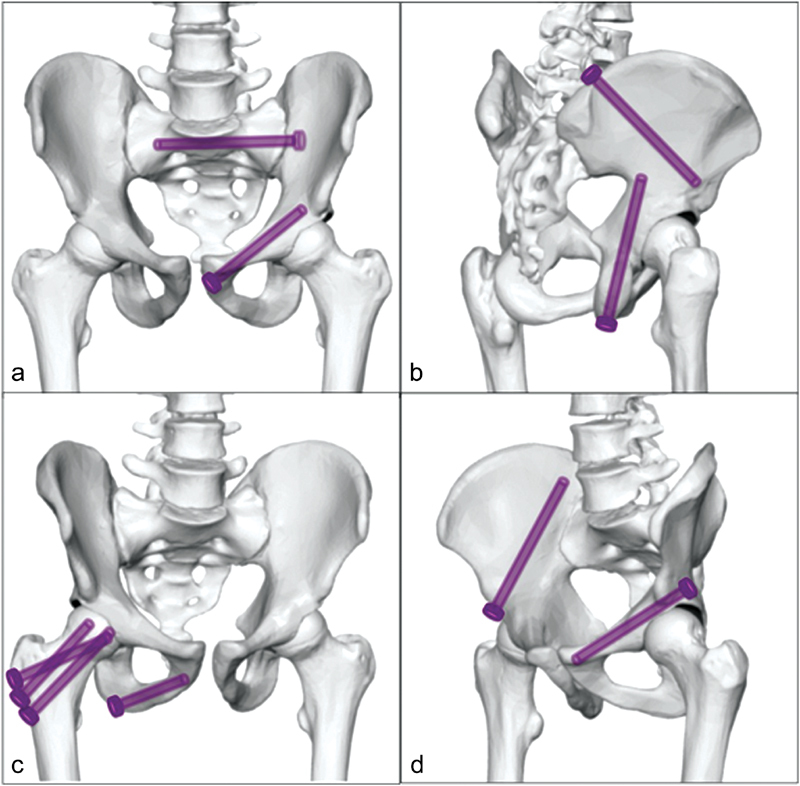
The screw insertion track is chosen based on the location of the fracture or lytic osseous metastasis: lateral sacroiliac track and anterior iliopubic track ( a ), posterior transiliac track and caudal to cranial transischial track ( b ), lateral to medial ischiopubic track and femoral inverted triangulation track ( c ), posterior iliopubic track and anterior transiliac track ( d ).
Fig. 3.
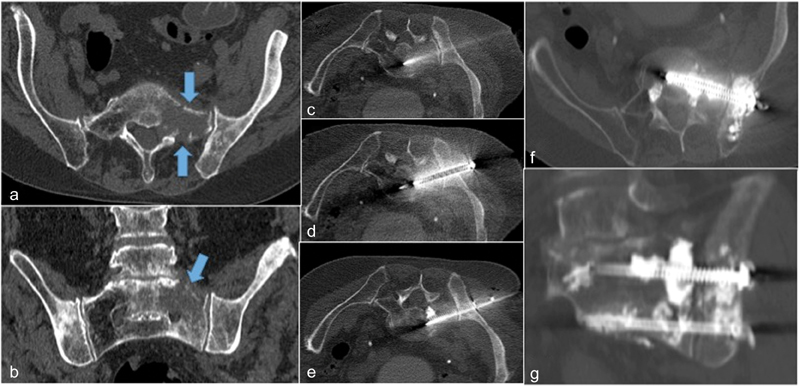
( a, b ) Lytic prostate metastasis to the left sacral ala and iliac bone that caused 10/10 mechanical pain during weight bearing. Pelvic FICS with CT-guided advancement of two cannulated, fully threaded, 6.5-mm diameter stainless steel screws ( c – e ) with tips anchored into the S1 and S2 vertebral bodies followed by consolidation cementoplasty ( f ). Final oblique coronal view demonstrates screw bridging lytic mass ( g ).
Fig. 4.
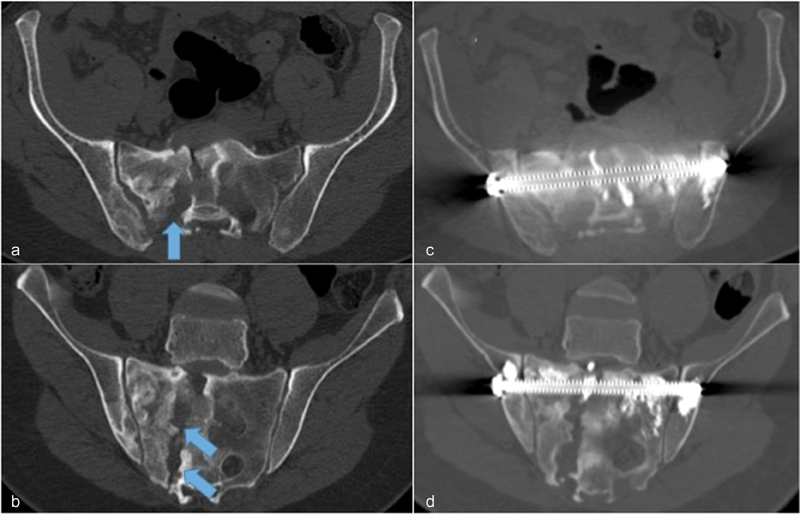
Lytic prostate metastasis resulted in extensive nonunion sacral fracture ( a : axial view, b : oblique coronal view) that caused 7/10 mechanical pain with some radicular pain from sacral neuroforaminal involvement (arrows). Pelvic FICS with CT-guided advancement of single cannulated, fully-threaded, 8-mm diameter stainless steel screw spanning the entire sacrum and bilateral sacroiliac joints through the S1 vertebral body followed by cement consolidation ( c : axial view, d : oblique coronal view).
To perform FICS in iliac pelvic brim disease, an anterior or posterior transiliac track is chosen based on the location of the disease and the iliac dimensions specific to the individual. Fractures or osseus tumor in this region may greatly destabilize the load-bearing portion of the iliac bone and result in marked pain during ambulation. The posterior transiliac approach may allow the placement of two parallel screws for added reinforcement ( Fig. 5 ). If iliac anatomy or osseous destruction does not provide adequate healthy bone to anchor two screws, then one screw may be advanced. In this case, consider the use of a larger diameter (8 mm) screw and the liberal application of consolidative cementoplasty for added structural support ( Fig. 6 ).
Fig. 5.
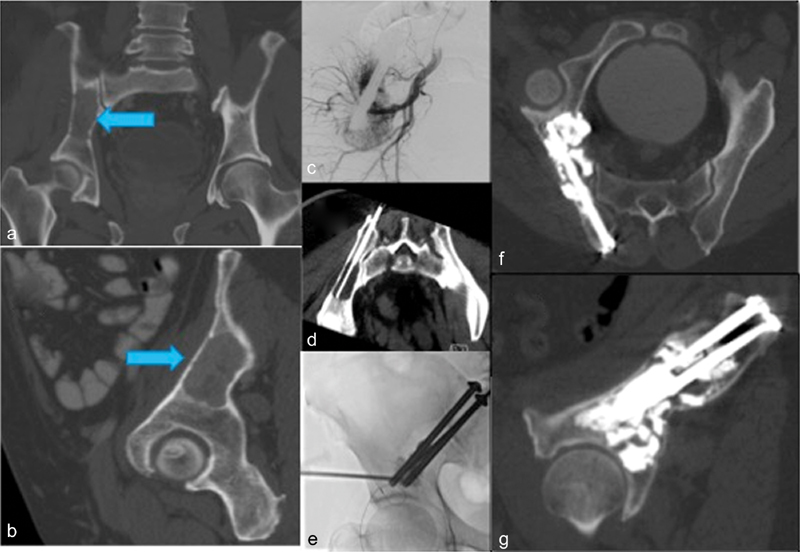
Lytic renal cell metastasis to the iliac pelvic brim (Blue arrow) resulted in severe pain limiting weight bearing ( a : oblique coronal view, b : oblique sagittal view). Locoregional treatment provided with embolization ( c ) and cryoablation ( d ) before pelvic FICS with fluoroscopy-guided advancement of two parallel cannulated, partially threaded, 6.5-mm diameter titanium screws from a posterior transiliac approach ( e ) and consolidative cementoplasty ( f and g ).
Fig. 6.
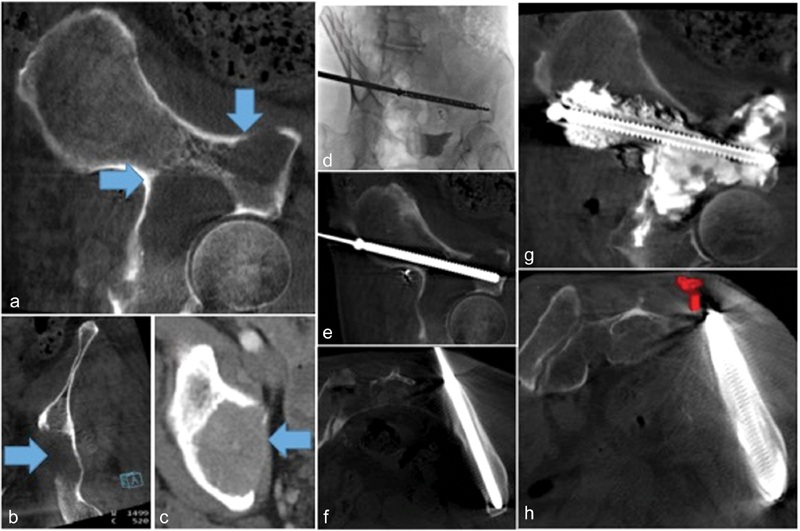
Lytic renal cell metastasis to the iliac pelvic brim, posterior acetabulum, and superior acetabulum (Blue arrows) resulted in severe hip pain limiting weight bearing ( a : oblique sagittal view, b : oblique coronal view, c : axial view). Pelvic FICS with fluoroscopy-guided advancement of single cannulated, fully-threaded, 8-mm diameter stainless steel screw from a posterior transiliac approach ( d : intraprocedural advancement; e and f : screw placement confirmation on CBCT). Liberal consolidative cementoplasty performed to fill lytic tumor and provide added structural support ( g and h ).
For superior acetabular disease, the approach may be varied based on the lesion presentation. If lytic tumor is confined to the superior acetabulum and is small in size (>3 cm), then consideration for cementoplasty alone may be sufficient as this location is subjected to predominantly axial compression forces. If lytic size is large (>2 cm) and extends outside of the superior acetabulum, then the additional screw fixation may provide added stabilization. An anterior or posterior transiliac track may be taken depending on the location and extent of the disease.
The approach to pubic rami disease should take into account patient presentation and lesion characteristics. Fractures or lesions in these locations are not technically in load-bearing portions of the pelvis. Locoregional treatment including ablation or radiotherapy may be sufficient for pain control. Despite excellent locoregional treatments, mechanical pain may still be present. Pelvic movement may elicit pain through bone-on-bone friction or by bone inflammation of adjacent muscles and nerves. To perform FICS for the treatment of pubic rami disease, either lateral or medial pubic tracks may be chosen based on the disease extent and location ( Fig. 7 ). Cementoplasty technique is typically anchorage due to the small diameter of the pubic rami.
Fig. 7.
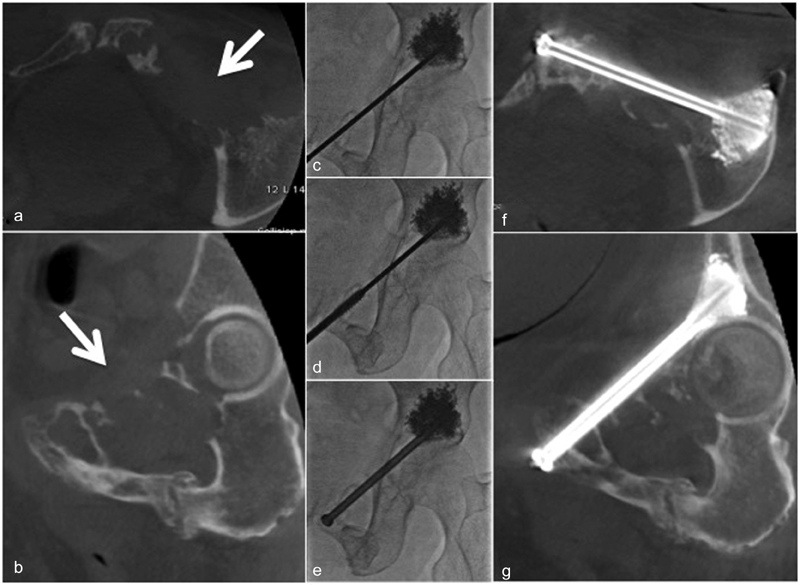
Lytic breast cancer metastasis to the iliopubic branch (white arrow) resulted in severe pain and high risk of fracture ( a : oblique coronal view, b : oblique sagittal view). Pelvic FICS with CBCT advancement of one cannulated, 8-mm diameter stainless steel screws ( c – e ) with tips anchored into the acetabulum ( f – g ).
To perform FICS for the treatment of femoral neck disease, screws may be placed in a two- or three-screw configuration along the long axis of the femoral neck ( Fig. 8 ). The tracks are parallel when two screws are inserted. When three screws are inserted, planning should allow for an inverted triangle configuration due to spatial confinements and to improve structural support. 23 The anchorage technique through an 8-gauge needle may be most applicable when performing femoral neck FICS, as access may be limited after all screws have been placed.
Fig. 8.
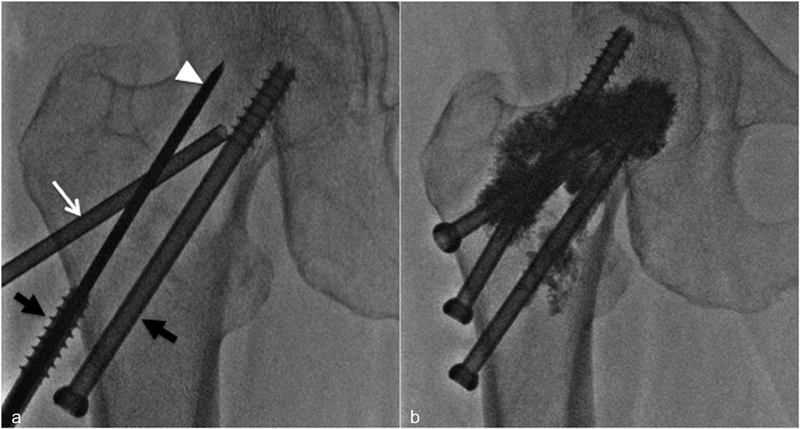
Percutaneous FICS of osteolytic metastasis located in the proximal femur. Three screws are inserted through the long axis of the femoral neck in an inverted triangle configuration. Kirschner's wires ( white arrow-head ) are coaxially advanced through 8-gauge needle ( white arrow ) and screws ( black arrow ) are advanced over the Kirschner wires ( a ). The metallic screws provide a resistance to torque and tension stresses that complement the compression resistance of cement ( b ).
Postprocedural Care
Routine postprocedural care includes planned hospitalization for 1 to 3 days for procedure-related pain control with analgesic adapted to numerical rating scale score. Prophylactic antibiotics to cover skin flora may be administered for 5 to 7 days. Physical therapy is initiated after hospitalization for patients. Postprocedural exercise or activity are overseen by a physical therapist and are guided by pain tolerance. Imaging follow-up and focused clinical evaluation should be obtained in 1 to 2 months to verify stability of hardware.
Conclusion
A recent paradigm shift applies interventional radiology techniques to enable highly accurate and minimally invasive osseous fixation for the treatment of unstable osseous disease in cancer patients. Many advanced locoregional disease presentations, particularly in the pelvis and proximal femur, may require minimally invasive stabilization techniques that are more durable than cementoplasty. Advanced image guidance capabilities facilitate percutaneous FICS procedures by interventional radiology to treat pathologic and cancer-related osteoporotic fractures. Furthermore, the technique may be applied for large osseous metastases as a pain palliative measure or for the prevention of impending pathologic fractures. Treatment by interventional radiology technique also allows the addition of locoregional control measures to be applied immediately before fixation for a more comprehensive treatment.
References
- 1.Tsuzuki S, Park S H, Eber M R, Peters C M, Shiozawa Y. Skeletal complications in cancer patients with bone metastases. Int J Urol. 2016;23(10):825–832. doi: 10.1111/iju.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill T, D'Alessandro P, Murray K, Yates P. Prognostic factors following pathological fractures. ANZ J Surg. 2015;85(03):159–163. doi: 10.1111/ans.12830. [DOI] [PubMed] [Google Scholar]
- 3.Kaemmerlen P, Thiesse P, Bouvard H, Biron P, Mornex F, Jonas P. [Percutaneous vertebroplasty in the treatment of metastases. Technic and results] J Radiol. 1989;70(10):557–562. [PubMed] [Google Scholar]
- 4.Anselmetti G C, Marcia S, Saba L et al. Percutaneous vertebroplasty: multi-centric results from EVEREST experience in large cohort of patients. Eur J Radiol. 2012;81(12):4083–4086. doi: 10.1016/j.ejrad.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Zhen Y, Wu C et al. CT fluoroscopy-guided percutaneous osteoplasty for the treatment of osteolytic lung cancer bone metastases to the spine and pelvis. J Vasc Interv Radiol. 2012;23(09):1135–1142. doi: 10.1016/j.jvir.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Deschamps F, Farouil G, Hakime A et al. Cementoplasty of metastases of the proximal femur: is it a safe palliative option? J Vasc Interv Radiol. 2012;23(10):1311–1316. doi: 10.1016/j.jvir.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Corcos G, Dbjay J, Mastier C et al. Cement leakage in percutaneous vertebroplasty for spinal metastases: a retrospective evaluation of incidence and risk factors. Spine. 2014;39(05):E332–E338. doi: 10.1097/BRS.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 8.Wong J M-L, Bucknill A. Fractures of the pelvic ring. Injury. 2017;48(04):795–802. doi: 10.1016/j.injury.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Iorio J A, Jakoi A M, Rehman S. Percutaneous sacroiliac screw fixation of the posterior pelvic ring. Orthop Clin North Am. 2015;46(04):511–521. doi: 10.1016/j.ocl.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Hopf J C, Krieglstein C F, Müller L P, Koslowsky T C. Percutaneous iliosacral screw fixation after osteoporotic posterior ring fractures of the pelvis reduces pain significantly in elderly patients. Injury. 2015;46(08):1631–1636. doi: 10.1016/j.injury.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Khaled S A, Soliman O, Wahed M A. Functional outcome of unstable pelvic ring injuries after iliosacral screw fixation: single versus two screw fixation. Eur J Trauma Emerg Surg. 2015;41(04):387–392. doi: 10.1007/s00068-014-0456-x. [DOI] [PubMed] [Google Scholar]
- 12.van den Bosch E W, van Zwienen C MA, van Vugt A B. Fluoroscopic positioning of sacroiliac screws in 88 patients. J Trauma. 2002;53(01):44–48. doi: 10.1097/00005373-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Pieske O, Landersdorfer C, Trumm C et al. CT-guided sacroiliac percutaneous screw placement in unstable posterior pelvic ring injuries: accuracy of screw position, injury reduction and complications in 71 patients with 136 screws. Injury. 2015;46(02):333–339. doi: 10.1016/j.injury.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Pishnamaz M, Dienstknecht T, Hoppe B et al. Assessment of pelvic injuries treated with ilio-sacral screws: injury severity and accuracy of screw positioning. Int Orthop. 2016;40(07):1495–1501. doi: 10.1007/s00264-015-2933-1. [DOI] [PubMed] [Google Scholar]
- 15.Wong J M-L, Bewsher S, Yew J, Bucknill A, de Steiger R. Fluoroscopically assisted computer navigation enables accurate percutaneous screw placement for pelvic and acetabular fracture fixation. Injury. 2015;46(06):1064–1068. doi: 10.1016/j.injury.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 16.Coste C, Asloum Y, Marcheix P S, Dijoux P, Charissoux J L, Mabit C.Percutaneous iliosacral screw fixation in unstable pelvic ring lesions: the interest of O-ARM CT-guided navigation Orthop Traumatol Surg Res 201399(4, Suppl):S273–S278. [DOI] [PubMed] [Google Scholar]
- 17.Deschamps F, de Baere T, Hakime A et al. Percutaneous osteosynthesis in the pelvis in cancer patients. Eur Radiol. 2016;26(06):1631–1639. doi: 10.1007/s00330-015-3971-1. [DOI] [PubMed] [Google Scholar]
- 18.Strobl F F, Haeussler S M, Paprottka P M et al. Technical and clinical outcome of percutaneous CT fluoroscopy-guided screw placement in unstable injuries of the posterior pelvic ring. Skeletal Radiol. 2014;43(08):1093–1100. doi: 10.1007/s00256-014-1890-x. [DOI] [PubMed] [Google Scholar]
- 19.Amoretti N, Huwart L, Hauger O et al. Percutaneous screw fixation of acetabular roof fractures by radiologists under CT and fluoroscopy guidance. AJR Am J Roentgenol. 2013;200(02):447–450. doi: 10.2214/AJR.11.7968. [DOI] [PubMed] [Google Scholar]
- 20.Pusceddu C, Fancellu A, Ballicu N, Fele R M, Sotgia B, Melis L. CT-guided percutaneous screw fixation plus cementoplasty in the treatment of painful bone metastases with fractures or a high risk of pathological fracture. Skeletal Radiol. 2017;46(04):539–545. doi: 10.1007/s00256-017-2584-y. [DOI] [PubMed] [Google Scholar]
- 21.Routt M L, Jr, Simonian P T, Grujic L. The retrograde medullary superior pubic ramus screw for the treatment of anterior pelvic ring disruptions: a new technique. J Orthop Trauma. 1995;9(01):35–44. doi: 10.1097/00005131-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Fischer S, Vogl T J, Marzi I et al. Percutaneous cannulated screw fixation of sacral fractures and sacroiliac joint disruptions with CT-controlled guidewires performed by interventionalists: single center experience in treating posterior pelvic instability. Eur J Radiol. 2015;84(02):290–294. doi: 10.1016/j.ejrad.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Deschamps F, Farouil G, Hakime A, Teriitehau C, Barah A, de Baere T. Percutaneous stabilization of impending pathological fracture of the proximal femur. Cardiovasc Intervent Radiol. 2012;35(06):1428–1432. doi: 10.1007/s00270-011-0330-8. [DOI] [PubMed] [Google Scholar]
- 24.Garnon J, Koch G, Ramamurthy N et al. Percutaneous CT and fluoroscopy-guided screw fixation of pathological fractures in the shoulder girdle: technical report of 3 cases. Cardiovasc Intervent Radiol. 2016;39(09):1332–1338. doi: 10.1007/s00270-016-1333-2. [DOI] [PubMed] [Google Scholar]
- 25.Harrington K D. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr Course Lect. 1986;35:357–381. [PubMed] [Google Scholar]
- 26.Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;(249):256–264. [PubMed] [Google Scholar]
- 27.Cazzato R L, Garnon J, Tsoumakidou G et al. Percutaneous image-guided screws meditated osteosynthesis of impeding and pathological/insufficiency fractures of the femoral neck in non-surgical cancer patients. Eur J Radiol. 2017;90:1–5. doi: 10.1016/j.ejrad.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 28.Mavrovi E, Pialat J B, Beji H, Kalenderian A C, Vaz G, Richioud B. Percutaneous osteosynthesis and cementoplasty for stabilization of malignant pathologic fractures of the proximal femur. Diagn Interv Imaging. 2017;98(06):483–489. doi: 10.1016/j.diii.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Cazzato R L, Koch G, Buy X et al. Percutaneous image-guided screw fixation of bone lesions in cancer patients: double-centre analysis of outcomes including local evolution of the treated focus. Cardiovasc Intervent Radiol. 2016;39(10):1455–1463. doi: 10.1007/s00270-016-1389-z. [DOI] [PubMed] [Google Scholar]


