Abstract
Carpal tunnel syndrome (CTS) may be treated surgically if medical treatment fails. The classical approach involves release of the flexor retinaculum by endoscopic or open surgery. Meta-analyses have shown that the risk of nerve injury may be higher with endoscopic treatment. The recent contribution of ultrasound to the diagnosis and therapeutic management of CTS opens new perspectives. Ultrasound-guided carpal tunnel release via a minimally invasive approach enables the whole operation to be performed as a percutaneous radiological procedure. The advantages are a smaller incision compared with classical techniques; great safety during the procedure by visualization of anatomic structures, particularly variations in the median nerve; and realization of the procedure under local anesthesia. These advantages lead to a reduction in postsurgical sequelae and more rapid resumption of daily activities and work. Dressings are removed by the third day postsurgery. Recent studies seem to confirm the medical, economic, and aesthetic benefits of this new approach.
Keywords: carpal tunnel syndrome, ultrasound-guided treatment, minimally invasive surgery, carpal tunnel release, interventional radiology, ultrasound-guided surgery
Objectives : Upon completion of this article, the reader will be able to identify the role of ultrasound-guided surgery for the treatment of carpal tunnel syndrome, as well as the patient selection process and potential complications arising from the treatment.
Carpal tunnel syndrome (CTS) is the most common musculoskeletal disorder in most Western countries and in the United States, with an annual incidence that varies depending on the study and the country. It affects around 5 women and 2 men in every 1,000 persons, with a peak between 40 and 60 years of age. Its prevalence is higher in diabetic patients and in women during pregnancy. 1 2 3
If medical treatment fails, carpal tunnel release is performed by surgery consisting of transection of the anterior annular ligament of the carpus (flexor retinaculum), by open or endoscopic surgery. A meta-analysis has shown that the risk of nerve injury is higher with endoscopic treatment. 4 Ultrasound has recently gained a key place in the diagnostic and therapeutic management of CTS. 5
The new technique of ultrasound-guided carpal tunnel release (UCTR) by minimally invasive surgery enables the whole operation to be performed as a percutaneous radiological interventional procedure. This opens new perspectives, by decreasing the overall cost of treatment, and promises greater safety with a more rapid resumption of daily activities. 6 7
We describe the classical treatments and compare them with the ultrasound-guided procedure of carpal tunnel release that we currently use.
Classical Treatments
Medical treatment consists of the removal of any microtraumatic factors; immobilization of the wrist with a splint, particularly during the night; and one or two cortisone injections into the carpal tunnel, possibly guided by ultrasound. The results are good in uncomplicated forms of CTS. 1
Surgical treatment is proposed in cases of medical treatment failure or aggravation of CTS despite treatment, and also in severe forms of the disease. Treatment consists of neurolysis by transection of the retinaculum.
In the United States, the number of surgical procedures has increased over the past 20 years, but has recently stabilized. In 2006, a total of 576,924 surgical carpal tunnel procedures were performed. 3 By comparison, in France, the number of interventions increased from 95,370 in 1995 to 142,405 in 2005. 1 3 The costs of CTS are linked to anesthesia, surgical treatment, and absence from work. The intervention is almost always performed as day surgery under local anesthesia with a pneumatic tourniquet. In addition, a complementary procedure of synovectomy may be performed.
Several surgical techniques exist and employ different surgical instruments for transection. These include classical open surgery (and its variant, miniopen surgery) and endoscopy.
Open Surgery
A short incision, 3 to 4 cm in length, is made in the palm in the axis of the fourth finger without passing Kaplan's cardinal line. In miniopen surgery, the size of the incision is reduced to 2 cm. Dissection is performed layer by layer and transection of the retinaculum is performed with a lancet after having controlled the position of the thenar motor branch. Nerve release is checked and the layers reclosed. It is necessary for the patient to stop work and daily activities for a period of approximately 3 to 4 weeks.
Endoscopic Procedure
Several possible endoscopic procedures exist, using either single or double access. This technique has resulted in a reduction in morbidity and an acceleration in recovery time due to the smaller incision size. 8 Transection of the retinaculum takes place after introduction of an endoscope with a retractable blade through a 1.5- to 2-cm incision in the wrist flexion crease. A break from work and daily activities of around 2 to 3 weeks is then recommended.
A meta-analysis recently demonstrated that although the medium- to long-term results are identical irrespective of the surgical procedure, endoscopy results in more rapid recovery of motor strength. Conversely, the risk of transitory nerve injury is higher than with open surgery. 4
Ultrasound-Guided Surgery
Several studies have recently demonstrated the interest of ultrasound-guided transection of the retinaculum. 2 3 4 5 6 7 This technique has the advantages of (1) great safety, due to continuous ultrasound monitoring and visualization of variants of the median nerve, 8 9 10 and (2) a decrease in size of the incision, enabling a more rapid return to work and a more aesthetic scar. 6 11
Percutaneous access by ultrasound-guided surgery (UCTR) is, at least, 10 times smaller than with classical surgical techniques. Open surgery requires an incision of 4 to 5 cm, miniopen surgery requires an incision of 2 cm, and endoscopic treatment requires an incision of 1.5 to 2 cm. More recent techniques of UCTR 6 7 require an incision of 0.1 to 0.3 cm only. 11 Evidently, the advantage is aesthetic, but the speed of scar formation is also accelerated allowing a more rapid resumption of daily activities and work. 6 12 The progression of classical open techniques with a large incision toward miniopen or endoscopic surgical techniques has already halved the time in which patients can resume work and daily activities. 13 14 Future progress in the miniaturization of access will improve these results even further.
Rojo-Manaute et al compared UCTR, with an incision of 1 mm, to miniopen surgery, with an incision of 20 mm, in 128 patients. 6 Algofunctional score, grip strength, disappearance of paraesthesia, and return to daily activities and work were evaluated. Algofunctional score clearly improved much faster in the group treated by UCTR than in the group treated by miniopen surgery. It was 2-fold less severe after only 1 week (23.6 vs. 52.6), and 3.3-fold less severe 6 months after the intervention (4.9 vs. 13.0). Daily activities were also resumed significantly earlier in the group treated by UCTR (4.9 vs. 25.4 days).
Technique of Carpal Tunnel Release by Ultrasound-Guided Surgery
The protocol that we have published previously 6 and have used routinely in our unit is described below.
Preoperative Consultation
The following information was checked systematically during the preoperative consultation: history of diabetes, hypothyroidism, wrist fracture, or inflammatory arthritis that could lead to medical treatment or alternative surgery. A history of cardiovascular disease could lead to adaptation of anticoagulant treatment. In the case of doubt or important comorbidities, an anesthesia consultation is recommended.
In our unit, good indications for UCTR include a CTS evolution of more than 6 months or rapid aggravation of the disease, the failure of at least one corticosteroid injection, and diagnostic confirmation by electromyography and/or ultrasound. An informed consent form is given to the patients systematically, explaining the benefits and risks of the procedure and the risks of failure, particularly in severe forms of CTS. The important step in this pretherapeutic consultation is to carry out preoperative and diagnostic ultrasound.
Ultrasound has become a simple examination with limited cost implications related to lengthening of the clinical examination. 5 An increase in cross-sectional area (CSA) of the median nerve at the proximal end of the carpal tunnel is the most classic parameter. A value of greater than 12 mm 2 indicates compression of the median nerve. 15 16
Klauser has demonstrated the interest of taking two measurements, which we also practice. The distal CSA is compared with the proximal CSA. 17 One ultrasound measurement is taken at the level of the carpal tunnel and the second is taken more proximally at the level of the pronator quadratus muscle. In addition to confirming the diagnosis of CTS, ultrasound can also be used to identify pathology of the surrounding structures that may compress the median nerve: tenosynovitis, ganglion cyst, tumors, lipoma, or muscular variants. 18 19 20
We have demonstrated that ultrasound is pertinent for analyzing the thenar motor branch of the median nerve. Ultrasound assessment of a transligament pathway at risk of iatrogenic injury during transection of the retinaculum 15 is a clear advantage of UCTR. 9 10 The position of the vascular arch is located. Finally, location of a safe zone between the ulnar artery and the median nerve enables visualization of the virtual path of the surgical instruments. 12
Installation and Instrumentation
We carry out this CTS procedure in an interventional radiography room or in our day surgery department. The patient is not required to have an empty stomach ( Fig. 1 ).
Fig. 1.
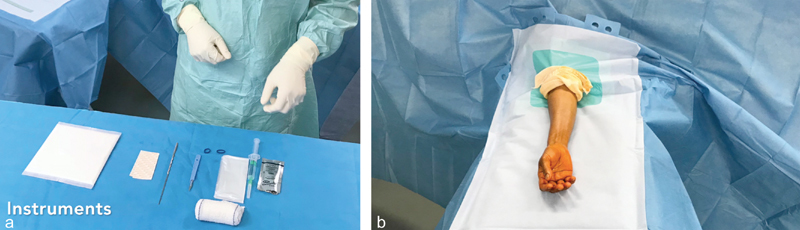
( a ) Instruments used for transection of the flexor retinaculum with ultrasound guidance and ( b ) patient position. The patient is lying down in the supine position, without a tourniquet, under strict aseptic conditions, with an open palm.
The patient is placed in the dorsal decubitus position, with the arm held at 90 degrees to the body on a support, palm facing up. Strict aseptic conditions are recommended with the radiologist wearing a mask, sterile gloves, and a gown. A high-frequency linear ultrasound probe is recommended (>16 MHz), protected with a probe shield. The patient's arm is disinfected three times and then covered with a sterile drape.
We use two needles (25G and 21G), a size 11 scalpel, and a hook knife. 7
Anesthesia and Hydrodissection
Ultrasound-guided mapping of the wrist is performed to locate the safe zone corresponding to the space between the ulnar artery and the median nerve. 12
Subcutaneous local anesthesia is performed with a 25G needle and 2 mL of lidocaine 1%, approximately 1.5 cm above the flexion crease on the ulnar side of the wrist. A second deeper injection is then performed around the median nerve with 3 mL of lidocaine and physiological saline using the same needle or a much longer 21G needle, depending on the diameter of the wrist. This fundamental step of our protocol provides both nerve block and hydrodissection, enlarging the safe zone within the tunnel and creating a chamber for passage of the surgical instruments ( Fig. 2 ).
Fig. 2.
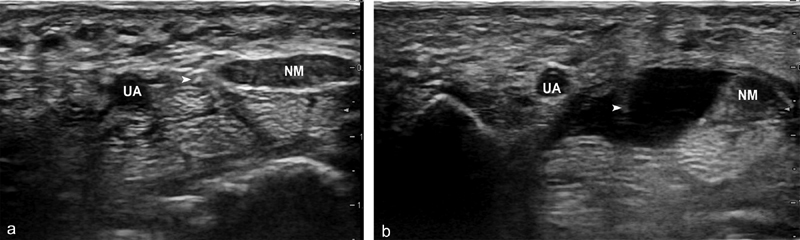
Hydrodissection. ( a ) Subcutaneous anesthesia is introduced and the needle ( arrow ) is then advanced slowly through the middle of the safe zone between the median nerve (MN) and ulnar artery (UA), with continuous axial ultrasound monitoring. ( b ) Using injected anesthetic, hydrodissection prepares the carpal tunnel for the action of the hook knife.
Placement of the Instruments
A punctiform percutaneous incision is made under ultrasound guidance using a scalpel (size 11 blade), passing the skin and palmar carpal ligament, which will have been previously completely detached by hydrodissection of the deeper tendon layer. The blade is kept tangential to the cutaneous layer in order not to accidentally cut any deeper. The instrument is then introduced under ultrasound guidance and advanced progressively under the retinaculum taking care to stay away from the median nerve and sensory branches such as the Berrettini branch distally. The apex of the hook of the hamate is used to estimate the position of the distal fibers of retinaculum which lies approximately 11 to 18.2 mm distally ( Fig. 3 ). 21
Fig. 3.
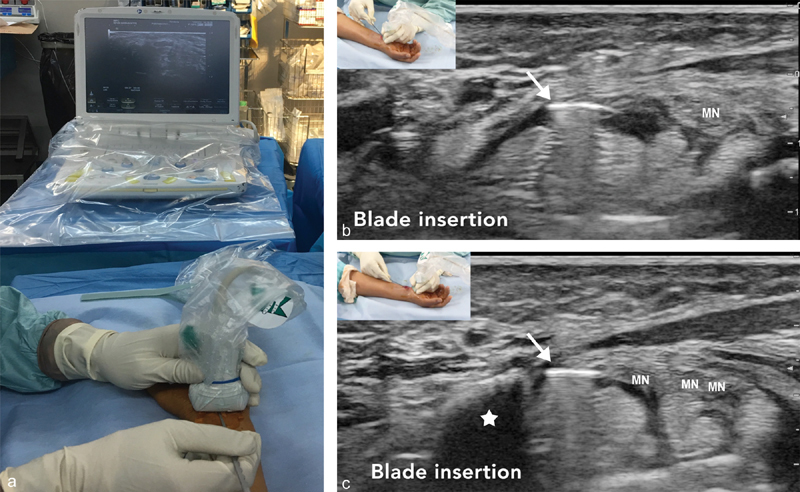
( a ) The hook knife is positioned horizontally. Corresponding axial ultrasound images at ( b ) the proximal and ( c ) distal carpal tunnel. Hook knife artifact ( arrow ) under the flexor retinaculum. Hook of Hamate bone (white star). Median nerve (MN) before and after division.
Flexor Retinaculum Release
The hook knife, which has been advanced horizontally so as not to damage the surrounding tissue, is turned to the vertical position. The retinaculum is then cut in a retrograde manner by progressive withdrawal of the hook knife under continuous ultrasound guidance ( Fig. 4 ).
Fig. 4.
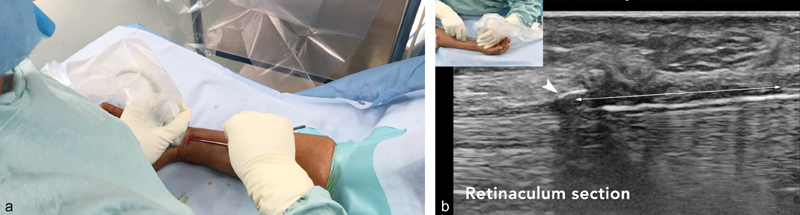
( a ) The hook is turned vertically and the retinaculum is then transected in a retrograde manner with continuous ultrasound monitoring. Corresponding sagittal ultrasound image ( b , white arrow ) during progressive retrograde transection of the flexor retinaculum. Flexor retinaculum ( double arrow ).
Fig. 5.
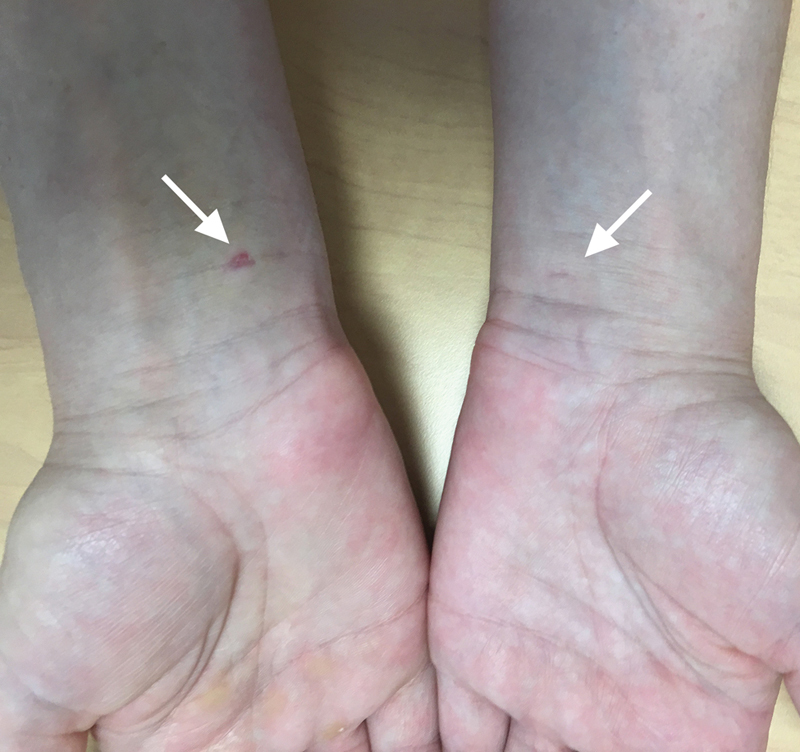
Percutaneous ultrasound-guided carpal tunnel release. Three-day postoperative appearance (right hand) and 6-week postoperative scar on the contralateral hand (left hand).
Control
At the end of the procedure, carpal tunnel release is verified using an injection of physiological saline which distends the opening of the retinaculum. The introduction of a Canula into the tunnel also confirms release.
Duration
The procedure of CTS release, including the percutaneous incision, is rapid. In our series, it was estimated to be 5.8 ± 2.4 minutes depending on the anatomy and the response to anesthesia, and the time of occupation of the room was estimated to be 23.2 ± 4.8 minutes. 7 The patient could return home within 1 hour of the end of the procedure.
Dressings
The incision is only 2 millimeters in size and is dressed with a compression bandage which the patient can remove himself or herself 3 days after the surgery.
Contraindications
To our knowledge, there are no contraindications to UCTR. The absence of visualization of the thenar motor branch, in particular when it is covered by the thenar muscle, should alert the radiologist to the existence of anatomic variation and open surgery should be preferred. In the case of secondary CTS, the absence of treatment of the primary cause (inflammatory tenosynovitis, cyst formation or compressive tissue, etc.) should alert the interventional radiologist to the possibility of failure and should lead to discussions about a two-step release or even immediate release by open surgery.
Interest of the Technique
Many UCTR techniques have been described 2 3 4 5 6 7 12 ( Table 1 ). To our knowledge, our original technique is the first description published by interventional radiologists. The protocol appears to be much simpler and cheaper in terms of materials than the different series published previously ( Table 1 ). The use of an ultrasound-guided hydrodissection step had enabled us to eliminate the need for dissecting scissors, retractors, trocars, or a Kirchner guide. The whole procedure is performed under ultrasound guidance and local anesthesia until final withdrawal of the instruments.
Table 1. Summary of publications reporting UCTR for the treatment of carpal tunnel syndrome.
| Reference | No. of patients | Type of study | Surgical approach | Instruments | Incision size | Postsurgical analysis | Follow-up | Complications |
|---|---|---|---|---|---|---|---|---|
| Nakamichi et al 1997 | 103 | Prospective, comparative, monocentric, randomized (50 vs. 53) | Mixed (miniopen surgery + UCTR) Access palmar Anterograde section under the FR versus miniopen (distal flexion crease) |
Dissecting scissors Basket punch Trocar |
15 vs. 10 mm | Pain Paraesthesia Grip test Sensitivity |
24 mo | 0 |
| Nakamichi et al 2010 | 74 | Prospective, comparative, monocentric, randomized (35 vs. 39) vs. miniopen | Mixed (surgery + UCTR) Access palmar Anterograde section under the FR |
Hook knife + guide + trocar | 4 vs. 10–15 mm | Pain Paraesthesia Grip test Sensitivity |
24 mo | 0 |
| Petrover et al 2017 7 | 39 | Monocentric, open, noncontrolled Interventional ultrasound room |
Dissection with scissors UCTR under the FR Retrograde Wrist flexion crease 19.0 ± 4.6 min |
Dissecting Scissors Endoscope Trocar Specific hook knife |
5 mm | Pain score Paraesthesia Resumption of activity |
3 mo | 0 |
| Capa-Grasa et al 2014 5 | 57 | Pilot study of feasibility, monocentric, randomized, comparative vs. miniopen (office-based surgery) | UCTR Wrist flexion crease Under the FR Retrograde Interval between patients 19.1 ± 1.41 min |
Hook knife + Kirschner's guide | 1–2 mm vs. 2 cm | Quick-Dash questionnaire Grip test |
3 mo | 0 |
| Rojo-Manaute et al 2016 6 | 128 | Monocentric, randomized, comparative vs. miniopen (office-based surgery) | UCTR Retrograde Under the FR Vs. miniopen Wrist flexion crease |
Hook knife + Kirschner's guide | 1–2 mm vs. 2 cm | Quick-Dash questionnaire Grip test Paraesthesia Resumption of daily activity and work |
12 mo | 0 |
| McShane et al 2012 22 | 17 | Retrospective Monocentric |
Needle fenestration Under the FR Wrist flexion crease |
18G needle xylo + betamethasone |
1 mm | DASH score Grip test Ultrasound median nerve diameter |
9.2 mo | 0 |
| Chern et al 2015 4 | 91 | Monocentric | Superficial route Below the FR Retrograde Wrist flexion crease |
Specific hook knife + Kirschner's guide | 2 mm | Boston score Grip test |
22.5 mo | 0 (1 recurrence 14 mo) |
| Petrover et al 2017 9 | 129 | Monocentric, open Interventional ultrasound block |
UCTR Under the FR Retrograde section Wrist flexion crease 5.8 ± 2.4 min |
Hook knife | 2 mm | Boston score MRI |
6 mo | 0 |
Abbreviations: FR, flexor retinaculum; MRI, magnetic resonance imaging; UCTR, ultrasound-guided carpal tunnel release.
Comparison of the Techniques
Most studies have been published by surgeons, which explain the earlier use of ultrasound as an aid to good positioning of the instruments and the need for dissection of the skin layers to insert the instrumentation ( Table 1 ). The whole percutaneous protocol does not require any scissors or retractors, in contrast to previous reports.
Nakamichi was the first to describe a hybrid technique releasing the distal portion of the retinaculum by open surgery and the proximal portion of the ligament under ultrasound guidance with very good results when compared with classical surgery. 2 The clinical results in terms of paraesthesia were identical, but regain of grip strength and scar-related pain were better in the group where surgery was performed partially using ultrasound, from the third week and confirmed at 6 weeks. In contrast, the incision was palmar and was relatively large (15 mm). In a more recent series, the same team changed the type of access for proximal access, and the material used, comparing this mixed technique to open surgery and confirming their initial results. 12
We did not use the palmar route of access because this requires anesthesia of the palm which could be more painful and risks hampering ultrasound analysis of the distal branches and the position of the tendons as well as visualization of the superficial vascular arch. Lecoq et al described a technique requiring a proximal incision of 5 mm and then dissection of the subcutaneous layers with scissors and finally the insertion of an endoscopic trocar to introduce the blade for retrograde section. The mean duration of the intervention described was 19.0 ± 4.5 minutes with a duration of occupation of the room of 38 ± 8 minutes. The algofunctional score was reduced significantly in the 39 patients from the 15th day in this series. Return to work was possible in one-third of patients on the 10th day and daily activities were resumed from the seventh day. 3
Rojo-Manaute et al described a technique termed “ultra-mini-invasive.” After an incision of 1 mm and preparation of a tunnel with the help of a Kirchner pin, an Acufex blade was inserted and then sectioning was performed in a retrograde manner. 6 16
The technique described by Chern et al carrying out retrograde section under ultrasound guidance but via the superficial route, that is above the retinaculum, in 91 patients also gave good results with, however, one failure linked to partial section. 4 The position, over the retinaculum, seems to carry the risk of partial release by not releasing the deeper fibers which will maintain compression of the median nerve. This argument, the immediate use of a guide to insert the hook knife and the artifact of the instrument placed over the retinaculum, which may mask the nerve, led us ignore this type of access.
Our 129 consecutively treated patients were significantly improved with a Boston score decreasing from 3.3 ± 0.7 to 1.7 ± 0.4 at 1 month and to 1.3 ± 0.3 at 6 months after the intervention. Magnetic resonance imaging performed systematically at 1 month showed complete transection of the retinaculum in 100% of cases with decompression of the medial nerve in nearly 90% of cases. 7
Other original percutaneous procedures have been described with good results, some using hydrodissection, but only allowing partial needle release of the retinaculum, 22 others enabling complete transection but specific materials requiring double access. 23 24 To our knowledge, no complications have been reported in the literature. A large multicenter, randomized, comparative study with endoscopy and miniopen surgery is still needed to confirm these optimistic results.
Conclusion
UCTR of the carpal tunnel has a certain future at the crossroads of radiology, rheumatology, and surgery. UCTR is a new step in the evolution of surgery toward miniaturization of access and endoscopic instrumentation, and interventional radiology is now able to benefit from these advantages.
Continuous safety of the procedure by ultrasound guidance and a reduction in the incision and dissection of the carpal tunnel enables a more rapid postoperative recovery. The procedure performed by ambulatory surgery under simple local anesthesia appears to give results that are at least as good medium-term as classical surgical, but with better short-term sequelae. These optimistic results need to be confirmed in large randomized clinical trials to make this technique the new gold standard for the treatment of CTS.
References
- 1.Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(02):CD001554. doi: 10.1002/14651858.CD001554.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Nakamichi K, Tachibana S. Ultrasonographically assisted carpal tunnel release. J Hand Surg Am. 1997;22(05):853–862. doi: 10.1016/s0363-5023(97)80081-0. [DOI] [PubMed] [Google Scholar]
- 3.Lecoq B, Hanouz N, Morello R et al. Ultrasound-assisted surgical release of carpal tunnel syndrome: results of a pilot open-label uncontrolled trial conducted outside the operating theatre. Joint Bone Spine. 2015;82(06):442–445. doi: 10.1016/j.jbspin.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Chern T C, Kuo L C, Shao C J, Wu T T, Wu K C, Jou I M. Ultrasonographically guided percutaneous carpal tunnel release: early clinical experiences and outcomes. Arthroscopy. 2015;31(12):2400–2410. doi: 10.1016/j.arthro.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Capa-Grasa A, Rojo-Manaute J M, Rodríguez F C, Martín J V. Ultra minimally invasive sonographically guided carpal tunnel release: an external pilot study. Orthop Traumatol Surg Res. 2014;100(03):287–292. doi: 10.1016/j.otsr.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Rojo-Manaute J M, Capa-Grasa A, Chana-Rodríguez F et al. Ultra-minimally invasive ultrasound-guided carpal tunnel release: a randomized clinical trial. J Ultrasound Med. 2016;35(06):1149–1157. doi: 10.7863/ultra.15.07001. [DOI] [PubMed] [Google Scholar]
- 7.Petrover D, Silvera J, De Baere T, Vigan M, Hakimé A. Percutaneous ultrasound-guided carpal tunnel release: study upon clinical efficacy and safety. Cardiovasc Intervent Radiol. 2017;40(04):568–575. doi: 10.1007/s00270-016-1545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith J, Barnes D E, Barnes K J et al. Sonographic visualization of thenar motor branch of the median nerve: a cadaveric validation study. PM R. 2017;9(02):159–169. doi: 10.1016/j.pmrj.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Petrover D, Bellity J, Vigan M, Nizard R, Hakime A. Ultrasound imaging of the thenar motor branch of the median nerve: a cadaveric study. Eur Radiol. 2017;27(11):4883–4888. doi: 10.1007/s00330-017-4882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riegler G, Pivec C, Platzgummer H et al. High-resolution ultrasound visualization of the recurrent motor branch of the median nerve: normal and first pathological findings. Eur Radiol. 2017;27(07):2941–2949. doi: 10.1007/s00330-016-4671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrover D, Richette P. Treatment of carpal tunnel syndrome: from ultrasonography to ultrasound guided carpal tunnel release. Joint Bone Spine. 2018;85(03):243–250. doi: 10.1016/j.jbspin.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Nakamichi K, Tachibana S, Yamamoto S, Ida M. Percutaneous carpal tunnel release compared with mini-open release using ultrasonographic guidance for both techniques. J Hand Surg Am. 2010;35(03):437–445. doi: 10.1016/j.jhsa.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Jugovac I, Burgić N, Mićović V et al. Carpal tunnel release by limited palmar incision vs traditional open technique: randomized controlled trial. Croat Med J. 2002;43(01):33–36. [PubMed] [Google Scholar]
- 14.Cellocco P, Rossi C, Bizzarri F, Patrizio L, Costanzo G. Mini-open blind procedure versus limited open technique for carpal tunnel release: a 30-month follow-up study. J Hand Surg Am. 2005;30(03):493–499. doi: 10.1016/j.jhsa.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan P, Mishra R, Jena M, Das A. Transligamentous thenar branch of the median nerve: the million dollar nerve. Neurol India. 2013;61(03):311–312. doi: 10.4103/0028-3886.115078. [DOI] [PubMed] [Google Scholar]
- 16.Rojo-Manaute J M, Capa-Grasa A, Rodríguez-Maruri G E, Moran L M, Martínez M V, Martín J V. Ultra-minimally invasive sonographically guided carpal tunnel release: anatomic study of a new technique. J Ultrasound Med. 2013;32(01):131–142. doi: 10.7863/jum.2013.32.1.131. [DOI] [PubMed] [Google Scholar]
- 17.Klauser A S, Abd Ellah M M, Halpern E J et al. Sonographic cross-sectional area measurement in carpal tunnel syndrome patients: can delta and ratio calculations predict severity compared to nerve conduction studies? Eur Radiol. 2015;25(08):2419–2427. doi: 10.1007/s00330-015-3649-8. [DOI] [PubMed] [Google Scholar]
- 18.Cossey A J, Stranks G J. Intramuscular lipoma in an anomalous muscle belly of the middle finger lumbrical as a cause of carpal tunnel syndrome and trigger wrist. Orthopedics. 2003;26(01):85–86. doi: 10.3928/0147-7447-20030101-21. [DOI] [PubMed] [Google Scholar]
- 19.Kerasnoudis A. Elongated muscle belly of the flexor digitorum superficial causing carpal tunnel syndrome. Hand (NY) 2012;7(03):333–334. doi: 10.1007/s11552-012-9435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Smet L. Median and ulnar nerve compression at the wrist caused by anomalous muscles. Acta Orthop Belg. 2002;68(05):431–438. [PubMed] [Google Scholar]
- 21.Sytsma T T, Ryan H S, Lachman N, Kakar S, Smith J. Anatomic relationship between the hook of the hamate and the distal transverse carpal ligament: implications for ultrasound-guided carpal tunnel release. Am J Phys Med Rehabil. 2018;97(07):482–487. doi: 10.1097/PHM.0000000000000902. [DOI] [PubMed] [Google Scholar]
- 22.McShane J M, Slaff S, Gold J E, Nazarian L N. Sonographically guided percutaneous needle release of the carpal tunnel for treatment of carpal tunnel syndrome: preliminary report. J Ultrasound Med. 2012;31(09):1341–1349. doi: 10.7863/jum.2012.31.9.1341. [DOI] [PubMed] [Google Scholar]
- 23.Markison R E. Percutaneous ultrasound-guided MANOS carpal tunnel release technique. Hand (NY) 2013;8(04):445–449. doi: 10.1007/s11552-013-9554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo D, Guo D, Guo J, Schmidt S C, Lytie R M. A clinical study of the modified thread carpal tunnel release (TCTR) Hand (NY) 2017;12(05):453–460. doi: 10.1177/1558944716668831. [DOI] [PMC free article] [PubMed] [Google Scholar]


