Abstract
Benign bone tumors consist of a wide variety of neoplasms that do not metastasize but can still cause local complications. Historical management of these tumors has included surgical treatment for lesion resection and possible mechanical stabilization. Initial percutaneous ablation techniques were described for osteoid osteoma management. The successful experience from these resulted in further percutaneous image-guided techniques being attempted, and in other benign bone tumor types. In this article, we present the most common benign bone tumors and describe the available results for the percutaneous treatment of these lesions.
Keywords: benign bone tumor, radiofrequency ablation, cryoablation, osteoid osteoma, vertebral hemangioma, interventional radiology
Objectives : Upon completion of this article, the reader will be able to (1) identify common benign bone tumors and their respective percutaneous treatment options; (2) discuss various ablation procedure techniques and current results for the treatment of these lesions.
Benign bone tumors consist of a wide variety of mesenchymal neoplasms that generally do not metastasize to other regions of the body, although they can still cause local complications as they grow, potentially compressing healthy bone or surrounding tissue. Historical management of benign bone tumors has included surgical treatment for lesion resection and possible mechanical stabilization. Since the early 1990s, percutaneous ablation techniques have been described for benign bone tumor management, initially for osteoid osteomas. 1 Percutaneous treatment for this indication is usually curative after a single treatment with very low complication rate. 2 This successful experience with radiofrequency has resulted in other percutaneous image-guided techniques being attempted with other benign bone tumor types. In this article, we present the most common benign bone tumors and describe the available results for the percutaneous treatment of these lesions.
Osteoid Osteoma
Epidemiology, Imaging Findings
Osteoid osteomas represent 12% of all skeletal neoplasms. They are small, painful bone tumors, usually measuring less than 1.5 cm in diameter. They typically occur in young male patients in the first or second decade of life. Patients often present with pain, which is characteristically more pronounced at night and relieved by nonsteroidal anti-inflammatory drugs, especially acetylsalicylic acid. 3
The most common location of osteoid osteomas is the cortex of long bones with 50% of them found in the fibula or tibia. 4 Osteoid osteomas consist of a vascularized soft-tissue nidus with variable central mineralization and also variable surrounding bony reaction. 5 Four types of osteoid osteomas have been described with specific characteristics: intracortical, with dense sclerosis around the nidus; periosteal, with thick periosteal reaction; spongiosal, which produces very little reactive bone; and subarticular, which induces arthritis as it produces synovial reaction. 4 5
The typical cortical osteoid osteoma appears as a round or oval radiolucent nidus, which may be partially mineralized, surrounded by thickened cortex or periosteal reaction ( Fig. 1 ). Due to its hypervascular composition, the nidus shows an increase in radiotracer activity on technetium-99m methylene diphosphonate skeletal scintigraphy and an early intense contrast enhancement on magnetic resonance imaging (MRI) after gadolinium administration. 3
Fig. 1.
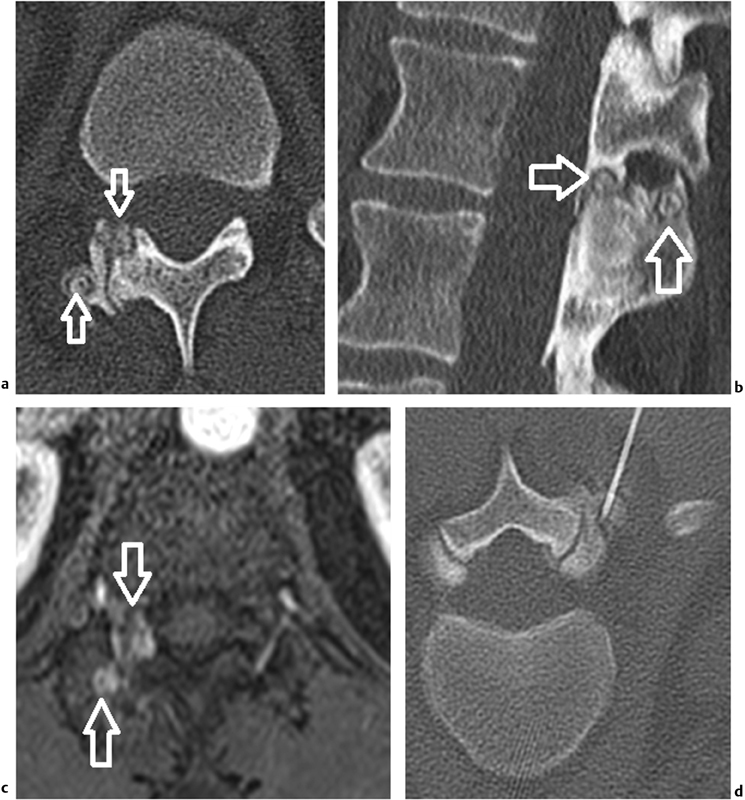
A 32-year-old man with back pain caused by two osteoid osteomas of the right upper articular process of L1. Axial ( a ) and oblique ( b ) unenhanced CT scan identifies two round dense niduses ( white arrows ) surrounded by thickened cortical reaction. Note the induced arthritis of the adjacent joint. ( c ) axial fat-suppressed T1-weighted contrast-enhanced MR image demonstrates early intense contrast enhancement of the two niduses ( white arrows ). ( d ) Axial prone per-procedure CT scan shows the position of a laser fiber in the nidus.
Treatment
Complete surgical excision of the nidus was considered for years as the gold standard treatment for osteoid osteomas. This treatment was effective for most of the patients but due to potentially poor visualization of the tumor during open surgery, there was a risk of local recurrence from mistargeting the tumor. 6
The development of computed tomography (CT) guidance has helped in the minimally invasive treatment of this tumor by percutaneous bone drilling and resection. This surgical technique allows “en bloc” tumor resection in a 1-cm diameter bone cylinder to obtain histopathological diagnosis and treatment, but exposes the patient to the risks of secondary fracture due to bone weakening and local recurrence in case of more than 1-cm large nidus. 7
Percutaneous ablation of osteoid osteoma may be performed using any thermal ablation techniques, either by heating or freezing methods. Complications can be minimized if the smallest possible volume of normal tissue is damaged in the ablation by using controlled small-size techniques. 8 The small size of osteoid osteoma allows either laser or radiofrequency ablation as suitable options. For lesions up to 15 mm in maximal dimension, the use of one laser fiber is sufficient to achieve successful ablation. 2 In this scenario, a 2-W continuous power laser is used for a mean delivered energy of 1,271 Joules 2 (the minimum targeted delivered energy can be calculated according to the equation: E (Joules) = (nidus size in millimeters × 100) + 200). 9
Radiofrequency ablation is the most commonly used technique for percutaneous osteoid osteoma ablation and can be performed by using low-powered generators (35–50 W) and simple straight electrodes with exposed tips measuring between 5 and 8 mm. Electrodes producing a larger ablation zone and which are designed for cancer ablation (such as multi-tined or internally cooled electrodes) are not recommended in this indication due to the potential to cause more tissue damage than is required, exposing the patient to greater risk of complication and postoperative pain. 8 However, the use of small ablation zones requires precise electrode placement into the center of the nidus when using only one application of energy, although sometimes two or more electrode placements might be necessary. Adequate image guidance is mandatory to achieve sufficient precision for applicator placement and intraprocedural ablation zone monitoring. CT, with or without fluoroscopy, is the most commonly used imaging modality for osteoid osteoma ablations. CT provides quick acquisition, thin slices, high-image resolution, and possibility for 3D reconstructions. 10 Access of the nidus may be the most challenging part of the procedure due to the hardness of the cortical bone in children and young adults, frequently aggravated by the local bony reaction and cortical thickening induced by the osteoid osteoma. In such cases, a bone biopsy needle or a sturdy drill might be used to access the nidus.
Laser photocoagulation and radiofrequency ablation have similar success rates (range, 80–100%), 11 12 and are reference-standard treatments to achieve short-term pain relief (within 2 weeks). 2 Lasting freedom from symptoms has been reported in 96% of patients. 13 Lanza et al 14 mentioned that in more than 1,700 patients treated percutaneously with either radiofrequency or laser ablation, the registered success rate was 90 to 100%, the complication rate was less than 2%, and the recurrence rate was approximately 5%. Most recurrences are seen relatively early (within 6 months of treatment) and are most likely due to incomplete ablation of the nidus. 8 13
Osteoblastoma
Epidemiology, Imaging Findings
Osteoblastoma is a rare benign bone tumor accounting for 14% of all tumor tumors. 4 It affects people mainly in their second or third decade of life, also with a male predilection (male:female ratio of 2.5:1). 15 Osteoblastomas are histologically similar to osteoid osteoma. But compared with osteoid osteomas macroscopically, they are larger (typically >2 cm in diameter) and expansile with less sclerotic components, although they may have thin peripheral sclerosis and they can be associated with aneurysmal bone cyst (ABC). On MR imaging, they demonstrate intense osseous and extraosseous enhancement. 15 Although any bone can be involved, osteoblastoma arises predominantly in the axial skeleton with spinal lesions constituting one-third of reported cases. 4 They often involve the posterior elements and are in the cervical spine in 10 to 40% of cases.
Treatment
The options for image-guided ablation techniques using CT are similar to those used for osteoid osteoma. However, given the larger size of osteoblastomas and especially with the involvement of posterior elements and potential soft-tissue components, cryoablation could also be considered as a safer option, with simultaneous placement of multiple probes and visualization of the ice ball to protect adjacent neurovascular structures. 10 15 Considering the large size of osteoblastomas, if using laser ablation, simultaneous multiple laser fibers may be necessary to achieve complete ablation. When radiofrequency ablation is considered, multiple applications or the use of bipolar systems, cooled electrodes, and longer ablation times may be needed to cover the entire tumor. An intense inflammatory postablation reaction may occur following thermal ablation, and nonsteroidal anti-inflammatory drugs (e.g., COX-2 inhibitor therapy) may be administered to limit the immediate posttreatment symptoms. 10 Weber et al compared radiofrequency ablation to surgery for the treatment of osteoblastomas. 16 They reported a technical success rate of 95%, with primary and secondary success rates of 89.5 and 100% for radiofrequency ablation. All patients were pain free 1 month after the procedure, whereas a longer duration (1–6 months) was needed after surgery to obtain a complete pain relief.
Hemangioma
Epidemiology, Imaging Findings
Vertebral hemangiomas (VH) are benign vascular lesions involving the spine with an incidence of 10 to 12%, usually found in young adults, with a slight female predilection. 17
Most VH are latent and do not require specific treatment; only 1% of VH become symptomatic. 18 Pain and aggressiveness, by its extension into the spinal canal or to the paravertebral space, are indications of a symptomatic VH. 18
Most of these lesions are asymptomatic and are seen as an incidental finding during imaging exams. 18 Their different imaging findings are related to the histological composition of VH: the amount of adipocytes, vessels, and interstitial edema. 15 18 Imaging findings for typical VH on radiographic images are coarsened vertical trabeculae, represented by a vertical striation and/or palisade pattern in the vertebral body. 19 CT images can demonstrate vertically oriented, sparse vertebral trabeculae separated by fatty tissue, with subsequent typical hyperintensity on T1- and T2-weighted MRI. 18 20 The diagnosis of atypical or aggressive VH may be difficult, as they can mimic primary bone tumor or metastases. Aggressive VH contain less fat and more vascular stroma, resulting in lower signal intensity on T1-weighted MRI. 19
Treatment
Numerous tumor ablation techniques have been described including ethanol treatment, embolization, cryoablation, vertebroplasty, radiotherapy, and radiosurgery. 15 17 18 21
Surgical intervention is reserved for VH associated with neurologic compromise and spinal instability, and radiation therapy is used when immobilization fails to resolve neurologic symptoms or for lesion progression after immobilization. 19 22 However, surgical intervention may be complicated by profuse intraoperative bleeding and postoperative epidural hematoma. 19
Aggressive VH are now frequently treated successfully with vertebroplasty. This technique aims to percutaneously inject cement (polymethylmethacrylate, PMMA) directly into the lesion under fluoroscopic guidance. PMMA polymerization causes irreversible sclerosis of the venous component of the VH and reinforces the bone's mechanical stability, thus obtaining an antalgic effect and preventing vertebral collapse. 19 Guarnieri et al 21 and Liu et al 19 demonstrated, in their studies of, respectively, 24 and 33 patients, the efficacy of vertebroplasty on pain control for all treated patients with aggressive VH, without symptomatic complication. The main risk of vertebroplasty for VH treatment is cement leakage. In this indication, the incidence of cement leakage may increase due to an anomalous intravertebral vascularization, wide high-flow blood in dilated vascular channels, formation of venous neoanastomosis, and with an incomplete vertebral cortex. 19 Vertebroplasty achieves thrombosis of the VH and consolidation of the vertebral body in a single procedure but is not suitable to treat any potential extraosseous extension of VH, which would require prior sclerotherapy.
Sclerotherapy is a percutaneous VH ablation technique that causes necrosis directly through cellular dehydration, and indirectly through vascular thrombosis and tissue ischemia ( Fig. 2 ). 23 The venous drainage of the VH must be carefully analyzed before sclerotherapy, particularly to identify the possible origin of the artery of Adamkiewicz or of any other vascular anastomosis to the spinal cord vascularization, which would then contraindicate this treatment. 23 24 To obtain a higher viscosity and to gain better control during injection, we use in our institution a mixture of ethiodized oil and alcoholic solution of zein. Sclerotherapy can result in an intense acute inflammatory reaction that might worsen compression on neurological structures in the first 3 days after the treatment. 25 To prevent neurological impairment, high doses of intravenous corticosteroids should be administered to the patient over 4 days of duration, starting the day before sclerotherapy. Due to the opacity of the sclerosant mixture, PMMA injection could not be injected safely during the same procedure but might be performed in the 10 to 15 days after sclerotherapy. In our unpublished series of 61 VH treated by sclerotherapy and vertebroplasty, in only one patient (1.7%) did a major complication occur. The patient presented 12 hours following sclerotherapy with a transient medullary cord compression syndrome due to the intense inflammatory response in the epidural component of an aggressive VH of T9. Seven patients (11.5%) had minor complications consisting of asymptomatic PMMA venous leakage. We reached clinical success in 92.1 and 80%, respectively, for aggressive VH type III and type IV.
Fig. 2.
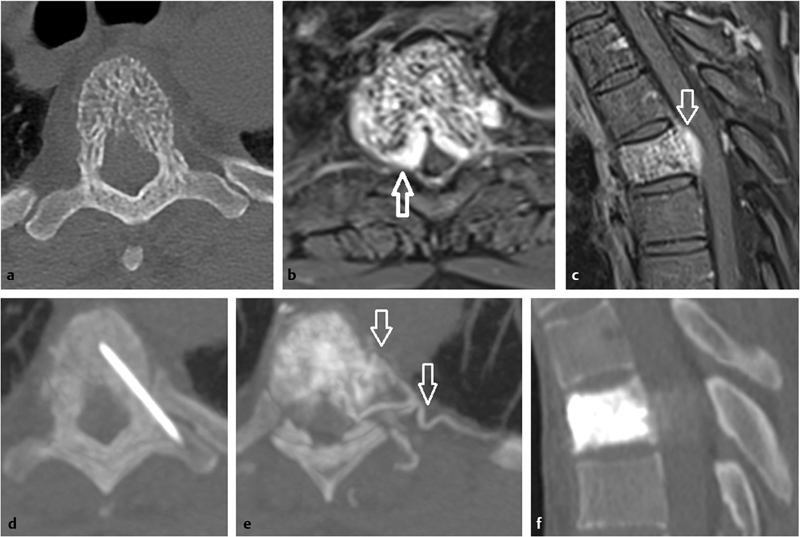
A 35-year-old woman with upper back pain due to an aggressive vertebral hemangioma of Th5. ( a ) Axial unenhanced CT scan shows an aggressive vertebral hemangioma of T5 with typical sparse and coarsened vertebral trabeculae. ( b, c ) Axial and sagittal fat-suppressed T1-weighted contrast-enhanced MR images identify a large extraosseous and epidural component ( white arrows ) leading to a severe thoracic canal stenosis. ( d ) Axial unenhanced intraprocedural CT scan shows the position of an 18-gauge needle in the vertebral body. ( e ) Axial intraprocedural CT scan during a venogram identifies the extensive vascularity of the hemangioma inside the vertebra and the surrounding soft tissue ( arrows ). ( f ) Postprocedure sagittal CT scan demonstrates cement filling the vertebral body.
Chondroblastoma
Epidemiology, Imaging Findings
Chondroblastomas are rare tumors accounting for approximately 1% of all benign bone lesions. They are more common in male patients aged between 3 and 25 years. Most chondroblastomas occur in the epiphysis or apophysis of long bones. They incite inflammatory changes in the surrounding tissues, resulting in pain and decreased range of motion. 26
Treatment
The classic treatment for chondroblastoma is surgical resection. Because of its periarticular location, the risks of surgery include injury to the articular surface or adjacent unfused growth plates leading to growth disturbances, decreased range of motion, and premature arthritis. 26 27 Surgery is curative in most cases, but recurrence rates of 10 to 35% have been reported in the literature. 28 Because of its small size and its frequent location close to joint surface and growth plates, percutaneous radiofrequency ablation is an alternative to surgery for treatment of selected chondroblastomas. Radiofrequency of chondroblastoma has been described using multi-tined expandable radiofrequency electrode 29 and single-tip monopolar electrodes with or without internally cooling system; with the number of simultaneously used electrodes varied between 1 and 3. 26 30 Most patients experience rapid pain relief on postprocedure day 1, 26 all patients had become symptomatically improved within 1 week and symptom-free within 4 months. 30 Complications are rare but consist in articular surface collapse, 26 chondrolysis, and osteonecrosis. 29 Reported recurrence rates after radiofrequency ablation of chondroblastoma are 6 to 12%. 26 29 30 Thus, larger lesions beneath weight-bearing surfaces should be approached with caution due to an increased risk of articular collapse and recurrence.
Giant Cell Tumor of Bone
Epidemiology, Imaging Findings
Giant cell tumor (GCT) represents 20% of all benign bone tumors and 5% of primary bone neoplasms. 4 They typically appear between the ages of 20 and 40 and are often solitary lesions located in long bones, predominantly around the knees (50–65%). 31 GCT is a locally aggressive, usually benign (80%) neoplasia. However, recurrence after treatment may occur in 20 to 50%, with 10% becoming malignant on recurrence. 32 Patients often experience localized pain, joint effusion, or pathological fracture of the affected bone.
A diagnosis of GCT is suggested by a lytic, eccentric lesion involving a long bone in a skeletally mature patient. 33 GCTs arise on the metaphyseal side of the epiphyseal plate and extend to within 1 cm of subarticular bone. 33 GCT is typically solitary and appears as a well-defined lytic lesion, with nonsclerotic margins and eccentric growth patterns. Other common features include cortical thinning or destruction, expansile remodeling of the bone, and prominent trabeculation. 33 Soft-tissue extension is common at CT and MR imaging. MR imaging of GCT frequently reveals a relatively well-defined lesion with a low-signal intensity margin representing either osseous sclerosis or a pseudocapsule. The solid components of GCT demonstrate low to intermediate signal intensity on T1- and T2-weighted MR imaging. ABC components within GCT are relatively common (14% of lesions), and GCT with prominent ABC elements may have a more aggressive radiographic appearance, reflecting the expansile cystic component. 33
Treatment
Historically, curettage and bone grafting has been the treatment of choice for GCT, although marginal resection is associated with a high recurrence rate. Wide resection shows a reduced recurrence rate. However, this treatment may cause significant compromise of limb function, which may be difficult to justify in the treatment of a benign lesion. 33 To decrease recurrence, combined surgical resection and open cryotherapy of the cavity margin has been described in combination with lumbopelvic surgical reconstruction. 34
Systemic therapy with bisphosphonates or RANKL inhibitors (i.e., denosumab) is reserved for patients with bad prognostic factors and for the treatment of nonresectable GCT. 35 Radiotherapy is used in selected nonresectable cases that are unresponsive to drugs used for systemic therapy. 31
Percutaneous CT-guided cryoablation has been described as an alternative treatment option for a case of GCT with extensive pelvic bone involvement, including the acetabular region. 31 Due to the tumor location, encompassing the obturator foramen, and its wide local extension, a radical surgical approach was not an option. After 1 year of medical treatment, denosumab stopped tumor growth, but there was no sign of local regression, leading to percutaneous cryoablation indication. Complete necrosis of the lesion was obtained after two procedures and the patient remains recurrence free after 31 months.
Aneurysmal Bone Cyst
Epidemiology, Imaging Findings
Aneurysmal bone cyst is a rare benign cystic lesion, accounting for approximately 9% of all bone tumors. 4 Connective tissue septa divide the blood-filled cysts that contain a mix of osteoclasts, giant cells, and reactive woven bone. In 30% of the cases, a predisposing lesion is identified, a finding that might suggests that ABC is more a reactive process rather than a distinct tumor type. GCT is the most common preexisting lesion. 36 ABC is an intraosseous lesion that can result in a blowout distension of the bone. 37 ABC is the most common in young patients, between the first and second decades of life. About 10 to 30% occur in the spine, while other commonly affected sites are the femur, tibia, humerus, and fibula. 4 38
On radiographs, ABC appears as radiolucent lesions of eccentric origin in the metaphysis of long bones. An erosion of the cortex of the bone and an elevation of the periosteum produce a characteristic “soap bubble” shape. CT imaging helps in identifying the margins of the cysts. MRI allows identification of the thin septa dividing the cyst, as well as demonstrating fluid–fluid levels within the cyst. 4 36
Treatment
Multiple treatment options for ABC have been described, including surgical resection or curettage, with or without bone grafting, and optional adjuvant therapies including selective arterial embolization, denosumab administration, and external beam radiotherapy. 39 40 41 42
Percutaneous image-guided therapies consist of sclerotherapy, bisphosphonate or doxycycline injections, cementoplasty, and ablation. For sclerotherapy, a sclerosing agent (e.g., alcohol of zein, hydroxy-polyethoxy-dodecane) is injected directly inside the lesion. Reported complications include pulmonary embolism, aseptic abscess formation, and fistula formation. 43 44 The injection of the sclerosing agent might be associated with transient local and general inflammatory side effects. Similar efficacy and a lower complication rate have been described with polidocanol sclerotherapy compared with curettage resection. 45 Repeated intracystic injection with absolute alcohol shows good results with fewer complications than other sclerosing agents. 37 Intralesional injection of doxycycline has been used with a good response and a low recurrence rate (5%). 46
Consolidation and ossification of the osseous part of ABC might be achieved by cement or bisphosphonate injection. 41 47 Consolidation using cementoplasty is indicated for bones under compression stress.
When there is a surrounding soft-tissue involvement and decompression is required, ablation techniques might be preferable to surgical resection. Cryoablation has been used for extra osseous tumor size reduction in symptomatic ABC. Although no large studies exist to evaluate the efficacy of percutaneous thermal ablation for the treatment of ABC, postablation tumor size reduction has been demonstrated. 38 In case of large lesion, embolization might be used to reduce the risk of hemorrhage and heat–sink effect prior to thermal ablation. 48
Chondromyxoid Fibroma
Epidemiology, Imaging Findings
Chondromyxoid fibroma is a benign cartilaginous neoplasm representing less than 1% of all bone tumors. Despite its benign classification, it is a locally aggressive and painful tumor.
The typical radiographic presentation of this tumor is an eccentric and well-circumscribed lesion with a sclerotic periphery, located in the metaphysis of long bones. The most common locations are the proximal tibia, distal femur, foot, and pelvis. 49
Treatment
Treatment options for chondromyxoid fibroma are surgical curettage (with and without bone grafting or PMMA filling) and “en bloc” excision. High recurrence rates of 20 to 25% have been described after the surgical treatments. 50
Only one case of radiofrequency ablation for chondromyxoid fibroma has been reported. 49 It was a 4-cm lesion of the distal fibula successfully treated using a 20-mm electrode with water-cooled tip. Two successive 6-minute ablations were performed after repositioning to cover the whole lesion. The child was discharged at day 1 postprocedure and returned to normal daily activities without recurrence of pain after 1 week.
Fibrous Dysplasia
Epidemiology, Imaging Findings
Fibrous dysplasia (FD) represents 5 to 7% among all benign bone tumors, 75% of cases are monostotic. FD typically affects patients in their third decade of life, commonly involving craniofacial bones, ribs, femur, or tibia. 4 51
FD consists of fibrous stroma with a cellular component, containing abnormal fibroblast cells and osteoblasts, which produce abnormally shaped trabeculae of woven bone. 4
FD appears as an abnormal “ground-glass” opacity within bone. Other features such as endosteal scalloping, bony expansion, and a thick reactive bone “rind” may also be present.
Treatment
FD is usually treated conservatively, but medication such as bisphosphonates, particularly pamidronate, may be effective for painful FD 52 and may also reduce the occurrence of secondary fractures, and even induce a partial radiographic resolution of lesions. 53 It has been suggested that other medical therapies such as denosumab and pregabalin may be useful treatments for symptomatic FD. 53 54
Surgery, consisting of curettage and autologous bone graft, might be considered in patients with progressive symptoms or when important anatomical structures are threatened. Corrective surgery (i.e., osteotomy or intramedullary fixation) may be required in case of FD-induced deformity. 51 After surgery, reported recurrence rates are around 18%. 55 Only four cases of percutaneous treatment of FD have been described, using cementoplasty mainly into the vertebral bodies ( Fig. 3 ). 56 57 58 59 In these cases, rapid pain relief was achieved for all patients with no recurrence of the symptoms.
Fig. 3.
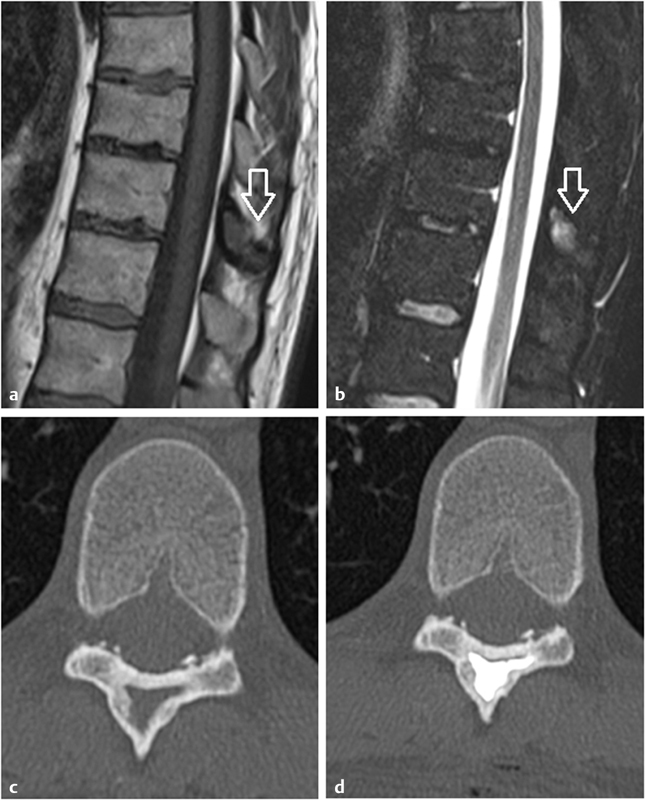
A 28-year-old man with persistent back pain caused by fibrous dysplasia of the spinous process of T11. ( a, b ) Unenhanced sagittal T1- and T2-weighted MR images demonstrate fibrous dysplasia of T11 as a hypo-T1 hyper-T2 lesion ( white arrows ) of the spinous process. ( c ) Axial unenhanced CT scan of T11 shows endosteal scalloping, opaque ground-glass abnormal bone, and a peripheral thick reactive bone. ( d ) Postprocedure CT scan demonstrates cement filling the lytic portion of the fibrous dysplasia.
Enchondroma
Enchondroma represents 2.6% of all benign bone tumors. These asymptomatic lesions may present at any age and consist of hyaline cartilage in a lobular formation, typically in long tubular bones, most commonly the hands and feet. 4 Enchondromas do not require treatment, unless they are symptomatic, increasing in size, or there is a risk of pathological fracture. Treatment of enchondromas consists of intralesional excision, followed by filling with an autologous bone graft or synthetic filling. 60
Only one study described the treatment of two intracortical enchondromas, preoperatively misdiagnosed as osteoid osteomas and treated with radiofrequency ablation. Radiofrequency appears to have cured the tumor in one patient, whereas the other required surgery. 61 62
How to Do It
Percutaneous treatment of benign bone tumors should be undertaken with a multidisciplinary approach, a preprocedural consultation, optimized image guidance, and appropriate anesthesia.
In our institution, a patient's case is discussed between the interventional radiologist, the orthopaedic surgeon, and the referring doctor (oncologist, rheumatologist, etc.) to collaboratively choose the best treatment for each patient.
Preprocedural consultation with the patient is mandatory to assess treatment indication and technique, and to explain to the patient the benefits, risks, and potential related complications of the procedure. Alternative treatment options should also be presented before collecting the written informed consent of the patient. During this consultation, routine clinical workup should include at least a neurologic examination, pain origin detection, and preprocedural laboratory results with blood cell count, CRP, and coagulation tests.
There are many imaging modalities available for percutaneous treatment of bone lesion. CT, ultrasound, conventional fluoroscopy, and MRI are currently used for different cases of percutaneous musculoskeletal lesion access. CT is the most commonly used modality for applicator placement and intraprocedural ablation zone monitoring, because it can be employed for nearly all bone locations in the axial and appendicular skeleton demonstrating compatibility with multiple devices. It provides quick images, thin slices, and high image resolution. In case of oblique or off plane approach, it is possible to use 3D reconstructions with or without combined fluoroscopy.
Intraprocedural pain control during bone tumor ablation most often requires sedation and analgesia. We use general anesthesia for ablation procedure as often as possible to limit patient discomfort during prolonged interventions, and to avoid patient movement during technically challenging ablations. During the procedure, there is no difference between radiofrequency and cryoablation in terms of analgesic intake; however, a significant difference appears during the first 24 hours postprocedure in favor of cryoablation. 63
Conclusion
Minimally invasive percutaneous imaging-guided techniques have been shown to be safe and effective for the treatment of benign bone tumors. Percutaneous ablation of some benign bone tumors such as osteoid osteomas has supplanted surgical resection as standard care. With the improvement of image guidance quality, the increase in experience of interventional radiologists, and the development of new ablative modalities, it is likely that the indications for percutaneous procedures will be extended to a wider variety of benign bone lesion types.
References
- 1.Rosenthal D I, Alexander A, Rosenberg A E, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(01):29–33. doi: 10.1148/radiology.183.1.1549690. [DOI] [PubMed] [Google Scholar]
- 2.Tsoumakidou G, Thénint M A, Garnon J, Buy X, Steib J P, Gangi A. Percutaneous image-guided laser photocoagulation of spinal osteoid osteoma: a single-institution series. Radiology. 2016;278(03):936–943. doi: 10.1148/radiol.2015150491. [DOI] [PubMed] [Google Scholar]
- 3.Huang A J. Radiofrequency ablation of osteoid osteoma: difficult-to-reach places. Semin Musculoskelet Radiol. 2016;20(05):486–495. doi: 10.1055/s-0036-1594280. [DOI] [PubMed] [Google Scholar]
- 4.Hakim D N, Pelly T, Kulendran M, Caris J A. Benign tumours of the bone: a review. J Bone Oncol. 2015;4(02):37–41. doi: 10.1016/j.jbo.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein M H, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(01):23–31. doi: 10.1007/BF00243089. [DOI] [PubMed] [Google Scholar]
- 6.Garnon J, Koch G, Caudrelier J, Tsoumakidou G, Cazzato R L, Gangi A. Expanding the borders: image-guided procedures for the treatment of musculoskeletal tumors. Diagn Interv Imaging. 2017;98(09):635–644. doi: 10.1016/j.diii.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Raux S, Abelin-Genevois K, Canterino I, Chotel F, Kohler R. Osteoid osteoma of the proximal femur: treatment by percutaneous bone resection and drilling (PBRD). A report of 44 cases. Orthop Traumatol Surg Res. 2014;100(06):641–645. doi: 10.1016/j.otsr.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal D, Callstrom M R. Critical review and state of the art in interventional oncology: benign and metastatic disease involving bone. Radiology. 2012;262(03):765–780. doi: 10.1148/radiol.11101384. [DOI] [PubMed] [Google Scholar]
- 9.Gangi A, Gasser B, De Unamuno S et al. New trends in interstitial laser photocoagulation of bones. Semin Musculoskelet Radiol. 1997;1(02):331–338. doi: 10.1055/s-2008-1080157. [DOI] [PubMed] [Google Scholar]
- 10.Tsoumakidou G, Koch G, Caudrelier J et al. Image-guided spinal ablation: a review. Cardiovasc Intervent Radiol. 2016;39(09):1229–1238. doi: 10.1007/s00270-016-1402-6. [DOI] [PubMed] [Google Scholar]
- 11.Gangi A, Alizadeh H, Wong L, Buy X, Dietemann J L, Roy C. Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology. 2007;242(01):293–301. doi: 10.1148/radiol.2421041404. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann R T, Jakobs T F, Kubisch C H et al. Radiofrequency ablation in the treatment of osteoid osteoma-5-year experience. Eur J Radiol. 2010;73(02):374–379. doi: 10.1016/j.ejrad.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Rimondi E, Mavrogenis A F, Rossi G et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(01):181–188. doi: 10.1007/s00330-011-2240-1. [DOI] [PubMed] [Google Scholar]
- 14.Lanza E, Thouvenin Y, Viala P et al. Osteoid osteoma treated by percutaneous thermal ablation: when do we fail? A systematic review and guidelines for future reporting. Cardiovasc Intervent Radiol. 2014;37(06):1530–1539. doi: 10.1007/s00270-013-0815-8. [DOI] [PubMed] [Google Scholar]
- 15.Tomasian A, Wallace A N, Jennings J W. Benign spine lesions: advances in techniques for minimally invasive percutaneous treatment. AJNR Am J Neuroradiol. 2017;38(05):852–861. doi: 10.3174/ajnr.A5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber M A, Sprengel S D, Omlor G W et al. Clinical long-term outcome, technical success, and cost analysis of radiofrequency ablation for the treatment of osteoblastomas and spinal osteoid osteomas in comparison to open surgical resection. Skeletal Radiol. 2015;44(07):981–993. doi: 10.1007/s00256-015-2139-z. [DOI] [PubMed] [Google Scholar]
- 17.Fox M W, Onofrio B M. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg. 1993;78(01):36–45. doi: 10.3171/jns.1993.78.1.0036. [DOI] [PubMed] [Google Scholar]
- 18.Jiang L, Liu X G, Yuan H S et al. Diagnosis and treatment of vertebral hemangiomas with neurologic deficit: a report of 29 cases and literature review. Spine J. 2014;14(06):944–954. doi: 10.1016/j.spinee.2013.07.450. [DOI] [PubMed] [Google Scholar]
- 19.Liu X W, Jin P, Wang L J, Li M, Sun G. Vertebroplasty in the treatment of symptomatic vertebral haemangiomas without neurological deficit. Eur Radiol. 2013;23(09):2575–2581. doi: 10.1007/s00330-013-2843-9. [DOI] [PubMed] [Google Scholar]
- 20.Kelley S P, Ashford R U, Rao A S, Dickson R A. Primary bone tumours of the spine: a 42-year survey from the Leeds Regional Bone Tumour Registry. Eur Spine J. 2007;16(03):405–409. doi: 10.1007/s00586-006-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guarnieri G, Ambrosanio G, Vassallo P et al. Vertebroplasty as treatment of aggressive and symptomatic vertebral hemangiomas: up to 4 years of follow-up. Neuroradiology. 2009;51(07):471–476. doi: 10.1007/s00234-009-0520-0. [DOI] [PubMed] [Google Scholar]
- 22.Hao J, Hu Z. Percutaneous cement vertebroplasty in the treatment of symptomatic vertebral hemangiomas. Pain Physician. 2012;15(01):43–49. [PubMed] [Google Scholar]
- 23.Gangi A, Buy X. Percutaneous bone tumor management. Semin Intervent Radiol. 2010;27(02):124–136. doi: 10.1055/s-0030-1253511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabal A M. Percutaneous technique for sclerotherapy of vertebral hemangioma compressing spinal cord. Cardiovasc Intervent Radiol. 2002;25(06):494–500. doi: 10.1007/s00270-002-1944-7. [DOI] [PubMed] [Google Scholar]
- 25.Heiss J D, Doppman J L, Oldfield E H. Treatment of vertebral hemangioma by intralesional injection of absolute ethanol. N Engl J Med. 1996;334(20):1340. doi: 10.1056/NEJM199605163342017. [DOI] [PubMed] [Google Scholar]
- 26.Rybak L D, Rosenthal D I, Wittig J C. Chondroblastoma: radiofrequency ablation--alternative to surgical resection in selected cases. Radiology. 2009;251(02):599–604. doi: 10.1148/radiol.2512080500. [DOI] [PubMed] [Google Scholar]
- 27.Christie-Large M, Evans N, Davies A M, James S L. Radiofrequency ablation of chondroblastoma: procedure technique, clinical and MR imaging follow up of four cases. Skeletal Radiol. 2008;37(11):1011–1017. doi: 10.1007/s00256-008-0526-4. [DOI] [PubMed] [Google Scholar]
- 28.Ramappa A J, Lee F Y, Tang P, Carlson J R, Gebhardt M C, Mankin H J.Chondroblastoma of bone J Bone Joint Surg Am 2000;82-A(081140–1145. [PubMed] [Google Scholar]
- 29.Tins B, Cassar-Pullicino V, McCall I, Cool P, Williams D, Mangham D. Radiofrequency ablation of chondroblastoma using a multi-tined expandable electrode system: initial results. Eur Radiol. 2006;16(04):804–810. doi: 10.1007/s00330-005-0022-3. [DOI] [PubMed] [Google Scholar]
- 30.Xie C, Jeys L, James S L. Radiofrequency ablation of chondroblastoma: long-term clinical and imaging outcomes. Eur Radiol. 2015;25(04):1127–1134. doi: 10.1007/s00330-014-3506-1. [DOI] [PubMed] [Google Scholar]
- 31.Panizza P S, de Albuquerque Cavalcanti C F, Yamaguchi N H, Leite C C, Cerri G G, de Menezes M R. Percutaneous CT-guided cryoablation as an alternative treatment for an extensive pelvic bone giant cell tumor. Cardiovasc Intervent Radiol. 2016;39(02):299–303. doi: 10.1007/s00270-015-1160-x. [DOI] [PubMed] [Google Scholar]
- 32.Szendröi M. Giant-cell tumour of bone. J Bone Joint Surg Br. 2004;86(01):5–12. [PubMed] [Google Scholar]
- 33.Murphey M D, Nomikos G C, Flemming D J, Gannon F H, Temple H T, Kransdorf M J. From the archives of AFIP. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation. Radiographics. 2001;21(05):1283–1309. doi: 10.1148/radiographics.21.5.g01se251283. [DOI] [PubMed] [Google Scholar]
- 34.Althausen P L, Schneider P D, Bold R J, Gupta M C, Goodnight J E, Jr, Khatri V P. Multimodality management of a giant cell tumor arising in the proximal sacrum: case report. Spine. 2002;27(15):E361–E365. doi: 10.1097/00007632-200208010-00020. [DOI] [PubMed] [Google Scholar]
- 35.Fritzsche H, Schaser K D, Hofbauer C. [Benign tumours and tumour-like lesions of the bone: general treatment principles] Orthopade. 2017;46(06):484–497. doi: 10.1007/s00132-017-3429-z. [DOI] [PubMed] [Google Scholar]
- 36.Kransdorf M J, Sweet D E. Aneurysmal bone cyst: concept, controversy, clinical presentation, and imaging. AJR Am J Roentgenol. 1995;164(03):573–580. doi: 10.2214/ajr.164.3.7863874. [DOI] [PubMed] [Google Scholar]
- 37.Ulici A, Florea D C, Carp M, Ladaru A, Tevanov I. Treatment of the aneurysmal bone cyst by percutaneous intracystic sclerotherapy using ethanol ninety five percent in children. Int Orthop. 2018;42(06):1413–1419. doi: 10.1007/s00264-018-3841-y. [DOI] [PubMed] [Google Scholar]
- 38.Tsoumakidou G, Too C W, Garnon J, Steib J P, Gangi A. Treatment of a spinal aneurysmal bone cyst using combined image-guided cryoablation and cementoplasty. Skeletal Radiol. 2015;44(02):285–289. doi: 10.1007/s00256-014-1967-6. [DOI] [PubMed] [Google Scholar]
- 39.Amendola L, Simonetti L, Simoes C E, Bandiera S, De Iure F, Boriani S. Aneurysmal bone cyst of the mobile spine: the therapeutic role of embolization. Eur Spine J. 2013;22(03):533–541. doi: 10.1007/s00586-012-2566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flont P, Kolacinska-Flont M, Niedzielski K. A comparison of cyst wall curettage and en bloc excision in the treatment of aneurysmal bone cysts. World J Surg Oncol. 2013;11:109. doi: 10.1186/1477-7819-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cornelis F, Truchetet M E, Amoretti N et al. Bisphosphonate therapy for unresectable symptomatic benign bone tumors: a long-term prospective study of tolerance and efficacy. Bone. 2014;58:11–16. doi: 10.1016/j.bone.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Lange T, Stehling C, Fröhlich B et al. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J. 2013;22(06):1417–1422. doi: 10.1007/s00586-013-2715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adamsbaum C, Mascard E, Guinebretière J M, Kalifa G, Dubousset J. Intralesional Ethibloc injections in primary aneurysmal bone cysts: an efficient and safe treatment. Skeletal Radiol. 2003;32(10):559–566. doi: 10.1007/s00256-003-0653-x. [DOI] [PubMed] [Google Scholar]
- 44.Topouchian V, Mazda K, Hamze B, Laredo J D, Penneçot G F. Aneurysmal bone cysts in children: complications of fibrosing agent injection. Radiology. 2004;232(02):522–526. doi: 10.1148/radiol.2322031157. [DOI] [PubMed] [Google Scholar]
- 45.Varshney M K, Rastogi S, Khan S A, Trikha V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin Orthop Relat Res. 2010;468(06):1649–1659. doi: 10.1007/s11999-009-1144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shiels W E, II, Mayerson J L. Percutaneous doxycycline treatment of aneurysmal bone cysts with low recurrence rate: a preliminary report. Clin Orthop Relat Res. 2013;471(08):2675–2683. doi: 10.1007/s11999-013-3043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guarnieri G, Vassallo P, Muto M, Muto M. Percutaneous treatment of symptomatic aneurysmal bone cyst of L5 by percutaneous injection of osteoconductive material (Cerament) J Neurointerv Surg. 2014;6(08):e43. doi: 10.1136/neurintsurg-2013-010912.rep. [DOI] [PubMed] [Google Scholar]
- 48.Griauzde J, Gemmete J J, Farley F. Successful treatment of a Musculoskeletal Tumor Society grade 3 aneurysmal bone cyst with N-butyl cyanoacrylate embolization and percutaneous cryoablation. J Vasc Interv Radiol. 2015;26(06):905–909. doi: 10.1016/j.jvir.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Berenstein-Weyel T, Lebel E, Katz D, Applbaum Y, Peyser A. Chondromyxoid fibroma of the distal fibula treated by percutaneous radiofrequency ablation. J Orthop Surg (Hong Kong) 2017;25(02):2.30949901772083E15. doi: 10.1177/2309499017720830. [DOI] [PubMed] [Google Scholar]
- 50.Dürr H R, Lienemann A, Nerlich A, Stumpenhausen B, Refior H J.Chondromyxoid fibroma of bone Arch Orthop Trauma Surg 2000120(1-2):42–47. [DOI] [PubMed] [Google Scholar]
- 51.DiCaprio M R, Enneking W F. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. 2005;87(08):1848–1864. doi: 10.2106/JBJS.D.02942. [DOI] [PubMed] [Google Scholar]
- 52.Riddle N D, Bui M M. Fibrous dysplasia. Arch Pathol Lab Med. 2013;137(01):134–138. doi: 10.5858/arpa.2012.0013-RS. [DOI] [PubMed] [Google Scholar]
- 53.Nogueira Drumond J M. Benign bone tumors and tumor-like bone lesions: treatment update and new trends. Rev Bras Ortop. 2015;44(05):386–390. doi: 10.1016/S2255-4971(15)30267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chapurlat R D, Gensburger D, Jimenez-Andrade J M, Ghilardi J R, Kelly M, Mantyh P. Pathophysiology and medical treatment of pain in fibrous dysplasia of bone. Orphanet J Rare Dis. 2012;7 01:S3. doi: 10.1186/1750-1172-7-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacDonald-Jankowski D. Fibrous dysplasia: a systematic review. Dentomaxillofac Radiol. 2009;38(04):196–215. doi: 10.1259/dmfr/16645318. [DOI] [PubMed] [Google Scholar]
- 56.Dang D, Baig M N, Christoforidis G, Chiocca E A, Gabriel J. C2/C3 pathologic fractures from polyostotic fibrous dysplasia of the cervical spine treated with percutaneous vertebroplasty. Eur Spine J. 2007;16 03:250–254. doi: 10.1007/s00586-007-0434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kotil K, Ozyuvaci E. Fibrous dysplasia in axis treated with vertebroplasty. J Craniovertebr Junction Spine. 2010;1(02):118–121. doi: 10.4103/0974-8237.77676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun H Y, Lee J W, Kim K J, Yeom J S, Kang H S. Percutaneous intervention of the C2 vertebral body using a CT-guided posterolateral approach. AJR Am J Roentgenol. 2009;193(06):1703–1705. doi: 10.2214/AJR.09.2783. [DOI] [PubMed] [Google Scholar]
- 59.Christoforidis G, Dang D, Gabriel J. Catheter-directed percutaneous transpedicular C2/C3 vertebroplasty in a patient with fibrous dysplasia using Seldinger technique. AJNR Am J Neuroradiol. 2006;27(08):1738–1740. [PMC free article] [PubMed] [Google Scholar]
- 60.Marco R A, Gitelis S, Brebach G T, Healey J H. Cartilage tumors: evaluation and treatment. J Am Acad Orthop Surg. 2000;8(05):292–304. doi: 10.5435/00124635-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Santiago F R, Del Mar Castellano García M, Montes J L, García M R, Fernández J M. Treatment of bone tumours by radiofrequency thermal ablation. Curr Rev Musculoskelet Med. 2009;2(01):43–50. doi: 10.1007/s12178-008-9042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramnath R R, Rosenthal D I, Cates J, Gebhardt M, Quinn R H. Intracortical chondroma simulating osteoid osteoma treated by radiofrequency. Skeletal Radiol. 2002;31(10):597–602. doi: 10.1007/s00256-002-0501-4. [DOI] [PubMed] [Google Scholar]
- 63.Thacker P G, Callstrom M R, Curry T B et al. Palliation of painful metastatic disease involving bone with imaging-guided treatment: comparison of patients' immediate response to radiofrequency ablation and cryoablation. AJR Am J Roentgenol. 2011;197(02):510–515. doi: 10.2214/AJR.10.6029. [DOI] [PubMed] [Google Scholar]


