Abstract
The prevalence of patients with painful bone metastases is constantly increasing. This is related to the rising incidence of cancer and increasing life expectancy of patients with metastatic stage. Advances in imaging and development of percutaneous techniques have gradually allowed offering minimally invasive acts on these metastases: cementing, vertebral augmentation, osteosynthesis, percutaneous thermal ablation, neurolysis, embolization. The purpose of this article is to present the main tools available to date for the interventional radiologist so that each participant can understand their functioning, indications, and limits.
Keywords: interventional radiology, metastases, cementoplasty, percutaneous ablation
Objectives : Upon completion of this article, the reader will be able to describe the role of interventional radiology in the treatment of osseous metastatic disease, including patient selection and technical considerations.
According to the World Health Organization (WHO), cancer is the leading global cause of death, with increasing prevalence of 12 million cases in 2012 to 24 million estimated in 2035. 1 At the same time, life expectancy for cancer patients is growing, especially in patients with metastatic disease. Half of them will suffer from bone metastases, of which 30% from severe pain. 2 3
Initially with a poor prognosis, the management of metastatic patients was essentially medical and palliative. The radiotherapy usually permits à partial pain improvement. A literature review of 12 studies involving 1,580 patients estimated that after radiotherapy, only 41% of patients experience a pain reduction of more than 50% at 1 month, and 25% presented a complete pain palliation. 4 Because of the complexity and the morbidity of the intervention, surgery is usually not performed.
Thanks to the improvement of systemic therapy, their prognosis is now constantly increasing. In the near future, millions of patients will live several years with bone metastases and their pain. Therefore, it appears necessary to develop new care networks and “tools” that improve their quality of life.
Advances in imaging and the development of different techniques of percutaneous treatment have progressively made it possible to propose the so-called minimally invasive procedures, such as cementation, vertebral augmentation, osteosynthesis, percutaneous thermoablation, the neurolysis, and embolization. The development of these techniques over the last two decades has permitted to extent the indications toward early management in metastatic disease. In fact, the treatment not only concerns the management of a terminal symptoms but also to prevent regional complications, and in some cases even to treat with a curative aim in selected oligometastatic patients. 5
It is essential to adapt these indications to the exact clinical context, which requires cooperation between physicians (oncologist, onco-rheumatologist, radiotherapist, surgeon, interventional radiologist) and the realization of multidisciplinary reunions.
The end point(s) can be both—pain palliation and/or prevention of complication related to the bone metastases:
-
For pain palliation, the main mechanism of the pain must be considered to choose the most appropriate first-line treatment:
Pain related to a fracture requires stabilization of the fracture.
Pain related to the tumor volume or to the inflammatory process requires tumor cells destruction, embolization, or neurolysis.
For prevention of complication in asymptomatic patients, the risk related to the bone metastasis (pathological fracture, tumor growth) must be balanced with the risk of the procedure itself and with oncological consideration, namely, the life expectancy.
The aim of this article is to present current tool for interventional radiologist so that physicians can understand their functioning, indications, and limitations.
Cementoplasty, Vertebral Augmentation
Cementoplasty is a technique of injecting cement (polymethylacrylate) through a canula inserted into a metastatic bone lysis. It is indicated for painful vertebral metastases with pathological fracture, or to prevent it. Typically, mechanical pain worsens with activity and decreases with rest.
Each vertebra can be treated. The most common introductory route is the posterior pedicular approach. It can be posterolateral or inter-costotransverse on the thoracic vertebrae, but also anterolateral and transoral for the upper cervical vertebrae. The procedure can be performed under simple sedation, especially when the treatment concerns only one vertebra. General anesthesia is used when several vertebral lesions are to be treated during the same procedure, usually the case with cancer patients.
The great advantage of vertebroplasty is the rapidity of its analgesic effect, observed early after the procedure in case of typical mechanical pain. Alvarez et al showed a pain palliation in more than 80% of patients treated with vertebroplasty, an average improvement of 5.9 points on the VAS and a resumption of mobility at 77% of bedridden patients following the intervention. 6
The technique is nowadays used also for treatment of impending fracture caused by lytic extraspinal lesions in bones such as acetabulum, femoral neck, and flat bones (ribs, sternum; Fig. 1 ). Studies on peripheral lesions find similar efficiency with a large and rapid pain improvement in a few days. Anselmetti et al prospectively showed an average improvement of 7.1 points on the VAS in the first week after the procedure in 50 patients. 7
Fig. 1.

Secondary fracture of the sternum body before ( a ) and after ( b ) cementoplasty. The average pain decreased from 8 to 2 in visual analog scale in 48 hours.
Vertebral augmentation techniques have been developed to treat vertebral compression fractures by restoring the vertebral height. Before cement injection, a kyphoplasty balloon or an implant is deployed through the same canula. The indication of vertebral augmentation procedures in comparison with cementoplasty only remains to be clarified. Many teams nevertheless use kyphoplasty as a tool that can facilitate the diffusion of cement by creating a cavity especially in case of significant vertebral compression and thus to reduce the risk of cement leakage. Most often, these cement leaks are asymptomatic. 8
Percutaneous Osteosynthesis
In case of lytic damage in an area subject to great mechanical stress, cementoplasty alone may be sufficient for pain palliation, but may be insufficient to prevent the risk of an impending fracture. For example, in upper femoral extremity lesions, a Mirel score superior to 8 suggests the necessity for a prophylactic stabilization, but in cancer patients with uncertain life expectancy, surgical intervention is refuted. Deschamps et al described for the first time in 2012 a percutaneous technique associating cementoplasty and osteosynthesis by screw fixation, for treating a femoral neck lytic lesion to prevent an impending fracture ( Fig. 2a ). In this pilot study, a mean VAS decrease of 5.5 points was observed and no fracture appeared after 145 days of follow-up. 9 Other series are underway to better evaluate the technique, especially for pelvic ( Fig. 2b ), spine, and humerus fixation. The osteosynthesis permitted to extend the indications of percutaneous techniques for stabilization of lytic bone metastasis. Even if pain relief is very satisfactory after cementoplasty, there is a risk of fracture due to insufficient consolidation of weight-bearing bone.
Fig. 2.

( a ) Osteosynthesis ( transparent arrowheads ) associated with a cementoplasty ( white arrowheads ) of a lytic lesion of the femoral neck. ( b ) Osteosynthesis ( transparent arrows ) of the ischio and ilio pubic branches of a postradiation fracture with no consolidation at 3 months. Immediate functional improvement and support on the right leg was obtained in the morning of the procedure.
Percutaneous Destruction (Radiofrequency, Cryoablation, Laser, Microwave, High-Intensity Focused Ultrasound)
Percutaneous destruction procedures, used for the treatment of small “soft” organs (liver, lung, kidney, etc.) lesions are now used for the treatment of bone and soft-tissue metastases. The initial indication was the curative treatment of small lesions such as osteoid osteoma, as well as the pain management of lesions that blow the bone and due to the absence of a container, the use of cementoplasty is not possible. The increasing learning of this technique has allowed today not only a pain relief but also a curative treatment in some patients with controlled oligometastatic disease and good life expectancy.
Different physical processes are used. The most commonly used is radiofrequency, which consists of positioning a needle within the tumor, and delivering an alternating current of between 400 and 500 kHz to create an ionic agitation of the adjacent tissue and to reach the necessary 60°C to irreversible destruction. Several generators are available, offering various sizes and shapes of ablation. Nevertheless, there is no direct visualization of the destruction zone on computed tomography (CT), which can represent a limit when treating metastases close to sensitive organs such as nerves, spinal cord, and digestive tract. In this context, cryotherapy, a recently developed technic, allows a direct visualization of the ice cube during the procedure under CT and therefore a better instantaneous control of ablation margins. The cold destruction is allowed thanks to the Joule-Thomson effect: the rapid decompression of argon gas at the end of the needle inserted in the tumor makes it possible to reach −180°C in a few minutes. The advantage of this technique is the use of several needles at the same time, making possible to carve the size and the shape of the ice cube, and thus to destroy a greater tumor volume ( Fig. 3 ).
Fig. 3.

Six-needle analgesic cryotherapy under CT scan of a lytic metastasis of the entire roof of the left acetabulum. Coronal ( a ) and axial ( b ) view maximum intensity projection indicates the placement of the needles ( transparent arrowheads ) to cover the tumor volume. Coronal ( c ) and sagittal ( d ) view showing the volume of the ice cube and the controlled margins toward the bottom and at the level of the coxofemoral articulation ( white arrowheads ).
In all cases, the radiologist will anticipate the complications by evaluating the organs adjacent to the metastasis and, if necessary, protect them by performing active (water or CO 2 ) or passive (thermocouple, evoked potential, and neurostimulation) thermal protection maneuvers.
The technique is essentially performed under CT guidance, or rarely under MRI with guide sequences or dedicated thermometry.
Compared with radiofrequency, the analgesic action of cryotherapy is delayed but remains prolonged. Callstrom et al 10 showed that 82% of patients have an average decrease of 3 points of VAS at 1 month after radiofrequency. The same authors reported on 61 patients treated with cryotherapy an average decrease of 3.5 points of VAS at 1 month and 5.5 points at 6 months. 11 Randomized trials evaluating the effectiveness of both techniques are underway.
Healing indications are rarer. McMenomy et al 12 reported local control for 45 of 52 treated tumors (87%) and after 21 months of average follow-up.
Other techniques that may be cited are as follows:
Laser, which offers a small area of destruction. This method is of little used in oncology, and applies mainly to benign osteoid osteoma tumors.
Microwave generators that allow destruction of greater power, by stirring water molecules.
High-intensity focused ultrasound.
Neurolysis
When the tumor directly invades a sensitive nerve, neuropathic pain can be difficult to relieve.
Sacrifice of the sensory pathway is described for all sympathetic plexuses (stellate ganglion, thoracic chain, celiac and lumbar plexus, hypogastric and sacrococcygeal plexus) and peripheral sensory nerves (thoracic roots, palatine, glossopharyngeal, phrenic, pudendal).
Neurolysis of mixed nerves (or even of the spinal cord) can be considered during extreme palliative situations, where motor loss is considered irreversible and therapeutic alternatives are nonexistent.
On the one hand, when a relatively extensive destruction is needed, the destruction agent employed can be chemical such as alcohol diffusing along a plexus. On the other hand, when very focal and limited destruction at the end of the inserted needle is required, a physical method such as radiofrequency or cryoablation will be preferred ( Fig. 4 ).
Fig. 4.

( a ) Intercostal metastasis left ( transparent arrowheads ) responsible for posterior parietal nociceptive pain and neuropathic metameric (VAS = 6 after grade III analgesic). T4–T6 xylocaine infiltration bloc-test positive. ( b ) Cryotherapy under CT of the intercostal lesion ( white arrowhead ) and cryoneurolysis staged from T4 to T6 ( white arrow ). ( c ) oblique coronal MIP reconstruction with each needle for cryoneurolysis ( white arrows ) at the exit of the foramens.
Embolization
Often unknown in oncology because it was initially intended for a hemostatic goal or tumor ischemia, embolization can also have an analgesic effect. All bone tumors present neoangiogenesis that theoretically makes embolization possible for all histological types. However, in practice, embolization is more favorable for hypervascular metastases (renal tumor, neuroendocrine, thyroid, etc.) with hypertrophied feeder arteries.
A prior angio CT scan is often useful to identify the origin of these arteries, to evaluate any accessory vascularization, to evaluate the risk of off-target embolization on close organs, and more generally to prepare the adapted material for the patient anatomy.
Distal embolizing agents (microbeads, biological glues) are most often used because the therapeutic target is the tumor microvascularization itself. After devascularization of the tumor microarterial, unembolization of the supply vessels is possible, knowing that in case of recurrence of tumor neovascularization this artery cannot be reused for embolization ( Fig. 5 ).
Fig. 5.
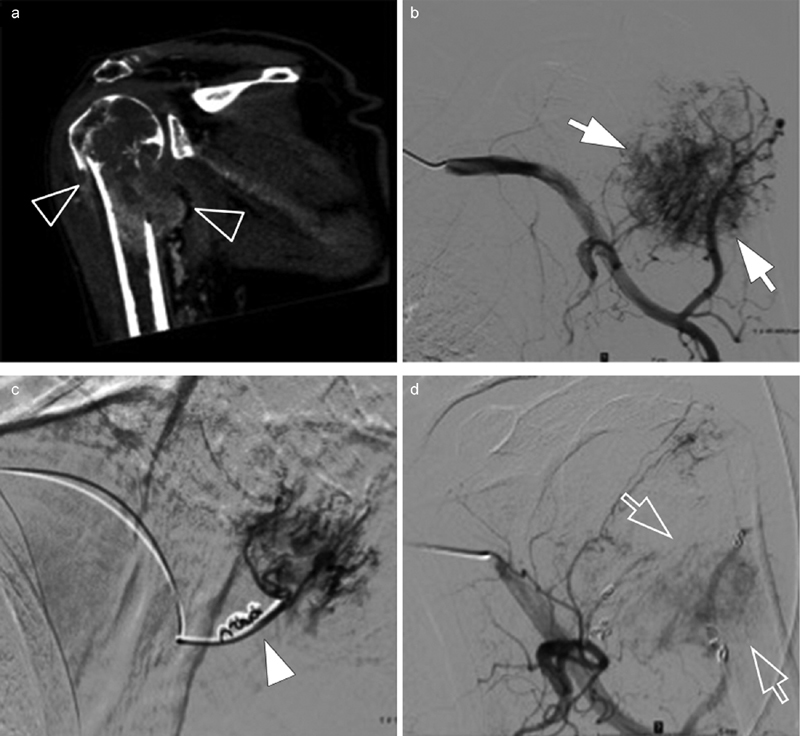
( a ) Hypervascular lytic metastasis ( transparent arrowhead ) of renal cell carcinoma with secondary humerus fracture. ( b ) Arteriography of the subclavian artery identifying the multiple supply arteries of the tumor associated with a tumor blush ( white arrows ). ( c ) One-to-one catheterization of the pedicles for distal tumor embolization using calibrated microparticles, then proximal by coils ( white arrowhead ). ( d ) Control at the end of the procedure with minimal residual tumor vascularization ( transparent arrow ). Embolization performed 48 hours before surgery to reduce the risk of bleeding and reduce pain (from 8 to 3 at the VAS).
Mavrogenis et al 13 summarized the literature by finding after tumor embolization a significant improvement of the VAS in 97% of cases but with a transient effect of only 1 to 9 months. In case of initial effectiveness, the embolization can be renewed. The postembolization syndrome (fever, vomiting) is frequent from 18 to 86% and requires a short-term management.
Combined Techniques
Percutaneous radiological techniques may be combined for the treatment of metastasis. The best example is the treatment of pathological vertebral fracture. In patients with favorable oncological live expectancy greater than 6 months or few metastases, Wallace et al 14 integrated in their algorithm the combination of cementoplasty allowing an immediate antalgic effect likely and radiotherapy or radiological destruction to optimize local control and reduce the risk of compressive complication in case of progression ( Fig. 6 ).
Fig. 6.

( a ) Scanner showing a lytic metastasis with early fracture of the vertebral body of L2 ( white arrowhead ). ( b ) The metastasis being unique after extension assessment (choroidal melanoma treated 3 years ago), bipedicular radiofrequency destruction (white arrows); ( c ) Consolidation cementoplasty ( transparent arrows ) in the same operating time.
These strategies are currently common with a level of evidence often modest that should be better assessed in the future to justify an increase in the immediate cost inherent in cumulative techniques.
Contraindications and Complications
It is difficult to consider all the possible complications, mostly because of the wide range of techniques, treating different anatomical sites of the bone metastases. The principle of these techniques is identical: percutaneously or endovascularly targeting a metastasis, to deliver a physical or chemical agent.
The complications are therefore related to the lesion site and nontarget damage, on the needle track, or close to the tumor (medullary compression, proximity of a lumbar plexus, etc.). Most of the nontarget lesion can be anticipated, and this notion therefore remains relative according to the teams and the ability to optimize their ballistics and the protection of adjacent organs. For example, epidural involvement was considered to be an absolute contraindication to cementoplasty. Hentschel et al 15 proved that there were no more complications of vertebroplasty in epidural cases. Anticipation by prior anatomical analysis is the most important point to avoid complications for the interventional radiologist.
To this we must add the usual contraindications (uncorrectable hemostasis disorders, leukopenia, ongoing sepsis, etc.).
Conclusion
Interventional radiology now offers many minimally invasive and low-risk options for the management of bone metastasis pain, or to prevent any musculoskeletal event, and should no longer be considered as a last resort treatment.
To be able to integrate these treatments in the patient's care path, it is most likely that we will need to improve access to specialized team: some techniques are doable in each structure (cementoplasty) and other techniques require expertise and a more advanced interventional radiology technical platform (cryotherapy, embolization, etc.).
The reader is referred to multiple prior Seminars in Interventional Radiology articles that focus on the treatment of musculoskeletal metastatic disease. 16 17 18 19 20
References
- 1.Stewart B W, Wild C P. Lyon, France: IARC Nonserial Publication; 2014. World Cancer Report 2014 (Print) [Google Scholar]
- 2.Smith H S. Painful osseous metastases. Pain Physician. 2011;14(04):E373–E403. [PubMed] [Google Scholar]
- 3.Zhu X C, Zhang J L, Ge C T et al. Advances in cancer pain from bone metastasis. Drug Des Devel Ther. 2015;9:4239–4245. doi: 10.2147/DDDT.S87568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McQuay H J, Collins S L, Carroll D, Moore R A. Radiotherapy for the palliation of painful bone metastases. Cochrane Database Syst Rev. 2000;(02):CD001793. doi: 10.1002/14651858.CD001793. [DOI] [PubMed] [Google Scholar]
- 5.Kurup A N, Callstrom M R. Expanding role of percutaneous ablative and consolidative treatments for musculoskeletal tumours. Clin Radiol. 2017;72(08):645–656. doi: 10.1016/j.crad.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez L, Pérez-Higueras A, Quiñones D, Calvo E, Rossi R E. Vertebroplasty in the treatment of vertebral tumors: postprocedural outcome and quality of life. Eur Spine J. 2003;12(04):356–360. doi: 10.1007/s00586-003-0525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anselmetti G C, Manca A, Ortega C, Grignani G, Debernardi F, Regge D. Treatment of extraspinal painful bone metastases with percutaneous cementoplasty: a prospective study of 50 patients. Cardiovasc Intervent Radiol. 2008;31(06):1165–1173. doi: 10.1007/s00270-008-9396-3. [DOI] [PubMed] [Google Scholar]
- 8.Phillips F M, Todd Wetzel F, Lieberman I, Campbell-Hupp M.An in vivo comparison of the potential for extravertebral cement leak after vertebroplasty and kyphoplasty Spine 200227192173–2178., discussion 2178–2179 [DOI] [PubMed] [Google Scholar]
- 9.Deschamps F, Farouil G, Hakime A, Teriitehau C, Barah A, de Baere T. Percutaneous stabilization of impending pathological fracture of the proximal femur. Cardiovasc Intervent Radiol. 2012;35(06):1428–1432. doi: 10.1007/s00270-011-0330-8. [DOI] [PubMed] [Google Scholar]
- 10.Callstrom M R, Charboneau J W, Goetz M P et al. Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radio-frequency ablation. Radiology. 2002;224(01):87–97. doi: 10.1148/radiol.2241011613. [DOI] [PubMed] [Google Scholar]
- 11.Callstrom M R, Dupuy D E, Solomon S B et al. Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer. 2013;119(05):1033–1041. doi: 10.1002/cncr.27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMenomy B P, Kurup A N, Johnson G B et al. Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission. J Vasc Interv Radiol. 2013;24(02):207–213. doi: 10.1016/j.jvir.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Mavrogenis A F, Angelini A, Vottis C et al. Modern palliative treatments for metastatic bone disease: awareness of advantages, disadvantages, and guidance. Clin J Pain. 2016;32(04):337–350. doi: 10.1097/AJP.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 14.Wallace A N, Robinson C G, Meyer J et al. The metastatic spine disease multidisciplinary working group algorithms. Oncologist. 2015;20(10):1205–1215. doi: 10.1634/theoncologist.2015-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hentschel S J, Burton A W, Fourney D R, Rhines L D, Mendel E. Percutaneous vertebroplasty and kyphoplasty performed at a cancer center: refuting proposed contraindications. J Neurosurg Spine. 2005;2(04):436–440. doi: 10.3171/spi.2005.2.4.0436. [DOI] [PubMed] [Google Scholar]
- 16.Jay B, Ahn S H. Vertebroplasty. Semin Intervent Radiol. 2013;30(03):297–306. doi: 10.1055/s-0033-1353483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasper D M. Kyphoplasty. Semin Intervent Radiol. 2010;27(02):172–184. doi: 10.1055/s-0030-1253515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsanos K, Sabharwal T, Adam A. Percutaneous cementoplasty. Semin Intervent Radiol. 2010;27(02):137–147. doi: 10.1055/s-0030-1253512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prince E A, Ahn S H. Interventional management of vertebral body metastases. Semin Intervent Radiol. 2013;30(03):278–281. doi: 10.1055/s-0033-1353480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lea W, Tutton S. Decision making; osteoplasty, ablation, or combined therapy for spinal metastases. Semin Intervent Radiol. 2017;34(02):121–131. doi: 10.1055/s-0037-1602707. [DOI] [PMC free article] [PubMed] [Google Scholar]


