Abstract
Image-guided, minimally invasive, percutaneous thermal ablation of bone metastases has unique advantages compared with surgery or radiation therapy. Thermal ablation of osseous metastases may result in significant pain palliation, prevention of skeletal-related events, and durable local tumor control. This article will describe current thermal ablation techniques utilized to treat bone metastases, summarize contemporary evidence supporting such thermal ablation treatments, and outline an approach to percutaneous ablative treatment.
Keywords: osseous metastases, thermal ablation, cancer pain, interventional radiology, pain palliation
Objectives : Upon completion of this article, the reader will be able to discuss the current thermal ablation modalities utilized to treat osseous metastases, summarize contemporary evidence supporting thermal ablation, and describe practical approaches to percutaneous ablative treatment, including patient selection, procedural techniques, and follow-up.
Bone is a common site of metastatic disease, with a higher prevalence in those patients with lung, breast, or prostate cancer primaries. Osseous metastases (OMs) have the potential to cause serious morbidity in the form of bone-related cancer pain, metabolic effects, or critical skeletal-related events (SREs), such as pathological fracture, spinal cord compromise, or major neurovascular structure compression. 1 Bone-related cancer pain is frequently undertreated with up to 80 percent of patients experiencing severe pain prior to initiation of a sufficient palliative treatment plan. 2 In patients with metastatic bone disease, an initial SRE is associated with an increased risk of subsequent SREs, increased health care–related costs, and shortened life expectancy. 3 4 Current management typically requires a multifaceted approach and may involve opioid and nonopioid analgesia, bisphosphonates, chemotherapy, radiation therapy (RT), surgery, or ablation. 5 6
External beam RT is the current gold standard locoregional treatment for pain palliation from OM, with pain reduction achievable in 50 to 80% of patients and complete in up to one-third of patients. 1 7 However, efficacy and applications of RT maybe limited due to irradiated tissue dose limits, delayed onset of pain relief, insufficient duration of symptom relief, and radio-resistant tumor subtypes. 8 9 Surgical resection, reconstruction, or stabilization can be technically challenging in this potentially frail group of patients. As perioperative morbidity and prolonged recovery times may delay or interrupt systemic therapy, surgery is typically reserved for patients with spinal cord compromise, existing pathologic fracture, or impending pathological fracture. 10
Percutaneous thermal ablation of OM is an important minimally invasive treatment option that has become increasingly integrated into interventional oncology (IO) practice over the past several years. Main indications for OM thermal ablation include treatment of painful metastases refractory or unsuitable to conventional therapies, local control of limited metastatic disease, prevention of critical SREs, or ablation of hormonally active metastases. When appropriate, ablation can be combined with percutaneous bone consolidation therapies (cementoplasty and osteosynthesis) with the intention of bone reinforcement. Consolidation techniques will be discussed elsewhere in this issue and not specifically discussed in this article. Excellent ablation outcomes depend on appropriate patient selection, device selection, ablation technique, and follow-up.
Thermal Ablation Techniques
Current percutaneous thermal ablation techniques utilized for treating OM are primarily cryoablation (CA) and radiofrequency ablation (RFA), whereas microwave ablation (MWA) and laser ablation (LA) are additional emerging techniques. MRI-guided focused ultrasound (MR-FUS) is a newer noninvasive technique in which highly focused ultrasound energy is delivered to the targeted tissue, causing heating (65–85°C) with resultant coagulative necrosis; this technique will be addressed elsewhere in this issue. The primary intent of thermal ablation is to cause substantially decreased (less than −20 to −40°C) or increased (>60°C) temperatures within the targeted tumor to induce tissue necrosis and cell death. 11
Cryoablation
Current CA systems use pressurized, room-temperature argon and helium gases for tissue freezing and heating. Using the Joule-Thompson effect, the expansion of argon gas within the distal cryoprobe chamber enables rapid cooling of the soft tissues about the uninsulated probe tip to less than −100°C within seconds with surrounding ice ball growth. A single 13 to 17G cryoprobe can typically produce an ice ball measuring 5.5 cm in length and 3.5 cm in diameter when used alone. Current systems allow for either 8 or 20 cryoprobes to be used simultaneously, with considerably larger ice balls achievable when probes are used synchronously. The resultant ice ball is visible in soft tissues as a region of decreased attenuation on CT using standard body window/level or a region of low signal intensity on MRI sequences. Soft tissues peripheral to the visualized 0°C ice ball margin are typically safe from thermal injury, with lethal ice (−20 to −40°C) usually occurring 3 to 5 mm deep to ice ball outer margin. 12 13 14 15
Radiofrequency Ablation
RFA is another widely used method of percutaneous tumor ablation, with treatment of malignant bone lesions originally reported in 1998. 16 High-frequency alternating current induces focal ionic agitation in the target tissues around the active tips of probes with resultant rapid heat generation and lethal temperatures greater than 60°C. 17 To expand the extent of tissue achieving lethal temperatures, probes circulate cooled fluid to minimize carbonization and charring effects that can limit electrical conduction. With traditional monopolar RFA systems, a maximum of three probes may be used, acting in sequence with a switching generator and requiring grounding pads to complete the electrical circuit and dissipate the deposited energy. 18 Newer bipolar RFA (bRFA) systems eliminate the need for grounding pads and utilize incorporated thermocouples. One system enables the use of two probes simultaneously without switching. Articulating bRFA probes in another system can be used for targets requiring curved access strategies in the spine or pelvis. 19 20 bRFA probes use defined treatment algorithms to create precise, predictable ablation zones up to 2 × 3 cm in size, which can be vital when treating lesions adjacent to nondisplaceable critical structures. 21 22
Microwave Ablation
MWA is a newer heat-based method, which causes oscillation of water molecules in tissue to create elevated temperatures and cause tissue necrosis even more rapidly. 23 Microwave applicators—termed “antennas”—range from 13 to 17G in caliber and are linear in configuration. Using current systems, up to three antennae may be operated simultaneously and synergistically to create ablation zones up to 6 cm in size as reported for musculoskeletal tumors. 24 Microwave energy has theoretical advantages over RF energy, including faster heating of a larger volume of tissue with decreased heat sink effects. 23 Due to bone's low conductivity, microwave energy should theoretically provide better tissue penetration when treating OM than RF energy. 17
Laser Ablation
LA, also called laser interstitial thermal therapy or interstitial laser photocoagulation, can be performed using flexible, small-caliber systems with a central fiberoptic core and an outer 17G cooling catheter. LA uses infrared photons to achieve lethal temperatures in a small area (∼1.5 cm) at the targeted soft tissues. 25 Importantly, LA is MRI compatible; so, MR thermal mapping can be used to assess ablation progress. 26 Thus far, LA has typically been restricted to treating benign bone tumors. 27
Ablation Modality Comparison
Each ablation modality has advantages and disadvantages with device selection based on several factors, including evidence supporting outcomes, tumor size/morphology, tumor location, local device availability, and overall preference of the performing interventional radiologist. Apart from MR-FUS, all thermal ablation techniques are invasive and require the placement of 11 to 17 G percutaneous applicators into the target tissue. Heat-based technologies (RFA and MWA) are fast, typically a 5- to 15-minute ablation cycle, which can rapidly produce ablation zones with minimal bleeding risk. CA takes longer, utilizing a typical freeze–thaw–refreeze treatment cycle lasting 20 to 25 minutes before the probes are actively thawed for another 5 to 15 minutes to enable safe removal. CA and MWA both have theoretical advantages over RFA in energy conduction through cortical or very sclerotic bone with potential to produce larger ablation zones. Both RFA and MWA are generally limited to treating smaller tumors, around 3 and 5 cm threshold, respectively, with bRFA systems currently offering a precise and predictable ablation zone of up to 3 × 4 cm with set algorithms. 17 28 CA can generate the largest ablation zones, and multiple probes can be placed in variable configurations to create overlapping ablation zones conforming to the target tumor size and shape. 29 30 A larger ablation zone achieved with CA, however, comes with the disadvantage of greater procedural expense and longer procedure times. 31
Neither RFA nor MWA can be visually monitored without MRI thermometry techniques, whereas the ice ball ablation zone with CA can be readily monitored with intermittent CT or using standard MRI pulse sequences 26 ( Fig. 1 ). Notably, as the ice ball 0°C margin is nonlethal, the ice ball should extend at least 0.3 to 0.5 cm beyond the target tumor to achieve a sufficient treatment margin. 14 15 The width of sublethally treated tissue with MWA or RFA is less certain, but heat-based ablation should also ideally include a margin. When treating tumors in very close proximity to nondisplaceable critical structures, such as vertebral body tumors adjacent to the spinal cord, the low conductivity of RFA through intact cortical bone may have some advantage in avoiding collateral damage. RFA devices may not be capable of ablating extremely sclerotic metastases due to the lack of water content and associated impedance. 17 A recent retrospective propensity-matched analysis of patients treated with painful osteolytic OM showed that complete pain response rate and reduction in narcotic medication requirements were superior with CA compared with RFA. 32 Patients treated with CA have also been shown to have significantly decreased periprocedural analgesia requirements and shorter hospital stays in comparison to monopolar RFA, which may increase pain in the early postprocedure period. 33
Fig. 1.

Imaging of the ablation zone. ( a ) On MRI, the ablation zone during cryoablation (CA) appears as an area of hypointensity ( black arrows ) on all sequences. ( b ) CA zone is readily visible with CT as a hypoattenuating region in the soft tissue surrounding bone or in an osteolytic defect as well as within the marrow cavity ( arrows ). Its margins are not evident in cortical or other dense bone. ( c ) The ablation zone during radiofrequency ablation is not visible on CT. Instead, only a few bubbles of gas ( arrow ) are seen within the treated tumor from vaporization of intralesional fluid.
Clinical Applications and Expected Outcomes
Bone Pain Palliation
Several prior multicenter trials have shown both CA and RFA to be highly effective and safe for alleviating pain from symptomatic bone metastases. 34 35 36 37 38 39 Ablation in these cases is ideally performed in patients with refractory or recurrent pain rated at least moderate in severity (>4/10 on a 0- to 10-point scale in a 24-hour period). 1 35 Early multicenter studies were primarily in patient groups treating painful OM in nonspinal locations. 40 Recent studies, including a multicenter prospective study using bRFA 37 and a smaller retrospective study using CA, 41 have shown both of these ablation modalities to be safe and effective in reducing pain in patients with painful vertebral metastases 42 ( Fig. 2 ). No prospective randomized control trials have compared either CA or RFA to RT. A single matched cohort study demonstrated superior pain control with combined RFA and RT compared with RT alone. 43 Results from contemporary studies are summarized in Table 1 . 44 Overall, these studies show favorable pain responses in 68 to 100% of patients with mean pain score reductions of 3.8 to 6.5 using 10-point scales.
Fig. 2.
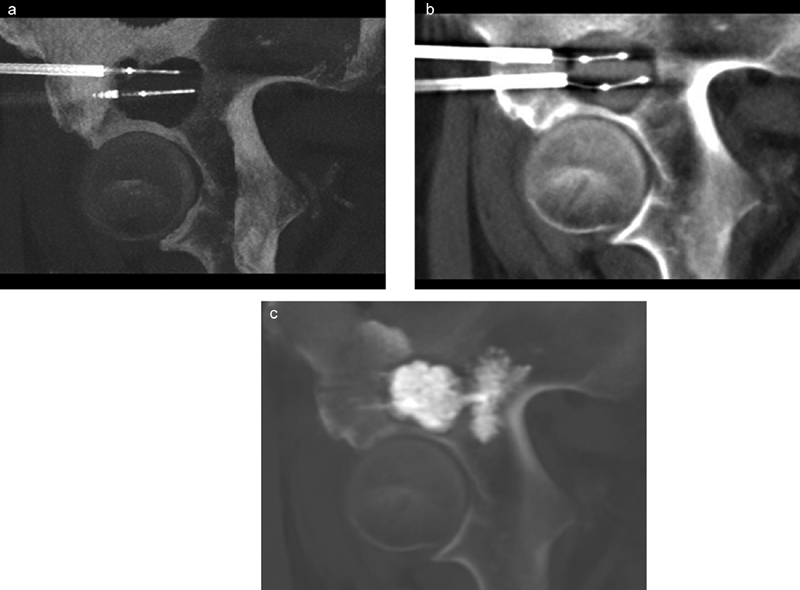
Palliative radiofrequency ablation (RFA) and cementoplasty of a painful right supra-acetabular renal cell carcinoma metastasis that progressed despite prior radiation therapy in a 64-year-old man. ( a ) Intraprocedural sagittal maximum intensity projection (MIP) image shows two bipolar RFA electrodes within the osteolytic metastasis. Note the electrodes were biased away from the hip joint during placement to avoid injury to the femoral head. ( b ) Using coaxial technique, the electrodes were replaced with vertebral augmentation balloons following ablation to create an adequate cavity for cement filling, given the tumor location in a weight-bearing bone. ( c ) Final sagittal MIP image shows excellent filling of the cavity, with cement tied into normal bone to create a solid construct.
Table 1. Outcomes of percutaneous ablation of bone metastases for pain palliation.
| First author | Year | Ablation device | No. of patients (no. of tumors) | Mean tumor size (cm) | Mean pain score change a | No. of patients with reduced pain (%) | Follow-up (mo) |
|---|---|---|---|---|---|---|---|
| Tanigawa 39 | 2018 | RFA | 33 (33) | 6.1 | 6.1–1.3 (4.8/10) | 23 (70) | 12 |
| Bagla 37 | 2016 | RFA | 50 (69) | NR | 5.9–2.1 (3.8/10) | 34 (68) | 3 |
| Tomasian 41 | 2016 | CA | 14 (31) | NR | 8–3 (5/10) | 14 (100) | 10 |
| Wallace 42 | 2015 | RFA | 72 (110) | NR | 8.0–2.9 (5.1/10) | 45 of 58 (78) | 1 |
| Prologo 40 | 2014 | CA | 50 (54) | NR | 8 to 3 (5/10) | 47 (94) | 3 |
| Callstrom 35 | 2013 | CA | 61 (69) | 4.8 | 7.1–1.4 (5.7/10) | 42 (69) | 6 |
| Pusceddu 44 | 2013 | MWA | 18 (21) | 5.3 | 5.6–0.5 (5.1/10) | 17 (94) | 3 |
| Kastler 46 | 2013 | MWA | 15 (25) | 4.7 | 7.2–1.8 (5.4/10) | 14 (93) | 6 |
| Dupuy 36 | 2010 | RFA | 55 (55) | 5.2 | NR (14.2/100) | NR | 3 |
| Goetz 34 | 2004 | RFA | 43 (43) | 6.3 | 7.9–1.4 (6.5/10) | 41 (95) | 6 |
Abbreviations: CA, cryoablation; MWA, microwave ablation; NR, not reported; RFA, radiofrequency ablation.
Mean score for maximal pain (mean score reduction/total points of pain scale used).
A paucity of data currently exists for evaluating the benefits of MWA or LA for palliation of OM. A single, prospective, pilot study and smaller retrospective case series have shown MWA to produce safe pain palliation and effective tumor necrosis. 24 44 45 46 47 48 Evidence for LA is limited to a few reported cases. 26 49
Local Control of Oligometastatic Disease
Minimally invasive ablation treatment of limited metastatic disease continues to have an increasing role in oncologic practice, with the intent of targeting solitary or oligometastatic disease to achieve complete remission ( Fig. 3 ). In a focused approach analogous to stereotactic body radiotherapy treatment strategies, 50 51 52 several studies have recently shown that thermal ablation may produce safe, effective, and durable local control of OM in appropriately selected patients utilizing CA, RFA, or MWA. 24 41 53 54 55 56 57 58 59 60 61 62 This targeted ablation approach can delay or avoid the need for systemic adjuvant therapies and avoid any associated side effects. 55 However, evidence evaluating the role of thermal ablation in treating oligometastatic OM remains limited to multiple retrospective case series and a solitary prospective MWA pilot study for mixed indications. Meta-analysis of outcomes, therefore, would be challenging, as studies may be confounded by differences in technique, adjuvant consolidation therapies, heterogeneous tumor primaries, local control assessment methodology, and variable postablation follow-up.
Fig. 3.
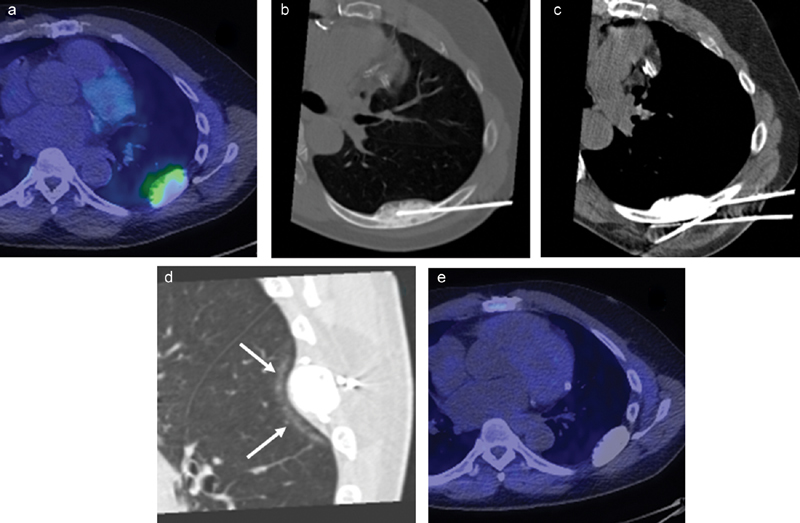
Cryoablation (CA) of oligometastatic disease from prostate carcinoma in a 52-year-old man with persistent PSA following prostatectomy and systemic therapy. ( a ) C11-choline PET/CT shows uptake within a sclerotic left 7th rib oligometastasis. CA was offered to obtain local tumor control. ( b ) Oblique axial CT image shows a cryoprobe advanced partially through the metastasis. Due to the tumor's dense sclerosis, a deeper tract could not be created with either a bone biopsy needle or handheld drill; so, the cryoprobe could not be placed deep enough to cover the posterior margin of the metastasis. ( c ) Instead, cryoprobes were placed along the margins of the rib to shape an ablation zone that would cover the entire tumor. As ice easily crosses through intact cortical and sclerotic bone, this strategy is acceptable even for a goal of local tumor control. ( d ) Intraprocedural sagittal CT image shows interstitial changes in the lung ( arrows ) deep to the metastasis along the edge of the ice ball, confirming adequate tumor coverage. ( e ) Follow-up PET/CT 1 year after ablation shows no uptake in the treated rib metastasis consistent with no residual tumor.
Recent studies are summarized in Table 2 . Overall, these studies have shown highly variable local tumor control rates of 36 to 97% and major complication rates of 0 to 11%. A 2014 single-center retrospective study of 89 consecutive patients with 122 bone metastases from different primaries (48 treated with CA and 74 treated with RFA) demonstrated local control in 67% of patients at 1-year follow-up. 57 Favorable treatment prognostic factors from this study included small size of metastases (<2 cm), metastases metachronous from the primary tumor, and absence of cortical bone erosion or closely adjacent neurological structures. More recently, retrospective studies have aimed to specifically review single metastatic tumor histologies and outcomes of OM ablation. A 2017 study reviewing CA to treat RCC bone metastases showed a local control rate per lesion of 82%, with less favorable treatment outcomes in patients with more than five metastases. 54 Most recently, a 2018 study assessing ablation (RF, CA, and MWA) in 45 patients with 76 non-small cell lung cancer OM demonstrated local control rates of 83% at 3 months and 68% at 12 months. 53
Table 2. Outcomes of percutaneous ablation for local tumor control of bone metastases.
| Author | Year | Tumor histology | Sites | Ablation modality | Average size (cm) | No. of patients (no. of tumors) | Local control No. (%) or %/y |
Survival (%/y) | Follow-up (mo) | Major comp (no., %) a |
|---|---|---|---|---|---|---|---|---|---|---|
| Vaswani 62 | 2018 | Sarcoma | MSK | RFA, CA | 3.0 b | 13 (13) b | 100/y b | NR | 12 b | 3 (5) |
| Ma 53 | 2018 | NSCLC | Bone | RFA, CA | 3.6 | 45 (76) | 17 (68) | NR | 12 | 2 (2.6) |
| Gardner 54 | 2017 | RCC | Bone | CA | 3.4 | 40 (50) | 41/50 (82) | 31(77/y 26/5 y) | 35 | 4 (8) |
| Erie 55 | 2017 | Prostate | MSK | RFA, CA | 1.6 | 16 (18) | 15 (83) | 100/2 y | 27 | 0 |
| Aubry 24 | 2017 | Mixed | MSK | MWA | 5.5 | 13 (16) | 4 (36.3) | NR | 12 | 0 |
| Tomasian 41 | 2016 | Mixed | Spine | CA | NR | 14 (31) | 30 (96.7) | NR | 10 | 0 |
| Wallace 56 | 2016 | Mixed | Spine | RFA | NR | NR (55) | 70/y | NR | 7.9 | 0 |
| Deschamps 57 | 2014 | Mixed | Bone | RFA, CA | NR | 89 (122) | 67/y | 91/1 | 22.8 | 11 (9) |
| Welch 58 | 2014 | Renal | c | RFA, CA | NR | NR (46) | 43 (93) | NR | 22.5 | 0 |
| McMenomy 59 | 2013 | Mixed | MSK | CA | 2 | 40 (52) | 45 (87) | 91/y, 84/2 y | 21 | 2 (5) |
| Bang 60 | 2012 | NSCLC | c | CA | 3.1 | 8 (18) | 17 (94) | NR | 11 | 2 (11) |
| Bang 61 | 2012 | Renal | c | CA | 3.7 | 27 (48) | 47 (97) | NR | 16 | 1 (2) |
Abbreviations: CA, cryoablation; Comp, complications; MSK, musculoskeletal; MWA, microwave ablation; NR, not reported; RFA, radiofrequency ablation.
Per procedure.
Data from subpopulation treated for local tumor control are reported here.
Study includes metastatic sites beyond bone and soft tissue; only data related to bone and soft-tissue metastases are reported here.
Prevention of Skeletal-Related Events
Few studies have specifically evaluated the prevention of critical SREs with thermal ablation. Outcomes from a 2018 single-center retrospective review evaluating the prevention of SREs in 28 patients with malignant paraganglioma and pheochromocytoma metastases suggest that the occurrence of a first serious SRE may be delayed in patients treated with thermal ablation and/or percutaneous consolidation techniques. 63 Ablation may be indicated to prevent critical SREs, such as tumor progression and encroachment upon the spinal cord, major motor nerves, or neve plexuses. In OM at high risk of fracture, ablation may be used primarily to sterilize and prepare a cavity for subsequent consolidation with cementoplasty ( Fig. 4 ).
Fig. 4.

Combined cryoablation (CA) and radiofrequency ablation (RFA) followed by cementoplasty for pain palliation. ( a ) Intraprocedural CT image shows an osteolytic left periacetabular metastasis from renal cell carcinoma in a 65-year-old man with pain rated as 9/10 in severity, causing loss of sleep. ( b ) CA was performed on the superior aspect of the tumor, given relatively large size. ( c ) Bipolar RFA with an articulating electrode was used to treat the lower portion of the metastasis while avoiding the femoral head. ( d and e ) Cementoplasty was then performed to consolidate this metastasis at risk for pathologic fracture.
How We Do It
Patient Selection and Preprocedural Workup
The collaboration of a multidisciplinary team consisting of an interventional radiologist, neurologic and/or orthopedic surgeon, radiation oncologist, and medical oncologist is ideal in coordinating decision plans to treat a patient with OM. All types of bone metastases may potentially be treated with thermal ablation. There are few absolute contraindications to percutaneous ablation. These include uncorrectable bleeding diatheses, inability of the patient to tolerate required level of anesthesia, or inaccessibility of the target lesion from an appropriate percutaneous approach. Active bone or bloodstream infection are additional contraindications.
Treatment aims and goals should be clearly identified and discussed with the patient at initial consultation, in addition to determining pain levels and functional status. When performing for pain palliation, ideally patients should have a solitary or limited number (<5) of symptomatic lesions with pain of at least moderate intensity. Focused physical examination is mandatory to confirm correlation of the painful site(s) with relevant imaging findings.
Appropriate high-quality preablation imaging is essential for a variety of factors including full characterization of tumor number, location, and extent; confirmation of safe percutaneous access route; and assessment of nearby vital neural or vascular structures to be monitored or avoided during ablation. Preprocedural imaging should be ideally within 4 weeks of the intended procedural date given the potential for tumor growth. CT, MRI, and PET all have useful and complementary roles. CT is excellent for delineating tumor mineralization, status of overlying cortex, and presence of any bone insufficiency which may drive access device selection and necessity for adjunctive consolidation treatments. 64 65 66 MRI offers the additional benefit of better delineation of tumor extent and adjacent soft tissue and neuroanatomical structures. 67 Metabolic imaging with PET is extremely useful in identifying metastases or recurrent disease (post-RT or prior ablation) that may be occult on routine CT imaging. 68 Absence of critical structures within a radius of 1 to 1.5 cm around the target ablation site is preferable to prevent nontarget ablation collateral damage. Desire for preprocedural myelogram or intraprocedural neural monitoring is further determined at this preprocedural imaging review based on proximity of vital neural structures.
Anesthesia and Physiologic Monitoring Considerations
We perform the great majority of our cases with patients under general anesthesia, though some centers routinely use conscious sedation for OM ablation. General anesthesia offers maximal patient comfort and physiologic control, expanding the complexity and size of tumors that may be treated and broadening the range of medically complex patients who can tolerate the procedure.
Some patients are treated under conscious sedation to allow them to provide feedback when the target tumors are in close proximity to vital neural structures. If patients report pain in the distribution of a sensory nerve or nerve root at risk, the ablation may be terminated. Neurophysiologic monitoring with somatosensory or motor-evoked potentials or direct nerve stimulation is also very useful. 69 70 In these cases, the patients must be placed under total intravenous general anesthesia and with close attention to mean arterial blood pressure intraprocedurally to maintain appropriate neural responses.
Ablation Technique
The patient is then optimally positioned in the intervention suite to allow percutaneous access and facilitate displacement of nontarget structures by gravity. Routine preprocedural CT or MRI scans of the target area are obtained, and acquired multiplanar images are then directly correlated to preprocedural images. We spend several minutes confirming ablation plan, access, and ablation devices.
We select an appropriate ablation device based on factors described in an earlier section of this article. In our practice, we predominantly use CA to treat OM arising in the pelvis and appendicular skeleton, and typically use CT guidance. For tumors involving the spine, we utilize either CA or bRFA, and use either fluoroscopic or CT guidance. For CA cases, we aim to place probes within 1.5 to 2 cm of each other and 1 cm inside the tumor margin, typically along the long axis of the lesion and positioned to allow ice ball growth to efficiently cover the targeted lesion. 13
Tumors within intact bone require a suitable pathway to be predrilled to allow safe placement of ablation probes. Densely sclerotic OM can be challenging to access (see Fig. 3 ). Handheld 10 to 14G manual or automatic drills are required to facilitate access to allow treatment of these metastases. 71 It may be necessary to sequentially remove small bone cores along the intended insertion tract to enable access in extremely osteoblastic lesions, similar to performing a bone biopsy.
Vulnerable structures may be displaced from the targeted ablation zone using sterile fluid (hydrodisplacement), gas (ideally CO 2 ) or less commonly balloon catheters. 72 73 We aim to create a 1.5-cm safety zone around target tumors through these techniques prior to commencing ablation, though that margin is almost always smaller in the spine. When treating tumors in superficial locations, the overlying skin can be displaced and physically warmed by a fluid-filled glove, operator's hand, or warming lights and the ice ball's extent may be evaluated with ultrasound.
CA treatment typically consists of two freeze cycles of 10 minutes each separated by a 5- to 8-minute thaw, although cycle lengths may be varied to allow for adequate tumor coverage and avoidance of adjacent vital structures. Several minutes of active thaw are required before cryoprobe removal. bRFA treatment parameters are selected according to manufacturer's guidelines and operator's experience. As with surgery or RT, treatment should extend beyond the visible tumor margin, particularly for tumors with infiltrative margins, to help prevent residual disease.
Intraprocedurally, the ablation zone may be monitored visually with CT or MR imaging or physiologically using thermocouples and/or neural monitoring techniques. For CA, intermittent imaging should be performed every 2 to 5 minutes during freeze cycles to monitor ice ball growth. Notably using CA to treat sclerotic bone lesions, particularly in the spine, can be challenging due to poor CT visualization of the hypoattenuating ice ball within intact or sclerotic bone. Determination of ice ball growth in these cases often requires extrapolation from visualization within adjacent disc space or paravertebral soft tissues. MRI guidance allows for very precise monitoring and maximal visualization of tumor margins. Disadvantages include the limited range of MRI-compatible ablation and bone access devices currently available.
Periprocedural short-course steroid administration should be considered when ablating in close proximity to critical neural structures or when ablation may involve treating substantial amount of adjacent nontarget skeletal muscle, to minimize postablation edema-related pain or neuropraxia.
Follow-up
Postablation care for most patients treated with OM is guided by each patient's prognosis and clinical symptoms. In addition to routine imaging, assessment ideally should include a subjective pain assessment scale and/or quality of life assessment scores. Examples include the Roland-Morris Disability Questionnaire for evaluation of back pain or the Musculoskeletal Tumor Society scoring system for assessment of functional outcomes after treatment in the pelvis or limbs. 65 74 75
Patients treated for local tumor should have more consistent imaging follow-up, which we typically perform at 3, 6, and 12 months postablation, and 6 to 12 months thereafter. We have found PET/CT to be particularly useful in the detection of local tumor progression following ablation of OM. 68 The radiologist should be familiar with the typical appearance of ablated musculoskeletal metastases as they can be misinterpreted as tumor progression or infection. 76
Conclusion
The utility of minimally invasive approach of thermal ablation has continued to expand and adds to the armamentarium of the treating IO team. Percutaneous thermal ablation of OM may be performed for palliation of pain, prevention of SREs, and durable local tumor control in nonsurgical patients. RFA offers fast, effective ablation with minimal bleeding risk for smaller tumors (< 3 cm). Disadvantages include inability to easily monitor procedure, and patients often experience increased periprocedural pain. CA provides safe, effective ablation with easier monitoring of the ablation zone with CT or MRI, less periprocedural pain, and simultaneous activation of numerous probes to treat large tumors. Its disadvantages include greater expense and longer procedure times. MWA and LA remain emerging ablation alternatives.
Successful thermal ablation of OM requires appropriate patient selection, preprocedural planning, careful intraprocedural technique designed to avoid complications, and judicious postprocedure follow-up.
References
- 1.Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24(02):112–124. doi: 10.1016/j.clon.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Janjan N. Bone metastases: approaches to management. Semin Oncol. 2001;28(04) 11:28–34. doi: 10.1016/s0093-7754(01)90229-5. [DOI] [PubMed] [Google Scholar]
- 3.So A, Chin J, Fleshner N, Saad F. Management of skeletal-related events in patients with advanced prostate cancer and bone metastases: incorporating new agents into clinical practice. Can Urol Assoc J. 2012;6(06):465–470. doi: 10.5489/cuaj.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saad F, Lipton A, Cook R, Chen Y M, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110(08):1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 5.Kurup A N, Callstrom M R. Ablation of musculoskeletal metastases: pain palliation, fracture risk reduction, and oligometastatic disease. Tech Vasc Interv Radiol. 2013;16(04):253–261. doi: 10.1053/j.tvir.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Deschamps F, Farouil G, de Baere T.Percutaneous ablation of bone tumors Diagn Interv Imaging 201495(7-8):659–663. [DOI] [PubMed] [Google Scholar]
- 7.Chow E, Harris K, Fan G, Tsao M, Sze W M. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 8.Lutz S, Berk L, Chang E et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79(04):965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Laufer I, Rubin D G, Lis E et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18(06):744–751. doi: 10.1634/theoncologist.2012-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccioli A, Spinelli M S, Maccauro G. Impending fracture: a difficult diagnosis. Injury. 2014;45 06:S138–S141. doi: 10.1016/j.injury.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed M, Brace C L, Lee F T, Jr, Goldberg S N. Principles of and advances in percutaneous ablation. Radiology. 2011;258(02):351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chosy S G, Nakada S Y, Lee F T, Jr, Warner T F. Monitoring renal cryosurgery: predictors of tissue necrosis in swine. J Urol. 1998;159(04):1370–1374. doi: 10.1016/s0022-5347(01)63618-8. [DOI] [PubMed] [Google Scholar]
- 13.Lee F T, Jr, Chosy S G, Littrup P J, Warner T F, Kuhlman J E, Mahvi D M. CT-monitored percutaneous cryoablation in a pig liver model: pilot study. Radiology. 1999;211(03):687–692. doi: 10.1148/radiology.211.3.r99jn29687. [DOI] [PubMed] [Google Scholar]
- 14.Littrup P J, Jallad B, Vorugu V et al. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J Vasc Interv Radiol. 2009;20(10):1343–1351. doi: 10.1016/j.jvir.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandison G A, Loye M P, Rewcastle J C et al. X-ray CT monitoring of iceball growth and thermal distribution during cryosurgery. Phys Med Biol. 1998;43(11):3309–3324. doi: 10.1088/0031-9155/43/11/010. [DOI] [PubMed] [Google Scholar]
- 16.Dupuy D E, Safran H, Mayo-Smith W W, Goldberg S N. Radiofrequency ablation of painful osseous metastatic disease. Radiology. 1998;209:389. [Google Scholar]
- 17.Brace C L. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38(03):135–143. doi: 10.1067/j.cpradiol.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisbrod A J, Atwell T D, Callstrom M R, Farrell M A, Mandrekar J N, Charboneau J W. Percutaneous radiofrequency ablation with a multiple-electrode switching-generator system. J Vasc Interv Radiol. 2007;18(12):1528–1532. doi: 10.1016/j.jvir.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Anchala P R, Irving W D, Hillen T J et al. Treatment of metastatic spinal lesions with a navigational bipolar radiofrequency ablation device: a multicenter retrospective study. Pain Physician. 2014;17(04):317–327. [PubMed] [Google Scholar]
- 20.Hillen T J, Anchala P, Friedman M V, Jennings J W. Treatment of metastatic posterior vertebral body osseous tumors by using a targeted bipolar radiofrequency ablation device: technical note. Radiology. 2014;273(01):261–267. doi: 10.1148/radiol.14131664. [DOI] [PubMed] [Google Scholar]
- 21.Gazis A N, Beuing O, Franke J, Jöllenbeck B, Skalej M. Bipolar radiofrequency ablation of spinal tumors: predictability, safety and outcome. Spine J. 2014;14(04):604–608. doi: 10.1016/j.spinee.2013.06.081. [DOI] [PubMed] [Google Scholar]
- 22.Buy X, Basile A, Bierry G, Cupelli J, Gangi A.Saline-infused bipolar radiofrequency ablation of high-risk spinal and paraspinal neoplasms AJR Am J Roentgenol 2006186(5, Suppl):S322–S326. [DOI] [PubMed] [Google Scholar]
- 23.Brace C L. Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol. 2009;38(02):61–67. doi: 10.1067/j.cpradiol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aubry S, Dubut J, Nueffer J P, Chaigneau L, Vidal C, Kastler B. Prospective 1-year follow-up pilot study of CT-guided microwave ablation in the treatment of bone and soft-tissue malignant tumours. Eur Radiol. 2017;27(04):1477–1485. doi: 10.1007/s00330-016-4528-7. [DOI] [PubMed] [Google Scholar]
- 25.Gangi A, Buy X. Percutaneous bone tumor management. Semin Intervent Radiol. 2010;27(02):124–136. doi: 10.1055/s-0030-1253511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahrar K, Stafford R J. Magnetic resonance imaging-guided laser ablation of bone tumors. Tech Vasc Interv Radiol. 2011;14(03):177–182. doi: 10.1053/j.tvir.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Gangi A, Alizadeh H, Wong L, Buy X, Dietemann J L, Roy C. Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology. 2007;242(01):293–301. doi: 10.1148/radiol.2421041404. [DOI] [PubMed] [Google Scholar]
- 28.Eckmann M S, Martinez M A, Lindauer S, Khan A, Ramamurthy S. Radiofrequency ablation near the bone-muscle interface alters soft tissue lesion dimensions. Reg Anesth Pain Med. 2015;40(03):270–275. doi: 10.1097/AAP.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 29.Kurup A N, Morris J M, Schmit G D et al. Balloon-assisted osteoplasty of periacetabular tumors following percutaneous cryoablation. J Vasc Interv Radiol. 2015;26(04):588–594. doi: 10.1016/j.jvir.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Kurup A N, Callstrom M R.Ablation of skeletal metastases: current status J Vasc Interv Radiol 201021(8, Suppl):S242–S250. [DOI] [PubMed] [Google Scholar]
- 31.Callstrom M R, Kurup A N. Percutaneous ablation for bone and soft tissue metastases--why cryoablation? Skeletal Radiol. 2009;38(09):835–839. doi: 10.1007/s00256-009-0736-4. [DOI] [PubMed] [Google Scholar]
- 32.Zugaro L, DI Staso M, Gravina G L et al. Treatment of osteolytic solitary painful osseous metastases with radiofrequency ablation or cryoablation: a retrospective study by propensity analysis. Oncol Lett. 2016;11(03):1948–1954. doi: 10.3892/ol.2016.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thacker P G, Callstrom M R, Curry T B et al. Palliation of painful metastatic disease involving bone with imaging-guided treatment: comparison of patients' immediate response to radiofrequency ablation and cryoablation. AJR Am J Roentgenol. 2011;197(02):510–515. doi: 10.2214/AJR.10.6029. [DOI] [PubMed] [Google Scholar]
- 34.Goetz M P, Callstrom M R, Charboneau J W et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22(02):300–306. doi: 10.1200/JCO.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 35.Callstrom M R, Dupuy D E, Solomon S B et al. Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer. 2013;119(05):1033–1041. doi: 10.1002/cncr.27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dupuy D E, Liu D, Hartfeil D et al. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer. 2010;116(04):989–997. doi: 10.1002/cncr.24837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bagla S, Sayed D, Smirniotopoulos J et al. Multicenter prospective clinical series evaluating radiofrequency ablation in the treatment of painful spine metastases. Cardiovasc Intervent Radiol. 2016;39(09):1289–1297. doi: 10.1007/s00270-016-1400-8. [DOI] [PubMed] [Google Scholar]
- 38.Callstrom M R, Charboneau J W, Goetz M P et al. Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radio-frequency ablation. Radiology. 2002;224(01):87–97. doi: 10.1148/radiol.2241011613. [DOI] [PubMed] [Google Scholar]
- 39.Tanigawa N, Arai Y, Yamakado K et al. Phase I/II study of radiofrequency ablation for painful bone metastases: Japan Interventional Radiology in Oncology Study Group 0208. Cardiovasc Intervent Radiol. 2018;41(07):1043–1048. doi: 10.1007/s00270-018-1944-x. [DOI] [PubMed] [Google Scholar]
- 40.Prologo J D, Passalacqua M, Patel I, Bohnert N, Corn D J. Image-guided cryoablation for the treatment of painful musculoskeletal metastatic disease: a single-center experience. Skeletal Radiol. 2014;43(11):1551–1559. doi: 10.1007/s00256-014-1939-x. [DOI] [PubMed] [Google Scholar]
- 41.Tomasian A, Wallace A, Northrup B, Hillen T J, Jennings J W. Spine cryoablation: pain palliation and local tumor control for vertebral metastases. AJNR Am J Neuroradiol. 2016;37(01):189–195. doi: 10.3174/ajnr.A4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallace A N, Greenwood T J, Jennings J W. Radiofrequency ablation and vertebral augmentation for palliation of painful spinal metastases. J Neurooncol. 2015;124(01):111–118. doi: 10.1007/s11060-015-1813-2. [DOI] [PubMed] [Google Scholar]
- 43.Di Staso M, Zugaro L, Gravina G L et al. A feasibility study of percutaneous radiofrequency ablation followed by radiotherapy in the management of painful osteolytic bone metastases. Eur Radiol. 2011;21(09):2004–2010. doi: 10.1007/s00330-011-2133-3. [DOI] [PubMed] [Google Scholar]
- 44.Pusceddu C, Sotgia B, Fele R M, Melis L. Treatment of bone metastases with microwave thermal ablation. J Vasc Interv Radiol. 2013;24(02):229–233. doi: 10.1016/j.jvir.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Kastler A, Alnassan H, Pereira P L et al. Analgesic effects of microwave ablation of bone and soft tissue tumors under local anesthesia. Pain Med. 2013;14(12):1873–1881. doi: 10.1111/pme.12242. [DOI] [PubMed] [Google Scholar]
- 46.Kastler A, Alnassan H, Aubry S, Kastler B. Microwave thermal ablation of spinal metastatic bone tumors. J Vasc Interv Radiol. 2014;25(09):1470–1475. doi: 10.1016/j.jvir.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Kastler A, Krainik A, Sakhri L, Mousseau M, Kastler B. Feasibility of real-time intraprocedural temperature control during bone metastasis thermal microwave ablation: a bicentric retrospective study. J Vasc Interv Radiol. 2017;28(03):366–371. doi: 10.1016/j.jvir.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 48.Pusceddu C, Sotgia B, Fele R M, Ballicu N, Melis L. Combined microwave ablation and cementoplasty in patients with painful bone metastases at high risk of fracture. Cardiovasc Intervent Radiol. 2016;39(01):74–80. doi: 10.1007/s00270-015-1151-y. [DOI] [PubMed] [Google Scholar]
- 49.Williams B J, Karas P J, Rao G, Rhines L D, Tatsui C E. Laser interstitial thermal therapy for palliative ablation of a chordoma metastasis to the spine: case report. J Neurosurg Spine. 2017;26(06):722–724. doi: 10.3171/2016.11.SPINE16897. [DOI] [PubMed] [Google Scholar]
- 50.Macdermed D M, Weichselbaum R R, Salama J K. A rationale for the targeted treatment of oligometastases with radiotherapy. J Surg Oncol. 2008;98(03):202–206. doi: 10.1002/jso.21102. [DOI] [PubMed] [Google Scholar]
- 51.Lo S S, Teh B S, Mayr N A et al. Stereotactic body radiation therapy for oligometastases. Discov Med. 2010;10(52):247–254. [PubMed] [Google Scholar]
- 52.Salama J K, Hasselle M D, Chmura S J et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118(11):2962–2970. doi: 10.1002/cncr.26611. [DOI] [PubMed] [Google Scholar]
- 53.Ma Y, Wallace A N, Waqar S N et al. Percutaneous image-guided ablation in the treatment of osseous metastases from non-small cell lung cancer. Cardiovasc Intervent Radiol. 2018;41(05):726–733. doi: 10.1007/s00270-017-1843-6. [DOI] [PubMed] [Google Scholar]
- 54.Gardner C S, Ensor J E, Ahrar K et al. Cryoablation of bone metastases from renal cell carcinoma for local tumor control. J Bone Joint Surg Am. 2017;99(22):1916–1926. doi: 10.2106/JBJS.16.01182. [DOI] [PubMed] [Google Scholar]
- 55.Erie A J, Morris J M, Welch B T et al. Retrospective review of percutaneous image-guided ablation of oligometastatic prostate cancer: a single-institution experience. J Vasc Interv Radiol. 2017;28(07):987–992. doi: 10.1016/j.jvir.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Wallace A N, Tomasian A, Vaswani D, Vyhmeister R, Chang R O, Jennings J W. Radiographic local control of spinal metastases with percutaneous radiofrequency ablation and vertebral augmentation. AJNR Am J Neuroradiol. 2016;37(04):759–765. doi: 10.3174/ajnr.A4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deschamps F, Farouil G, Ternes N et al. Thermal ablation techniques: a curative treatment of bone metastases in selected patients? Eur Radiol. 2014;24(08):1971–1980. doi: 10.1007/s00330-014-3202-1. [DOI] [PubMed] [Google Scholar]
- 58.Welch B T, Callstrom M R, Morris J M et al. Feasibility and oncologic control after percutaneous image guided ablation of metastatic renal cell carcinoma. J Urol. 2014;192(02):357–363. doi: 10.1016/j.juro.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 59.McMenomy B P, Kurup A N, Johnson G B et al. Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission. J Vasc Interv Radiol. 2013;24(02):207–213. doi: 10.1016/j.jvir.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 60.Bang H J, Littrup P J, Currier B P et al. Percutaneous cryoablation of metastatic lesions from non-small-cell lung carcinoma: initial survival, local control, and cost observations. J Vasc Interv Radiol. 2012;23(06):761–769. doi: 10.1016/j.jvir.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bang H J, Littrup P J, Goodrich D J et al. Percutaneous cryoablation of metastatic renal cell carcinoma for local tumor control: feasibility, outcomes, and estimated cost-effectiveness for palliation. J Vasc Interv Radiol. 2012;23(06):770–777. doi: 10.1016/j.jvir.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaswani D, Wallace A N, Eiswirth P S et al. Radiographic local tumor control and pain palliation of sarcoma metastases within the musculoskeletal system with percutaneous thermal ablation. Cardiovasc Intervent Radiol. 2018;41(08):1223–1232. doi: 10.1007/s00270-018-1932-1. [DOI] [PubMed] [Google Scholar]
- 63.Gravel G, Leboulleux S, Tselikas L et al. Prevention of serious skeletal-related events by interventional radiology techniques in patients with malignant paraganglioma and pheochromocytoma. Endocrine. 2018;59(03):547–554. doi: 10.1007/s12020-017-1515-y. [DOI] [PubMed] [Google Scholar]
- 64.Deschamps F, Farouil G, Hakime A, Teriitehau C, Barah A, de Baere T. Percutaneous stabilization of impending pathological fracture of the proximal femur. Cardiovasc Intervent Radiol. 2012;35(06):1428–1432. doi: 10.1007/s00270-011-0330-8. [DOI] [PubMed] [Google Scholar]
- 65.Hartung M P, Tutton S M, Hohenwalter E J, King D M, Neilson J C. Safety and efficacy of minimally invasive acetabular stabilization for periacetabular metastatic disease with thermal ablation and augmented screw fixation. J Vasc Interv Radiol. 2016;27(05):682–6880. doi: 10.1016/j.jvir.2016.01.142. [DOI] [PubMed] [Google Scholar]
- 66.Kelekis A, Filippiadis D K, Kelekis N L, Martin J B. Percutaneous augmented osteoplasty of the humeral bone using a combination of microneedles mesh and cement. J Vasc Interv Radiol. 2015;26(04):595–597. doi: 10.1016/j.jvir.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 67.Kurup A N, Morris J M, Schmit G D et al. Neuroanatomic considerations in percutaneous tumor ablation. Radiographics. 2013;33(04):1195–1215. doi: 10.1148/rg.334125141. [DOI] [PubMed] [Google Scholar]
- 68.Packard A T, Broski S M, Callstrom M R et al. Utility of PET/CT after cryoablation for early identification of local tumor progression in osseous metastatic disease. AJR Am J Roentgenol. 2017;208(06):1342–1351. doi: 10.2214/AJR.16.17222. [DOI] [PubMed] [Google Scholar]
- 69.Kurup A N, Morris J M, Boon A J et al. Motor evoked potential monitoring during cryoablation of musculoskeletal tumors. J Vasc Interv Radiol. 2014;25(11):1657–1664. doi: 10.1016/j.jvir.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Tsoumakidou G, Garnon J, Ramamurthy N, Buy X, Gangi A. Interest of electrostimulation of peripheral motor nerves during percutaneous thermal ablation. Cardiovasc Intervent Radiol. 2013;36(06):1624–1628. doi: 10.1007/s00270-013-0641-z. [DOI] [PubMed] [Google Scholar]
- 71.Wallace A N, Chang R O, Tomasian A, Jennings J W. Drill-assisted, fluoroscopy-guided vertebral body access for radiofrequency ablation: technical case series. Interv Neuroradiol. 2015;21(05):631–634. doi: 10.1177/1591019915594329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurup A N, Schmit G D, Morris J M et al. Avoiding complications in bone and soft tissue ablation. Cardiovasc Intervent Radiol. 2017;40(02):166–176. doi: 10.1007/s00270-016-1487-y. [DOI] [PubMed] [Google Scholar]
- 73.Tsoumakidou G, Buy X, Garnon J, Enescu J, Gangi A. Percutaneous thermal ablation: how to protect the surrounding organs. Tech Vasc Interv Radiol. 2011;14(03):170–176. doi: 10.1053/j.tvir.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 74.Enneking W F, Dunham W, Gebhardt M C, Malawar M, Pritchard D J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;(286):241–246. [PubMed] [Google Scholar]
- 75.Roland M, Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine. 2000;25(24):3115–3124. doi: 10.1097/00007632-200012150-00006. [DOI] [PubMed] [Google Scholar]
- 76.Wallace A N, Greenwood T J, Jennings J W. Use of imaging in the management of metastatic spine disease with percutaneous ablation and vertebral augmentation. AJR Am J Roentgenol. 2015;205(02):434–441. doi: 10.2214/AJR.14.14199. [DOI] [PubMed] [Google Scholar]


