Abstract
Transitional cell carcinoma is the most common cancer of the canine urinary tract. The inconsistent appearance of transitional cell carcinoma in patients introduces error if applying mathematic models for extrapolating total tumor volume from linear measurements. Reliable techniques to assess tumor size are important for monitoring treatment response. A method comparison study was performed comparing four techniques for calculating tumor volume were compared: 1 & 2) contoured tracing of tumor margins using serial computed tomography (CT) images using pre-(1) and post-intravenous (2) contrast medium studies, 3) longest three linear dimensions using CT, and 4) longest three linear dimensions on abdominal ultrasound (US). Volumes of the transitional cell carcinoma tumor calculated by CT tracing techniques were significantly smaller than volumes calculated with an ellipsoid mathematic model using the linear measurements (p < 0.01). Intravenous contrast medium did not significantly change the volumes calculated from tracing tumor margins on CT for observer B; however, volumes differed for observer A. The volumes extrapolated from linear measurements using CT and US did not differ significantly. The interobserver reliability was highest for the pre-contrast CT contoured technique and was lowest using the ultrasound linear technique. Tumor volumes differed significantly between techniques of contoured tracing of the tumor margins on serial CT images compared to calculation of tumor volume from linear dimensions. The calculated volume of a transitional cell carcinoma depends upon the technique used. Characterizing the response of urinary bladder transitional cell carcinoma tumor size to therapy differs based on the method and modality used.
Keywords: urinary bladder cancer, dog, imaging, measurement, RECIST
INTRODUCTION
Transitional cell carcinoma is the most common cancer of the canine urinary tract.1 The prevalence of naturally occurring transitional cell carcinoma in dogs is not fully understood, but it is estimated to be approximately 1 – 2% of all canine cancers.2 Urinary bladder transitional cell carcinomas have different sizes, shapes, and distributions in dogs. These characteristics may change in an individual dog throughout the course of the disease particularly in response to treatment. Treatment for dogs with non-surgical transitional cell carcinoma most commonly consists of a combination of non-steroidal anti-inflammatory drugs, chemopharamceuticals, small molecular inhibitors, and/or radiation therapy. These therapies are aimed at reducing the tumor volume to palliate clinical signs, thus improving quality of life. Surgical intervention is often difficult because the disease is frequently in a trigonal location and may have a multifocal distribution.3 Mapping of the bladder to characterize the location of the transitional cell carcinoma tumor is poignant in the clinical evaluation and staging if disease to determine if surgery is an option.4 Even when surgical excision is possible, the goal of surgery may be limited to diagnostic purposes or for palliation of clinical signs due to the inability to obtain appropriate surgical margins and the effect of field carcinogenesis on the remaining urinary bladder tissue.4,5
Response evaluation criteria in solid tumors (RECIST) guidelines provide established objective measures to dictate and categorize effectiveness of chemotherapy drugs.6 The RECIST guidelines are important for testing efficacy of novel therapeutics in clinical trials and the response of an individual patient to treatment. The RECIST guidelines use linear measurements in the longest dimension of the tumor and percentage changes in this length to monitor response to treatment or progression of disease: progressive disease is an increase of 20%, partial response is a decrease of 30%, and stable disease is an increase of less than 20% and/or decrease of less than 30%. A complete response is considered to be the absence of tumor at the recheck. Ultrasound is commonly used for tumor monitoring and measurement in veterinary medicine. However, US in considered an inappropriate tool in human medicine due to the high user dependency and low interobserver reliability.6 Previous veterinary studies demonstrated that intraobserver repeatability of US-based linear tumor measurements can be excellent, but interobserver measurements had a significantly high variation.5 Intraobserver two-dimensional ultrasonographic measurements of transitional cell carcinoma tumors in dogs vary based on the degree of bladder distension with a poor agreement when interobserver reliability is compared between ultrasonographers.7 There is evidence that a single highly experienced user guided by a specific approach to urinary bladder tumors can have a high repeatability for linear measurements; however, the interobserver reliability within this study was poor.8 The same study also found that CT volume measurements were more reliable than US, as well as, more consistent for tumor measurements at varying volumes of urinary bladder distention.8 In people, cross-sectional modalities like magnetic resonance imaging (MRI) and CT are used for assessing the treatment response of solid tumors.9 There have been multiple clinical trials performed in a variety of different tumor types utilizing RECIST guidelines to evaluate chemotherapy and radiation therapy protocols.5,10–16 However, the inconsistent, irregularly shaped urinary bladder transitional cell carcinoma tumors do not allow for accurate calculations of tumor volumes based on linear measurements. Utilization of unidimensional RECIST guidelines for assessment and monitoring of canine bladder transitional cell carcinoma is flawed.
Accurate measurements are critical for evaluating response to treatment. The objective of the study is to compare the tumor volumes measured using four volume methodologies. A second objective of this study was to evaluate if a tumor’s response to therapy, as defined by the unidimensional RECIST guidelines, would change if using US and CT linear dimensions or CT tracing methodologies to calculate tumor volume. It is hypothesized that volumes based on linear measurements made using CT and US will yield significantly different volumes than CT tracing volumes for urinary bladder transitional cell carcinoma. It is also hypothesized that the linear measurements will result in significantly greater calculated tumor volumes when compared to volumes calculated from tracing the margins of the tumors on CT images.
METHODS
This clinical trial was approved by the Clinical Research and Advising Committee at the College of Veterinary Medicine and Institutional Animal Care and Use Committee (IACUC) at Ohio State University. Images were collected from 10 dogs that were concurrently enrolled in a parent study evaluating the combination of toceranib and vinblastine for canine transitional cell carcinoma. A priori power analysis was used to compute the sample size of paired measurements to detect a significant difference in the tumor volume between techniques (G*Power, v.3.1.9.2., http://www.gpower.hhu.de/en.html). The data used for the power analysis were sampled from a subset of the dogs with urinary bladder transitional cell carcinoma. The volumes were generated from linear US measurements and tracing volumes on CT post-contrast with a two-tailed matched-pair analysis to calculate a correlation of 0.6789327 between techniques. From this analysis, 22 match paired measurements provides sufficient power (1-β=0.8) to identify a significant (α=0.05) difference in a method comparison study. Cytologic confirmation of transitional cell carcinoma from either a urine cytospin or traumatic catheterization was required for study entry.5 The imaging studies, US and CT, were performed before chemotherapeutic treatment (week 0), then at weeks 8 and 16 of the study. The patients were under general anesthesia for all imaging studies and measurement acquisitions at week 0, week 8, and week 16. Some dogs were removed from the study before either the week 8 and week 16 rechecks due to criteria defined by the parent study. In total, 24 total tumors were measured. Five dogs were imaged at week 0, week 8, and week 16, four dogs were imaged at week 0 and week 8, and one dog was imaged only at week 0.
Tumor measurements: US
After placement of a Foley catheter (Bardex® Foley Catheter, C.R. Bard, Inc, Covington, GA 30014), the urinary bladder was emptied. Sterile 0.9% saline was instilled into the urinary bladder at a dose of 2 ml/kg. The urinary catheter was withdrawn into the proximal urethra. All exams were performed with the patient in dorsal recumbency using a 3-12 MHz microconvex transducer (Toshiba Aplio 500, Toshiba America Medical Systems, Tustin, CA 92780). The frequency and depth of imaging were determined by the size of the urinary bladder. Neither harmonics nor image compounding were used. The greatest cranial-caudal length of the urinary bladder was determined by locating the neck of the urinary bladder and finding the scan plane with the longest urinary bladder dimension. The plane was marked on the patient’s skin using a pen. While maintaining the transducer parallel to the marked sagittal plane, the image with the greatest tumor length was determined (Figure 1A). The length of the tumor was measured using the ultrasound machine calipers to the nearest tenth of a centimeter. To measure the maximum tumor height and width, the transducer was oriented 90° to the sagittal plane of the urinary bladder (using the mark on the dog’s skin as a guide). Maintaining the transverse plane, the maximum tumor width and maximum height of the tumor were recorded (Figure 1B). For each case, two observers independently located the tumor within the urinary bladder and made measurements. One observer (DMA) made images at all 24 time points. One person (WTD) was the second observer on 21 time points and a third observer (EMG) was the observer on the remaining 3 time points. All observers had at least 16 years of veterinary ultrasound experience.
Figure 1.
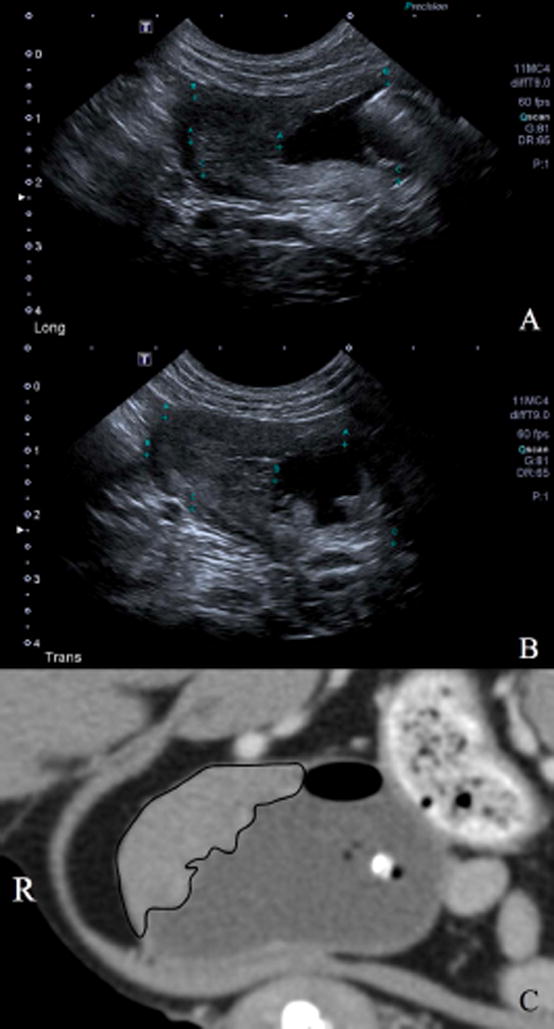
Ultrasonographic images of urinary bladder transitional cell carcinoma. Figure 1A shows the tumor in the longitudinal plane (Long) with calipers to measure the maximum length. Figure 1B is a 90° orientation to the Figure 1A image with calipers measuring the maximum width of the urinary bladder tumor in a transverse plane (Trans). Figure 1C is the same tumor on computed tomography with the margins traced (black line).
Tumor volumes were calculated using equation 1 and the maximum tumor dimension in three orthogonal planes (length, width, and height).17
| [1] |
This formula assumes the tumor has an ellipsoid shape. Each tumor volume was calculated twice using the measurements obtained by each observer at each evaluation and an average tumor volume was calculated using the mean value of the three measurements obtained by both observers at each evaluation.
Tumor measurements: CT
The CT images were acquired following completion of the US examination. The dog was placed in ventral recumbency on the CT (GE Light Speed, 8-detector, Milwaukee, WI) table. Scout images were acquired. Helical images with a slice thickness of 1.25 mm and pitch of 1.35 were acquired from the diaphragm through the perineum. The tube rotation was 0.8 seconds with a fluctuating mA that had a maximum of 400 and a set kVp of 120. The field of view was 500 mm with a matrix of 512 × 512.
Three separate series were acquired and are as follows: (1) empty urinary bladder; (2) infusion of 2 ml/kg of 0.9% sterile saline into the urinary bladder; (3) following a hand injection of intravenous administration of 2 mL/kg of iohexol 240 (Omnipaque, GE Healthcare, Princeton, NJ 08540) via a cephalic vein catheter within the urinary bladder distended as in series 2. Transverse images were then reconstructed into sagittal and dorsal plane images for both bone and standard algorithms; however, the standard algorithm with a window width of 400 and window level of 40 was used for review. The tumor volumes were measured on the pre-contrast and post-contrast series that had infused 2 mL/kg saline (Figure 2).
Figure 2.
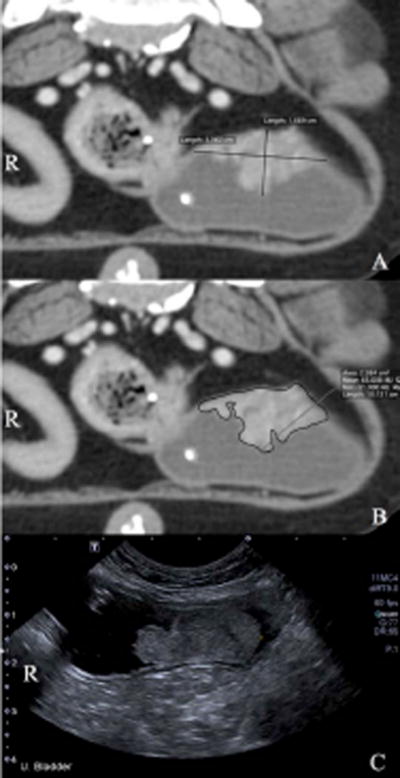
Transverse images of a urinary bladder transitional cell carcinoma along the dorsal margin of the urinary bladder. Figure 2A demonstrates computed tomography (CT) linear measurements with the maximum width and height of the tumor. Figure 2B shows the CT tracing of the tumor. Figure 2C is a transverse image of the same tumor on ultrasound. The right side of the patient is to the left of the image indicated by an ‘R’.
Linear measurements were made on post-contrast CT images. The dorsal plane images were initially reviewed to evaluate the position of the urinary bladder relative to the sagittal plane (long axis) of the patient. If the urinary bladder was parallel to the sagittal plane of the patient, the maximum length of the tumor was measured using dorsal or sagittal plane images. The maximum width of the tumor was measured using dorsal or transverse plane images. The maximum height of the tumor was measured using sagittal or transverse plane images. Measurements were made using a DICOM viewer (eFilm Workstation 3.2, Merge Healthcare, Chicago, IL 60654). If the sagittal plane of the urinary bladder was not parallel to the sagittal plane of the patient, orthogonal multi-planar reformats of the urinary bladder were made using the DICOM viewer using the sagittal plane of the urinary bladder as a point of reference. Length, width and height measurements were of the tumor were made using the multi-planar reformatted images to the nearest tenth of a millimeter. Two observers (ETH, WTD) independently made measurements of each tumor at each time point.
Tumor volume tracing measurements were made using a commercial DICOM viewer (Horos2k v. 2.0.2, https://www.horosproject.org). The tumor volumes were measured on the pre-contrast and post-contrast series that had infused 2 mL/kg saline. Two observers (AJL, ETH) independently evaluated each transverse slice and traced the outline of the tumor with the pencil tool on each slice in which a portion of the transitional cell carcinoma tumor was present. In the cases in which multiple non-contiguous pieces of a tumor were present on a single transverse slice, each individual piece of the tumor was traced with the pencil tool and their areas were added together. For tracing purposes, the assumption was made that the transitional cell carcinoma tumors had infiltrated the urinary bladder wall to the level of the serosal. Observer A was a fourth-year veterinary student (AJL) and observer B was a board-certified veterinary radiologist (ETH). The tumor area (mm2) on each CT slice was multiplied by the slice thickness (1.25 mm) to yield a volume for each individual transverse slice (mm3). The volume of the tumor from each CT slice was then added together to yield a total tumor volume (mm3). This process was applied to both pre-contrast and post-contrast images from each dog obtained on week 0, week 8, and week 16. To help minimize bias, each observer measured the images in the following systematic format: week 0 pre-contrast, week 8 pre-contrast, week 16 pre-contrast, week 0 post-contrast, week 8 post-contrast, week 16 post-contrast. After a temporal recess (a minimum of 30 days), measurements were repeated utilizing this same method.
Statistical Analysis
Statistical analysis was performed by an epidemiologist (GGT) using SAS v. 9.4 (Cary, North Carolina).
Overall differences in the calculated volumes made using the four techniques (CT-linear, CT-tracing pre-contrast, CT-tracing post-contrast, and US-linear) were tested using a mixed effects model (PROC MIXED, SAS, v. 9.4). To correct for the lack of independence between observations, dog was included as a random intercept, and the variables for week of observation and observer (A or B) were included as fixed effects. The differences in calculated volumes made using the four techniques were tested by including technique as a fixed effect in the model. The calculated volumes made using each technique were log-transformed, and the derived values for the least-squared means and 95% confidence intervals were subsequently back-transformed. Significance was considered at an alpha value of 0.05.
The intraclass correlation coefficient was calculated using separate 2-level random-effects mixed models for each technique. Calculated volume was the dependent variable, and a random intercept was included for dog. Variables for week of observation and observation number (first or second) were included as fixed effects in the model. The intraclass correlation coefficient was calculated as the covariance parameter estimate for the random intercept (dog), divided by the total variance ( ), and was interpreted as the proportion of variance explained within dogs. Thus, a high intraclass correlation coefficient indicates small amounts of variation between observers. Each intraclass correlation coefficient estimate was graded into three categories: high (>0.90), moderate (0.75 – 0.90), and poor (< 0.75).18,19
RESULTS
Ten dogs were enrolled in the parent study between 2011 and 2014. The median age was 11.5 years (mean 11.5, range 9–14) with a median weight of 13.6 kg (mean 17.1, range 8.1–29.2). Eight dogs were spayed females and two were castrated males. There were five mixed breed dogs, two West Highland white terriers, one Shetland sheepdog, one Scottish terrier, and one greyhound.
The tumor volumes calculated by linear measurement and tracing methods differed significantly (all p < 0.05) (Table 1 & Figure 3). The volumes calculated from the linear measurements made on the CT and US images did not differ. Tumor volumes calculated based on pre-contrast CT tracings and post-contrast CT tracings did not differ significantly when the volumes calculated by the two observers were averaged (p = 0.0022). There was a significant difference in the tumor volumes calculated on the pre-contrast CT and post-contrast CT tracings for observer A (p = 0.0003), but not for observer B (p = 0.9952).
Table 1.
Descriptive statistics for volumes of the urinary bladder transitional cell carcinoma tumors.
| Mean Volume (mm3) | Median Volume (mm3) | Standard deviation | Range (mm3) | |
|---|---|---|---|---|
| Computed Tomography: Pre-Contrast Tracing * | 4,768.93 | 3,261.51 | 4,270.68 | 449.36 – 18,194.64 |
| Computed Tomography: Post-Contrast Tracing * | 4,591.56 | 2,949.44 | 4,286.48 | 907.52 – 18,481.29 |
| Computed Tomography: Linear Measurements | 34,999.17 | 25,415.17 | 46,187.93 | 544.54 – 211,584.17 |
| Ultrasound: Linear Measurements | 32,928.57 | 15,845.54 | 48,658.57 | 524.10 – 230,660.99 |
The pre-contrast tracing and post-contrast tracing-based volumes were significantly smaller (*) than the linear measurement based volumes for both computed tomography and ultrasound.
Figure 3.
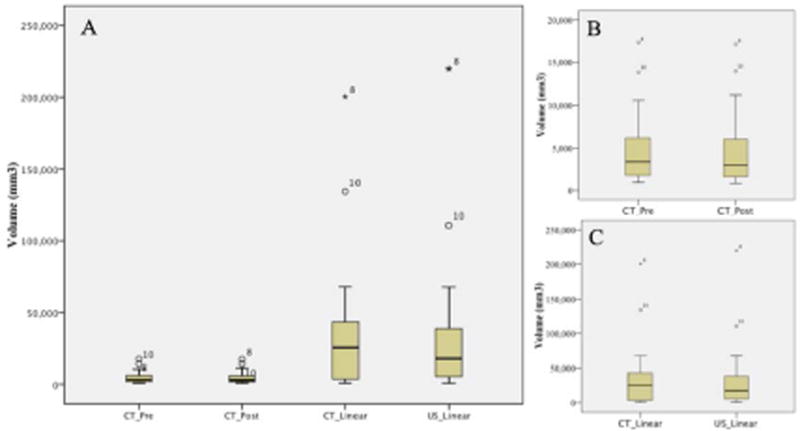
Box and whisker plots for the volumes of urinary bladder transitional cell carcinoma tumors measured using different techniques (A). Figure 3B isolates the computed tomography (CT) tracing techniques for pre-contrast (_Pre) and post-contrast (_Post) and 3C shows the linear measurements on CT and ultrasound (US). The horizontal line in each box represents the mean. Boxes represent the interquartile range (25th to 75th percentile). Whiskers represent the 5th and 95th percentiles. Outliers and extreme outliers are plotted separately as a circle and star, respectively.
The volumes calculated from linear measurements using CT and US were significantly greater (range: 1.42× – 16.22×) than the volumes calculated by tracing tumor margins on CT images (p < 0.01). When the volumes calculated by the four methodologies were averaged between the observers, the linear methods were significantly larger when compared to the average tumor volume for each tumor. The volumes generated by tracing tumor margins on CT were significantly smaller (Table 1 & Figure 3). Of the 24 total calculated tumor volumes, only four volumes calculated using US linear measurements and five volumes calculated using CT linear measurements were less than the average volume of the four methods (Figure 4, Figure 5). Four calculated tumor volumes using the tracing technique on CT images had a greater volume for both the pre-contrast and post-contrast CT series compared to the average volume of the four methods (Figure 4, Figure 5).
Figure 4.
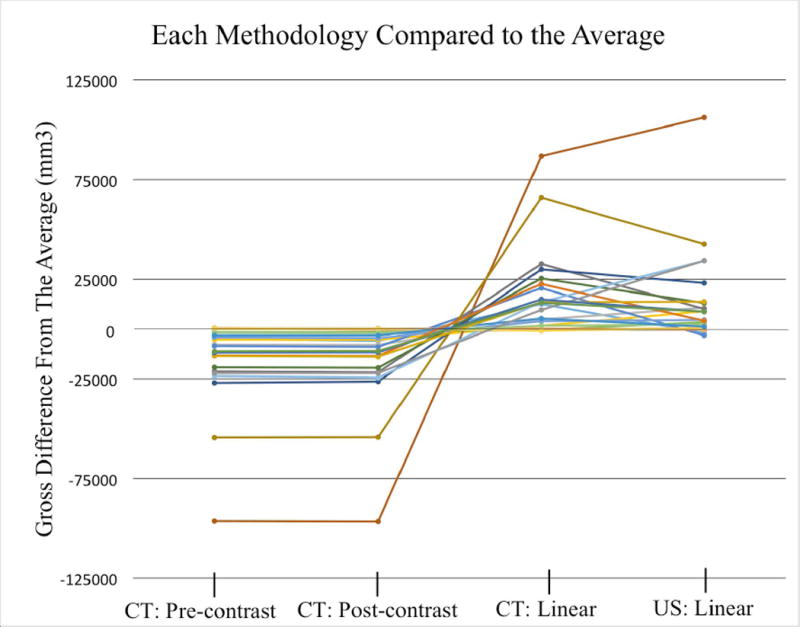
Line graph displaying the four methods for generating a volume (mm3) as a difference in volume compared to the average of all modalities. The average of all four modalities was standardized to 0. Each line indicates a tumor at a unique time point. Positive values indicate that the modality average was greater than the average of the four modalities. The tumor volumes from CT: Pre-contrast and CT: Post-contrast are from tracing tumor margins.
Figure 5.
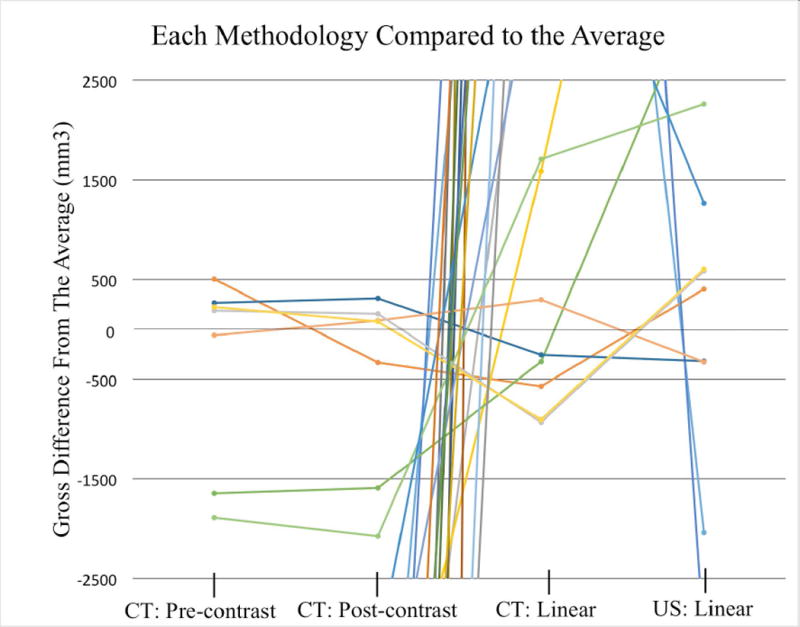
Line graph displaying the four methods for generating a volume (mm3) as a difference in volume compared to the average of all modalities. The graph is a magnification of Figure 4 centered on the average of the four modalities at 0. The four tumors that had tracing-based volumes greater than the average volumes were near 0 indicating that there was little deviation from the average.
The intraclass correlation was calculated for reliability between the observers (Table 2). The highest intraclass correlation coefficient (0.868, moderate correlation) was found when comparing volumes calculated based on tracing the tumor margins on the pre-contrast CT series. The lowest intraclass correlation coefficient (0.611, poor correlation) was found when comparing volumes calculated using linear measurements on US.
Table 2.
Intraclasss correlation coefficient for each measuring methodology.
| Interobserver - Intraclass Correlation Coefficient | |
|---|---|
| Computed Tomography: Pre-Contrast Tracing | 0.868 |
| Computed Tomography: Post-Contrast Tracing | 0.854 |
| Computed Tomography: Linear Measurements | 0.808 |
| Ultrasound: Linear Measurements | 0.611 |
Fourteen tumors at unique time points in the nine dogs were evaluated for response to therapy using the RECIST guidelines as part of the parent study (Table 3). This study applied the unidimensional RECIST guidelines to the volumes calculated from the CT tracing and three linear dimensional measurement-based methods in order to demonstrate how the RECIST status may differ from the current unidimensional based method (Table 3). No cases were considered complete response in this study. Only one tumor had agreement between the two unidimensional methods, two methods using the three linear dimensions for volume, and two tracing volumetric methods; this tumor was defined as stable disease based on the RECIST guidelines. Volumes based on CT pre- and post-contrast tracings of the tumor agreed for 12/14 cases; the two cases that disagreed were near the thresholds (+41.8% vs −29.3% and +17.2% vs +43.7%). The US linear measurement method resulted in the least amount of detectable progression or response to therapy with 3 cases (two partial response, one progressive disease). The CT unidimensional linear measurement method detected partial response or progressive disease to therapy in 4 cases (three partial response, one progressive disease). One tumor had a conflicting therapeutic response between partial response and progressive disease with the CT tracing (pre- and post-contrast) calculated volumes disagreeing with the CT and US based linear measurement calculated volumes; the tracing of tumors were classified as progressive disease, while the linear measurements (three linear dimensional and unidimensional) were all partial response. The disagreement highlights that categorization of the longest length(s) may not be a true reflection of the internal volume of a mass determined by tracing Eleven tumors had a mixed methodology response of either partial response and stable disease or progressive disease and stable disease.
Table 3.
RECIST categorization of the tumors at rechecks.
| Volumetric Analysis | Unidimensional | |||||
|---|---|---|---|---|---|---|
| Pre-Trace CT | Post-Trace CT | Linear CT | Linear US | CT | US | |
| Tumor 1: Week8 | PR | PR | PR | PR | PR | SD |
| Tumor 2: Week8 | PR | PR | PR | SD | SD | SD |
| Tumor 3: Week8 | PR | PR | PR | SD | SD | SD |
| Tumor 3: Week16 | PD | PD | PR | PR | PR | PR |
| Tumor 4: Week8 | PR | PR | PR | PR | SD | PR |
| Tumor 5: Week8 | PR | PR | SD | PR | SD | SD |
| Tumor 5: Week16 | PD | PD | SD | PD | SD | SD |
| Tumor 6: Week8 | PD | SD | PR | PD | SD | SD |
| Tumor 6: Week16 | SD | SD | SD | SD | PR | SD |
| Tumor 7: Week8 | PR | PR | PR | PR | SD | SD |
| Tumor 7: Week16 | SD | PD | PD | SD | SD | SD |
| Tumor 8: Week8 | PD | PD | SD | PD | SD | PD |
| Tumor 9: Week8 | SD | SD | SD | SD | PD | SD |
| Tumor 9: Week16 | SD | SD | SD | SD | SD | SD |
RECIST was applied to tumor volumes to compare to the unidimensional linear methods. A total of 9 tumors at 14 time points were available for rechecks. PR = partial response (solid gray), PD = progressive disease (solid black, white lettering), and SD = stable disease (solid white). One dog was not included in this table because it was only measured at one time point.
DISCUSSION
The tumor volumes calculated using the CT tracing methodology were significantly smaller than volumes calculated using CT and US linear measurements. This study compares different CT and US techniques, including the tracing of tumor margins on serial CT images using free open source DICOM software, to quantify the volume of urinary bladder transitional cell carcinoma. These findings impact the current guidelines for tumor treatment response as it pertains to urinary bladder transitional cell carcinoma. The current RECIST guidelines use a unidimensional analysis that significantly differs compared to the three linear dimensional or tracing methods as demonstrated by this study. Tracing methods are a more representative method for monitoring response to therapy.
The tracing method contours to the intricate shape of the tumor on each CT slice. When the calculated areas on all the CT slices are summed, the resulting volume is typically smaller than the volume of an ellipse calculated based on the greatest tumor length, width and height. If the volume of a tumor changes, but not in the longest dimension, then guidelines based on unidimensional RECIST criteria or other linear methodologies will not reflect measurable differences that could be demonstrated using a tracing technique. Objectively assessing the changes in tumor volume are critical to assess response to therapy to direct clinical treatment decisions and when evaluating investigational therapies in clinical trials.
The RECIST guidelines use the longest unidimensional diameter to assess for treatment response. Once the longest diameter of a solid tumor is established, the same measurement plane is then used for follow up assessment. Limitations to the longest unidimensional diameter methodology assumes that the response or progression of a tumor is will result in a change in that dimension. The RECIST guidelines also assume a tumor is a three-dimensional geometric shape like a sphere or ellipsoid. A limitation of applying RECIST guidelines to urinary bladder transitional cell carcinoma is that they are not a three-dimensional geometric shape, often having multiple frondular projections (Figure 6). Assessing for response using a three linear dimensional method to calculate a tumor volume will be more representative. However, the three linear dimensional measuring technique used in this study does not overcome the limitation of an irregular shape. The ellipsoid volumes calculated from the linear measurements did not represent the actual shape of the tumors; however, there are no relatively straightforward mathematic models that embody the wide array of tumor shapes for transitional cell carcinoma.17 The calculated volumes based on the three linear dimensions were significantly different, and larger, than those volumes calculated by tracing the tumor margins on serial images (Figure 4, Figure 5).
Figure 6.
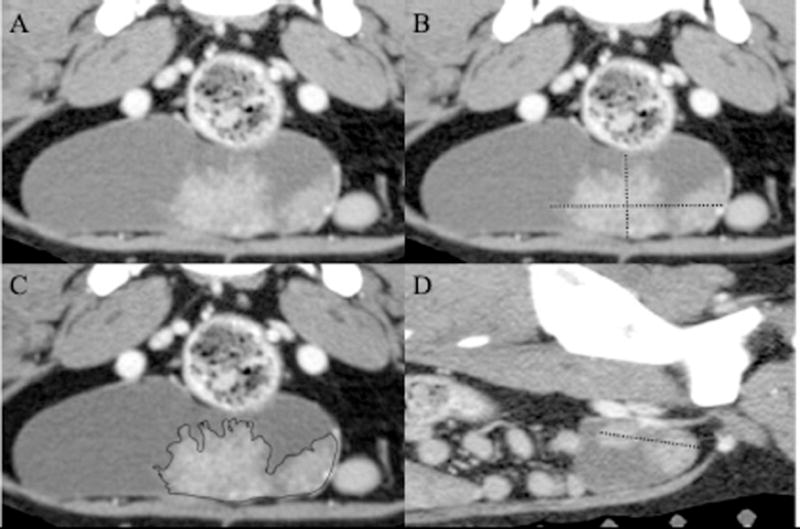
Post-contrast images of a urinary bladder transitional cell carcinoma along the ventral urinary bladder wall in the transverse plane (A-C) and a para-sagittal plane (D). Figures 3B & 3D show maximum linear measurements for length (3D), width and height (3B) used to calculate volume. Figure 3C is an example of the tracing of the tumor margin on a single transverse image. Calculations based on the linear measurements overestimate the volume of the mass due to the complex shape of the tumor; however, the tracing of the margins more accurately fits the shape of the tumor. For images A-C, the right side of the patient is to the left of the image. For image D, the cranial aspect of the patient is to the left of the image.
Greater changes in tumor size are required to identify tumor response to therapy when linear measurement-based volume calculations are used versus more accurate methods of volume calculation.20 The tracing method is able to identify smaller volumetric tumor changes because of its contoured nature, potentially improving sensitivity and accuracy for the assessment of tumor response to therapy.20 Utilizing a unidimensional or other linear measuring scheme may falsely designate a tumor as stable or progressive, potentially overlooking efficacious therapeutic agents, as these methods typically overestimate tumor volume. In our study, 11 dogs had stable disease using linear US methods and 10 dogs had stable disease using linear CT methods, while volume calculation identified 4 dogs designated as stable disease using tracing methods on pre-contrast and post-contrast CT and 6 dogs had stable disease using three linear dimensional measuring methods.
The linear measurement and tracing-based volume calculation methodologies used in this study resulted in differing tumor volumes. Overestimating tumor size using linear measurement-based calculations can impact assessment of response to therapy classification. Inaccurate assessment of response can result in a therapy being discontinued early or continued despite not having the desired effect. It is especially important that tumor measurements be accurate and repeatable when making clinical therapeutic decisions or in clinical trials assessing the efficacy of a new treatment strategy. In canine urinary bladder transitional cell carcinoma, the use of US for monitoring response may result in a novel therapy inaccurately being labeled as a failure and not pursued further. Three-dimensional volumetric cross-sectional MRI and the unidimensional RECIST method have been used to evaluate the response to therapy of breast cancer lesions in women, which are more uniform shaped masses. It was determined that there was good agreement in the category of response in patients responding to therapy determined by either method; however, only a moderate agreement existed when categorizing the patient as a responder or non-responder to therapy using the two methods. A disagreement between partial response and progressive disease was observed in 18.75% of breast cancer cases.21 Two of our 14 (14.3%) canine transitional cell carcinoma had a disagreement between partial response and progressive disease depending on the method used; however, none of the 14 tumors had a unanimous agreement on an assignment of partial response and progressive disease. One tumor of 14 (7.1%) was categorized as stable disease using all methods.
Volume calculations based on tumor tracings are more representative for the evaluation of response to therapeutics due to the overestimation and inaccuracy inherent in assessments and volume calculations based on unidimensional or multidimensional linear measurements. Changing the methods for assessing tumor response from a unidimensional length to a volume measurement method based on CT images will require reassessing the RECIST guidelines used to classify partial response, progressive disease, and stable disease. Computed tomography has the added benefit of acquiring a landscape of anatomy whereas the field of view for ultrasonography is a narrow projection. Computed tomography captures a region of anatomy that can be reconstructed into multiple planes. The acquisition of CT images is less dependent on operator skill. The challenge for CT is more focused on the ability to identify that pathology. Although CT images are planar, CT includes the entire volume of the tumor unlike ultrasound images that only include the planes acquired. Summing the tumor areas on CT slices makes the calculation of volume independent of the scan plane. While an entire tumor volume can be captured in a US video loop, calculating the volume of the tumor cannot be performed on the loop given the lack of normalization of sweep speed. Three-dimensional US techniques can allow volume calculations but have yet to be fully validated.23 Although more labor-intensive, tracing tumor outlines on CT images results in calculated volumes that are more consistent as this study also showed that the interobserver reliability was highest for the for calculated volumes based on CT tracings of tumor margins. The veterinary RECIST guidelines from the Veterinary Cooperative Oncology Group (VCOG) state that ultrasonographic examination is not ideal for repeated assessment of target lesions. The VCOG recommends that CT is the preferred method for assessment of urinary bladder lesions.23 Computed tomography is underutilized in the assessment of urinary bladder wall tumors, regional nodal metastasis, or response to therapy in veterinary medicine.
Observer experience appears to correlate positively to the reliability of tracing transitional cell carcinoma tumor margins. A significant difference in the tumor volume was identified between the repeat measurements for the less experienced observer. The measurements of the more experienced, board-certified veterinary radiologist did not differ for the repeat measures. The data suggests that familiarity with CT image review and urinary bladder wall pathology will likely improve the reliability of using the tracing method. This study is limited in only two observers recorded tracing volumes using CT; the assertion that experience is a contributing factor should be tested with additional observers of different experience levels. The overall intraclass correlation coefficient was highest for the two tracing methods; therefore the tracing methods should be further investigated for relevance in evaluating tumor response to treatment in clinical trials and in standard care.
This study is a retrospective analysis of a prospectively gathered data set. Though the retrospective nature is a limitation, the protocol was relatively standardized enabling evaluation of the different modalities. The gold standard for determining accuracy of the methods would be histopathologic measurements of transitional cell carcinoma masses for comparison; however, response to therapy was a goal of the parent study, thereby limiting gross measurements. Three-dimensional US has been used to evaluate tumor size with high reliability; three-dimensional US was not included in this project.21 The costs comparing abdominal US and CT at our clinic do not differ enough to favor one modality; therefore, the limitation of cost difference between the two modalities will be dependent on the hospital. Though the cases within the study were performed with general anesthesia, the majority of clinical cases for abdominal CT are performed under enough sedation to place a urinary catheter; therefore, additional costs related to general anesthesia are avoided. The small number of cases with serial follow up is a limitation to compare the different methodologies.
The major benefit of the current RECIST model is that the unidimensional linear measurement requires minimal effort. Anecdotally, once observers were comfortable with the method, the tumor tracing required 5 – 15 minutes depending on size and complexity of the mass. However, given the large financial investment made by clients pursuing therapy for their pets and pharmaceutical companies developing new drugs; the tracing method, though more labor-intensive and time consuming, is likely better suited for accurate clinical assessment. Developing RECIST guidelines for tumor volume based on CT images for monitoring response to therapy in clinical trials should be considered.
Volume calculations based on tracing the urinary bladder transitional cell carcinoma margins on CT are highly repeatable and minimize the overestimation of tumor size. This method is more suitable for tumors with irregular shapes like urinary bladder transitional cell carcinoma. Using free open source DICOM software is not specific to a single CT scanner and can provide a means for standardization of a technique between hospitals. Tracing tumor margins on serial CT images to calculate volume as an alternative method to assess response to therapy in clinical trials needs further investigation. Volumetric measurements using CT should be considered when establishing future veterinary RECIST guidelines and clinical trials.
Acknowledgments
The Clinical Trials Office (CTO) at the Ohio State University College of Veterinary Medicine coordinated all aspects of this study including generation of case report forms, collection of samples, collation of data, quality assurance, coordination of other clinical sites and final quality control on all data from all study sites. This project was supported by the following grants: UL1TR001070 from the National Center for Advancing Translational Sciences and P30CA016058 from the National Cancer Institute to The Ohio State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences, National Cancer Institute or the National Institutes of Health or Zoetis.
FUNDING: Funding for the study was provided by the Canine Research Funds and Jesse
Funds at the Ohio State University.
Footnotes
ETHICAL APPROVAL
The Ohio State University Clinical Research and Advisory Committee (CRAC) and IACUC approved this study protocol. This study was conducted in agreement with the VCH guidelines on Good Clinical Practice (GCP). Prior to entry into this study, all dog owners were required to sign a standard consent form outlining the details of the study, as well as the potential risks and benefits of this clinical trial.
COMPETING INTERESTS
Toceranib was provided by Zoetis for this clinical trial.
LIST OF AUTHOR CONTRIBUTIONS
Category 1
(a) Conception and Design: Author name (s): Andrew J. Leffler, Eric T. Hostnik, Emma E. Warry, Wm Tod Drost
(b) Acquisition of Data: Author name (s): Andrew J. Leffler, Eric T. Hostnik, Danelle M. Auld, Eric M. Green, Wm Tod Drost
(c) Analysis and Interpretation of Data: Andrew J. Leffler, Eric T. Hostnik, Emma E. Warry, Gregory G. Habing, Danelle M. Auld, Eric M. Green, Wm Tod Drost
Category 2
(a) Drafting the Article: Author name (s): Andrew J. Leffler, Eric T. Hostnik, Emma E. Warry, Gregory G. Habing, Danelle M. Auld, Eric M. Green, Wm Tod Drost
(b) Revising Article for Intellectual Content: Andrew J. Leffler, Eric T. Hostnik, Emma E. Warry, Gregory G. Habing, Danelle M. Auld, Eric M. Green, Wm Tod Drost
Category 3
(a) Final Approval of the Completed Article: Andrew J. Leffler, Eric T. Hostnik, Emma E. Warry, Gregory G. Habing, Danelle M. Auld, Eric M. Green, Wm Tod Drost
References
- 1.Mutsaers AJ, Widmer WR, Knapp DW. Canine transitional cell carcinoma. J Vet Intern Med. 2003;17:136–144. doi: 10.1892/0891-6640(2003)017<0136:ctcc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Knapp DW, Glickman NW, Denicola DB, Bonney PL, Lin TL, Glickman LT. Naturally-occurring canine transitional cell carcinoma of the urinary bladder A relevant model of human invasive bladder cancer. Urol Oncol. 2000;5:47–59. doi: 10.1016/s1078-1439(99)00006-x. [DOI] [PubMed] [Google Scholar]
- 3.Hanazono K, Fukumoto S, Endo Y, Ueno H, Kadosawa T, Uchide T. Ultrasonographic findings related to prognosis in canine transitional cell carcinoma. Vet Radiol Ultrasound. 2014;55(1):79–84. doi: 10.1111/vru.12085. [DOI] [PubMed] [Google Scholar]
- 4.Fulkerson CM, Knapp DW. Management of transitional cell carcinoma of the urinary bladder in dogs: A review. The Veterinary Journal. 2015;205:217–225. doi: 10.1016/j.tvjl.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Rippy SB, Gardner HL, Nguyen SM, Warry EE, Portela RA, Drost WT, Hostnik ET, Green EM, Chew DJ, Peng J, London CA. A pilot study of toceranib/vinblastine therapy for canine transitional cell carcinoma. BMC Vet Res. 2016;12:257. doi: 10.1186/s12917-016-0882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Hume C, Seiler G, Porat-Mosenco Y, Caceres A, Shofer F, Sorenmo K. Cystosonographic measurements of canine bladder tumours. Vet Comp Oncol. 2010;8:122–126. doi: 10.1111/j.1476-5829.2010.00212.x. [DOI] [PubMed] [Google Scholar]
- 8.Honkisz SI, Naughton JF, Weng HY, Fourez LM, Knapp DW. Evaluation of two-dimensional ultrasonography and computed tomography in the mapping and measuring of canine urinary bladder tumors. The Veterinary Journal. 2018;232:23–26. doi: 10.1016/j.tvjl.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Browne RF, Meehan CP, Colville J, Power R, Torreggiani WC. Transitional cell carcinoma of the upper urinary tract: spectrum of imaging findings. Radiographics. 2005;25:1609–1627. doi: 10.1148/rg.256045517. [DOI] [PubMed] [Google Scholar]
- 10.Knapp DW, Ruple-Czerniak A, Ramos-Vara JA, Naughton JF, Fulkerson CM, Honkisz SI. A Nonselective Cyclooxygenase Inhibitor Enhances the Activity of Vinblastine in a Naturally-Occurring Canine Model of Invasive Urothelial Carcinoma. Bladder Cancer. 2016;2:241–250. doi: 10.3233/BLC-150044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulkerson CM, Knapp DW. Management of transitional cell carcinoma of the urinary bladder in dogs: a review. Vet J. 2015;205:217–225. doi: 10.1016/j.tvjl.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Schrempp DR, Childress MO, Stewart JC, Leach TN, Tan KM, Abbo AH, de Gortari AE, Bonney PL, Knapp DW. Metronomic administration of chlorambucil for treatment of dogs with urinary bladder transitional cell carcinoma. J Am Vet Med Assoc. 2013;242:1534–1538. doi: 10.2460/javma.242.11.1534. [DOI] [PubMed] [Google Scholar]
- 13.Arnold EJ, Childress MO, Fourez LM, Tan KM, Stewart JC, Bonney PL, Knapp DW. Clinical trial of vinblastine in dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med. 2011;25:1385–1390. doi: 10.1111/j.1939-1676.2011.00796.x. [DOI] [PubMed] [Google Scholar]
- 14.Poirier VJ, Forrest LJ, Adams WM, Vall DM. Piroxicam, mitoxantrone, and coarse fraction radiotherapy for the treatment of transitional cell carcinoma of the bladder in 10 dogs: a pilot study. J Am Anim Hosp Assoc. 2004;40:131–136. doi: 10.5326/0400131. [DOI] [PubMed] [Google Scholar]
- 15.Choy K, Fidel J. Tolerability and Tumor Response of a Novel Low-Dose Palliative Radiation Therapy Protocol in Dogs with Transitional Cell Carcinoma of the Bladder and Urethra. Vet Radiol Ultrasound. 2016;57:341–351. doi: 10.1111/vru.12339. [DOI] [PubMed] [Google Scholar]
- 16.Allstadt SD, Rodriguez CO, Jr, Boostrom B, Rebhum RB, Skorupski KA. Randomized phase III trial of piroxicam in combination with mitoxantrone or carboplatin for first-line treatment of urogenital tract transitional cell carcinoma in dogs. J Vet Intern Med. 2015;29:261–267. doi: 10.1111/jvim.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somville J, De Beuckeleer L, De Schepper A, Verstreken J, Taminiau A. Reliability of measuring volume by different methods for tumors of the musculoskeletal system. Acta Orthop Belg. 2001;67:338–343. [PubMed] [Google Scholar]
- 18.Shrout PE, Fleiss JL. Intraclass Correlations - Uses in Assessing Rater Reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Koh D, Ong CN. Statistical Evaluation of Agreement between 2 Methods for Measuring a Quantitative Variable. Computers in Biology and Medicine. 1989;19:61–70. doi: 10.1016/0010-4825(89)90036-x. [DOI] [PubMed] [Google Scholar]
- 20.Welsh JL, Bodeker K, Fallon E, Bhatia SK, Buatti JM, Cullen JJ. Comparison of response evaluation criteria in solid tumors with volumetric measurements for estimation of tumor burden in pancreatic adenocarcinoma and hepatocellular carcinoma. Am J Surg. 2012;204:580–585. doi: 10.1016/j.amjsurg.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An YY, Kim SH, Kang BJ, Lee AW, Song BJ. MRI volume measurements compared with the RECIST 1.1 for evaluating the response to neoadjuvant chemotherapy for mass-type lesions. Breast Cancer. 2014;21:316–324. doi: 10.1007/s12282-012-0388-4. [DOI] [PubMed] [Google Scholar]
- 22.Naughton JF, Widmer WR, Constable PD, Knapp DW. Accuracy of three-dimensional and two-dimensional ultrasonography for measurement of tumor volume in dogs with transitional cell carcinoma of the urinary bladder. Am J Vet Res. 2012:1919–1924. doi: 10.2460/ajvr.73.12.1919. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Veterinary and Comparative Oncology. 2015;13:176–183. doi: 10.1111/vco.12032. [DOI] [PubMed] [Google Scholar]


