Abstract
Objective:
Delivery of pediatric critical care in low-income countries is limited by a lack of infrastructure, resources and providers. Few studies have analyzed the epidemiology of disease associated with a pediatric intensive care unit (PICU) in a low-income country. The aim of this study was to document the primary diagnoses and the associated mortality rates of patients presenting to a tertiary PICU in Mozambique in order to formulate quality improvement projects through an international academic partnership. We hypothesized that the PICU mortality rate would be high and that sepsis would be a common cause of death.
Design:
Retrospective, observational study
Setting:
Tertiary academic PICU
Patients:
All admitted PICU patients
Interventions:
All available data collection forms containing demographic and clinical data of patients admitted to the PICU at Hospital Central de Maputo, Mozambique from January-December 2013 were analyzed retrospectively.
Measurements and Main Results:
The patient median age was two years (57% male). The most common primary diagnoses were malaria (22%), sepsis (18%), respiratory tract infections (12%), and trauma (6%). The mortality rate was 25%. Mortality rates were highest among patients with sepsis (59%), encephalopathy (56%), non-infectious CNS pathologies (33%), neoplastic diseases (33%), meningitis/encephalitis (29%), burns (26%) and cardiovascular pathologies (26%). The median length of PICU stay was 2 days. HIV exposure/infection had a non-statistically significant association with mortality. Patients admitted for burns had the highest median length of PICU stay (4 days). Most trauma admissions were male (75%), and approximately half of all trauma admissions had an associated head injury (55%).
Conclusions:
Infectious disease and trauma were highly represented in this Mozambican PICU, and overall mortality was high compared to high-income countries. With this knowledge, targeted collaborative projects in Mozambique can now be created and modified. Further research is needed to monitor the potential benefits of such interventions.
Keywords: Sepsis, Burns, Trauma, Pediatric, HIV, Malaria
INTRODUCTION
Child mortality remains high in sub-Saharan Africa. Since 2000, the United Nations Millennium Development Goals and Sustainable Development Goals have guided aid funding and health outcome goals for ministries of health (1). During this time, global under-five child mortality decreased by approximately half, from 12.6 million in 1990 to 5.6 million in 2016 (2). In the World Health Organization (WHO) African Region, however, the average under-five mortality rate is still the highest at 81 per 1,000 live births, with great variability by region and country (3).
Mozambique is a low-income country in sub-Saharan Africa with a gross national income per capita of $480 in 2016 (4). It ranked 181 out of 188 countries in the 2016 United Nations Development Program Human Development Index (5). The country has made significant strides in reducing newborn, infant and under-5 mortality through health system strengthening efforts, but many challenges remain (6). In 2015, infant and neonatal mortality rates were 57 per 1,000 live births and 27 per 1,000 live births, respectively (7), and national HIV prevalence in people 15–49 years of age was estimated to be 13.2% (8).
The Mozambican health system has tiered levels of care, including health posts, health centers, district hospitals, provincial hospitals and three central/referral hospitals. Only the central/referral hospitals have pediatric intensive care units. There were four doctors per 100,000 people in Mozambique based on a recent WHO report (9) and approximately 50 pediatric specialists in the public sector to serve a population of over 10 million children under 15 years of age. According to the WHO, 73% of the nation’s health budget in 2008 came from outside the country (10).
Hospital Central de Maputo (HCM) is the primary referral and academic hospital in Mozambique and has the most advanced pediatric intensive care unit (PICU) in the country. When this study was conducted, the pediatric department was a training site for 27 pediatric resident physicians and three pediatric surgery resident physicians. Although there were no strict criteria for PICU admission, children needing assisted ventilation, vasopressor therapy or more intensive nursing care are generally admitted to the PICU. Occasionally, patients with terminal diseases or requiring palliative care are admitted to non-PICU beds at HCM. All services at HCM are available to patients free of charge.
The PICU has 21 beds. An average PICU day shift has two pediatricians/intensivists, two pediatric residents, four-five nurses and three medical students. An average PICU evening shift has one-two pediatricians/intensivists, two pediatric residents, three nurses and two medical students. An average PICU night shift has one pediatrician/intensivist, one pediatric resident, three nurses and two medical students. There were three attending pediatric intensivists on staff at the time of the study (one Mozambican and two foreign nationals).
Pulse oximetry, central venous access, intravenous pumps and five ventilators were available. Oxygen blending, heating and humidification are only available with mechanical ventilation, thereby precluding the use of high-flow nasal oxygen. Oxygen can be delivered via face mask. Continuous positive airway pressure and bilevel positive airway pressure were not used during this period in the PICU. Dialysis for PICU patients was not routinely available at the time of this study. Recently, the PICU started building the capacity to offer peritoneal dialysis to patients. Laboratory tests available include point-of-care blood gas, lactate and hemoglobin testing, routine hematology and electrolyte panels, urinalysis and cerebrospinal fluid studies (Gram stain, cell counts, glucose and protein, Cryptococcus staining), rapid testing and smear microscopy for malaria, rapid HIV testing and CD4 counts. HCM does have the capacity to perform blood, urine and CSF cultures, but the microbiology lab does not receive specimens at all hours, and slow turn-around times for cultures make them less useful for early clinical decision-making in the PICU. Imaging services include portable radiography, computed tomography and ultrasound. Blood products available include packed red blood cells, fresh frozen plasma and platelets. Routinely available antibiotics include ampicillin, gentamicin, cotrimoxazole, metronidazole, ciprofloxacin and ceftriaxone, with vancomycin and imipenem available on request based on culture results. Antimalarials used for complicated malaria in 2013 included both intravenous quinine and artesunate.
In 2007, the David Geffen School of Medicine at UCLA formed an academic partnership with HCM to strengthen the Department of Pediatrics and the Department of Pediatric Surgery and their associated residency programs. This partnership has supported pediatric faculty, physician trainee and nursing education through in-country training and bilateral exchanges. There have also been several areas of more in-depth subspecialty support, including pediatric critical care. In addition, the partnership has secured donations and has made strategic investments in pediatric advanced life support equipment while developing longitudinal support and training in the PICU.
To date, there has not been a published description of the epidemiology of disease managed in the HCM PICU. The aim of this study was to document the primary diagnoses and the associated mortality rates of patients presenting to the tertiary HCM PICU and to use this data to identify priorities for future collaborative work to improve outcomes through our academic partnership. We hypothesized that the PICU mortality rate would be high and that sepsis would be a common cause of death.
MATERIALS AND METHODS:
Study Design
A data collection form designed to capture PICU patient data, including demographics, length of PICU stay, patient origin, procedures, HIV status, diagnoses, outcomes and causes of mortality for all patients admitted to the PICU at HCM, was being used in 2013. Forms were completed by HCM medical students, pediatric resident physicians or intensivists at the completion of a patient’s PICU stay.
This study represented a retrospective review of the data from these data collection forms from January 2013 to December 2013 to characterize disease occurrence rates and mortality in the HCM PICU. Incomplete records were available from July and August. Data from the PICU data collection forms was entered into a Microsoft Excel database. No patient-level chart review was done as part of this study, and no other data sources were used.
For patients who had multiple diagnoses, a senior clinician assigned the primary diagnosis to be either the most severe of those listed or the main diagnosis from which others were considered complications. When questions arose, the PICU chief physician was consulted for a final determination. Records were reviewed and adjudicated by members of the study team. When documented, sepsis was noted as the primary diagnosis regardless of the underlying etiology. Due to extremely limited microbiology laboratory capacity at HCM, sepsis was primarily a clinical diagnosis based on signs and symptoms of hypoperfusion and infection. Malaria was diagnosed by smear microscopy and/or rapid antigen tests, and cerebral malaria was diagnosed in patients with confirmed Plasmodium falciparum malaria with coma (Glasgow coma scale <11) or coma persisting >30 minutes after a seizure per the standard WHO definition (11).
Positive rapid antibody tests were only used to confirm HIV-exposure in patients less than 18 months. At the time of this study, timely virologic HIV testing by DNA PCR for definitive diagnosis of infants was not available. For the purposes of this study, all patients less than 18 months with positive rapid antibody tests were considered HIV-exposed. Patients greater than 18 months of age were classified as HIV-infected if they had a positive rapid antibody test and HIV-uninfected if they had a negative rapid antibody test. Patients with no indication of a rapid antibody test being conducted or those with an inconclusive result were considered to have an unknown HIV status.
Statistical Analyses
Comparisons between groups were performed using Fisher’s exact test for categorical variables and Wilcoxon rank sum test for continuous variables. The Mantel-Haenszel test was used to perform the bivariate analysis. Potential predictors of mortality analyzed included age, gender, HIV status and sepsis. Age under one year was used as a predictor based on the National Institute of Health and Child Development Pediatric Terminology definition of a neonate and infant (age <28 days and age less than 12 months, respectively) (12). Multivariable analysis was performed using logistic regression. Stata 12.1 was used for analyses (Stata Corp, College Station, TX, USA). Statistical significance was defined as p<0.05.
Ethical Considerations
This study was approved by the UCLA Institutional Review Board, the Bioethics Committee at Universidade Eduardo Mondlane (Maputo, Mozambique) and the Scientific Committee at HCM. Need for informed consent was waived.
RESULTS
PICU Patient Demographics
In 2013 there were 340 pediatric beds and 8581 admissions to the Department of Pediatrics at HCM. The hospital’s overall pediatric mortality rate was 9%. Non-study departmental statistics for the PICU reported a total of 1287 admissions, with 957 children (74%) transferred to the wards after stabilization and 330 deaths (26%). Of these 1287 admissions, data collection forms for 987 patients (77%) were available for analysis. Incomplete records were available from July and August. Most patients were male (57%). The median age was 2 years (range, 0–15 years). Median length of stay was 2 days (range, 0–30 days).
HIV Status
HIV rapid antibody testing was performed in 620 patients (63%). Of those patients tested, 149 (24%) had positive results, 461 (74%) had negative results, and 10 (2%) had indeterminant results. Of patients with a positive HIV antibody result, 62 (42%) were <18 months of age and were potentially infected but were classified as exposed for this study. Of the remaining patients with positive tests, 84 (56%) were ≥18 months of age and categorized as HIV-infected. Three patients (2%) did not have age recorded. More than one-third of patients (37%) were either not tested or did not have information available on their data collection form.
Patients who had malnutrition listed as a diagnosis had a higher HIV exposure/infection likelihood than those without documented malnutrition (16% v. 4%, p<0.0001). Patients with a primary diagnosis of a respiratory tract infection had the highest co-morbidity with HIV. Of the 70 (12%) patients who had a primary diagnosis of a respiratory tract infection, 28 (40%) were HIV-exposed/infected (Table 1).
Table 1.
Pediatric intensive care unit demographics, length of stay, and mortality by discharge diagnosis
| Primary Diagnosis | Patient Totals |
Female n (% of known) |
Age Median (Range) |
Length of Stay Median (Range) |
Mortality n (% of known) |
HIV-positive or exposed n (% of known) |
|
|---|---|---|---|---|---|---|---|
| n | % | ||||||
| Total | 987 | 100% | 401 (43%) | 2 (0-15) | 2 (0-30) | 242 (25%) | 149 (24%) |
| Missing Data, n (%) | N/A | N/A | 61 (6%) | 27 (3%) | 127 (13%) | 42 (4%) | 367 (37%) |
| Malaria | 217 | 22% | 94 (45%) | 5 (0-14) | 2 (0-27) | 30 (14%) | 30 (22%) |
| Sepsis | 182 | 18% | 86 (49%) | 0.8 (0-14) | 2 (0-30) | 104 (59%) | 34 (29%) |
| Respiratory Tract Infection | 117 | 12% | 42 (38%) | 0.8 (0.1-13) | 2 (0-10) | 23 (21%) | 28 (40%) |
| Trauma | 63 | 6% | 15 (25%) | 5 (0.1-14) | 2 (0-16) | 5 (8%) | 0 (0%) |
| Meningitis/Encephalitis | 62 | 6% | 21 (38%) | 3 (0-14) | 3 (0-15) | 17 (29%) | 18 (38%) |
| Gastroenteritis | 63 | 6% | 27 (45%) | 0.7 (0.4-1.1) | 2 (1-2) | 11 (18%) | 8 (23%) |
| Surgical Abdomen | 52 | 5% | 16 (36%) | 1.2 (0-15) | 3 (1-8) | 6 (13%) | 5 (15%) |
| Cardiovascular Pathologies | 33 | 3% | 14 (44%) | 3 (0.1-14) | 1 (0-7) | 8 (26%) | 7 (33%) |
| Burns | 29 | 3% | 17 (63%) | 2 (0.1-12) | 4 (0-28) | 7 (26%) | 0 (0%) |
| Diabetic Ketoacidosis | 26 | 3% | 11 (44%) | 12 (1.1-15) | 1 (1-4) | 1 (4%) | 1 (8%) |
| Seizures | 22 | 2% | 9 (47%) | 5 (0-14) | 1 (0-22) | 3 (14%) | 2 (13%) |
| Non-infectious CNS Pathologies |
21 | 2% | 11 (55%) | 6 (0-12) | 1 (0-4) | 7 (33%) | 0 (0%) |
| Other | 18 | 2% | 6 (38%) | 7 (0-14) | 2.5 (0-28) | 4 (24%) | 2 (20%) |
| Intoxication | 15 | 2% | 8 (53%) | 2 (0.3-15) | 1 (0-12) | 3 (20%) | 1 (11%) |
| Renal Pathologies | 14 | 1% | 7 (58%) | 6 (0.3-14) | 2 (1-7) | 3 (21%) | 2 (25%) |
| Other Respiratory Pathologies |
14 | 1% | 2 (15%) | 1.4 (0.3-11) | 2 (0-8) | 3 (25%) | 4 (44%) |
| Encephalopathy | 9 | 1% | 4 (50%) | 1.5 (0-14) | 1 (0-12) | 5 (56%) | 2 (33%) |
| Asthma | 7 | 1% | 2 (29%) | 3.1 (1.9-11) | 1 (0-2) | 0 (0%) | 0 (0%) |
| Neoplastic Diseases | 6 | 1% | 4 (67%) | 11.5 (2-14) | 2 (1-27) | 2 (33%) | 1 (16%) |
| Malnutrition | 5 | 1% | 2 (40%) | 1.2 (0.4-1.8) | 3 (1-6) | 0 (0%) | 3 (75%) |
| Skin/Soft Tissue Infection/Abscess |
4 | <1% | 2 (50%) | 3.2 (0-7) | 1 (1-4) | 0 (0%) | 0 (0%) |
| Anemia | 4 | <1% | 1 (100%) | 1.6 (0.1-11) | 1 (0-5) | 0 (0%) | 1 (33%) |
| GI Bleeding | 3 | <1% | 0 (0%) | 5 (2-13) | 1 (0-2) | 0 (0%) | 0 (0%) |
| Mental Illness | 1 | <1% | 0 (0%) | Missing | 3 (3-3) | 0 (0%) | 0 (0%) |
Primary Diagnoses
Malaria was the most frequent primary diagnosis (22%), followed by sepsis, respiratory tract infections, trauma, meningitis/encephalitis, gastroenteritis and surgical abdomen (Table 1). Diagnoses differed for children less than 1 year of age (Table 2). The diagnoses with the lowest median ages were gastroenteritis (median, 0.7 years; range, 0–8 years), sepsis (median, 0.8 years; range, 0–14 years) and respiratory tract infection (median, 0.8 years; range 0.1–13 years). The diagnoses with the highest median ages were diabetic ketoacidosis (median, 12 years; range, 1.1–15 years) and neoplastic diseases (median, 11.5 years; range, 2–14 years) (Table 1). The diagnosis of neoplastic diseases was used to refer to patients presenting to HCM with complications of their oncologic diagnoses. One patient received hemodialysis in this study.
Table 2.
Most frequent discharge diagnoses and associated mortality by age (< 1 year vs. >= 1 year)
| Age | |||||
|---|---|---|---|---|---|
| < 1 year | >= 1 year | ||||
| Diagnoses | Number (%) |
Mortality Rate |
Diagnoses | Number (%) |
Mortality Rate |
| Sepsis | 95 (31%) | 60% | Malaria | 196 (30%) | 14% |
| Respiratory Tract Infection | 63 (20%) | 22% | Sepsis | 80 (13%) | 59% |
| Gastroenteritis | 44 (14%) | 19% | Trauma | 51 (8%) | 8% |
| Surgical Abdomen | 24 (8%) | 19% | Respiratory Tract Infection | 49 (8%) | 21% |
| Other | 83 (27%) | 24% | Other | 275 (41%) | 20% |
Sepsis
Patients with a primary diagnosis of sepsis had a higher mortality rate than non-septic patients (56% v. 18%; p<0.001). Underlying etiologies of sepsis differed significantly between patients <1 year of age and those ≥1 year of age (p<0.001) (Figure 1). In patients <1 year of age, the most prevalent causes of sepsis were respiratory tract infection (28%), gastroenteritis (22%), sepsis of unknown etiology (13%) and meningitis/encephalitis (10%). The most prevalent causes of sepsis in children ≥1 year of age were burns (22%), respiratory tract infections (16%), sepsis of unknown etiology (16%) and gastroenteritis (15%).
Figure 1.
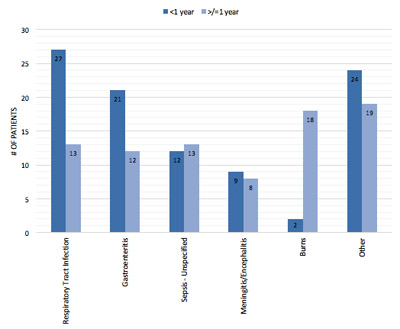
Conditions associated with sepsis diagnosis among children <1 year of age and ≥1 year of age.
Malaria
Of the 237 (24%) patients who had a diagnosis of malaria, 172 patients (60%) had documented cerebral malaria. No mortality difference was observed between patients with cerebral malaria and those without. With respect to age, 90% of those patients with a primary diagnosis of malaria were ≥1 year of age.
Trauma
Trauma accounted for 6% (63/987) of all primary diagnoses in the PICU (Table 1). Seventy-seven percent of all trauma cases were traumatic brain injuries (TBI). Mortality was 8% among patients with a TBI and 13% among all other trauma patients (p=0.615). Patients admitted with trauma were significantly more likely to be male (75% v. 25%; p<0.005).
Burns
A total of 49 patients had burn injury listed as one of their PICU diagnoses. Fifty-nine percent of those patients had a primary diagnosis of burns. The remaining 41% of patients had a primary diagnosis of sepsis. The mortality rate for all burn patients was 45% compared to a mortality rate of 24% for patients without a burn diagnosis (p=0.001). In burn patients who had a primary diagnosis of sepsis, the mortality rate was 75%. Burn patients without a primary diagnosis of sepsis had a mortality rate of 24%. Of patients with a documented burn degree, seven (17%) had third-degree burns, and the remaining 83% had second-degree burns. The mortality rate was 86% in patients with documented third-degree burns. There was no significant difference in median percent total body surface area burned between survivors and non-survivors (23%, 25%, respectively). Seven patients among the non-survivors were mechanically ventilated, and no survivors were mechanically ventilated (p=0.002).
Mortality
The PICU mortality rate for the 987 patients was 25% (Table 1). Of these 987 patients, 114 patients (12%) were transferred from the HCM ward with a mortality rate of 28%, 317 patients (32%) were admitted through the HCM emergency department without transfer from another facility with a mortality rate of 19%, 518 patients (52%) were transferred or referred to the PICU from outside HCM with a mortality rate of 27%, and 38 patients (4%) did not have an origin noted with a mortality rate of 29%. Of the 91 patients who received mechanical ventilation, 71 patients died (78% mortality).
A bivariate analysis was performed to determine whether age, gender, sepsis or HIV status were independent predictors of PICU mortality. Age <1 year and a positive sepsis diagnosis were each individually associated with mortality (p=0.001, p<0.001, respectively). Further analysis of these two predictors of interest showed that only sepsis was a significant predictor of mortality (aOR: 5.64; 95% CI: 3.56, 8.93; p<0.001) after accounting for age-related mortality. An association between HIV-exposure/infection and mortality neared significance (p=0.06) (Table 3).
Table 3.
Bivariate analysis of potential predictors of mortality in the HCM PICU
| Mortality | OR (95% CI) | P-value | |
|---|---|---|---|
| Risk Factors | % (number/total) | ||
| Age | |||
| < 1 year | 34% (99/294) | 1 | |
| >= 1 year | 22% (139/624) | 0.6 (0.42-0.77) | 0.001 |
| Gender | |||
| Male | 24% (121/504) | 1 | |
| Female | 28% (106/383) | 1.21 (0.89-1.64) | 0.215 |
| HIV Status | |||
| HIV not infected | 24% (106/445) | 1 | |
| HIV exposed/infected |
32% (46/145) | 1.49 (0.98-2.24) | 0.060 |
| Sepsis | |||
| No | 18% (138/766) | 1 | |
| Yes | 59% (104/176) | 6.57 (4.62-9.35) | <0.001 |
DISCUSSION
This study adds to the small body of literature describing the spectrum of disease and mortality for pediatric critical care patients in sub-Saharan Africa and an even smaller body of literature reporting results from this region from a dedicated PICU. There was a high occurrence rate of infectious disease and a high mortality rate of 25%.
Others have published pediatric critical care epidemiological reports from Africa also showing high mortality rates. Ilori and Kalu (2012) found the pediatric critical care mortality rate to be 45.5% at Calabar Teaching Hospital, Nigeria with common reasons for admission including burns, eclampsia, thoracic surgery and abdominal surgery (13). Embu and colleagues (2011) reported a pediatric critical care mortality rate of 36.1% at Jos University Hospital, Nigeria, with post-surgical and trauma patients constituting most of the admissions (14). The most recent report was from Ballot and colleagues (2016) who noted a pediatric mortality rate of 16.2% in a combined neonatal ICU/PICU in South Africa. Most of the admissions were surgical (15).
Healthcare worker staffing for pediatric critical care undoubtedly affects patient outcomes and mortality. In North America, where critical care physicians are more abundant, one study reported a mortality rate of 6.04% in surgical ICUs with intensivist staffing compared to a rate of 14.4% in non-critical care physician staffed surgical ICUs (16). At HCM at the time of this study, there were only 17 critical care nurses, one Mozambican pediatric intensivist and two foreign national pediatric critical care physicians. This can lead to a low provider-to-patient ratio as well as a low nurse-to-patient ratio that undoubtedly affects patient care and monitoring and likely mortality. In addition, there are no respiratory therapists in the HCM PICU which also likely affects outcomes. In the HCM PICU, critical care nurses receive on-the-job critical care training and do not recieve a formal degree or certificate classification. Critical care nursing staff are essential to assuring close monitoring, administration of therapies and early detection of clinical deterioration. All of these limitations may play a role in the high mortality seen among mechanically ventilated patients in our study. These challenges highlight the general problem of insufficient human resources for health care in low-income countries such as Mozambique.
Another likely contributing factor to the high PICU mortality rate at HCM is late presentation to critical care. Although this was not quantified, HCM intensivists report this anecdotally, and slightly higher mortality rates were seen for patients transferred from other facilities to HCM. There are many potential contributing factors to late presentation, including delays related to triage up Mozambique’s tiered healthcare system (17), late care-seeking by families due to a lack of health education, and socioeconomic factors and delays in accessing health care related to the geographic distribution of health centers and a poor transportation infrastructure. Anecdotally, we also speculate that systems issues with interfacility communication, resource limitations and challenges involved with interfacility transport lead to late presentation of critically ill children who have resultant short PICU lengths of stay due to high mortality rates. Improving access to care, building a more robust interfacility transport system and further promoting health education would likely result in an earlier presentation to definitive medical care. Further investigation is needed to evaluate these hypotheses. Determining the length of stay or the length of illness prior to PICU admission was beyond the scope of this study but could add additional information regarding the severity of disease, recognition of a change in acuity or timeliness of transfer to the HCM PICU.
Malaria was the most common primary PICU diagnosis in this study. Survivors of cerebral malaria often have significant, permanent morbidity and disability, and our dataset did not allow for estimation of this outcome. Review of malaria clinical management protocols in the HCM PICU show practice consistent with national and international guidelines, and, therefore, we believe that reducing the incidence and severity of disease seen in the PICU will depend primarily on improved public health malaria control measures to reduce the prevalence of disease in southern Mozambique (18).
Sepsis and burns were a significant cause of mortality in the HCM PICU. Similar findings have been described in both high- and low-income countries. For instance, pediatric mortality rates for sepsis in the United States have been reported to be as high as 25% (19). Sepsis may be an indicator of disease severity and can complicate other diagnoses seen, including malaria, HIV/AIDS and diabetes mellitus (20). HCM follows global guidelines for treating sepsis and has access to cardiorespiratory monitoring, mechanical ventilation and crystalloid fluid, but limitations in terms of access to broad spectrum antibiotics, timely microbiological culture and sensitivities, inotropes and low provider-to-patient ratios likely affect patient outcomes.
Burn injuries account for a significant proportion of pediatric deaths in low-income countries. At HCM the PICU mortality for all burn patients was 45%. Greater than 96% of fatal fire-related burns occur in low- and middle-income countries (21). The mortality rate of pediatric fire-related burns in Africa is 8.7 deaths per 100,000 children, compared to 0.4 deaths per 100,000 in high-income countries (22). Burns are a significant public health issue, and clearly burn prevention measures are needed in countries like Mozambique to reduce the incidence of these injuries. Although surgical debridement and antibiotics are available at HCM for burn patients, further analysis is required to determine the reasons for this high mortality. We did not capture any diagnoses specifically related to airway burn injury among the patients with burns, but we cannot state definitively that there were no airway injuries given that in-depth chart reviews were not conducted.
Trauma was the primary diagnosis for 6% of patients in this study and, surprisingly, mortality was lower for TBI than for those without TBI (although not statistically significant). Anecdotally, most pediatric head injuries seen at HCM are related to motor vehicle collisions with concomitant polytrauma. Given the lack of capacity for critical care transport stabilization at many of the referring medical facilities, there was likely a selection bias for less severe polytrauma with associated head injuries to be admitted to HCM which may help explain these findings.
The association between HIV exposure/infection and mortality approached significance in our cohort, and, if we had been able to differentiate HIV exposure from infection in children less than 18 months of age, we believe that HIV infection would have been significantly associated with mortality. HIV-exposed and infected children are likely over-represented in the PICU (24% of children with test results) compared to the estimated HIV prevalence in the general population (11.5% in people 15–49 years of age in 2009, no pediatric estimates available) (8). The national prevention of mother to child transmission program has moved to Option B+, and the pediatric antiretroviral treatment program has grown from 34,866 children on treatment in 2013 to 82,201 as of June 2017 (23). These strides should reduce the number of untreated children at risk for severe HIV-associated infections, and we suspect that future studies will show a reduced proportion of infected children admitted to the PICU.
Over one third of the children in this study did not have their HIV status documented on discharge from the PICU. WHO and national guidelines call for routine opt-out testing for all admitted children in HIV-endemic countries through provider-initiated testing and counseling (PITC) (24). Many children likely had testing done on the wards after stabilization in the PICU, but those data are not available, and more timely screening for HIV on arrival to the hospital is needed. HCM has received a point-of-care HIV DNA PCR machine which gives results in less than one hour, allowing HCM clinicians to move from presumptive to definitive diagnoses in exposed infants.
There are several other limitations to this study. Our study was retrospective and relied on PICU data collection forms completed at the time of care. These forms did not have the adequate breadth of data available, nor did the study team perform an in-depth chart review of specific diagnostic and treatment-related factors associated with mortality from various illnesses. Since our focus was to identify general disease demographics for targeted teaching, quality improvement and resource allocation purposes, we sought this approach for efficiency in an environment lacking an electronic medical record and robust medical documentation upon patient transfer to HCM.
Multiple data collection forms were missing from July and August which are winter months in Mozambique. This possibly led to an underestimation of viral infections and burns which are known to occur in higher frequencies in winter months as well as an overestimation of malaria, which is more common in summer months. Additionally, we only had access to 987 data collection forms from the 1287 admissions to the PICU in 2013. Furthermore, we also do not know how many children in the community may have died who may have benefited from PICU care at HCM. Although determining the severity of illness at PICU admission is desirable and should be investigated in a future study, this was beyond the scope of our project. Lastly, the actual mechanism of death was not consistently recorded or described with complete detail making it difficult to determine whether additional resources or training could have made an impact on mortality. Additional study is also needed to document the barriers to accessing medical care as well as the microeconomic and macroeconomic interactions of Mozambique and its citizens.
Despite these limitations, this retrospective review has been extremely helpful for our academic partnership in terms of identifying areas for training, quality improvement projects, investments in medical equipment and future research. We have initiated a comprehensive project to improve pediatric critical care transport with HCM and its principal referring hospitals. As part of this project, we plan to study inter-facility communication, patient demographics and PICU outcomes. Additional areas for future clinical research that were identified through this study include more detailed evaluations of disease specific outcomes based on severity at initial presentation and specific treatments, with analysis of the mechanisms of death.
CONCLUSIONS
We have summarized the typical PICU caseload for a large, academic, urban referral center in Mozambique. There was a high occurrence rate of HIV exposure/infection, malaria and sepsis as well as a high overall mortality rate compared to high-income countries. This initial descriptive study has helped determine priorities for training, quality improvement and future research and has offered an example of how an academic partnership can focus attention and resources on pediatric critical care in a low-income country.
ACKNOWLEDGEMENTS
We would like to recognize all of the HCM PICU staff, including the nurses, technicians and physicians who work daily in challenging conditions and are invested in projects like this that can lead to improvement in patient outcomes. Celma Issufo, Pedro Santos, Vanda Amado and Sergio Salvador all gave valuable background information for this study.
This partnership has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Health Resources and Services Administration (HRSA) under the terms of Cooperative Agreement U97HA04128. This partnership as well as this research study has been supported by Anadarko Petroleum, the UCLA Center for World Health, Mending Kids International, Sun West Mortgage, the UCLA AIDS Institute, the NIH/NCRR/NCATS UCLA CTSI Grant UL1TR000124 and the Department of Surgery at the David Geffen School of Medicine at UCLA. The findings and conclusions presented are those of the authors and do not necessarily represent the official position of the funding agencies.
Funding:
1. Anadarko Petroleum
2. UCLA Center for World Health
3. Mending Kids International
4. Sun West Mortgage
5. UCLA AIDS Institute
6. NIH/NCRR/NCATS UCLA CTSI Grant UL1TR000124
7. Department of Surgery, David Geffen School of Medicine at UCLA
Footnotes
Copyright form disclosure: Drs. Kelly, Hall, Seni and DeUgarte received support for article research from the National Institutes of Health (NIH). Drs. Kelly, Hall, Buck and Seni’s institutions received funding from President’s Emergency Plan for AIDS Relief (PEPFAR) through the Health Resources and Services Administration (HRSA) under the terms of Cooperative Agreement U97HA04128, Mending Kids International, Sun West Mortgage, Anadarko Petroleum, and Dr. Hartford disclosed that she was employed by the University of California Los Angeles as a pediatrician and partnership director for the partnership between UCLA and Maputo Central Hospital during the study data collection time frame. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Institution Where the Work Was Performed: Hospital Central de Maputo, Universidade Eduardo Mondlane, Maputo, Mozambique
REFERENCES
- 1.Taylor S, Williams B, Magnus D, et al. : From MDG to SDG: good news for global child health? Lancet 2015; 386:1213–1214 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization: Fact Sheet: Children: Reducing mortality, Version 31 October 2017. Available at: http://www.who.int/mediacentre/factsheets/fs178/en/. Accessed April 25, 2018
- 3.World Health Organization: Global Health Observatory data: Under-five mortality. Available at: http://www.who.int/gho/child_health/mortality/mortality_under_five_text/en/. Accessed April 25, 2018
- 4.The World Bank: World Development Indicators. Available at: http://databank.worldbank.org/data/reports.aspx?source=world-development-indicators. Accessed April 25, 2018
- 5.Human Development Report 2016: Human Development for Everyone, United Nations Development Programme, 2016 [Google Scholar]
- 6.Fernandes QF, Wagenaar BH, Anselmi L, et al. : Effects of health-system strengthening on under-5, infant, and neonatal mortality: 11-year provincial-level time-series analyses in Mozambique. Lancet Glob Health 2014; 2:e468–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The State of the World’s Children 2016: A Fair Chance for Every Child, United Nations Children’s Fund (UNICEF), 2016 [Google Scholar]
- 8.Inquérito de indicadores de imunização, malária e HIV/SIDA em Moçambique (IMASIDA) 2015: Relatório de Indicadores Básicos de HIV, Instituto Nacional de Saúde and Instituto Nacional de Estatística, 2017 [Google Scholar]
- 9.World Health Statistics 2015, World Health Organization, 2015 [Google Scholar]
- 10.World Health Organization: Mozambique: Mozambique’s health system. Available at: http://www.who.int/countries/moz/areas/health_system/en/index1.html. Accessed April 23, 2018
- 11.Guidelines for the treatment of malaria - 2nd Edition, World Health Organization, 2010 [PubMed] [Google Scholar]
- 12.Williams K, Thomson D, Seto I, et al. : Standard 6: age groups for pediatric trials. Pediatrics 2012; 129:S153–160 [DOI] [PubMed] [Google Scholar]
- 13.Ilori IU, Kalu QN: Intensive care admissions and outcome at the University of Calabar Teaching Hospital, Nigeria. J Crit Care 2012; 27:105–e1–4. [DOI] [PubMed] [Google Scholar]
- 14.Embu HY, Yiltok SJ, Isamade ES, et al. : Paediatric admissions and outcome in a general intensive care unit. Afr J Paediatr Surg 2011; 8:57–61 [DOI] [PubMed] [Google Scholar]
- 15.Ballot DE, Davies VA, Cooper PA, et al. : Retrospective cross-sectional review of survival rates in critically ill children admitted to a combined paediatric/neonatal intensive care unit in Johannesburg, South Africa, 2013–2015. BMJ Open 2016; 6:e010850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghorra S, Reinert SE, Cioffi W, et al. : Analysis of the effect of conversion from open to closed surgical intensive care unit. Ann Surg 1999; 229:163–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dos Anjos Luis A, Cabral P: Geographic accessibility to primary healthcare centers in Mozambique. Int J Equity Health 2016; 15:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Global Technical Strategy for Malaria 2016–2030, World Health Organization, 2015 [Google Scholar]
- 19.Weiss SL, Fitzgerald JC, Pappachan J, et al. : Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Care Med 2015; 191:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng AC, West TE, Limmathurotsakul D, et al. : Strategies to reduce mortality from bacterial sepsis in adults in developing countries. PLoS Med 2008; 5:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization: Violence and Injury Prevention: Burns. Available at: http://www.who.int/violence_injury_prevention/other_injury/burns/en/. Accessed August 20, 2017
- 22.Peden M, Oyegbite K, Ozanne-Smith J, et al. (Eds): World report on child injury prevention. Geneva, WHO and UNICEF, 2008 [PubMed] [Google Scholar]
- 23.Ministério da Saúde: Relatorio Semestral 2017: Relatório Semestral das Actividades Relacionadas ao HIV/SIDA. Available at: http://www.misau.gov.mz/index.php/relatorios-semestrais. Accessed April 25, 2018
- 24.Guidance on Provider-Initiated HIV Testing and Counselling in Health Facilities, World Health Organization, 2007 [Google Scholar]


