Abstract
Objective:
To determine the association between the Functional Status Scale (FSS) and Pediatric Functional Independence Measure (WeeFIM) scores during the rehabilitation stay in children who survive traumatic brain injury (TBI).
Design:
Secondary analysis of a prospective observational cohort study.
Setting:
Tertiary care children’s hospital with a level 1 trauma center and inpatient rehabilitation service.
Patients:
Sixty-five children less than 18-years-old admitted to an Intensive Care Unit with acute TBI and subsequently transferred to the inpatient rehabilitation service.
Interventions:
Not applicable.
Measurements and Main Results:
FSS and WeeFIM at transfer to rehabilitation and WeeFIM at discharge from rehabilitation. The median age of the cohort was 7.1 years (interquartile range (IQR) 0.8 – 12.3) and 29% were female. Nearly all of the children were healthy prior to the TBI: 6 (9.2%) patients had a baseline FSS score > 6. At the time of transfer to inpatient rehabilitation, total FSS and WeeFIM scores had the expected negative correlation due to increasing disability resulting in lower scores in WeeFIM and higher scores in FSS (r = −0.49, 95% confidence interval −0.62 to −0.35). Among subjects with less disability as measured by lower total FSS scores, we found substantial variability in the total WeeFIM scores. In contrast, WeeFIM scores were consistently low among subjects with a wide range of higher total FSS scores (more disability).
Conclusions:
Although proprietary and more time-intensive, the WeeFIM has advantages relative to the FSS for less severely injured patients and task-specific measurements. The FSS may have advantages relative to the WeeFIM for more severely injured patients. Further investigations are needed to characterize changes in the FSS during the rehabilitation stay and after discharge.
Keywords: pediatric; critical care outcomes; patient outcome assessment; brain injuries, traumatic; critical care; wounds and injury
Introduction
Traumatic brain injury (TBI) causes approximately 2,300 deaths and 42,000 hospitalizations in U.S. children annually. (1) Children who survive severe TBI often have substantial disability. (2, 3) Accurate quantification of disability is necessary in these children in order to understand the trajectory of their recovery and the impact of interventions on their long-term outcomes. The Pediatric TBI Outcomes Workgroup recommends use of the Pediatric Functional Independence Measure (WeeFIM) for this purpose. (4) The WeeFIM score measures a child’s performance in the domains of self-care, mobility, and cognition. It can be used to measure disability longitudinally and across inpatient, outpatient and community-based settings. (5) The WeeFIM is validated in children with developmental disabilities and also in survivors of TBI. (5–9) It has been used extensively in pediatric rehabilitation research, but only rarely in critical care outcome studies. (10) However, measuring the WeeFIM is time- and resource-intensive. It requires multi-disciplinary evaluation, performed at our institution by speech therapists, occupational therapists, physical therapists, psychiatrists, and nursing staff. Additionally, it is proprietary and cannot be used without subscription to the WeeFIM system. A more readily administered outcome measure in the public domain would facilitate pediatric TBI research, particularly multi-center studies.
In 2009, the Collaborative Pediatric Critical Care Research Network (CPCCRN) developed and validated the Functional Status Scale (FSS) as a new way to measure functional impairment in pediatric inpatients. (11) The FSS has been validated across a broad range of pediatric critical illness and injury and is now in wide use by critical care investigators. Although the FSS was developed and validated using a heterogeneous pediatric cohort drawn from hospitals with inpatient rehabilitation units, it has not been specifically evaluated in relation to the inpatient rehabilitation care of survivors of critical illness. (12–17)
The purpose of this study was to evaluate the performance of the FSS and the WeeFIM as measures of function in a population cared for by both critical care and rehabilitation providers: survivors of TBI. To do so, we sought to test for the association between 1) the FSS and WeeFIM at the time of transfer to inpatient rehabilitation, 2) the FSS at transfer to inpatient rehabilitation and the WeeFIM at discharge from inpatient rehabilitation, and 3) the FSS at transfer to inpatient rehabilitation and the change in WeeFIM during the rehabilitation stay.
Materials and Methods
Subjects
We previously conducted a prospective two-center cohort study of children with TBI and demonstrated that the FSS performed well as a measure of morbidity in that population. (12) Briefly, we evaluated children who were < 18 years old, admitted to an intensive care unit (ICU), and had acute TBI with Glasgow Coma Scale (GCS) score ≤ 12 or a neurosurgical procedure within 24 hours of hospital admission. We excluded patients who were discharged from the ICU within 24 hours of ICU admission without a neurosurgical or critical care intervention.
In this study, we linked the prospective cohort data of survivors admitted between September 2014 and December 2016 from a single center with WeeFIM scores collected as part of routine clinical care by the inpatient rehabilitation service at that institution. Both studies were performed under a waiver of consent and received Institutional Review Board approval.
Pediatric Functional Independence Measure (WeeFIM)
WeeFIM is an 18-item instrument that measures pediatric disability based on quantifying level of assistance or supervision required for daily tasks across three subscales (self-care, mobility, and cognition). Each item is scored on a 7-point scale with 1 indicating complete dependence and 7 indicating complete independence resulting in lower scores representing higher levels of disability (score range 18–126). (18) (Table 1) As an example, in children 4 years and older, a total score greater than 100 signifies the need for some supervision but no direct help. (19) The rehabilitation team including speech/language therapists, occupational therapists, physical therapists, rehabilitation psychologists, and nursing staff administer and score the WeeFIM at the time of admission to and discharge from the rehabilitation service. For this study, the WeeFIM scores were documented as part of inpatient rehabilitation care and later were abstracted from the electronic health record. When scores were incomplete, a member of the research team reviewed the medical record with the appropriate rehabilitation team member to retrospectively assess the items missing from the WeeFIM instrument. For example, an occupational therapist assessed toileting function.
Table 1.
Comparison of the Pediatric Functional Independence Measure (WeeFIM) and the Functional Status Score (FSS)
| Characteristics | Pediatric Functional Independence Measure (WeeFIM) | Functional Status Scale (FSS) |
|---|---|---|
| Functional score to measure usual performance to criterion standards of self-care, sphincter control, transfers, locomotion, communication, and social cognitive tasks (18) | Functional score based on activities of daily living that correlates to a more extensive measure of adaptive behavior, the Adaptive Behavior Assessment System II (10) | |
| Domains (number of criteria) | Self-care (8) Mobility (5) Cognition (5) |
Mental Status (1) Sensory (1) Communication (1) Motor (1) Feeding (1) Respiratory (1) |
| Age Range | 6 months – 7 yearsa (17) Infant module (0–3 years) available but is an indirect evaluation and not consistently used |
Newborn – 18 years (15) |
| Scoring | ||
| Total | 18–126 (higher score represents lower disability) | 6–30 (higher score represents greater disability) |
| Domain-specific scoring | Self-care: 8–56 Mobility: 5–35 Cognition: 5–35 Composite Motor (mobility + self-care): 13–91 |
All domains: 1–5 |
| Categorizations (12, 30) | Complete Dependence: 1–2 Modified Dependence: 3–5 Independent: 6–7 |
>21: very severely abnormal 16–21: severely abnormal 10–15: moderately abnormal 8–9: mildly abnormal 6–7: good |
| Advantages (4–10, 13, 30, 31) |
|
|
| Disadvantages (13, 18) |
|
|
If cognitively impaired, instrument is applicable to children up to 7 years cognitive age.
Functional Status Scale (FSS)
FSS is a rapid, reliable measure of functional status measured across six domains (mental status, sensory, communication, motor, feeding and respiratory). (11) Each domain is scored on a scale from 1 (normal) to 5 (very severe dysfunction) resulting in a total score range of 6–30. (Table 1) The FSS is designed such that scores can be collected from a child’s current hospital providers or through review of the medical record. (15) In the prospective study, we preferentially obtained the FSS from health care providers including bedside nurses or physicians and abstracted the data from the electronic health record only when necessary. Pre-admission (baseline) FSS was collected at the time of hospital admission and reflected pre-injury status. FSS and WeeFIM were collected at the time of transfer to inpatient rehabilitation. Only WeeFIM was collected at discharge from inpatient rehabilitation. The WeeFIM and FSS scores were obtained independently. Hospital length of stay represents the total duration of acute care including the inpatient rehabilitation stay.
Statistical Methods
We used descriptive statistics for all relevant demographic variables including age, gender, and injury mechanism, as well as hospital, ICU, and rehabilitation lengths of stay. We used Pearson correlations to examine the relationship between the FSS, WeeFIM, and WeeFIM change scores measured at transfer to inpatient rehabilitation (FSS and WeeFIM) and discharge from inpatient rehabilitation (WeeFIM). We evaluated the relationship between FSS at time of transfer to rehabilitation and WeeFIM at discharge in order to determine if the functional status at the time of PICU discharge is associated with disability at the time of discharge from the Rehabilitation Service. Hypotheses about, for example, the ability of FSS to predict a later WeeFIM score will be useful for planning future collaborative critical care and rehabilitation research. Due to the non-normality of the FSS and WeeFIM total scores, we used percentile bootstrapped confidence intervals with 10,000 bootstrap samples. We stratified the relationships between FSS at transfer and WeeFIM at discharge by age, gender, injury mechanism, and injury severity (Emergency Department GCS unless directly admitted to ICU, in which case the first ICU GCS was used) and plotted the data. We then visually inspected the plots for any potential moderating relationships. Statistical analysis was performed using R version 3.4.1 (2017-06-30).
Results
Cohort Characteristics
The cohort for this study included 65 children who survived TBI and were transferred from acute care to inpatient rehabilitation. The median age was 7.1 years (interquartile range (IQR) 0.8 – 12.3) and 29% were female. Nearly all of the children were healthy prior to the TBI: the median pre-injury FSS was 6 (IQR 6–6) and 6 (9.2%) of patients had a pre-injury FSS > 6. The most frequent mechanisms of injury were motor vehicle accidents and known or suspected inflicted injury. (Table 2) The median ICU length of stay was 10 days (IQR 5 – 11) and the median duration of rehabilitation stay was 24 days (IQR 13 – 47). (Table 2)
Table 2:
Patient Characteristics
| Variable | Cohort (n=65) |
|---|---|
| Age at Hospital Admission | |
| Years, Median (IQR) | 7.1 (0.8, 12.3) |
| Gender, n (%) | |
| Female | 19 (29.2) |
| Injury Mechanism, n (%) | |
| Motor Vehicle | 33 (50.8) |
| Fall | 5 (7.7) |
| Inflicted Injury | 18 (27.7) |
| Other | 9 (13.8) |
| Glasgow Coma Scalea | |
| Total, Median (IQR) | 6 (3.0, 8.0) |
| Motor, Median (IQR) | 4 (1.0, 5.0) |
| Pupil reactivity, n (%) | |
| Both Fixed | 12 (18.5) |
| Both Reactive | 45 (69.2) |
| One Reactive | 5 (7.7) |
| Unknown/Missing | 3 (4.6) |
| Pediatric Intensive Care Unit Length of Stay, days | |
| Median (IQR) | 10.0 (5.0, 11.0) |
| Rehabilitation Length of Stay, days | |
| Median (IQR) | 24.0 (13.0, 47.0) |
| Hospital Length of Stay, daysb | |
| Median (IQR) | 34.0 (17.0, 52.0) |
| Total FSS at Transfer to Rehabilitation, n (%) | |
| 6–7 (Good) | 3 (4.6) |
| 8–9 (Mildy Abnormal) | 4 (6.2) |
| 10–15 (Moderately Abnormal) | 40 (61.5) |
| 16–21 (Severely Abnormal) | 15 (23.1) |
| >21 (Very Severely Abnormal) | 3 (4.6) |
| Total WeeFIM at Transfer to Rehabilitation | |
| Mean ± SD | 30.3 ± 17.8 |
| Median (IQR) | 19 (18.0, 41.0) |
| Total WeeFIM at Discharge from Rehabilitation | |
| Mean ± SD | 65.9 ± 38.8 |
| Median (IQR) | 78 (24.0, 100.0) |
Emergency Department GCS unless directly admitted to ICU, in which case the first ICU GCS was used.
Hospital length of stay refers to total inpatient, acute care hospitalization.
Transfer to Rehabilitation
Complete WeeFIM scores at the time of transfer to rehabilitation were collected as part of clinical care in 55% of our cohort. The remaining patients had 2–3 individual items (of the 18 total) completed retrospectively by chart review, most frequently in the self-care domain including items related to toileting, bowel, and bladder function. At the time of transfer to rehabilitation, all patients had a total WeeFIM score of less than 100 (range 18–84), representing some level of disability resulting in dependency on others. The most common abnormal domain score was 5 (57% and 51% of mobility and cognition domain scores, respectively), representing modified dependence with supervision required.
At transfer to rehabilitation, 98% of the cohort had functional impairments based on a total FSS score > 6. Motor, communication, and feeding were the most affected domains as signified by a score of > 1 which was seen in 82%, 88%, and 88% of patients, respectively. In the motor and feeding domains, the most common abnormal score was 3 (51% and 49% of motor and feeding domain scores, respectively), representing moderate impairment. In the communication domain, the most common abnormal domain score was 2 (55% of communication domain scores), representing mild impairment.
Association between FSS and WeeFIM scores at transfer to rehabilitation
At the time of transfer from acute care to inpatient rehabilitation, total FSS and WeeFIM scores had the expected negative correlation: r = −0.49, 95% confidence interval (CI) −0.62 to −0.35 (Figure 1). However, among subjects with less functional impairment as measured by lower total FSS scores, we found substantial variability in the total WeeFIM scores (Figure 1). In contrast, WeeFIM scores were consistently low among subjects with more functional impairment (higher total FSS scores).
Figure 1.
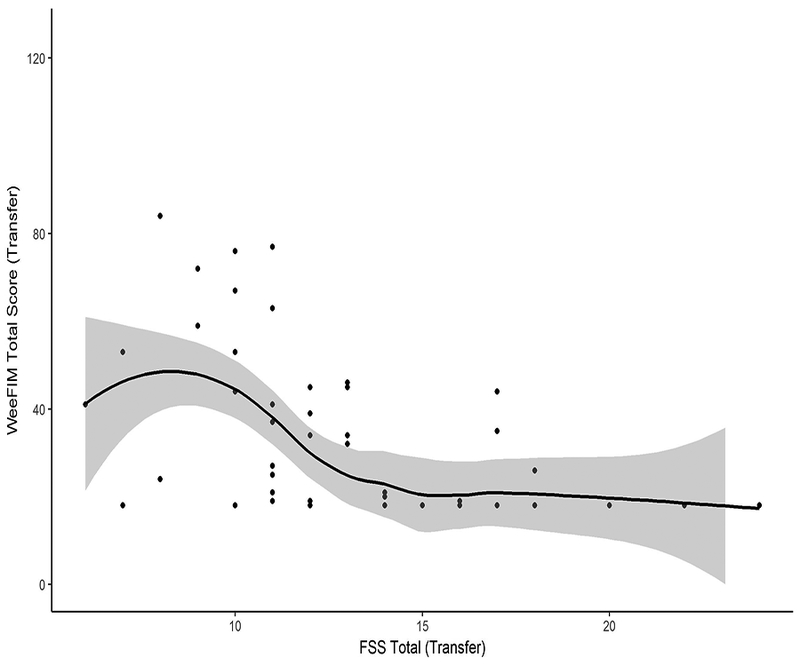
Functional Status Scale correlates with WeeFIM at transfer to rehabilitation
Functional change during Rehabilitation
WeeFIM scores improved during the rehabilitation stay. The median total WeeFIM score was 19 (IQR 18 – 41) at time of transfer to rehabilitation and 78 (IQR 24 – 100) at discharge. The median improvement in the WeeFIM score over the course of the rehabilitation stay was 37 (IQR 4 – 64). (Figure 2a) At the time of transfer to rehabilitation, 89% of subjects had a total WeeFIM < 54, representing average individual item scores of 3, or needing moderate assistance. (19) This improved to 43% of subjects with a total WeeFIM < 54 at the time of discharge and 26% displaying a total WeeFIM > 100, representing the need for some supervision but no direct help. (19)
Figure 2.
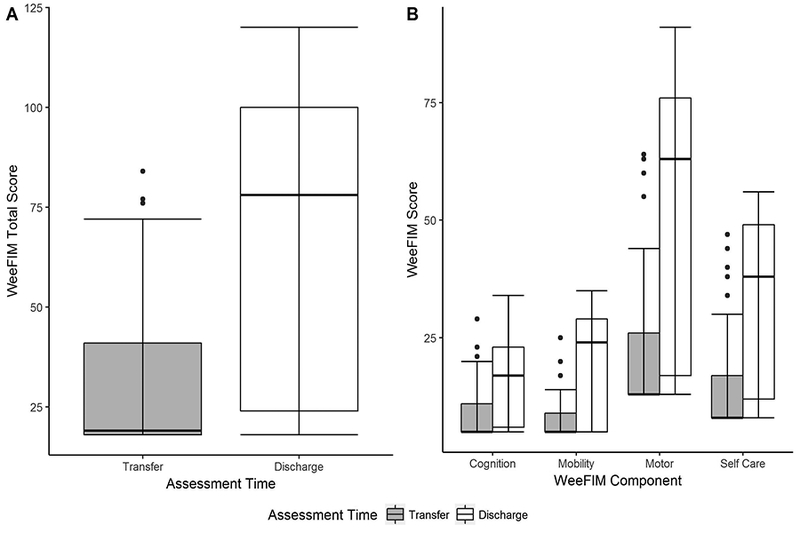
Change in WeeFIM score between transfer to rehabilitation and discharge. 2a) Total WeeFIM scores measured at the time of transfer to rehabilitation and discharge from rehabilitation. 2b) WeeFIM individual components (cognition, mobility, self-care) and composite motor (mobility + self-care) measured at the time of transfer to rehabilitation and discharge from rehabilitation.
Similarly, the individual components of the WeeFIM score improved throughout the rehabilitation stay. (Figure 2b) Of the individual domains including self-care, mobility, and cognition, self-care improved the most with a median increase of 16 (IQR 3 – 30). The cognition scores improved the least with a median increase of 7 (IQR 1 – 12).
Association between FSS at transfer to rehabilitation and WeeFIM at discharge
FSS measured at transfer to rehabilitation and WeeFIM measured at rehabilitation discharge were also negatively correlated (r = −0.29, 95% CI −0.49, −0.05), but less strongly than FSS and WeeFIM at transfer to rehabilitation. (Figure 3a) However, we did not find a consistent relationship for all subjects. To explore potential subgroups, we stratified this comparison by age, gender, injury severity as measured by GCS, and mechanism of injury. Young children (< 3 years old) cluster in the relatively flat fit lines in the lower portion of the figure, while older children cluster in the upper half of the figure (Figure 3b). The impact of age on the FSS-WeeFIM association was maintained across the subscales of each score. (Supplemental Figure 1) Similarly, children with inflicted injury cluster within the lower half of the figure. (Supplemental Figure 2) Gender and injury severity differences did not significantly modify the FSS-WeeFIM relationship (not shown). We found no association between FSS at transfer to rehabilitation and the change in WeeFIM during the rehabilitation stay (r =0.08, 95% CI −0.18, 0.3).
Figure 3.
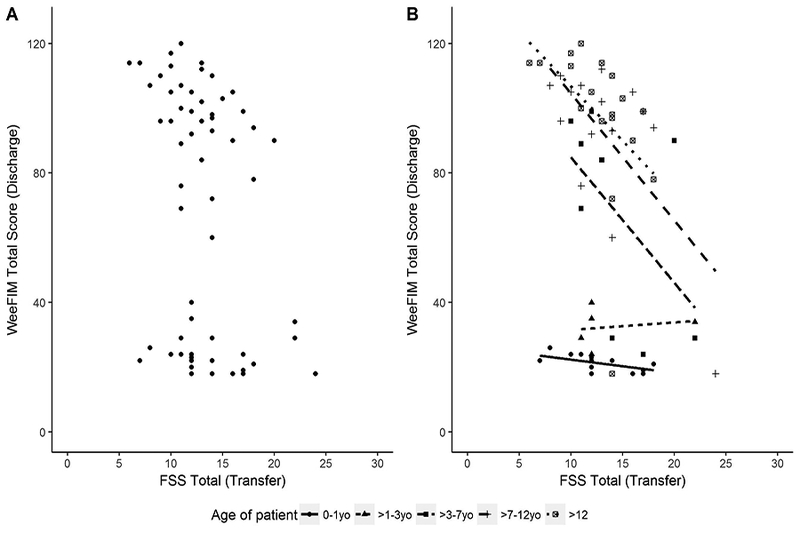
3a) Functional Status Scale at transfer to rehabilitation is associated with WeeFIM at discharge. 3b) Functional Status Scale at transfer to rehabilitation is associated with WeeFIM at discharge stratified by age.
Discussion
In this cohort of children who survived TBI, both the FSS and the WeeFIM total score had a linear relationship with degree of disability at the time of transfer to rehabilitation. Because the FSS measures functional impairment with an increasing scale and the WeeFIM measures disability with a decreasing scale, the two scores had a negative correlation. However, the relationship between the two scores varies by level of disability and by patient age and injury mechanism.
The WeeFIM had greater variability than the FSS at lower ranges of functional impairment, suggesting that the WeeFIM is more sensitive to differences in functional impairment level among less affected children. This might be expected given that the WeeFIM has more component items than the FSS and is more task-specific (bathing, toileting, etc.). Although proprietary and more time-intensive, the WeeFIM likely has advantages relative to the FSS for less severely injured patients and for task-specific assessments.
In contrast, the FSS displayed a broader range of scores than the WeeFIM at higher levels of functional impairment, suggesting that the FSS is more sensitive to differences in functional impairment level among severely injured children. This might be because the FSS was developed and validated for inpatient use, when more acute functional impairments might be expected. (11, 15, 16) The FSS is less time-intensive, is in the public domain, and may have advantages relative to the WeeFIM during the acute phase of illness and potentially beyond the acute phase for more severely injured patients.
Most of our cohort had significant disability at the time of transfer to rehabilitation. At the time of that transfer, every patient in our study had disability requiring dependency on others as measured by the WeeFIM and 98% had functional impairment as measured by the FSS. The types of functional disability in our cohort are similar to those reported in the literature for children with TBI. (7, 12, 20) Specifically, the FSS identified motor skills, feeding, and communication and the WeeFIM identified mobility and cognition as the most affected domains. (12)
Many children gained substantial functional ability during inpatient rehabilitation: the WeeFIM score increased by a median of 37 points during the rehabilitation stay. The largest improvements were seen in the self-care domain and the smallest in the cognitive domain. Unfortunately, many children had persistent disability at the conclusion of inpatient rehabilitation, as other investigators have reported. (7, 22) Also similar to other reports, a subgroup of children with more severe injuries did not have substantial improvements in their level of disability during inpatient rehabilitation. (7, 21–24) These children tended to be younger and more often were victims of inflicted injury. (5, 7, 22, 25)
Young, severely injured children pose a particular challenge. Children younger than 3 years old and those with inflicted injury have consistently low WeeFIM scores despite substantial variability in FSS. The WeeFIM is validated in children as young as 6 months-old and it is the practice of our rehabilitation team to use the WeeFIM across this broad age range. However, when the WeeFIM is sequentially measured in functionally normal young children, it displays an increase over time. It is developmentally appropriate for children 3 and under to have a high degree of dependence on caregivers across the domains of the WeeFIM, regardless of injury. (19) The developmental differences in children < 3 years-old may create a floor effect that is not displayed in the FSS. Also, the lack of change in the WeeFIM among young children during rehabilitation may be due to a higher percentage of inflicted injuries in this younger age group. Some studies suggest that young children with inflicted injury have less potential to respond to rehabilitation services. (20, 22, 26–28) However, worse outcomes in children who suffer inflicted injury as compared to non-inflicted injury are not universally supported in the literature. (29) The FSS may have advantages relative to the WeeFIM for young, severely injured children, many of whom are victims of inflicted injury.
Study Limitations
Our study has several important limitations to consider. First, our data collection was restricted to the acute inpatient and rehabilitation phases of care and may not predict ongoing disability or the recovery trajectory of children after discharge. Second, this study was conducted at a single center. The relationship between the WeeFIM and FSS should be further evaluated in larger multi-center cohorts to appropriately test for effects of confounding variables. Patient, injury, ICU, and rehabilitation service characteristics may affect the relationship between the two measures. Multi-center studies should have a particular focus on younger patients. Third, we did not collect the FSS at the completion of inpatient rehabilitation, and as such only measured FSS and WeeFIM simultaneously at a single time point, the transfer to rehabilitation from acute care. Also, we did not use the WeeFIM Infant module based on the current practice and preference of our Rehabilitation Service. The Infant Module is designed to be completed by parents or care-givers, primarily in Early Intervention or preschool settings. In an inpatient setting, our Rehabilitation service does not feel that the indirect evaluation provided by the Infant Module adds meaningfully to the direct, hands-on assessment performed by the Rehabilitation team. Finally, of the 18 items in the WeeFIM instrument, 45% of the cohort had only 15 or 16 items collected as part of clinical care. The remaining 2–3 items were most often in the self-care domain and were assessed retrospectively by the appropriate rehabilitation team member.
Conclusions
Although proprietary and more time-intensive, the WeeFIM has advantages relative to the FSS for less severely injured patients and task-specific measurements. The FSS may have advantages relative to the WeeFIM during the acute phase of illness, beyond the acute phase for more severely injured patients, and in tracking functional impairments among young, severely injured patients. Further investigations are needed to characterize changes in the FSS during the rehabilitation stay and after discharge. More cross-discipline collaboration is needed, and outcome standardization between fields would facilitate collaboration. (30)
Supplementary Material
Components of the Functional Status Scale at transfer to rehabilitation is associated with components of WeeFIM at discharge stratified by age. 1a) FSS mental score at transfer is associated with the WeeFIM cognition score at discharge stratified by age. 1b) FSS sensory score at transfer is associated with the WeeFIM cognition score at discharge stratified by age. 1c) FSS communication score at transfer is associated with the WeeFIM cognition score at discharge stratified by age. 1d) FSS motor score at transfer is associated with the WeeFIM motor score at discharge stratified by age. 1e) FSS feeding score at transfer is associated with the WeeFIM self-care score at discharge stratified by age.
Functional Status Scale at transfer to rehabilitation is associated with WeeFIM at discharge stratified by injury mechanism.
Acknowledgments
We acknowledge Yamila Sierra, MPH, CCRP and Diane Ladell, MPH for their contribution to this article through data collection.
Funding: This work was supported by the Eunice Kennedy Shriver National Institute for Child Health and Human Development (Grant K23HD074620 to TB).
Copyright form disclosure: Drs. Maddux and Bennett’s institution received funding from Eunice Kennedy Shriver National Institute for Child Health and Human Development (Grant K23HD074620 to TB), and they received support for article research from the National Institutes of Health. Dr. Bennett’s institution also received funding from the Colorado Department of Public Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
No reprints will be ordered.
Study Site: University of Colorado School of Medicine, Children’s Hospital Colorado, Departments of Pediatric Critical Care Medicine and Physical Medicine and Rehabilitation.
References:
- 1.Rutland-Brown W, Langlois JA, Thomas KE et al. : Incidence of traumatic brain injury in the United States, 2003. The Journal of head trauma rehabilitation 2006, 21(6):544–548. [DOI] [PubMed] [Google Scholar]
- 2.Anderson VA, Catroppa C, Haritou F et al. : Identifying factors contributing to child and family outcome 30 months after traumatic brain injury in children. Journal of neurology, neurosurgery, and psychiatry 2005, 76(3):401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraus JF, Fife D, Conroy C: Pediatric brain injuries: the nature, clinical course, and early outcomes in a defined United States’ population. Pediatrics 1987, 79(4):501–507. [PubMed] [Google Scholar]
- 4.McCauley SR, Wilde EA, Anderson VA et al. : Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. Journal of neurotrauma 2012, 29(4):678–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice SA, Blackman JA, Braun S et al. : Rehabilitation of children with traumatic brain injury: descriptive analysis of a nationwide sample using the WeeFIM. Archives of physical medicine and rehabilitation 2005, 86(4):834–836. [DOI] [PubMed] [Google Scholar]
- 6.McBride T: Neuropsychological scores and WeeFIM cognitive ratings of children with traumatic brain injury: A brief report. Brain injury 2015, 29(7–8):951–954. [DOI] [PubMed] [Google Scholar]
- 7.Suskauer SJ, Slomine BS, Inscore AB et al. : Injury severity variables as predictors of WeeFIM scores in pediatric TBI: Time to follow commands is best. Journal of pediatric rehabilitation medicine 2009, 2(4):297–307. [PMC free article] [PubMed] [Google Scholar]
- 8.Trovato MK, Bradley E, Slomine BS et al. : Physical Abilities and Mobility Scale: reliability and validity in children receiving inpatient rehabilitation for acquired brain injury. Archives of physical medicine and rehabilitation 2013, 94(7):1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziviani J, Ottenbacher KJ, Shephard K et al. : Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries. Physical & occupational therapy in pediatrics 2001, 21(2–3):91–101. [PubMed] [Google Scholar]
- 10.Limperopoulos C, Majnemer A, Shevell M et al. : Functional limitations in young children with congenital heart defects after cardiac surgery. Pediatrics 2001, 108(6):1325–1331. [DOI] [PubMed] [Google Scholar]
- 11.Pollack MM, Holubkov R, Glass P et al. : Functional Status Scale: new pediatric outcome measure. Pediatrics 2009, 124(1):e18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett TD, Dixon RR, Kartchner C et al. : Functional Status Scale in Children With Traumatic Brain Injury: A Prospective Cohort Study. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2016, 17(12):1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cashen K, Reeder R, Dalton HJ et al. : Functional Status of Neonatal and Pediatric Patients After Extracorporeal Membrane Oxygenation. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2017, 18(6):561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto NP, Rhinesmith EW, Kim TY et al. : Long-Term Function After Pediatric Critical Illness: Results From the Survivor Outcomes Study. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2017, 18(3):e122–e130. [DOI] [PubMed] [Google Scholar]
- 15.Pollack MM, Holubkov R, Funai T et al. : Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Critical care medicine 2015, 43(8):1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollack MM, Holubkov R, Funai T et al. : Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2014, 15(9):821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zinter MS, Holubkov R, Steurer MA et al. : Pediatric Hematopoietic Cell Transplant Patients Who Survive Critical Illness Frequently Have Significant but Recoverable Decline in Functional Status. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Msall ME, DiGaudio K, Rogers BT et al. : The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities. Clinical pediatrics 1994, 33(7):421–430. [DOI] [PubMed] [Google Scholar]
- 19.Msall ME, DiGaudio K, Duffy LC et al. : WeeFIM. Normative sample of an instrument for tracking functional independence in children. Clinical pediatrics 1994, 33(7):431–438. [DOI] [PubMed] [Google Scholar]
- 20.Zonfrillo MR, Durbin DR, Winston FK et al. : Physical disability after injury-related inpatient rehabilitation in children. Pediatrics 2013, 131(1):e206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aitken ME, McCarthy ML, Slomine BS et al. : Family burden after traumatic brain injury in children. Pediatrics 2009, 123(1):199–206. [DOI] [PubMed] [Google Scholar]
- 22.Babikian T, Asarnow R: Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology 2009, 23(3):283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy ML, MacKenzie EJ, Durbin DR et al. : Health-related quality of life during the first year after traumatic brain injury. Archives of pediatrics & adolescent medicine 2006, 160(3):252–260. [DOI] [PubMed] [Google Scholar]
- 24.Sesma HW, Slomine BS, Ding R et al. : Executive functioning in the first year after pediatric traumatic brain injury. Pediatrics 2008, 121(6):e1686–1695. [DOI] [PubMed] [Google Scholar]
- 25.Niedzwecki CM, Marwitz JH, Ketchum JM et al. : Traumatic brain injury: a comparison of inpatient functional outcomes between children and adults. The Journal of head trauma rehabilitation 2008, 23(4):209–219. [DOI] [PubMed] [Google Scholar]
- 26.Estroff JM, Foglia RP, Fuchs JR: A comparison of accidental and nonaccidental trauma: it is worse than you think. The Journal of emergency medicine 2015, 48(3):274–279. [DOI] [PubMed] [Google Scholar]
- 27.Keenan HT, Runyan DK, Marshall SW et al. : A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics 2004, 114(3):633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ewing-Cobbs L, Kramer L, Prasad M et al. : Neuroimaging, physical, and developmental findings after inflicted and noninflicted traumatic brain injury in young children. Pediatrics 1998, 102(2 Pt 1):300–307. [DOI] [PubMed] [Google Scholar]
- 29.Risen SR, Suskauer SJ, Dematt EJ et al. : Functional outcomes in children with abusive head trauma receiving inpatient rehabilitation compared with children with nonabusive head trauma. The Journal of pediatrics 2014, 164(3):613–619. e611–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett TD: Functional status after pediatric critical care: is it the disease, the cure, or both? Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2015, 16(4):377–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Components of the Functional Status Scale at transfer to rehabilitation is associated with components of WeeFIM at discharge stratified by age. 1a) FSS mental score at transfer is associated with the WeeFIM cognition score at discharge stratified by age. 1b) FSS sensory score at transfer is associated with the WeeFIM cognition score at discharge stratified by age. 1c) FSS communication score at transfer is associated with the WeeFIM cognition score at discharge stratified by age. 1d) FSS motor score at transfer is associated with the WeeFIM motor score at discharge stratified by age. 1e) FSS feeding score at transfer is associated with the WeeFIM self-care score at discharge stratified by age.
Functional Status Scale at transfer to rehabilitation is associated with WeeFIM at discharge stratified by injury mechanism.


