SUMMARY
Spirochetes possess a unique periplasmic flagellar motor component called the collar. However, little is known about the composition or function of the flagellar collar proteins. To identify a collar protein, we have inactivated almost all genes annotated as motility-related in the Borrelia burgdorferi genome and identified only FlbB, which comprises the base of the collar. Since the major components of the collar complex remained unidentified, we took advantage of a protein-protein interaction map developed in another spirochete, Treponema pallidum to identify proteins of unknown function that could be collar proteins. Subsequently, using various comprehensive approaches, we identified a tetratricopeptide repeat protein BB0236 as a potential candidate for the collar. Biochemical assays indicated that FlbB interacts with BB0236. Furthermore, Δbb0236 mutant analyses indicated that BB0236 is crucial for collar structure assembly, cellular morphology, motility, orientation of periplasmic flagella, and assembly of other flagellar structures. Moreover, using comparative motor analyses, we propose how the collar structure is assembled in B. burgdorferi. Together, our studies provide new insights into the organization and the complex assembly inherent to the unique spirochetal collar structure.
INTRODUCTION
Borrelia burgdorferi is the causative agent of Lyme disease, which is the most common vector-borne illness in the United States and Europe (Mead, 2015). Flagella-driven motility and chemotaxis are reported to be crucial for the pathogenesis of many bacteria including the spirochetes (Xu et al., 2017, Novak et al., 2016, Moon et al., 2016a, Sultan et al., 2015, Motaleb et al., 2015, Sultan et al., 2013b, Li et al., 2010, Lambert et al., 2012, Wunder et al., 2016, Butler & Camilli, 2005, Lertsethtakarn et al., 2011, Guyard et al., 2013, Sze et al., 2012, Lin et al., 2015). Unlike other externally flagellated bacteria, spirochetes’ flagella are located between the outer membrane and peptidoglycan layer i.e. in the periplasmic space (Kudryashev et al., 2009, Charon et al., 2009, Charon et al., 2012, Wolgemuth, 2015). B. burgdorferi possesses 7–11 periplasmic flagella that are inserted at each pole of the cell and extend toward the other pole as they wrap around the cell cylinder to produce the spirochete’s distinctive flat-wave morphology. This normal orientation of the periplasmic flagella toward the other pole of the cell is partly determined by FliL, which is also essential for the spirochetes morphology and smooth swimming of the bacteria (Motaleb et al., 2011). In motile cells, these periplasmic flagella form a ribbon-like structure. Periplasmic flagella are not only crucial for motility but also for morphology of B. burgdorferi, as flaB mutant cells are non-motile and exhibit a rod-shaped morphology (Kudryashev et al., 2009, Charon et al., 2009, Charon et al., 2012, Sultan et al., 2015, Motaleb et al., 2015, Sultan et al., 2013a, Motaleb et al., 2000).
The architectural structures and amino acid sequences of the proteins encoded by various components of the periplasmic flagella resemble those of external flagella including the MS-ring, C-ring, P-ring, stator, hook, export apparatus, and the filament (Ge & Charon, 1997, Heinzerling et al., 1997, Motaleb et al., 2000, Zhao et al., 2013, Zhao et al., 2014, Chen et al., 2011, Lin et al., 2015). However, due to the unique location in the periplasmic space and the large torque required for driving the cell body, periplasmic flagella possess a unique spirochete-specific component known as the collar that is absent in all other bacterial flagella reported to date (Chen et al., 2011, Zhao et al., 2014). While the periplasmic collar complex is specific to the spirochetes, we know very little about the proteins contributing to this novel structure or its assembly in any spirochete (Chen et al., 2011, Zhao et al., 2014). FlbB is the first and only protein that was shown to be involved in collar assembly (Moon et al., 2016b). However, FlbB is a small membrane protein, which likely forms the base of the collar. Based on its large structure (~71 nm in diameter and ~24 nm in height) and the fact that other large structures described below are comprised by multiple proteins, we hypothesized that the periplasmic collar complex is also constructed by several proteins (Moon et al., 2016b).
In this communication, we identified BB0236 as a potential candidate for inclusion in the periplasmic collar structure. This protein possesses tetratricopeptide repeat (TPR) domains, but it is currently annotated as a hypothetical protein with unknown function in B. burgdorferi genome (Fraser et al., 1997). Using mutational analysis followed by various approaches including cryo-electron tomography, we show that BB0236 is involved in collar assembly and is crucial for cell morphology, motility, orientation of periplasmic flagella and assembly of other important flagellar motor components. Additionally, BB0236 directly interacts with FlbB, supporting our model that the collar is a multi-protein complex. Moreover, using various flagellar mutants for motor analyses, we propose how the flagellar collar structure is assembled in B. burgdorferi.
RESULTS
Screening for a periplasmic flagellar collar protein in B. burgdorferi
We recently reported that FlbB is important for periplasmic flagellar collar assembly in B. burgdorferi (Moon et al., 2016b). FlbB is a small protein (205 amino acids) that is unlikely to make-up the large collar complex structure. Using green fluorescent protein fusion, we previously demonstrated that FlbB is located at the base of the collar and is embedded in the cytoplasmic membrane using its transmembrane domain (Moon et al., 2016b). We hypothesized that other unidentified collar proteins are assembled onto the FlbB base. Thus, deletion of flbB has a dramatic effect on the entire collar complex, as the whole structure is diminished in the ΔflbB mutant (Moon et al., 2016b). These data lead us to predict that the collar is comprised of multiple proteins. This proposition is supported by the fact that other relatively smaller complexes such as the flagellar C-ring, flagellar type III export apparatus or type IV secretion structures are composed of multiple proteins (Fronzes et al., 2009, Sowa & Berry, 2008, Minamino, 2014). To identify additional collar proteins, we employed various comprehensive strategies. Since we have analyzed almost all motility-related genes annotated in the B. burgdorferi genome, and found no collar proteins other than FlbB, we took advantage of a protein-protein interaction (PPI) map developed in another spirochete, Treponema pallidum, which identified 176 PPIs between known motility proteins and proteins of unknown functions using a comprehensive array-based yeast-two-hybrid screen (Rajagopala et al., 2007). We hypothesized that some of the proteins of unknown function could be a periplasmic collar protein in T. pallidum. We refined the list of candidates by removing all protein homologs that have been characterized in other non-spirochetal bacteria because the periplasmic collar is a spirochete-specific feature (Chen et al., 2011, Zhao et al., 2014). We found a FlbB homolog in the PPI map (TP0567) that is annotated as a hypothetical protein in T. pallidum. TP0567 shares 32% amino acid sequence identity with B. burgdorferi FlbB (E-value, 5e-17). Moreover, TP0567 was shown to interact with TP0421, TP0708, and TP0675 in that PPI map (Fig. 1A). TP0675 and TP0708 have no significant homologs in B. burgdorferi, whereas TP0421 shares 27% amino acid sequence identity with B. burgdorferi BB0236 (E-value, 6e-80; Fig. 1A). Since FlbB was found to be involved in periplasmic flagellar collar assembly and the FlbB homolog TP0567 is proposed to interact with TP0421, we hypothesized that TP0421 protein’s homolog BB0236 is a potential candidate for the periplasmic collar structure.
Figure 1.
(A) Identification of a collar protein in B. burgdorferi from a T. pallidum protein-protein interaction network. The PPI map was developed between known motility proteins and proteins of unknown function by a yeast two hybrid screen. T. pallidum protein TP0567 shares 32% amino acid sequence identity with B. burgdorferi FlbB (E-value, 5e-17). TP0567 is shown to interact with TP0421, TP0675, and TP0708. Only TP0421 shares significant amino acid sequence identity with B. burgdorferi BB0236 (27% identity; E-value, 6e-80). (B) Interaction between BB0236 and FlbB was determined by pull-down assay. Purified His-FlbB protein was incubated with resin-bound MBP-BB0236 followed by extensive washing and elution. The eluted proteins were separated by SDS-PAGE and then immunoblotted with anti-FlbB antibody. Lane 1, FlbB protein was detected only in the elution from the MBP-BB0236 bound amylose resin but not in the MBP itself (lane 2) or the empty amylose resin (lane 3). FlbB (~24 kDa) is indicated by an arrowhead. (C) Far-western or affinity blotting showing specific interaction between BB0236 and FlbB proteins. Approximately 2.0 μg of MBP proteins were subjected to SDS-PAGE and Coomassie staining (left panel) or transferred to a polyvinylidene difluoride membrane (right panel). The membrane was incubated with His-FlbB and then immunoblotted with anti-FlbB polyclonal antibodies.
bb0236 is located in an operon consisting of seven genes, bb0229—bb0236 (Fig. S1). None of the genes in this operon is predicted to be involved in motility or chemotaxis. BB0236 is annotated as a hypothetical protein (668 amino acids) that harbors TPR and NHL domains, and an uncharacterized ChID domain known as Mg-chelatase subunit ChID (proposed to be important for coenzyme transport and metabolism) (Marchler-Bauer et al., 2017) (Fig. S1). TPR domain-containing proteins are reported to be important for the assembly of various multi-protein complexes as well as serving as chaperones for type III secretion systems (T3SS) in other bacteria (Cerveny et al., 2013). NHL domain was originally identified in NCL-1, HT2A, and Lin-41 proteins and was also proposed to be important for protein-protein interactions (Slack & Ruvkun, 1998). These observations suggest that BB0236 could serve as a chaperone in the multi-protein collar complex in association with FlbB and other proteins.
Protein-protein interaction between FlbB and BB0236
To determine if BB0236 actually interacts with FlbB as suggested by the T. pallidum PPI map, we performed pull-down assays with recombinant proteins MBP-BB0236 and 6xHis-FlbB. MBP-BB0236 was allowed to bind with amylose resin, and this protein-bound resin was subsequently incubated with 6xHis-FlbB. After incubation and extensive washing, we collected the proteins that were bound to the MBP-BB0236 followed by SDS-PAGE and immunoblotting with anti-FlbB. MBP itself and amylose resin without any protein were used as controls. As shown in Fig. 1B, BB0236 was found to bind with FlbB (lane 1) but not with the resin or MBP (lanes 2 and 3). As an alternative, we performed far-western or affinity blotting to determine interactions between MBP-BB0236 and His-FlbB proteins. Our results shown in Fig. 1C indicate that FlbB interacts with MBP-BB0236 but not the control protein MBP-MCP5 or MBP. Together, these results indicate that FlbB specifically interacts with BB0236 and support our bioinformatics analysis and the PPI map developed in T. pallidum.
Construction of bb0236 mutant and complementation in trans
To determine the role of BB0236 in B. burgdorferi, we used allelic exchange to replace the endogenous bb0236 gene with a streptomycin resistance cassette (PflgB-aadA) (Fig. S1B) (Frank et al., 2003). PCR analysis of the streptomycin-resistant clones indicated that the bb0236 gene was replaced with the PflgB-aadA, resulting in successful construction of the Δbb0236 mutant (Fig. S1C).
To demonstrate that the Δbb0236 mutant’s phenotype described below is due solely to the mutation and not to a polar effect on downstream gene expression or a secondary alteration elsewhere, we complemented the mutant in trans using the shuttle vector pBSV2G containing an intact copy of bb0236 fused to the constitutive flgB promoter (PflgB-bb0236) (Elias et al., 2003, Sultan et al., 2015). Furthermore, for detection of the BB0236 protein in B. burgdorferi, we fused a 6xHistidine tag at the 3’-end of the gene (PflgB-bb0236-6xHis) (Fig. S2A). Subsequent analysis of the complemented cell lysates by western blotting using anti-His antibody detected the BB0236–6xHis in the bb0236 complemented cells (bb0236com), but not in wild-type or mutant cells (Fig. S2B). These results indicate the restoration of BB0236 protein synthesis in the bb0236com complemented cells.
Furthermore, we determined the expression of bb0236 and the genes located immediately upstream and downstream of bb0236 in the wild-type, Δbb0236, and bb0236com cells by qRT-PCR using gene-specific primers (Fig. S3). Our real-time PCR detected the bb0236 transcripts in wild-type and bb0236com cells, but not in the Δbb0236 mutant cells, indicating the restoration of bb0236 synthesis in the bb0236com cells (Fig. S3 middle panel). However, due to the in trans bb0236 complementation on the shuttle vector with flgB promoter, the level of bb0236 transcripts synthesized in the bb0236com cells was approximately 15.4-fold more than the wild-type cells (Fig. S3 middle panel; P=0.001). The expression of the genes upstream (bb0235) or downstream (bb0237) was not altered in the Δbb0236 mutant or bb0236com cells compared to the wild-type B. burgdorferi (Fig. S3 top and bottom panels). These results suggest that the mutant lacks any polar effect.
BB0236 is important for motility and bacterial morphology
To determine the Δbb0236 cells morphology and motility phenotypes, the spirochetes were analyzed using dark-field microscopy and swarm plate motility assays. While the wild-type cells show its characteristic flat-wave morphology and mobility, the Δbb0236 mutant cells were completely non-motile and they are mostly rod-shaped with slightly wave-like morphology near the tip of the mutant cells (Fig. 2). The swarm diameters of Δbb0236 are similar to those from other non-motile mutants such as ΔflaB and ΔflbB (Fig. S4) (Moon et al., 2016b, Sultan et al., 2013a, Motaleb et al., 2000). Complementation of the Δbb0236 mutant restored the distinctive flat-wave morphology (Fig. 2). Motility was also restored, albeit not completely when compared to the wild-type cells (Fig. S4, panels A, B). These results indicate that BB0236 is crucial for motility and morphology of B. burgdorferi.
Figure 2. Comparison of morphology of B. burgdorferi clones detected by dark-field microscopy.
Wild-type B. burgdorferi cells display a flat-wave morphology, whereas the Δbb0236 mutant exhibited a rod-shaped morphology with slight wave-like shape in the cell tips. Complemented bb0236com cells restored the morphology phenotype. Similar rod-shaped morphology of ΔflbB mutant cells was reported previously (Moon et al., 2016b).
The lack of BB0236 has an impact on orientation and assembly of periplasmic flagella
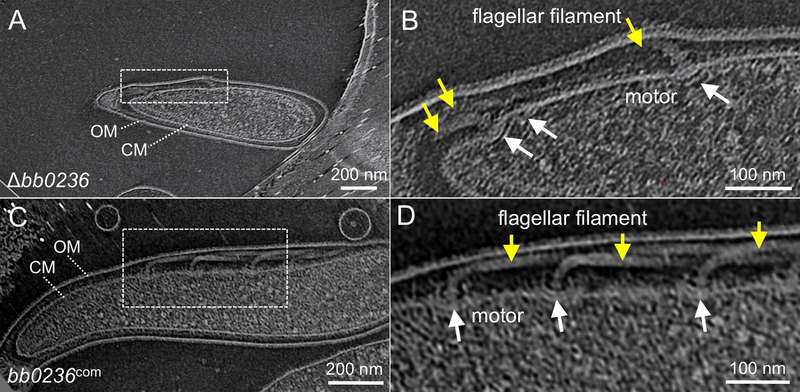
We utilized cryo-ET to visualize the periplasmic flagella in Δbb0236 mutants. We found that the Δbb0236 mutants assembled less flagellar motors per cell than the wild-type or complemented cells (Table 1, Fig. 3). On average, the motor numbers of Δbb0236 mutants were approximately 64% and 50% less than the wild-type and bb0236com cells, respectively (p ≥ 0.0001; Table 1). In addition, 45% of periplasmic flagella in the Δbb0236 cells were detected to be abnormally oriented toward the cell pole instead of their normal orientation toward the cell cylinder seen in the wild-type or bb0236com cells (Table 1, Fig. 3). Moreover, 69% of periplasmic flagella were also shorter than wild-type flagella (Table 1). Complemented bb0236com cells restored these phenotypes albeit not completely. Approximately 11% of periplasmic flagella were found to be abnormally oriented in the bb0236com cells compared to only 2% in the wild-type cells (Table 1). These abnormally oriented flagella were also detected in the ΔflbB cells (>82%) and ΔfliL cells (~26%) (Moon et al., 2016b, Motaleb et al., 2011). Together, these results indicate that BB0236 is important for motility, morphology, and orientation of periplasmic flagella in B. burgdorferi.
Table 1.
Phenotypes of Δbb0236 mutant’s periplasmic flagella detected by cryo-ET.
| B. burgdorferi clone | No. of cells analyzed | 1Total no. of flagella detected | No. of irregular flagella (%) | No. of short flagella (%) | 2Average no. of motors ± SD |
|---|---|---|---|---|---|
| Wild-type | 15 | 163 | 1 (0.6%) | 3 (2%) | 11 ± 4 |
| Δbb0236 | 23 | 101 | 46 (45%) | 70 (69%) | 4 ± 3 |
| bb0236com | 15 | 118 | 14 (11%) | 25 (21%) | 8 ± 4 |
Total number of periplasmic flagella (normal and abnormal) were detected from the ‘number of cells analyzed’ shown on the left column. Percentages were determined by dividing the number of irregular or short periplasmic flagella by the total number of periplasmic flagella x 100. A normal flagellum is defined as being oriented toward the cell body. An abnormal or irregular periplasmic flagellum is defined as being tilted toward the cell pole. Short periplasmic flagella are shorter than the wild-type flagella.
Statistical analysis by Student’s t-test suggests that the mutant cells assembled considerably fewer periplasmic flagellar motors per cell tip than wild-type (p = 0.0001). The bb0236com cells synthesized significantly more motors than the mutant cells (p=0.001) but less than the wild-type cells.
Figure 3. Representative tomographic slices of Δbb0236 mutant and bb0236com cells.
(A) A representative tomographic slice of a Δbb0236 cell shows three short and cell pole-oriented abnormal periplasmic flagella. The boxed area in (A) is zoomed and shown in (B). (C) A representative tomographic slice of a bb0236com cell shows several normal periplasmic flagella that are oriented toward the cell cylinder, characteristic of WT cells (WT, not shown). (D) The zoom-in view shows the motors and flagellar filaments. White arrows indicate motors, yellow arrows show the flagellar filaments.
BB0236 is important for the formation of the intact periplasmic collar structure
Interaction of BB0236 with FlbB and the phenotypes observed with the ΔflbB mutant leading to the hypothesis that bb0236 gene products may be involved in the assembly of the collar structure. To determine if bb0236 is actually involved in this process, we visualized the in situ motor structures by cryo-ET and sub-tomogram averaging (Fig. 4). The averaged structure of the Δbb0236 motors show major features of the periplasmic flagellar motor, such as the export apparatus, the C-ring, the MS-ring, the rod, and the P-ring (Fig. 4B). Interestingly, a bulge-like structure is detected on the periplasmic side of the MS-ring (Fig. 4B, D; indicated by red arrows) that is not detected in ΔflbB (Fig. 4A) (Moon et al., 2016b). However, the large structure surrounding the central rod and the P-ring are absent in the mutant, indicating that the Δbb0236 motors lack the flagellar collar structure detected in the wild-type motors (compare WT or bb0236com and Δbb0236 structures in Fig. 4). Moreover, structural densities associated with the stator (comprised of MotA and MotB) and FliL are also diminished in the Δbb0236 mutant cells even though the mutant cells synthesized MotB, FliL, as well as FlbB proteins (compare Fig. 4B-D with F-H; Fig. S5). Complementation of Δbb0236 restored the collar and all other associated flagellar structures such as FliL and the stator (Fig. 4F). These results suggest that BB0236 is important for the formation of the collar structure and the assembly of the stator and FliL.
Figure 4. Three dimensional structures of flagellar motors from wild-type and mutant cells.
A central slice of an averaged ΔflbB motor structure (A) is shown for comparing with Δbb0236 motor (B). A horizontal dashed line in panel B shown by C indicates location of a cross-section shown in panel C. (C) A cross-section of the averaged Δbb0236 motor structure showing the P-ring and rod. (D) A 3D surface rendering of the Δbb0236 motor structure. (E) A central slice of an averaged structure of the wild type motor. (F) A central slice of an averaged structure of bb0236com motor. A horizontal dashed line in panel F indicates location of the slices shown in panel G. (G) A slice of the averaged structure of bb0236com motor showing the complexity of the collar structure. (H) A 3D surface rendering of the bb0236com motor structure. Collar (yellow), stator (blue), cytoplasmic membrane (CM: light green), MS-ring (dark green), red (unknown/FlbB), P-ring (light blue), C-ring (aqua), export apparatus (gray).
The periplasmic collar structure assembles sequentially, in an ordered fashion
We performed comparative motor analysis from our previous and current mutants such as the ΔflbB, ΔfliL, and Δbb0236 that affected the periplasmic flagellar collar structure to understand the assembly of this complex (Moon et al., 2016b, Motaleb et al., 2011). FliL is a membrane protein associated with the collar complex as it directly interacts with FlbB (Moon et al., 2016b). Our previous report also showed that FliL forms a unique density at the interface between the collar and stator (compare Fig. 5E, J with D, I) (Motaleb et al., 2011). Interestingly, our classification of the ΔfliL motors indicate that ~80% of the motors possess an intact collar structure (Fig. 5D, I) while ~20% of the ΔfliL motors do not contain the top part of the collar (Fig. 5C, H), suggesting that FliL is important for the assembly of the collar complex, and that it interacts with not just FlbB but also with the (unidentified) collar proteins that make-up the top part of the collar structure (indicated by yellow in Fig. 5I). It is important to note that this type of non-homogenous phenotype seen in ΔfliL is common to the bacterial flagellar mutants. For example, in the ΔflgK, ΔflgL, ΔfliK, or ΔmotB mutant, not all hooks or filaments are similar in size or number (Muramoto et al., 1999, Miller et al., 2014, Sultan et al., 2015).
Figure 5. Comparative analysis of the collar structures from different flagellar mutants.
(A, F) A central section of the averaged ΔflbB motor structure and corresponding isosurface rendering. (B, G) A central section of the averaged Δbb0236 motor structure and corresponding isosurface rendering. (C, H) A central section of one class average of the ΔfliL motor and corresponding isosurface rendering. (D, I) A central section of another class average of the ΔfliL motor and corresponding isosurface rendering. Compared with the WT motor (E, J), the motor structures from different mutants are distinct from each other. The collar is a multiprotein complex that assembles sequentially and FlbB forms the base of the collar. Other collar proteins are predicted to assemble on top of each other as illustrated. The identity of the red spike structure in (G) is currently unknown but it could be FlbB because this density is missing in the ΔflbB mutant. The question marks indicate that the identity of these proteins is yet to be experimentally confirmed.
Since BB0236 and FlbB interacts and FlbB forms the base of the collar, we propose that BB0236 assembles on FlbB (Fig. 5C, H) because the Δbb0236 cells (Fig. 5B, G) lack a density detected in the ΔfliL (Fig. 5C, H). That structural density likely corresponds to BB0236 (indicated by a question mark in 5H). FlbB interacts with FliL (Moon et al., 2016b). FliL, therefore, either assembles simultaneously with FlbB or subsequently after FlbB to provide support for the assembly of BB0236 structure and the proteins that make the top part of the collar. Presumably, other unidentified proteins (indicated by a ? in 5I) then assemble on BB0236/FliL and so on to make an intact periplasmic collar complex (Fig. 5E, J).
DISCUSSION
The periplasmic collar provides a static framework for recruiting sixteen torque-generating units to produce an intact stator structure, which can produce larger torques to rotate the periplasmic flagellum. Consequently, the rotation of the flagella in the periplasm enables the spirochete to bore through viscous and complex environments in the vertebrate/tick hosts (Beeby et al., 2016, Moon et al., 2016b). Indeed, periplasmic flagellar motility is found to be crucial for every juncture of the spirochete’s life cycle (Sultan et al., 2015, Motaleb et al., 2015, Sultan et al., 2013a). The periplasmic collar also ensures that the flagella are oriented toward the cell body but not the cell pole (Table 1). This function is critical to the production of B. burgdorferi’s characteristic wave-like morphology and smooth swimming (Moon et al., 2016b, Motaleb et al., 2011).
The TPR-domain protein BB0236 identified in this study is essential for the assembly of periplasmic collar structure (Fig. 4). Proteins with TPR motifs are reported to function as chaperones of a T3SS in several species of bacteria such as Yersinia spp. (LcrH), Pseudomonas aeruginosa (PcrH), and Shigella spp. (LpgC) (Cerveny et al., 2013). B. burgdorferi contains several TPR domain proteins, however, only two of them—BB0238 and BB0324 (BamD)—have so far been investigated. The function of BB0238 is unknown but it is involved in interacting with BB0323 and stabilizing each other. BamD was reported to be involved in a β-barrel assembly machinery, which is an outer membrane porin protein complex (Thakur et al., 2017, Groshong et al., 2014, Kariu et al., 2015, Lenhart et al., 2012, Dunn et al., 2015).
Based on these observations, we anticipated that BB0236 may serve as a chaperone in the flagellar collar complex assembly. If this proposition is true, then in the absence of the chaperone (BB0236) the collar should not assemble. Indeed, our data indicate that the collar structure is not assembled in the Δbb0236 but is restored in the complemented bb0236com cells (Fig. 4). The periplasmic collar structure is considerably larger than other complex membrane structures such as the C-ring complex, flagellar type III export apparatus, or type IV secretion core structure, all of which are composed of multiple proteins (Fronzes et al., 2009, Sowa & Berry, 2008, Minamino, 2014, Lin et al., 2015). Moreover, in addition to its large structure, the finding of the TPR motifs in BB0236, and BB0236 interaction with FlbB strongly suggests that the collar is a multiprotein complex (Figs. 1, 5) (Moon et al., 2016b).
The Δbb0236 mutant cells are non-motile (Fig. S4), even though the periplasmic flagellar filament comprised of FlaB is synthesized (Fig. S5), but those periplasmic flagella are inactive due to their missing stators (Fig. 4). The number of flagellar motors and the level of FlaB proteins are also reduced in the mutant cells (Table 1; Fig. S5). The bb0236 gene is not part of a known flagellar or motility-related gene cluster and it is not genetically linked to any predicted flagellar gene, however, the Δbb0236 phenotypes are not unusual given the fact that other non-motile B. burgdorferi mutants such as ΔmotB or ΔflbB exhibit similar phenotypes (Moon et al., 2016b, Sultan et al., 2015). These data lead us to propose that the stator and/or collar-stator is likely involved in periplasmic flagellar filament assembly in B. burgdorferi.
Moreover, the Δbb0236 mutant cells lack the cellular densities associated with FliL and stator structures (Fig. 4) even though the FliL or stator (MotB) protein synthesis was not inhibited in this mutant (Fig. S5), suggesting that the collar/BB0236 is important for the assembly of stator and FliL structures. Since the collar structure is intact in the motB- or fliL-deletion mutant, we propose that the collar provides support for the assembly of the stator and FliL [(Motaleb et al., 2011); our unpublished data]. Similar results were also obtained with the FlbB (Moon et al., 2016b). While this is the first example showing that the TPR protein BB0236 is important for the periplasmic collar, stator, and FliL, chaperones are reported to be involved in the assembly of flagellar components in several species of bacteria (Macnab, 2003, Macnab, 1999). In Sinorhizobium meliloti, the periplasmic chaperone MotE is essential for MotC motor assembly (Eggenhofer et al., 2004). In Salmonella enterica, several substrate-specific chaperones are involved in various flagellar apparatus assembly, such as the FlgA periplasmic chaperone which is important for the assembly of the P-ring, FlgN for the hook protein, FliT for the filament capping protein, and FliS for the flagellin assembly (Auvray et al., 2001, Bennett et al., 2001, Nambu & Kutsukake, 2000, Yokoseki et al., 1995, Fraser et al., 1999). Taken together, experimental evidence from this and our previous FlbB studies support the hypothesis that the collar structure is assembled before the stator and FliL. In doing so, the collar provides a foundation for the assembly of the stator and FliL. This proposition is supported by the fact that BB0236 interacts with FlbB, FlbB binds to FliL, and FliL (and unidentified proteins) interact with the stator proteins MotA-MotB (Suaste-Olmos et al., 2010, Zhu et al., 2015, Moon et al., 2016b).
Moreover, Δbb0236 cells exhibit abnormally oriented flagellar filaments (Table 1; Fig. 3). 45% periplasmic flagella was found to be abnormally oriented in the Δbb0236 mutant, whereas 82% flagella was found to be abnormally tilted in the ΔflbB and only 21% of the flagella were tilted in the ΔfliL mutant cells (Table 1) (Moon et al., 2016b, Motaleb et al., 2011). In the Δbb0236 mutant, we assume the structure for FlbB is intact (see below), but FliL and stators are abolished, and that is likely why we observed fewer filaments pointed abnormally than in the ΔflbB mutant (Moon et al., 2016b). These arguments are supported by the fact that FlbB interacts with BB0236 as well as FliL. Based on these results, we propose that the collar and FliL are essential for the normal orientation of periplasmic flagella. We found a bulge appendage (it is a ring in three dimension) in the Δbb0236 cells which was not detected in the ΔflbB mutant (outlined in red in Fig. 4B, D, 5G). The identity of this appendage is currently unknown, however, we propose that this bulge could be composed of FlbB.
Despite the possession of periplasmic flagella, the Δbb0236 mutant cells are mostly rod-shaped but the tip of the cells is relatively wave-like. However, the mutant cells are missing the stator structure (Fig. 4). Diminished stators may explain why these cells are mostly rod-shaped because we observed a similar phenotype in a stator motB-deletion mutant (Sultan et al., 2015). While the complemented bb0236com cells restored the collar, stator, and FliL structures that are missing in the mutant cells, the motility phenotype was not fully reestablished, likely due to the use of the multi-copy shuttle vector for complementation (Frank et al., 2003).
In summary, this study hints not only the complexity of the collar structure, but also suggests that the B. burgdorferi genome possesses additional genes encoding the flagellar collar proteins that remain to be identified. A significant feature of this communication is the utilization of the T. pallidum protein-protein interaction map to directly identify the flagellar collar proteins in B. burgdorferi. Thus, the knowledge obtained in B. burgdorferi may be applicable to understand the structure and function of periplasmic flagellar motors of the syphilis-causing spirochete, which cannot yet be genetically manipulated.
EXPERIMENTAL PROCEDURES
Bacterial strains and growth conditions.
High-passage, avirulent B. burgdorferi strain B31-A was used as a wild-type clone throughout the study (Bono et al., 2000, Elias et al., 2002). Constructions of bb0236 mutants and its complemented strains are described below. B. burgdorferi cells were cultured in liquid Barbour-Stoenner-Kelly (BSK-II) medium, and plating BSK was prepared using 0.4% agarose (Sultan et al., 2013a, Motaleb et al., 2007). Cells were grown at 35°C in a 2.5% CO2 incubator as described previously (Motaleb et al., 2007). Antibiotics, when required, were included in the B. burgdorferi culture medium with the following concentrations: 100 μg ml−1 streptomycin, 40 μg ml−1 gentamicin. Escherichia coli cells were grown at 37°C in Luria-Bertani (LB) broth or LB agar (Bertani, 1951). Additional supplements, when required, were included in the E. coli culture medium with the following concentrations: 100 μg ml−1 ampicillin, 35 μg ml−1 chloramphenicol, 100 μg ml−1 spectinomycin, 0.2% glucose, 80 μg ml−1 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal), 0.5 mM isopropyl-β-D-thiogalactoside (IPTG).
Bioinformatics.
Basic local alignment search tool (BLAST) (Altschul et al., 1997) was used to identify a homolog of FlbB and its interacting partner in Treponema pallidum from a protein-protein interaction (PPI) map generated using a yeast-two hybrid system between known motility-related proteins and unknown proteins (Rajagopala et al., 2007). The lower an E-value (lower than 0), the more significant the score is.
Pull-down assay and Far-western.
BB0236 and FlbB protein-protein interaction was determined by pull-down assays using recombinant proteins. Briefly, B. burgdorferi FlbB was cloned in the expression vector pTrcHis-TOPO (Invitrogen) after removing the transmembrane binding domain (a.a. 1–50). E. coli codon plus cells harboring pTrcHis-TOPO::flbB was expressed and purified according to the manufacturer’s protocol (Invitrogen). The purified 6xHis-FlbB was dialyzed in Maltose binding protein (MBP)-Column buffer (20 mM Tris, 200 mM NaCl, 1 mM EDTA, pH 7.4) for subsequent assays. To recombinantly express bb0236 in E. coli, the gene was synthesized after codon optimization. Subsequently, the codon optimized bb0236 was cloned in pMAL c5x expression vector (NEB Inc.) after removing the signal sequence (a.a. 1–20). E. coli pLysS cells harboring plasmid BB0236-pMAL c5x or the empty vector pMAL c5x were induced with 1 mM of IPTG for four hours followed by harvesting the cells. The cell pellets were resuspended in 10 ml MBP-Column buffer and disrupted by using a French Pressure (SLM AMINCO Inc.) at 18,000 psi. Cell lysates were centrifuged at 22,000 x g at 4°C for 20 min to separate soluble fractions. The soluble fraction was incubated with 150 μl of amylose resin (NEB Inc.) at 4°C for overnight. Protein-bound amylose resins, i.e., MBP-BB0236 (~2.0 μg protein per reaction), or MBP itself were washed twice with 50 ml of MBP-Column buffer and mixed with 10 μg of 6xHis-FlbB. The total volume was adjusted to 500 μl with MBP-Column buffer. The mixture was then incubated for 1 hr at room temperature with constant agitation. After the incubation, the protein-bound amylose resins were washed twice with 50 ml of MBP-Column buffer. 500 μl SDS loading dye containing 10 mM of maltose was added to the protein-bound amylose resins after the washes, and then heated for 10 minutes in boiling water bath. The boiled samples were subjected to SDS-PAGE, transferred to a PVDF membrane, and immunoblotted using B. burgdorferi FlbB-specific antibodies. Far-western or affinity blotting was performed as described (Moon et al., 2016a). Construction, expression and purification of recombinant MBP-MCP5 have been previously reported (Moon et al., 2016a). Approximately 2.0 μg protein (codon optimized MBP-BB0236, MBP-MCP5 or MBP) was subjected to SDS-PAGE, transferred to a PVDF membrane, and then incubated with 4 μg His-FlbB in a blocking solution (5% skim milk, 150 mM NaCl, 10 mM Tris, 0.3% Tween 20, pH 7.4). After extensive washing with 150 mM NaCl, 10 mM Tris, 0.3% Tween-20, pH 7.4, the membrane was immunoblotted with the FlbB polyclonal antibodies. Detection was performed using ECL immunoblotting detection kit (GE Healthcare Inc.).
Construction and complementation of the bb0236 mutant.
Construction of the bb0236 inactivation plasmids, electroporation, and plating conditions were described previously (Sultan et al., 2015, Motaleb et al., 2011, Sultan et al., 2011). Briefly, the 5’- (1038 bp), and 3’-flaking (1072 bp) DNA of bb0236 gene were amplified by PCR from chromosomal DNA of B. burgdorferi strain B31-A using primers 236.KO-P1F (CCTCACAAGAATAAAACTTCTGCTTTAATAAA) and 236.KO-P1R (GTAGTCGGCAAATAAATTTCTTTTTGATTT), and 236.KO-P2F (TGAAGCTCGGGTAAATCCCCTTTACT) and 236.KO-P2R (TTTGAGGGAGCCCCAATGG), respectively. Streptomycin resistance cassette with the B. burgdorferi flgB promoter (PflgB-aadA, 1227 bp) was similarly amplified by PCR from the shuttle vector pKFSS1 using primers 236.KO-StrepF (AAATCAAAAAGAAATTTATTTGCCGACTAC) and 236.KO-StrepR (AGTAAAGGGGATTTACCCGAGCTTCA) (Frank et al., 2003). These three pieces of DNA fragments were linked by overlapping PCR, yielding bb0236_KO_PflgB-aadA, then cloned into the pGEM-T Easy (Promega Inc.), yielding plasmid Teasy::bb0236_KO_PflgB-aadA. Competent B31-A cells were electroporated with bb0236_KO_PflgB-aadA DNA that was linearized with NotI restriction enzyme digestion to remove the ampicillin restriction marker of the vector, preventing it from being introduced into B. burgdorferi (Sultan et al., 2015, Motaleb et al., 2011, Sultan et al., 2011). The transformants were selected with streptomycin. The streptomycin-resistant transformants were isolated and confirmed for the replacement of bb0236 gene with the PflgB-aadA by PCR.
To complement the Δbb0236 mutant, the flgB promoter (PflgB) and bb0236 gene were PCR amplified from chromosomal DNA of B. burgdorferi strain B31-A using primers PflgB-BamHI-F (GGATCCCGAGCTTCAAGGAAGATTTCC) and PflgB-R (ACCAAAAATTAACATATGGAAACCTCCCTC), and BB0236-F (GAGGGAGGTTTCCATATGTTAATTTTTGGT) and BB0236.His-PstI-R (CTGCAGCTAATGATGATGATGATGATGATTAATAAAATAT), respectively (restriction sites are in underlined, and 6xHis sites are in bold). A 6x histidine amino acid tag was linked at the C-terminal of bb0236 gene during the PCR. These two pieces of DNA fragments were linked by overlapping PCR, yielding PflgB-bb0236-6xHis. The amplified PflgB-bb0236-6xHis DNA was cloned into the pGEM-T Easy (Promega Inc.), yielding plasmid Teasy::PflgB-bb0236-6xHis. These and the B. burgdorferi shuttle vector pBSV2G were digested with BamHI and PstI, then ligated to yield pBSV2G::PflgB-bb0236-6xHis (Elias et al., 2003). Approximately 50 μg of pBSV2G::PflgB-bb0236-6xHis plasmid DNA was electroporated into the Δbb0236 cells (Sultan et al., 2015, Sultan et al., 2011). Potential transformants were selected with streptomycin and gentamicin. Resistant transformants were analyzed by PCR to confirm the presence of the plasmid in the transformants (complemented bb0236com cells). Furthermore, the expression of BB0236–6xHis proteins in complemented bb0236com cells were confirmed by western blot with Pierce™ His Antibody-HRP conjugate (Thermo Scientific) as described below. Restoration of bb0236 expression in the bb0236com cells, and polar effect on downstream (bb0235, ychF) or effect on upstream (bb0237, lnt) gene expressions were verified by quantitative reverse transcriptase-PCR (qRT-PCR) using gene-specific primers (Motaleb et al., 2000; Sultan et al., 2010; Sultan et al., 2013).
Reverse transcription-polymerase chain reaction (RT-PCR).
Exponentially growing B. burgdorferi wild-type cells (2 × 107 cells ml−1) were treated with RNAprotect™ followed by total RNA isolation using the RNeasy mini kit (Qiagen Inc.). Contaminating DNA in the RNA samples was removed by RNase-free Turbo® DNase I (Ambion Inc.) digestion for 3 hr. at 37°C followed by RNeasy mini purification. For RT-PCR, cDNA was prepared from 1 μg RNA using the AffinityScript QPCR cDNA synthesis kit according to the manufacturer’s protocol (Agilent Technologies Inc.). The iCycler detection system (Bio-Rad Inc.) was used to measure bb0236, and its upstream (ychF) and downstream (lnt) genes transcript levels according to the manufacturer’s instructions. B. burgdorferi enolase was used as a reference gene (Pitzer et al., 2011, Sultan et al., 2011, Sultan et al., 2010, Motaleb et al., 2004). The gene specific primers (5’−3’) were RT-enolase-F (TGGAGCGTACAAAGCCAACATT); RT-enolase-R (TGAAAAACCTCTGCTGCCATTC); ychF-qRT-F (GCCTTTCATCGGGAATC); ychF-qRT-R (CATCTAATGTTGAGATCGCG); bb0236-qRT-F (CAAGATTATTAGGATCAAAGCTTAA); bb0236-qRT-R (GGGCTCTTAATTCTTATAATAATGG); lnt-qRT-F (GTTGCTTATGTACCACTTTTTATAGC); and lnt-qRT-R (GTTGGCTATTATAAAGTAAAATACCG). The relative level of expression was calculated using the 2−ΔΔCT method (Livak; Schmittgen, 2001; Simm et al., 2009; Pitzer et al., 2011).
SDS-PAGE and immunoblot analyses.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with an enhanced chemiluminescent detection method (GE Health Inc.) were carried out as reported previously (Sultan et al., 2013a, Motaleb et al., 2000). The concentration of protein in cell lysates was determined by a Bio-Rad protein assay kit. Unless otherwise noted, 10 μg of lysate protein was subjected to SDS-PAGE and immunoblotting using proper antibodies.
Dark-field microscopy and swarm plate assays.
Growing B. burgdorferi clones were imaged using a Zeiss Imager M1 dark-field microscope connected to a digital camera to determine bacterial morphology (and motility). To determine B. burgdorferi motility, we performed swarm plate assays (Xu et al., 2017, Moon et al., 2016b, Moon et al., 2016a). Briefly, approximately 1 × 108 cells in a 5 μl volume were spotted into 0.35% agarose plates containing BSK medium diluted in 1:10 in Dulbecco’s phosphate-buffered saline. Since B. burgdorferi is a slow-growing organism with a 5 to 12 h generation time, swarm plates were incubated for 7 days to measure swarm diameters (Sultan et al., 2015, Sultan et al., 2013a, Rosa et al., 2005).
Cryo-ET sample preparation.
Frozen-hydrated specimens were prepared as described previously (Sultan et al., 2015, Zhao et al., 2014, Zhao et al., 2013, Liu et al., 2009). Briefly, late-exponential phase B. burgdorferi wild-type, bb0236 mutant, and bb0236com complemented cells’ pellets were harvested, and then resuspended in 40 μl phosphate buffered saline (PBS, pH 7.4) at a final concentration of ~2 × 109 cells ml−1. Cell suspensions were mixed with 15 nm gold clusters, then 5 μl was deposited onto freshly glow-discharged holey carbon grids (Quantifoil Micro Tools GmbH). Grids were blotted with a filter paper to remove excess fluid, followed by rapid freezing in liquid ethane using a homemade gravity-driven plunger apparatus (Sultan et al., 2015, Zhao et al., 2014, Zhao et al., 2013, Liu et al., 2009).
Cryo-electron tomography.
Tilt series acquisitions were conducted as previously described (Lin et al., 2015, Zhao et al., 2014, Zhao et al., 2013). Cryo-samples were imaged at −170°C using a 300 keV Polara G2 electron microscope (FEI Company) equipped with a field emission gun. Tilt series of B. burgdorferi cells were collected by SerialEM software, under low dose mode (Mastronarde, 2005) and were recorded by Direct Detection Camera (Gatan K2 Summit). EM images were acquired at X 9,400 magnification (pixel size of ~4.5 Å), and at ~7 μm defocus. Single axis tilt series were recorded from −60° to +60° with 2°angular increments. The cumulative dose was ~60 e−/Å2 distributed over 61 images. Dose fractionation mode was used during tilt series acquisition with each tilt image being fractionated into 8 frames. The stack of 8 frames were aligned and drift-corrected using Motioncorr (Li et al., 2013).Tilt series were automatically aligned and reconstructed using a combination of IMOD (Kremer et al., 1996) and TOMO3D packages (Agulleiro & Fernandez, 2011).
Subtomogram average.
Subtomograms containing a flagellar motor were extracted, aligned, and averaged as described previously (Zhao et al., 2014, Zhao et al., 2013, Liu et al., 2009). Briefly, the flagellar motor subtomograms (256 × 256 × 256 voxels) were visually identified by two coordinates at the C-ring and the hook positions, and then computationally extracted from the tomographic reconstructions. To accelerate image processing, 4 × 4 × 4 binned subtomograms (64 × 64 × 64 voxels) were used for initial alignment. The alignment and the averaging were then carried out to 2 × 2 × 2 binned subtomograms.
3D visualization.
The averaged maps were visualized by using UCSF Chimera (Pettersen et al., 2004). Two-dimensional tomographic slices were visualized by using the IMOD software (Kremer et al., 1996).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. P. Stewart for critical reading of the manuscript. This work was supported by National Institute of Allergy and Infectious Diseases grants 2R01AI087946 (J.L.) and 1R01AI132818 (M.A.M) and by Welch Foundation grant AU-1714 (J.L.).
REFERENCES
- Agulleiro JI & Fernandez JJ, (2011) Fast tomographic reconstruction on multicore computers. Bioinformatics 27: 582–583. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W & Lipman DJ, (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvray F, Thomas J, Fraser GM & Hughes C, (2001) Flagellin polymerisation control by a cytosolic export chaperone. J Mol Biol 308: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeby M, Ribardo DA, Brennan CA, Ruby EG, Jensen GJ & Hendrixson DR, (2016) Diverse high-torque bacterial flagellar motors assemble wider stator rings using a conserved protein scaffold. Proc Natl Acad Sci U S A 113: E1917–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JC, Thomas J, Fraser GM & Hughes C, (2001) Substrate complexes and domain organization of the Salmonella flagellar export chaperones FlgN and FliT. Mol Microbiol 39: 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G, (1951) Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono JL, Elias AF, Kupko Iii JJ, Stevenson B, Tilly K & Rosa P, (2000) Efficient targeted mutagenesis in Borrelia burgdorferi. . Journal of Bacteriology 182: 2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SM & Camilli A, (2005) Going against the grain: chemotaxis and infection in Vibrio cholerae Nat.Rev.Microbiol 3: 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon NW, Cockburn A, Li C, Liu J, Miller KA, Miller MR, Motaleb MA & Wolgemuth CW, (2012) The unique paradigm of spirochete motility and chemotaxis. Annual Review of Microbiology 66: 349–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon NW, Goldstein SF, Marko M, Hsieh C, Gebhardt LL, Motaleb MA, Wolgemuth CW, Limberger RJ & Rowe N, (2009) The flat ribbon configuration of the periplasmic flagella of Borrelia burgdorferi and its relationship to motility and morphology. Journal of Bacteriology 191: 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Beeby M, Murphy GE, Leadbetter JR, Hendrixson DR, Briegel A, Li Z, Shi J, Tocheva EI, Muller A, Dobro MJ & Jensen GJ, (2011) Structural diversity of bacterial flagellar motors. EMBO Journal 30: 2972–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JP, Kenedy MR, Iqbal H & Akins DR, (2015) Characterization of the beta-barrel assembly machine accessory lipoproteins from Borrelia burgdorferi. BMC Microbiol 15: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias AF, Bono JL, Kupko JJ III, Stewart PE, Krum JG & Rosa PA, (2003) New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi J.Mol.Microbiol.Biotechnol 6: 29–40. [DOI] [PubMed] [Google Scholar]
- Elias AF, Schmutzhard J, Stewart PE, Schwan TG & Rosa P, (2002) Population dynamics of a heterogeneous Borrelia burgdorferi B31 strain in an experimental mouse-tick infectious cycle. Wien Klin Wochenschr 114: 557–561. [PubMed] [Google Scholar]
- Frank KL, Bundle SF, Kresge ME, Eggers CH & Samuels DS, (2003) aadA confers streptomycin resistance in Borrelia burgdorferi Journal of Bacteriology 185: 6723–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD & Gocayne J, (1997) Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi Nature 390: 580–586. [DOI] [PubMed] [Google Scholar]
- Fraser GM, Bennett JC & Hughes C, (1999) Substrate-specific binding of hook-associated proteins by FlgN and FliT, putative chaperones for flagellum assembly. Mol Microbiol 32: 569–580. [DOI] [PubMed] [Google Scholar]
- Fronzes R, Schafer E, Wang L, Saibil HR, Orlova EV & Waksman G, (2009) Structure of a type IV secretion system core complex. Science 323: 266–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y & Charon NW, (1997) Identification of a large motility operon in Borrelia burgdorferi by semi-random PCR chromosome walking. Gene 189: 195–201. [DOI] [PubMed] [Google Scholar]
- Groshong AM, Fortune DE, Moore BP, Spencer HJ, Skinner RA, Bellamy WT & Blevins JS, (2014) BB0238, a presumed tetratricopeptide repeat-containing protein, is required during Borrelia burgdorferi mammalian infection. Infect Immun 82: 4292–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyard C, Raffel SJ, Schrumpf ME, Dahlstrom E, Sturdevant D, Ricklefs SM, Martens C, Hayes SF, Fischer ER, Hansen BT, Porcella SF & Schwan TG, (2013) Periplasmic Flagellar Export Apparatus Protein, FliH, Is Involved in Post-Transcriptional Regulation of FlaB, Motility and Virulence of the Relapsing Fever Spirochete Borrelia hermsii PLoS One. 8: e72550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling HF, Olivares M & Burne RA, (1997) Genetic and transcriptional analysis of flgB flagellar operon constituents in the oral spirochete Treponema denticola and their heterologous expression in enteric bacteria. Infection and Immunity 65: 2041–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariu T, Sharma K, Singh P, Smith AA, Backstedt B, Buyuktanir O & Pal U, (2015) BB0323 and novel virulence determinant BB0238: Borrelia burgdorferi proteins that interact with and stabilize each other and are critical for infectivity. J Infect Dis 211: 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN & McIntosh JR, (1996) Computer visualization of three-dimensional image data using IMOD. Journal of Structural Biology 116: 71–76. [DOI] [PubMed] [Google Scholar]
- Kudryashev M, Cyrklaff M, Baumeister W, Simon MM, Wallich R & Frischknecht F, (2009) Comparative cryo-electron tomography of pathogenic Lyme disease spirochetes. Molecular Microbiology 71: 1415–1434. [DOI] [PubMed] [Google Scholar]
- Lambert A, Picardeau M, Haake DA, Sermswan RW, Srikram A, Adler B & Murray GA, (2012) FlaA proteins in Leptospira interrogans are essential for motility and virulence but are not required for formation of the flagellum sheath. Infect Immun 80: 2019–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart TR, Kenedy MR, Yang X, Pal U & Akins DR, (2012) BB0324 and BB0028 are constituents of the Borrelia burgdorferi beta-barrel assembly machine (BAM) complex. BMC.Microbiol. 12: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertsethtakarn P, Ottemann KM & Hendrixson DR, (2011) Motility and chemotaxis in Campylobacter and Helicobacter Annual Review of Microbiology 65: 389–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xu H, Zhang K & Liang FT, (2010) Inactivation of a putative flagellar motor switch protein FliG1 prevents Borrelia burgdorferi from swimming in highly viscous media and blocks its infectivity. Molecular Microbiology 75: 1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Mooney P, Zheng S, Booth CR, Braunfeld MB, Gubbens S, Agard DA & Cheng Y, (2013) Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat Methods 10: 584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Gao L, Zhao X, Liu J & Norris SJ, (2015) Mutations in the Borrelia burgdorferi Flagellar Type III Secretion System Genes fliH and fliI Profoundly Affect Spirochete Flagellar Assembly, Morphology, Motility, Structure, and Cell Division. MBio 6: e00579–00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lin T, Botkin DJ, McCrum E, Winkler H & Norris SJ, (2009) Intact flagellar motor of Borrelia burgdorferi revealed by cryo-electron tomography: evidence for stator ring curvature and rotor/C-ring assembly flexion. Journal of Bacteriology 191: 5026–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab RM, (1999) The bacterial flagellum: reversible rotary propellor and type III export apparatus. Journal of Bacteriology 181: 7149–7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab RM, (2003) How bacteria assemble flagella. Annual Review of Microbiology 57: 77–100. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Geer LY & Bryant SH, (2017) CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res 45: D200–D203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN, (2005) Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol 152: 36–51. [DOI] [PubMed] [Google Scholar]
- Miller KA, Motaleb MA, Liu J, Hu B, Caimano MJ, Miller MR & Charon NW, (2014) Initial Characterization of the FlgE Hook High Molecular Weight Complex of Borrelia burgdorferi PLoS One. 9: e98338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, (2014) Protein export through the bacterial flagellar type III export pathway. Biochim Biophys Acta 1843: 1642–1648. [DOI] [PubMed] [Google Scholar]
- Moon KH, Hobbs G & Motaleb MA, (2016a) Borrelia burgdorferi CheD Promotes Various Functions in Chemotaxis and the Pathogenic Life Cycle of the Spirochete. Infect Immun 84: 1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KH, Zhao X, Manne A, Wang J, Yu Z, Liu J & Motaleb MA, (2016b) Spirochetes flagellar collar protein FlbB has astounding effects in orientation of periplasmic flagella, bacterial shape, motility, and assembly of motors in Borrelia burgdorferi. Mol Microbiol 102: 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaleb MA, Corum L, Bono JL, Elias AF, Rosa P, Samuels DS & Charon NW, (2000) Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc.Natl.Acad.Sci.U.S.A 97: 10899–10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaleb MA, Liu J & Wooten RM, (2015) Spirochetal motility and chemotaxis in the natural enzootic cycle and development of Lyme disease. Curr.Opin.Microbiol 28: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaleb MA, Miller MR, Bakker RG, Li C & Charon NW, (2007) Isolation and characterization of chemotaxis mutants of the Lyme disease Spirochete Borrelia burgdorferi using allelic exchange mutagenesis, flow cytometry, and cell tracking. Methods Enzymol. 422: 421–437. [DOI] [PubMed] [Google Scholar]
- Motaleb MA, Pitzer JE, Sultan SZ & Liu J, (2011) A novel gene inactivation system reveals an altered periplasmic flagellar orientation in a Borrelia burgdorferi fliL mutant. Journal of Bacteriology 193: 3324–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaleb MA, Sal MS & Charon NW, (2004) The decrease in FlaA observed in a flaB mutant of Borrelia burgdorferi occurs posttranscriptionally. Journal of Bacteriology 186: 3703–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto K, Makishima S, Aizawa S & Macnab RM, (1999) Effect of hook subunit concentration on assembly and control of length of the flagellar hook of Salmonella. J Bacteriol 181: 5808–5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu T & Kutsukake K, (2000) The Salmonella FlgA protein, a putativeve periplasmic chaperone essential for flagellar P ring formation. Microbiology 146 ( Pt 5): 1171–1178. [DOI] [PubMed] [Google Scholar]
- Novak EA, Sekar P, Xu H, Moon KH, Manne A, Wooten RM & Motaleb MA, (2016) The Borrelia burgdorferi CheY3 response regulator is essential for chemotaxis and completion of its natural infection cycle. Cell Microbiol 18: 1782–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC & Ferrin TE, (2004) UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612. [DOI] [PubMed] [Google Scholar]
- Pitzer JE, Sultan SZ, Hayakawa Y, Hobbs G, Miller MR & Motaleb MA, (2011) Analysis of the Borrelia burgdorferi cyclic-di-GMP binding protein PlzA reveals a role in motility and virulence. Infect.Immun 79: 1815–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopala SV, Titz B, Goll J, Parrish JR, Wohlbold K, McKevitt MT, Palzkill T, Mori H, Finley RL Jr. & Uetz P, (2007) The protein network of bacterial motility. Mol.Syst.Biol 3: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa PA, Tilly K & Stewart PE, (2005) The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol 3: 129–143. [DOI] [PubMed] [Google Scholar]
- Slack FJ & Ruvkun G, (1998) A novel repeat domain that is often associated with RING finger and B-box motifs. Trends Biochem Sci 23: 474–475. [DOI] [PubMed] [Google Scholar]
- Sowa Y & Berry RM, (2008) Bacterial flagellar motor. Q.Rev.Biophys 41: 103–132. [DOI] [PubMed] [Google Scholar]
- Suaste-Olmos F, Domenzain C, Mireles-Rodriguez JC, Poggio S, Osorio A, Dreyfus G & Camarena L, (2010) The flagellar protein FliL is essential for swimming in Rhodobacter sphaeroides. J Bacteriol 192: 6230–6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan SZ, Manne A, Stewart PE, Bestor A, Rosa PA, Charon NW & Motaleb MA, (2013a) Motility is crucial for the infectious life cycle of Borrelia burgdorferi Infect.Immun. 81: 2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan SZ, Manne A, Stewart PE, Bestor A, Rosa PA, Charon NW & Motaleb MA, (2013b) Motility is crucial for the infectious life cycle of Borrelia burgdorferi. Infect Immun 81: 2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan SZ, Pitzer JE, Boquoi T, Hobbs G, Miller MR & Motaleb MA, (2011) Analysis of the HD-GYP domain cyclic-di-GMP phosphodiesterase reveals a role in motility and enzootic life cycle of Borrelia burgdorferi Infect Immun 79 3273–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan SZ, Pitzer JE, Miller MR & Motaleb MA, (2010) Analysis of a Borrelia burgdorferi phosphodiesterase demonstrates a role for cyclic-di-guanosine monophosphate in motility and virulence. Molecular Microbiology 77: 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan SZ, Sekar P, Zhao X, Manne A, Liu J, Wooten RM & Motaleb MA, (2015) Motor rotation is essential for the formation of the periplasmic flagellar ribbon, cellular morphology, and Borrelia burgdorferi persistence within Ixodes scapularis tick and murine hosts. Infect.Immun 83: 1765–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze CW, Zhang K, Kariu T, Pal U & Li C, (2012) Borrelia burgdorferi needs chemotaxis to establish infection in mammals and to accomplish its enzootic cycle. Infect Immun 80: 2485–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur M, Sharma K, Chao K, Smith AA, Herzberg O & Pal U, (2017) A protein-protein interaction dictates Borrelial infectivity. Sci Rep 7: 2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth CW, (2015) Flagellar motility of the pathogenic spirochetes. Semin Cell Dev Biol 46: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunder EA Jr., Figueira CP, Benaroudj N, Hu B, Tong BA, Trajtenberg F, Liu J, Reis MG, Charon NW, Buschiazzo A, Picardeau M & Ko AI, (2016) A novel flagellar sheath protein, FcpA, determines filament coiling, translational motility and virulence for the Leptospira spirochete. Mol Microbiol 101: 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Sultan S, Yerke A, Moon KH, Wooten RM & Motaleb MA, (2017) Borrelia burgdorferi CheY2 Is Dispensable for Chemotaxis or Motility but Crucial for the Infectious Life Cycle of the Spirochete. Infect Immun 85 (1). pii: e00264–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoseki T, Kutsukake K, Ohnishi K & Iino T, (1995) Functional analysis of the flagellar genes in the fliD operon of Salmonella typhimurium. Microbiology 141 ( Pt 7): 1715–1722. [DOI] [PubMed] [Google Scholar]
- Zhao X, Norris SJ & Liu J, (2014) Molecular Architecture of Bacterial Flagellar Motor in Cells. Biochemistry 53: 4323–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhang K, Boquoi T, Hu B, Motaleb MA, Miller KA, James ME, Charon NW, Manson MD, Norris SJ, Li C & Liu J, (2013) Cryoelectron tomography reveals the sequential assembly of bacterial flagella in Borrelia burgdorferi Proc.Natl.Acad.Sci.U.S.A 110: 14390–14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Kumar A, Kojima S & Homma M, (2015) FliL associates with the stator to support torque generation of the sodium-driven polar flagellar motor of Vibrio. Mol Microbiol 98: 101–110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.