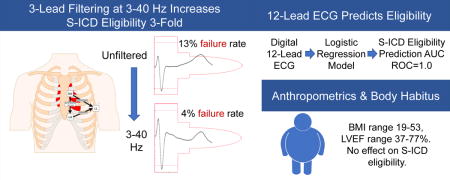
Abstract
Introduction
The subcutaneous implantable cardioverter-defibrillator (S-ICD) is a lifesaving device. Recording of a specialized 3-lead electrocardiogram (ECG) is required for S-ICD eligibility assessment. The goals of this study were: (1) evaluate the effect of ECG filtering on S-ICD eligibility, and (2) simplify S-ICD eligibility assessment by development of an S-ICD ineligibility prediction tool, which utilizes the widely available routine 12-lead ECG.
Methods and Results
Prospective cross-sectional study participants [n=68; 54% male; 94% white, with wide ranges of age (18-81 y), body mass index (19-53), QRS duration (66-150 ms), and left ventricular ejection fraction (37-77 %)] underwent 12-lead supine, 3-lead supine and standing ECG recording. All 3-lead ECG recordings were assessed using the standard S-ICD pre-implantation ECG morphology screening. Backward, stepwise, logistic regression was used to build a model for 12-lead prediction of S-ICD eligibility. Select electrocardiogram waves and complexes: QRS, R-, S-, and T- amplitudes on all 12 leads, averaged QT interval, QRS duration, and R/T ratio in the lead with the largest T wave (R/Tmax) were included as predictors. The effect of ECG filtering on ECG morphology was evaluated. A total of 9 participants (13%) failed S-ICD screening prior to filtering. Filtering at 3-40 Hertz, similar to the S-ICD default, reduced S-ICD ineligibility to 4%. A regression model that included RII, SII-aVL, TI, II, aVL, aVF, V3-V6, and R/Tmax perfectly predicted S-ICD eligibility, with an Area Under the Receiver Operating Characteristic Curve of 1.0.
Conclusion
Routine clinical 12-lead ECG can be used to predict S-ICD eligibility. ECG filtering may improve S-ICD eligibility.
Keywords: subcutaneous ICD, electrocardiogram, eligibility
Graphical abstract
Introduction
The subcutaneous implantable cardioverter defibrillator (S-ICD) technology is a groundbreaking step forward in the management of patients at risk of sudden cardiac death (SCD).[1] Most ICD candidates with a primary prevention indication can arguably benefit more from S-ICD than transvenous ICD due to less potential harm, in the absence of clear benefit from ICD for those who never sustained a life-threatening ventricular arrhythmia.[2] Pre-implant screening before device implantation is a crucial component of the S-ICD clinical application. Patients who fail the screening test cannot undergo S-ICD implantation. About 7-8% of a general ICD patient population with indications for an S-ICD are ineligible to receive the device.[3, 4] The proportion of potential S-ICD recipients who fail the screening test is even higher amongst special populations: hypertrophic cardiomyopathy (HCM; up to 40%)[5] and congenital heart disease (up to 60%)[6] patients. ECG filtering changes ECG morphology, and can potentially improve S-ICD eligibility. However, the effect of ECG filtering on S-ICD eligibility has not been studied in a prospective study.
The S-ICD’s ability to sense ECGs arises from a total of three sensing electrodes - two along the sternum and the device’s box – the “Can” – along the left chest. Thus, screening for S-ICD eligibility[7] necessitates the use of a specialized 3-lead ECG recorded on the Boston Scientific Zoom Latitude programmer (Boston Scientific, Natick, MA, USA) in locations mimicking the device electrodes (Figure 1) rather than the readily available 12-lead ECG. As a consequence, patients currently undergoing S-ICD eligibility screening do so without knowing their likelihood of passing or failing beforehand. Personalized - potentially automated - prediction of S-ICD eligibility from previously performed 12-lead ECGs in patients’ medical records could inform S-ICD eligibility expectations during pre-screening discussions of treatment options. The largest up-to-date S-ICD registry, which included 1,637 S-ICD patients found that body mass index (BMI) and left ventricular ejection fraction (LVEF) were the only patient characteristics significantly associated with the number of passed leads.[2]
Figure 1.
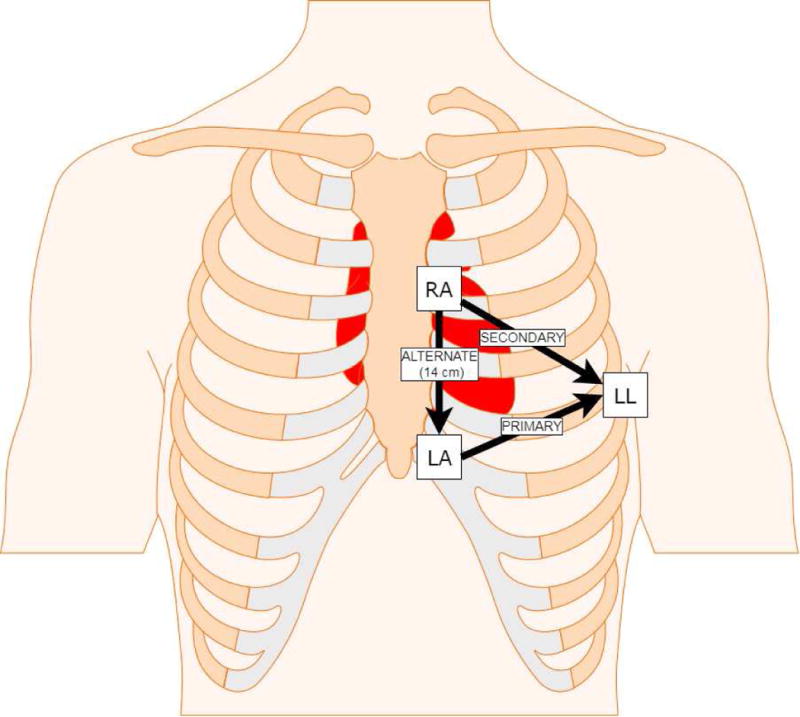
Location of Left Arm (LA), Left Leg (LL), and Right Arm (RA) electrodes placement for the 3-lead ECG to mimic the primary, secondary, and alternate sensing vectors of the S-ICD. Lead RL was placed on the lower right leg.
The goal of this study was to two-fold: (1) characterize the effect of ECG morphology changes due to filtering on S-ICD eligibility, and (2) utilize the widely available, routine 12-lead ECG to develop a tool for S-ICD eligibility prediction.
Methods
Study Population
We conducted a prospective cross-sectional study at Oregon Health & Science University (OHSU). The study was approved by the OHSU Institutional Review Board. All participants signed written informed consent before entering the study. Eligible adult OHSU patients undergoing clinically indicated 12-lead ECG in outpatient services clinics were invited to participate while awaiting their scheduled ECG examination. Inclusion criteria were: (1) ordered resting 12-lead ECG at an OHSU outpatient clinic, and (2) age ≥ 18 years. Exclusion criteria were: (1) acute medical condition, (2) pre-existing implanted pacemaker or defibrillator, (3) known pregnancy, (4) left bundle branch block, (5) end-stage organ failure.
Smoking history and alcohol use were recorded per a questionnaire. Clinical patient characteristics were recorded from the most recent available notes in the electronic medical record.
ECG Recording
ECGs were recorded using a MAC 5500 HD ECG system (General Electric (GE) Healthcare, Milwaukee, WI, USA). Three 10-second digital ECGs were recorded at the sweep speed 25 mm/sec (sampling rate 500 Hertz [Hz]) consecutively in the following order: standard 12-lead supine, left-sided 3-lead supine, left-sided 3-lead standing. Recorded 3-lead ECG electrodes were placed according to the Boston Scientific/Cameron Health Emblem™ S-ICD user manual[7] to mimic the sensing vectors of the device (Figure 1). Lead Left Leg (LL) was placed over the 5th intercostal space at the midaxillary line, Left Arm (LA) was placed 1 centimeter left lateral of the xiphoid midline, and Right Arm (RA) was placed 14 centimeters superior to the LA electrode with measurements performed using the Boston Scientific Model 4744 Screening Tool.[7] Lead Right Leg (RL) was placed on the lower right leg as the ground per the standard for limb lead ECG placement[8, 9]. All recordings were performed by a single study team member (JAT) to control for potential variability across multiple ECG technicians. Alternative screening positions were not evaluated as the study was designed as a prospective assessment of standard screening methodology.
Anthropometrics measurements
Standing hip, waist, lower (level of the xiphoid process) and upper (level of the armpits) chest circumference were measured using non-stretching measurement tape. The ratio of anteroposterior to lateral diameter of the chest, the angle of the slope of the ribs, and the subcostal angle were evaluated. Height and weight data were collected from the most recent visit in the medical record, and body mass index (BMI) was calculated.
S-ICD eligibility assessment
Raw digital ECG data underwent Butterworth filtering at 0.2-40 Hz to match filter settings on the Boston Scientific Zoom Latitude Programmer (Boston Scientific, Natick, MA, USA). Digital 3-Lead QRS-T morphologies in standing and supine position were evaluated using a digitized version of the Boston Scientific EMBLEM S-ICD Patient Screening tool (Figure 2) by two investigators (EAPA, CH). There was a disagreement in 3 cases, where the 3rd investigator (LGT) made the final adjudication. For every QRS-T complex, the left side of each of the six colored profiles was aligned with the QRS complex onset and the horizontal line aligned with the isoelectric line. The gain was adjusted to a minimum of 5 mm/mV and a maximum of 20 mm/mV to select an appropriate colored profile for QRS-T morphology analysis. A sensing vector was considered to pass if all beats of all morphologies in both standing and supine 10-second recording at 5-20 mm/mV gain passed the following criteria: maximum QRS amplitudes crossed the dotted line and all QRS complexes and trailing T waves fit within a profile. The reason for failure was recorded and categorized into 5 categories: (1) high T-wave voltage; (2) high R-wave voltage; (3) high S-wave voltage; (4) low QRS complex voltage; (5) high P-wave voltage.
Figure 2.
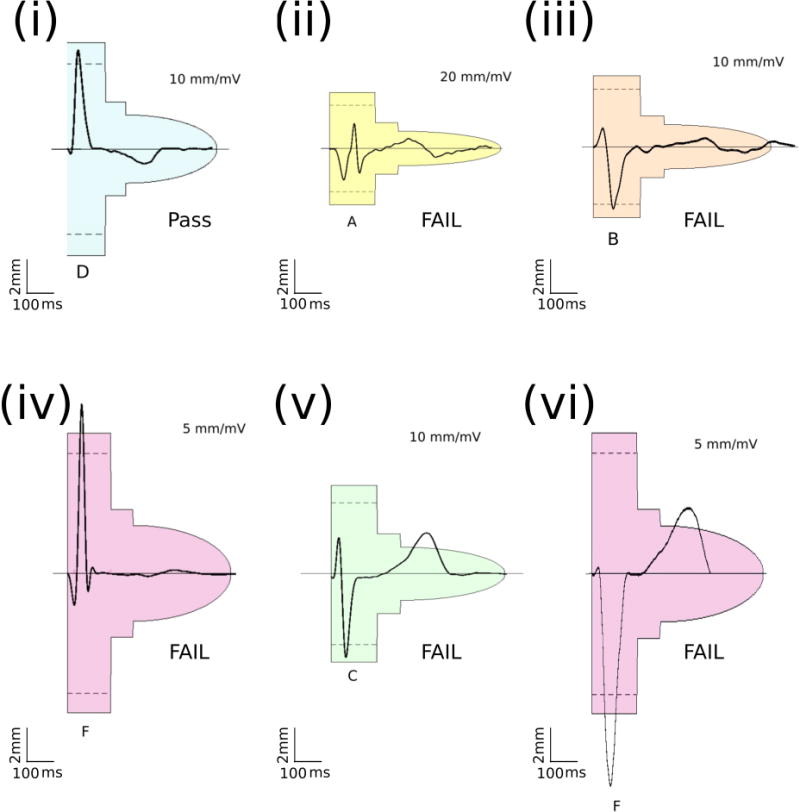
Representative examples of S-ICD screening template passing and failing QRS morphologies. (i) Passed QRS morphology; (ii) Failed due to small QRS; (iii) Failed due to large P-wave; (iv) Failed due to large R-wave; (v) Failed due to large T-wave; (vi) Failed due to large S and T waves. Color and size of screening templates corresponds to the physical Boston Scientific EMBLEM S-ICD Patient Screening tool.
Effect of the ECG Filtering on ECG morphology
To assess the effect of ECG filtering, that is similar to filtering implemented in the S-ICD, two other bandpass filter settings were used: 3-40Hz, and 9-40 Hz, and S-ICD eligibility was compared in 3 bandpass filter settings: 0.2-40 Hz (to mimic currently approved screening strategy), 3-40 Hz (similar to SMART Pass OFF S-ICD function), and 9-40 Hz (similar to SMART Pass ON).
ECG analysis
Resting 12-lead ECG (supine) and special S-ICD 3-lead ECGs (supine and standing) were analyzed. Averaged across 10-seconds and all leads RR’, PR, QT, QTc, and QRS intervals were measured on a median beat. R-, S-, peak-to-peak QRS-, and T- amplitudes on each of 12 (or 3, as appropriate) leads were measured automatically by the GE 12SL algorithm (GE Marquette, Milwaukee, WI). In addition, several indices were calculated on supine 12-lead ECG as previous reports showed their association with S-ICD eligibility: (1) presence of T-wave inversion[3] in lead I, II, aVF; (2) R/T ratio in the lead with the largest T wave[4] (R/Tmax); (3) presence of discordant R and T waves[4] in lead I, II, aVF.
Statistical analysis
Normally distributed continuous variables are presented as means ± standard deviation (SD). Variables with skewed distribution are presented as median and interquartile range (IQR). For variables with an IQR of zero (0-0), the 5th – 95th percentile range is reported. Normally distributed continuous variables in participants deemed S-ICD eligible vs. non-eligible were compared using t-test. The Wilcoxon rank-sum test was used to compare variables with a skewed distribution. Fisher’s exact test was used to compare categorical variables.
Unadjusted comparison of participants with 0, 1, 2, and 3 passing sensing vectors was performed using ANOVA (for normally distributed variables), or Kruskal-Wallis test (for non-normally distributed variables).
Paired comparison of 3-lead S-ICD ECG characteristics in supine vs. standing positions was performed using paired t-test (for normally distributed variables), or Wilcoxon matched-pairs signed-ranks test (for non-normally distributed variables).
Agnostic, backward, stepwise, logistic regression was used to build a model for prediction of S-ICD eligibility (passing at least one vector). Peak-to-peak amplitudes of QRS complex, R-, S-, and T- amplitudes on all 12 leads, averaged QT interval, QRS duration, and R/T ratio in the lead with the largest T wave[4] (51 variables) were initially included in the model. At each step, the least informative variable was removed, one-by-one. Selection of predictors was stopped at the last step of perfect prediction. Area Under the Receiver Operating Characteristic Curve (ROC AUC) was measured, and the best threshold was defined as the linear prediction value corresponding to 100% accuracy (100% sensitivity and 100% specificity).
Three-fold cross-validation was performed to evaluate a model’s ability to fit out-of-sample data. The dataset was split randomly into 3 partitions. Then, for each partition, the specified model was fitted using the other two groups. The resulting parameters were used to predict the S-ICD eligibility in the remaining group. Mean absolute errors were used to calculate 95% confidence intervals (CI) for cross-validation ROC AUCs.
Statistical analysis was performed using STATA MP 15.0 (StataCorp LP, College Station, TX). P-value < 0.05 was considered statistically significant.
Results
Study population
The study population (N=68; Table 1) was characterized by a wide range of age (18-81 y), BMI (19-53), QRS duration (66-150 ms), and LVEF (37-77 %). Half of the study population (n=36; 49%) was either diagnosed with cardiovascular disease (CVD) or had CVD risk factors (hypertension, diabetes). About a third of study population was on cancer chemotherapy, and about 20% of the study population was considered for bariatric surgery.
Table 1.
Clinical characteristics of study participants
| Characteristic | All (n=68) | Fail all 3 (n=9) | Pass at least one (n=59) | Pass/Fail P-value | Pass one (n=11) | Pass two (n=35) | Pass three (n=13) | ANOVA/Fisher P |
|---|---|---|---|---|---|---|---|---|
| Age (SD), y | 54.3(15.7) | 64.4(15.2) | 52.8(15.4) | 0.057 | 45.5(15.9) | 54.5(14.3) | 55.2(17.0) | 0.063 |
| Female, n(%) | 37(54) | 6(66.7) | 31(52.5) | 0.494 | 7(63.4) | 18(51.4) | 6(46.2) | 0.708 |
| White, n(%) | 64(94.1) | 9(100) | 55(93.2) | 1.00 | 10(90.9) | 33(94.3) | 12(92.3) | 0.633 |
| Height(SD), m | 1.7(0.1) | 1.7(0.1) | 1.7(0.1) | 0.643 | 1.7(0.1) | 1.7(0.1) | 1.7(0.9) | 0.742 |
| Weight(SD), kg | 90.1(27.8) | 94.8(27.4) | 89.4(28.1) | 0.591 | 82.7(24.3) | 92.2(29.7) | 87.4(27.3) | 0.722 |
| Body mass Index, kg/m2 | 30.0(8.8) | 31.0(7.0) | 30.0(8.8) | 0.709 | 28.5(8.5) | 30.6(9.2) | 29.9(8.6) | 0.900 |
| Barrel shaped chest, n(%) | 8(12.1) | 1(111) | 7(12.3) | 1.00 | 1(10.0) | 5(14.3) | 1(8.3) | 1.00 |
| Upper chest circumference(SD), cm | 109.0(18.7) | 113.2(18.2) | 108.3(18.9) | 0.472 | 98.5(12.9) | 112.3(19.9) | 106(18.1) | 0.151 |
| Lower chest circumference(SD), cm | 104.8(19.5) | 108.1(17.3) | 104.2(20.0) | 0.542 | 94.7(10.8) | 109.0(21.2) | 99.4(19.7) | 0.118 |
| Waist-to-Hip ratio(SD) | 0.96(0.10) | 1.0(0.08) | 0.95(0.10) | 0.077 | 0.92(0.08) | 0.97(0.08) | 0.93(0.09) | 0.167 |
| CHD, n(%) | 6(8.8) | 1(111) | 5(8.5) | 1.00 | 1(9.1) | 4(11.4) | 0(0) | 0.747 |
| LVEF(SD), % | 61.1(7.8) | 59.2(2.6) | 61.3(8.2) | 0.385 | 61.1(13.6) | 60.5(5.4) | 65.5(1.8) | 0.773 |
| Heart failure, n(%) | 5(7.4) | 0(0) | 4(6.8) | 1.00 | 1(9.1) | 3(8.6) | 0(0) | 0.894 |
| Diabetes mellitus, n(%) | 13(19.1) | 2(22.2) | 11(18.6) | 1.00 | 0(0) | 7(20.0) | 4(30.8) | 0.256 |
| Hypertension, n(%) | 28(41.2) | 3(33.3) | 25(42.4) | 0.727 | 3(27.3) | 17(48.6) | 5(38.5) | 0.581 |
| Valvular heart disease, n(%) | 3(4.4) | 0(0) | 3(5.1) | 1.00 | 2(18.2) | 1(2.9) | 0(0) | 0.172 |
| Cancer chemotherapy, n(%) | 23(33.8) | 2(22.2) | 21(35.6) | 0.707 | 4(36.4) | 12(34.3) | 5(38.5) | 0.924 |
| Bariatric surgery, n(%) | 5(7.1) | 0 | 5(8.5) | 1.00 | 0(0) | 4(11.4) | 1(7.7) | 0.763 |
| Never smoked, n(%) | 34(51.5) | 6(66.7) | 28(49.1) | 0.658 | 8(72.7) | 12(36.4) | 8(61.5) | 0.227 |
| Drinks per week(SD), n | 2.7(3.0) | 1(0) | 3.1(32) | 0.040 | 1.7(0.5) | 1.3(0.5) | 1.4(0.5) | 0.083 |
S-ICD screening test results
According to S-ICD screening criteria, 59 participants (87%) were suitable for S-ICD implantation: 11 (16%) participants had only one acceptable sensing vector, 35 (51%) had two acceptable sensing vectors, and 13 (19%) had all 3 sensing vectors acceptable. Figure 2 shows examples of passed and failed ECG morphologies. The primary sensing vector was the most appropriate (79%), followed by secondary sensing vector (72%). The alternate vector often (75%) failed.
The primary vector more frequently failed screening in standing than in supine position [10(15%) vs. 8(12%); P=0.013]. In contrast, the secondary vector [9(13%) vs. 16(24%); P=0.004], and the alternate vector [36(53%) vs. 43(63%); P=0.002] failed less frequently in standing vs. supine position (Figure 3). Reasons of inappropriate morphologies significantly differed in standing vs. supine positions, across all sensing vectors (for all comparisons Fisher’s exact test P<0.05). All possible reasons for failure were observed (Figure 3). Large T-wave was the main contributor to screening failure.
Figure 3.
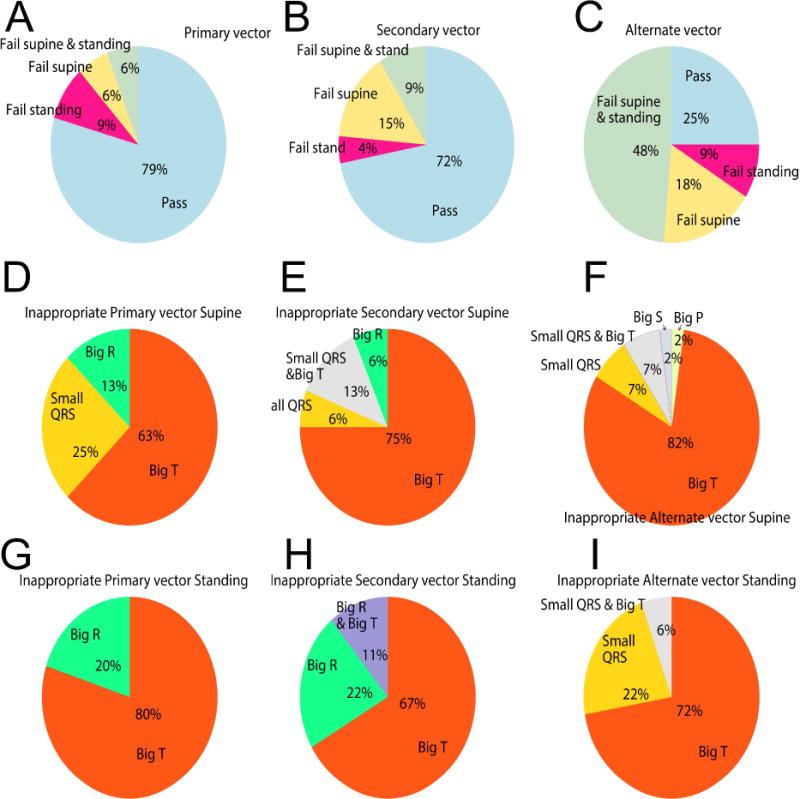
Failing sensing vectors in supine and standing positions, and the reasons for failure. A-C. The proportion of study participants with passing and failing Primary, Secondary, and Alternate sensing vectors in supine and standing positions. D-F. Reasons for inappropriate vectors in the supine position. G-I. Reasons for inappropriate vectors in standing position.
Characteristics of participants failing S-ICD screening
A total of 9 participants (13%) failed S-ICD screening. There was no significant difference in demographic, anthropometric, and clinical characteristics of participants who failed vs. pass the screening (Table 1).
Participants who failed the S-ICD screening were characterized by significantly smaller R-wave amplitude in lead II and significantly larger amplitudes of TI and TaVL (Table 2). T-wave inversion (TWI) and discordant R-T waves in aVF were significantly associated with the number of eligible sensing vectors.
Table 2.
ECG characteristics of study participants
| Characteristic | All (n=68) | Fail all 3 (n=9) | Pass ≥1 (n=59) |
Pass/ Fail P- value |
Pass one (n=11) |
Pass two (n=35) |
Pass three (n=13) |
ANOVA/ KW P-value |
|
|---|---|---|---|---|---|---|---|---|---|
| 12-lead ECG parameters | RR’ interval(SD), ms | 913(154) | 849(153) | 922(154) | 0.210 | 963(198) | 904(112) | 939(206) | 0.368 |
| PR interval(SD), ms | 155(36) | 155(15) | 155(38) | 0.943 | 130(70) | 159(24) | 166(25) | 0.066 | |
| QRS duration(SD), ms | 93(16) | 97(21) | 92(15) | 0.574 | 103(23) | 91(12) | 89(10) | 0.085 | |
| QT interval(SD), ms | 399(36) | 390(43) | 401(34) | 0.503 | 417(40) | 397(25) | 396(48) | 0.302 | |
| QTc(SD), ms | 419(18) | 424(20) | 418(18) | 0.451 | 428(21) | 418(17) | 410(15) | 0.102 | |
| R/Tmax(SD), ms | 2.19(1.47) | 2.15(1.24) | 2.19(1.51) | 0.928 | 2.14(1.50) | 2.00(1.44) | 2.53(1.71) | 0.669 | |
| TWII, n(%) | 4(5.9) | 0(0) | 4(6.5) | 1.00 | 2(18) | 2(6) | 0(0) | 0.296 | |
| TWIII, n(%) | 1(1.5) | 0(0) | 1(1.6) | 1.00 | 1(9) | 0(0) | 0(0) | 0.294 | |
| TWIaVF, n(%) | 24(35.3) | 2(22) | 22(37) | 0.476 | 8(73) | 9(26) | 5(38) | 0.038 | |
| R-T discordantI, n(%) | 4(5.9) | 0(0) | 4(6.5) | 1.00 | 2(18) | 2(6) | 0(0) | 0.296 | |
| R-T discordantII, n(%) | 1(1.5) | 0(0) | 1(1.6) | 1.00 | 1(9) | 0(0) | 0(0) | 0.294 | |
| R-T discordantaVF, n(%) | 24(35.3) | 2(22) | 22(37) | 0.476 | 8(73) | 9(26) | 5(38) | 0.038 | |
| RII(SD), mV | 0.77(0.48) | 0.34(0.25) | 0.84(0.47) | 0.0001 | 1.02(0.9) | 0.74(0.31) | 0.94(0.27) | 0.005 | |
| SII median(IQR), mV | 0.08(0-0.23) | 0.24(0.07-0.31) | 0.07(0-0.22) | 0.055 | 0.10(0-0.17) | 0.08(0-0.22) | 0.05(0-0.11) | 0.224 | |
| SaVL median(IQR), mV | 0.06(0-0.17) | 0.15(0.06-0.17) | 0.05(0-0.18) | 0.470 | 0.17(0.04-0.42) | 0.05(0-0.17) | 0(0-0.08) | 0.146 | |
| TI(SD), mV | 0.21(0.12) | 0.27(0.07) | 0.20(0.12) | 0.029 | 0.22(0.19) | 0.20(0.10) | 0.21(0.10) | 0.376 | |
| TII(SD), mV | 0.25(0.12) | 0.26(0.07) | 0.25(0.13) | 0.725 | 0.27(0.22) | 0.25(0.10) | 0.22(0.10) | 0.799 | |
| TaVL(SD), mV | 0.10(0.10) | 0.15(0.06) | 0.09(0.10) | 0.012 | 0.11(0.15) | 0.08(0.10) | 0.10(0.08) | 0.300 | |
| TaVF(SD), mV | 0.15(0.11) | 0.13(0.05) | 0.15(0.11) | 0.251 | 0.16(0.17) | 0.16(0.09) | 0.13(0.08) | 0.684 | |
| TV3(SD), mV | 0.38(0.27) | 0.39(0.14) | 0.38(0.29) | 0.951 | 0.27(0.51) | 0.42(0.21) | 0.37(0.24) | 0.465 | |
| TV4(SD), mV | 0.36(0.29) | 0.39(0.10) | 0.36(0.31) | 0.558 | 0.30(0.60) | 0.40(0.19) | 0.30(0.17) | 0.663 | |
| TV5(SD), mV | 0.31(0.26) | 0.38(0.10) | 0.29(0.27) | 0.078 | 0.30(0.52) | 0.30(0.21) | 0.27(0.12) | 0.792 | |
| TV6(SD), mV | 0.25(0.17) | 0.31(0.11) | 0.24(0.17) | 0.096 | 0.28(0.27) | 0.23(0.16) | 0.23(0.06) | 0.485 | |
| S-ICD 3-lead EC G parameters | Supine RC(SD), mV | 1.34(0.70) | 0.73(0.47) | 1.43(0.69) | 0.002 | 1.45(0.90) | 1.51(0.69) | 1.20(0.45) | 0.019 |
| Standing RC(SD), mV | 1.34(0.82) | 0.78(0.47) | 1.42(0.83) | 0.003 | 1.56(1.23) | 1.43(0.77) | 1.29(0.63) | 0.139 | |
| Supine RB(SD), mV | 1.61(0.70) | 0.82(0.53) | 1.72(0.65) | 0.0006 | 1.82(0.99) | 1.66(0.59) | 1.83(0.40) | 0.002 | |
| Standing RB(SD), mV | 1.53(0.71) | 0.77(0.44) | 1.65(0.67) | 0.0001 | 1.77(0.97) | 1.56(0.64) | 1.78(0.41) | 0.003 | |
| Supine RA(SD), mV | 0.61(0.46) | 0.32(0.22) | 0.66(0.47) | 0.002 | 0.81(0.42) | 0.46(0.26) | 1.07(0.64) | <0.0001 | |
| Standing RA(SD), mV | 0.52(0.45) | 0.29(0.26) | 0.56(0.47) | 0.021 | 0.79(0.46) | 0.35(0.27) | 0.94(0.58) | <0.0001 | |
| Supine SC(median, 5th-95th percentile), mV | 0(0-0.70) | 0(0-2.08) | 0(0-0.62) | 0.244 | 0(0-1.54) | 0(0-0.18) | 0(0-0) | 0.104 | |
| Standing SC(median, 5th- 95th percentile), mV | 0(0-0.68) | 0(0-1.75) | 0(0-0.51) | 0.012 | 0(0-1.54) | 0(0-0.06) | 0(0-0) | 0.004 | |
| Supine SB(median, IQR), mV | 0(0-0.14) | 0.16(0.08-0.27) | 0(0-0.09) | 0.019 | 0.09(0-0.50) | 0(0-0.09) | 0(0-0) | 0.004 | |
| Standing SB(median, IQR), mV | 0(0-0.13) | 0.11(0-0.25) | 0(0-0.07) | 0.114 | 0.16(0-0.63) | 0(0-0.06) | 0(0-0) | 0.011 | |
| Supine SA(median, IQR), mV | 0.35(0.11-0.65) | 0.35(0.16-0.36) | 0.36(0.1-0.69) | 0.657 | 0.19(0.04-1.11) | 0.42(0.17-0.69) | 0.25(0.08-0.39) | 0.453 | |
| Standing SA(median, IQR), mV | 0.29(0.04-0.55) | 0.21(0.07-0.31) | 0.31(0-0.56) | 0.855 | 0.32(0-1.03) | 0.37(0.04-0.59) | 0.18(0-0.45) | 0.825 | |
| Supine TC(median, IQR), mV | −0.07(−0.27-0.11) | 0.14(-0.07 to 0.22) | −0.10(−0.31 to 0.09) | 0.032 | 0(−0.46 to 0.30) | −0.12(−0.31 to −0.02) | −0.40(−0.16 to 0.09) | 0.090 | |
| Standing TC(median, IQR), mV | −0.05(−0.23-0.14) | 0.13(−0.07 to 0.31) | −0.05(−0.26 to 0.10) | 0.033 | 0.08(−0.38 to 0.20) | −0.09(−0.28 to 0.08) | −0.04(−0.08 to 0.04) | 0.058 | |
| Supine TB(median, IQR), mV | 0.20(−0.06-0.36) | 0.34(0.14-0.41) | 0.20(−0.04 to 0.33) | 0.247 | 0.42(0-0.61) | 0.20(0.06-0.30) | 0.13(−0.11 to 0.31) | 0.098 | |
| Standing TB(median, IQR), mV | 0.11(−0.07-0.28) | 0.25(0.15-0.33) | 0.04(−0.09 to 0.27) | 0.035 | 0.33(−0.04 to 0.36) | 0.05(−0.08 to 0.23) | −0.05(−0.13 to 0.04) | 0.010 | |
| Supine TA(median, IQR), mV | 0.25(0.11-0.38) | 0.16(0.12-0.34) | 0.25(0.11-0.38) | 0.814 | 0.39(0-0.54) | 0.26(0.11-0.37) | 0.12(0.06-0.25) | 0.125 | |
| Standing TA(median, IQR), mV | 0.29(0.04-0.55) | 0.15(0.04-0.21) | 0.15(0-0.28) | 0.807 | 0.28(0.06-0.44) | 0.17(0.06-0.28) | 0.07(-0.08 to 0.18) | 0.133 |
C=Primary sensing vector; B=Secondary sensing vector; A=altemate sensing vector. Amplitudes of R, S, and T waves are reported. KW=Kruskal- Wallis equality-of-populations rank test. TWI=T-wave inversion.
In participants deemed ineligible for S-ICD, R-wave amplitudes in all sensing vectors were significantly smaller, in both standing and supine positions (Table 2). Also, T-wave amplitude was markedly larger in primary and secondary sensing vectors of participants who failed S-ICD screening.
Standing vs. supine S-ICD ECG characteristics
Overall, as expected, standing as compared to supine position was characterized by shortening of RR’, QT, and QRS intervals, prolongation of QTc, and reduction of amplitudes on secondary and alternate vectors (Table 3). With the exception of T-wave amplitude on alternate vector, a significant decrease of R, S, and T amplitudes in standing position was observed only in S-ICD eligible participants.
Table 3.
Paired comparison of S-ICD 3-lead ECG parameters in supine and standing positions
| All participants (n=68) | Failed S-ICD eligibility test (n=9) | Passed S-ICD eligibility test (n=59) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ECG parameters | Supine | Standing | Paired P | Supine | Standing | Paired P | Supine | Standing | Paired P |
| RR’ interval(SD), ms | 918(157) | 807(144) | <0.0001 | 841(159) | 750(155) | 0.008 | 930(155) | 816(142) | <0.0001 |
| QT interval(SD), ms | 401(37) | 390(39) | <0.0001 | 393(46) | 383(50) | 0.245 | 402(36) | 391(37) | 0.0001 |
| QTc (SD), ms | 420(22) | 436(31) | <0.0001 | 429(26) | 444(33) | 0.136 | 418(21) | 435(31) | <0.0001 |
| QRS duration(SD), ms | 91.4(17) | 89.1(17) | 0.003 | 98.2(22) | 97.8(27) | 0.913 | 90(16) | 88(15) | 0.0001 |
| PR interval(SD), ms | 159(46) | 151(48) | 0.295 | 167(21) | 161(22) | 0.254 | 157(49) | 150(51) | 0.347 |
| RC(SD), μV | 1341(703) | 1336(820) | 0.248 | 734(470) | 776(465) | 0.757 | 1433(689) | 1421(832) | 0.798 |
| RB(SD), μV | 1606(701) | 1530(709) | 0.001 | 819(533) | 773(436) | 0.728 | 1726(646) | 1646(672) | 0.007 |
| RA(SD), μV | 614(460) | 524(453) | 0.0005 | 316(221) | 293(257) | 0.738 | 659(470) | 559(468) | 0.0004 |
| SC(median, 5th-95th percentile), μV | 0(0-703) | 0(0-653) | 0.980 | 0(0-2080) | 0(0-1752) | 0.895 | 0(0-650) | 0(0-507) | 0.994 |
| SB(median, IQR), μV | 0(0-139) | 0(0-129) | 0.598 | 161(83-268) | 112(0-253) | 1.00 | 0(0-87) | 0(0-73) | 0.638 |
| SA(median, IQR), μV | 346(110-654) | 285(41-554) | 0.008 | 346(161-361) | 214(73-307) | 0.767 | 356(102-688) | 312(0-556) | 0.006 |
| TC(median, IQR), μV | −71(-266-107) | −48(−234-136) | 0.198 | 136(−68 to 219) | 131(−68 to 307) | 0.635 | −102(−312 to 92) | −53(−258 to 102) | 0.193 |
| TB(median, IQR), μV | 200(58-356) | 114(−71-278) | <0.0001 | 336(136-405) | 253(146-327) | 0.214 | 200(−39 to 332) | 39(−87 to 273) | <0.0001 |
| TA(median, IQR), μV | 247(107-376) | 149(37-264) | <0.0001 | 161(122-336) | 146(39-205) | 0.024 | 249(107-380) | 151(0-278) | <0.0001 |
C=Primary sensing vector; B=Secondary sensing vector; A=alternate sensing vector. Amplitudes of R, S, and T waves are reported
Effect of ECG Filtering on ECG morphology
Filtering significantly improved S-ICD eligibility (Fisher’s test for all comparisons P<0.05). Only 3(4%) participants had all 3 sensing vectors fail at a bandpass 3-40Hz; 6(9%) had just one acceptable sensing vector, 25(37%) had two acceptable vectors, and 34(50%) had all 3 vectors acceptable (Figure 4). The ECG filtering at 9-40Hz did not improve S-ICD eligibility, as compared to 3-40Hz filtering. All 3 sensing vectors failed screening in 7(10%) participants; 5(7%) passed one vector; 15(22%) passed two vectors, and 41(60%) passed 3 vectors. Reasons for failure changed significantly (Figure 5). Small QRS amplitude was the main reason for ineligibility. At the same time, the number of participants who passed all 3 leads improved at 9-40Hz filtering, as compared to 3-40Hz filtering, which highlights the robustness of eligibility in filtered ECG.
Figure 4.
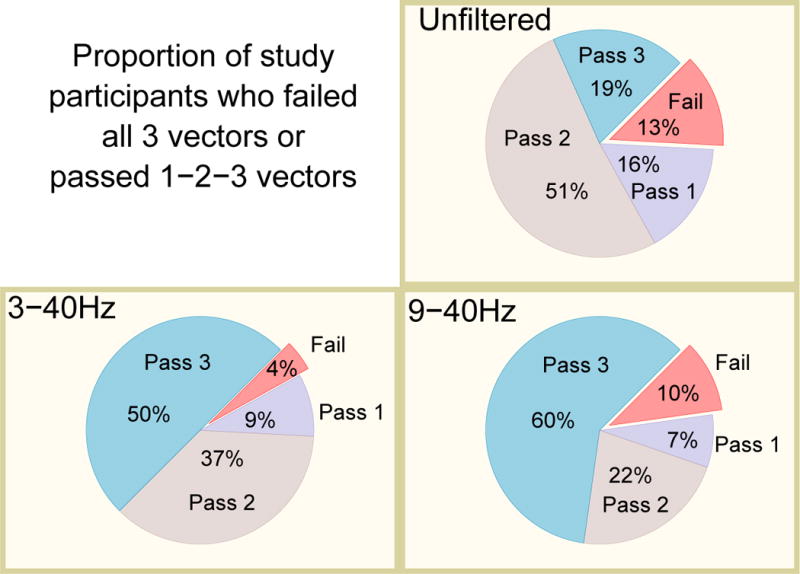
Proportion of the study participants who failed all 3 vectors, or passed 1-2-3 vectors in different filter settings.
Figure 5.
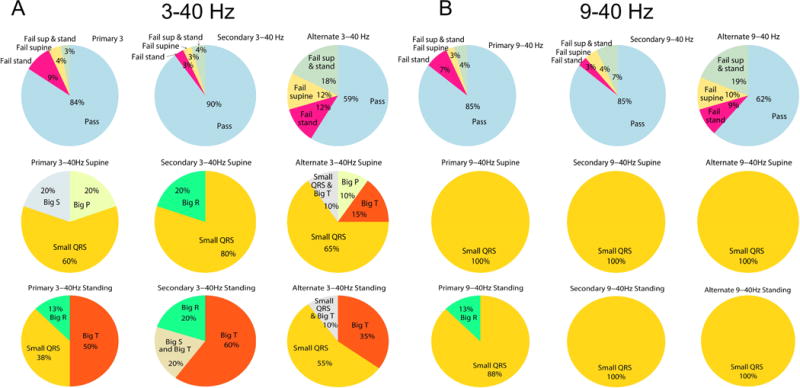
Failing sensing vectors in supine and standing positions, and the reasons of failure in ECG signal filtered at 3-40Hz (A), and 9-40Hz (B).
Development and validation of S-ICD screening tool
The final regression model included 12 ECG variables and an intercept (Table 4). S-ICD eligibility predictive model included R and S amplitudes in lead II, S amplitude in aVL, T amplitudes in leads I-II, aVL-aVF, V3-V6, and R/Tmax.[4] The model accurately predicted screening failure with AUC ROC equal to 1.0. Cross-validation confirmed the high likelihood of stable prediction of out-of-sample data (Table 5). The best threshold (100% accuracy) was identified at linear prediction function value of zero. A user-friendly risk calculator was developed; it is provided as a supplementary excel file.
Table 4.
Logistic regression model for prediction of S-ICD screening failure
| Supine 12-lead ECG parameter | Beta-coefficient |
|---|---|
| R-wave amplitude in lead II, μV | 2.418 |
| S-wave amplitude in lead II, μV | −0.747 |
| S-wave amplitude in aVL, μV | −2.485 |
| T-wave amplitude in lead I, μV | 58.411 |
| T-wave amplitude in lead II, μV | −94.181 |
| T-wave amplitude in lead aVL, μV | −18.308 |
| T-wave amplitude in lead aVF, μV | 89.520 |
| T-wave amplitude in V3, μV | −3.476 |
| T-wave amplitude in V4, μV | 5.280 |
| T-wave amplitude in V5, μV | −2.800 |
| T-wave amplitude in V6, μV | −3.097 |
| R/Tmax | −60.729 |
| Constant(intercept) | 300.692 |
Table 5.
Three-fold cross-validation of the S-ICD eligibility prediction tool
| ROC AUC | Mean absolute error | 95% Confidence Interval | |
|---|---|---|---|
| The whole population | 1.00 | <0.0001 | 1.00-1.00 |
| 1st partition | 1.00 | 0.174 | 0.659-1.00 |
| 2nd partition | 1.00 | 0.130 | 0.744-1.00 |
| 3rd partition | 1.00 | 0.182 | 0.643-1.00 |
Discussion
Our study demonstrated significant improvement of S-ICD eligibility after ECG signal filtering. The percentage of ineligible participants was reduced three-fold, and the percentage of participants with all three sensing vectors passing experienced a three-fold increase. In addition, we developed and internally validated an S-ICD eligibility prediction tool. The tool accurately predicted all S-ICD screen failures. The S-ICD eligibility prediction tool utilizes readily available 12-lead ECG parameters; it can be easily calculated in any healthcare settings. After external validation, implementation of this screening tool in a wide range of clinical practices can increase confidence in electrophysiology referral among primary care providers, internists, and cardiologists. Importantly, the developed S-ICD eligibility prediction tool is a preliminary step in S-ICD eligibility assessment. The final decision regarding S-ICD eligibility should be made by an electrophysiologist, after application of the Boston Scientific EMBLEM S-ICD Patient Screening tool,[7] as appropriate.
Effect of S-ICD screening filtering
Filtering of the ECG signal significantly improved S-ICD eligibility. ECG filtering efficiently removed large T-, P-, and QRS voltage as the reason of inappropriate ECG morphology, which is a main cause of P-(or F-)[10, 11] and T-wave oversensing.[12] However, both filters (3-40 Hz and 9-40Hz) reduced not only T-wave but also QRS complex amplitudes. Filtering with the bandpass 9-40Hz slightly increased the number of participants with inappropriately small QRS voltage, which can be associated with ventricular fibrillation under- sensing. Thus, activation of S-ICD filtering options requires individualized assessment of patient-specific ECG morphology.
Effect of postural changes on S-ICD electrocardiogram
We observed the effect of postural changes on the reason of inappropriate ECG morphology, which is specific for each sensing vector. It is well-known that standing causes shortening of RR’ intervals, which is associated with QTc prolongation, and in some cases - with ST-segment and T-wave morphology changes. However, in S-ICD patients postural changes in S-ICD ECG morphology are likely multifactorial. Our results are consistent with case reports of T-wave oversensing associated with particular body position and potential postural-driven movement of the “Can” – the dual purpose defibrillator and LL position sensing electrode in the S-ICD system - observed in HCM patients.[13] [14] Thus, evaluation of ECG morphology in different body positions should remain an essential part of the personalized patient evaluation protocol.
Body habitus does not affect S-ICD eligibility
In this study, we did not find an association between body habitus, anthropometric measurements, and S-ICD eligibility. This finding highlights complexity of the reasons for S-ICD ineligibility. Individuals of all body types can be eligible for S-ICD.
Development of the S-ICD eligibility prediction tool
S-ICD eligibility prediction tool utilizes readily available, routine clinical 12-lead ECG parameters. Many of them have been previously implicated in S-ICD eligibility. The R/Tmax has been shown independently associated with S-ICD eligibility in a general ICD population[4], and HCM population.[5] R, S, and T-wave amplitudes in leads I-II and aVL-aVF[3] were also shown to be associated with S-ICD eligibility in the general ICD population, and HCM.[15] T-wave amplitudes in the precordial area (leads V3-V6) characterize S-ICD sensing vectors voltages.
To provide broad applicability and generalizability of the tool, we included in our study participants with a wide range of LVEF and BMI.[2] Many participants of our study do not have indications for primary prevention ICD, per current guidelines. However, ongoing S-ICD clinical trials[16] are targeting patients with relatively preserved LVEF and diabetes (who are frequently overweight or obese). Thus, results of our study showing no association between such a wide range of LVEF and BMI with screening failure may have important implications for the future.
We used logistic regression for development of S-ICD ineligibility prediction tool. Our logistic regression model included 12 predictors (beta-coefficients) in a study with 9 outcomes, which seemingly contradicts the statistical “rule of ten”, and might be viewed by statisticians as an example of overfitting. However, it is important to emphasize that we are solving a mathematical problem of surface ECG signal transformation from 12-lead to special 3-lead ECG. Both predictor and outcome in our regression are ECG parameters, recorded in the same person. Regression analysis was used for development of Kors transformation matrix,[17] which is currently a gold standard of 12-lead ECG transformation into orthogonal Frank ECG.[18, 19] Similarly to development of the ECG transformation matrix, we developed an ECG transformation equation, which relays 12-lead ECG and special 3-lead ECG. We did not develop a full transformation matrix of 12-lead ECG to special 3-lead ECG because special 3-lead ECG is not used clinically. Instead, we developed a transformation equation, targeting binary thresholds of 3-lead ECG morphology parameters, serving as S-ICD eligibility thresholds. Validation of the developed tool in independent population of patients is needed before its implementation into clinical practice.
Limitations
Several study limitations should be considered. We did not test eligibility for right-sided parasternal placement of the lead. In some populations prone to SCD, lower risk patients with more normal ECGs have a higher probability of passing eligibility criteria, whereas high-risk patients with severe ECG abnormalities are less likely to pass.[5] Most of our study participants do not have indications for S-ICD. Thus, our study likely underestimated the rate of failing eligibility criteria. Similarly, our study did not screen patients during exercise, which can also increase the likelihood of ineligibility for S-ICD[5]. Nonetheless, we assumed that ECG morphologies of our study participants are not significantly different compared to potential S-ICD candidates, as characteristics of the study population (age, LVEF), and ECG predictors of S-ICD eligibility are similar to previously reported in general S-ICD population studies.[3, 4] Our study population is similar to a regular S-ICD population, as reported in international EFFORTLESS S-ICD registry,[20] a meta-analysis of S-ICD,[21] and the multi-center US experience.[2] Importantly, external validation of the prediction tool will be necessary for different populations of patients with S-ICD indications.
Supplementary Material
Highlights.
Electrocardiogram filtering at 3-40 Hz improves S-ICD eligibility threefold
Filtering (3-40 Hz and 9-40Hz) reduces both T-wave and QRS complex amplitudes
Body habitus and anthropometrics do not affect S-ICD eligibility
The 12-lead electrocardiogram predicts S-ICD eligibility
The primary vector fails screening more often in standing than in supine position
Acknowledgments
This physician-initiated study was partially supported by Boston-Scientific Center for the Advancement of Research. This work was partially supported by the National Institutes of Health R01HL118277 (LGT).
Biographies
Jason A. Thomas, BS, is a PhD student at the University of Washington studying Biomedical and Health Informatics as a recipient of the National Library of Medicine Pre-Doctoral Training Grant. His research interests include Clinical Informatics, Outcomes Research, and Quality Improvement. In 2016, Jason published an entire health system-wide screening for Deep Terminal Negativity in Lead V1 and ECG referral’s association with mortality in the International Journal of Cardiology. Since then, he has co-authored four more peer-reviewed Cardiology journal articles ranging from ECG-patch monitoring to global electric heterogeneity (GEH) and its longitudinal association with cardiac structure and function.
Dr. Erick Andres Perez Alday, PhD, received his Bachelor and Master degree in physics degree at Universidad de Guanajuato, Guanajuato, Mexico, in 2009 and 2011, respectively, and then the Ph.D. degree in Physics at the Biological Physics Group at the University of Manchester, Manchester, UK, in 2016. Currently, Dr. Perez-Alday is a post-doctoral fellow at the Knight Cardiovascular Institute at Oregon Health & Science University, Portland, USA. The multi-disciplinary work undertaken along the different research performed during his Ph.D. and Postdoctoral training focuses in applying mathematical models and physical methods to improve the understanding and diagnosis of cardiac arrhythmias.
Christopher Hamilton, BA, is a Senior Research Assistant at the Knight Cardiovascular Institute for the Tereshchenko Laboratory. He graduated in 2016 from the University of Colorado at Boulder with degree in Integrative Physiology. His research interests include electrocardiography and ventricular arrhythmias.
Dr. Muammar M Kabir, PhD, received his Bachelors of Engineering Degree with Honours in Electrical and Electronic Engineering in 2007 and his PhD in Biomedical Engineering with Dean’s recommendation for Doctoral Thesis Excellence in 2012 from The University of Adelaide, Australia. His major interests lie in the field of electrophysiology and biomedical signal processing. He was a Postdoctoral Fellow in Tereshchenko Laboratory at OHSU in 2014 – 2016. Currently, Muammar is Postdoctoral Fellow at the University of Toronto, Canada.
Eugene A Park, BS, graduated from the University Honors College at Oregon State University in 2015 with a major in Biology and minors in Chemistry and Medical Humanities. Currently a medical student in the OHSU School of Medicine with an expected graduation year of 2021. Specialty and research interests in internal medicine and general surgery.
Dr. Larisa G. Tereshchenko, MD, PhD, FACC, FAHA, FHRS, CCDS, is an Associate Professor of Medicine at the Oregon Health & Science University, Knight Cardiovascular Institute. Dr. Tereshchenko is leading the translational electrophysiology laboratory. Editorial Board Member of Circulation: Arrhythmia and Electrophysiology, Heart Rhythm Journal, BMJ Heart, Annals of Noninvasive Electrocardiology, section editor of the Journal of Electrocardiology. President of the International Congress of Electrocardiology (joint ISE-ISHNE meeting) in Portland in 2017.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burke MC, Gold MR, Knight BP, Barr CS, Theuns D, Boersma LVA, Knops RE, Weiss R, Leon AR, Herre JM, Husby M, Stein KM, Lambiase PD. Safety and Efficacy of the Totally Subcutaneous Implantable Defibrillator: 2-Year Results From a Pooled Analysis of the IDE Study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65:1605–1615. doi: 10.1016/j.jacc.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 2.Gold MR, Aasbo JD, El-Chami MF, Niebauer M, Herre J, Prutkin JM, Knight BP, Kutalek S, Hsu K, Weiss R, Bass E, Husby M, Stivland TM, Burke MC. Subcutaneous implantable cardioverter-defibrillator Post-Approval Study: Clinical characteristics and perioperative results. Heart rhythm. 2017;14:1456–1463. doi: 10.1016/j.hrthm.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Groh CA, Sharma S, Pelchovitz DJ, Bhave PD, Rhyner J, Verma N, Arora R, Chicos AB, Kim SS, Lin AC, Passman RS, Knight BP. Use of an electrocardiographic screening tool to determine candidacy for a subcutaneous implantable cardioverter-defibrillator. Heart rhythm. 2014;11:1361–1366. doi: 10.1016/j.hrthm.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Olde Nordkamp LR, Warnaars JL, Kooiman KM, de Groot JR, Rosenmoller BR, Wilde AA, Knops RE. Which patients are not suitable for a subcutaneous ICD: incidence and predictors of failed QRS-T-wave morphology screening. J Cardiovasc Electrophysiol. 2014;25:494–499. doi: 10.1111/jce.12343. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan NT, Patel KH, Qamar K, Taylor A, Baca M, Providencia R, Tome-Esteban M, Elliott PM, Lambiase PD. Disease Severity and Exercise Testing Reduce Subcutaneous Implantable Cardioverter-Defibrillator Left Sternal ECG Screening Success in Hypertrophic Cardiomyopathy. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.117.004801. [DOI] [PubMed] [Google Scholar]
- 6.Alonso P, Osca J, Rueda J, Cano O, Pimenta P, Andres A, Sancho MJ, Martinez L. Conventional and right-sided screening for subcutaneous ICD in a population with congenital heart disease at high risk of sudden cardiac death. Ann Noninvasive Electrocardiol. 2017;22 doi: 10.1111/anec.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulse Generator User’s Manual: Emblem™ S-ICD: Subcutaneous Implantable Cardioverter Defibrillator Model A209. Boston: Scientific/Cameron Health; [Google Scholar]
- 8.Batchvarov VN, Malik M, Camm AJ. Incorrect electrode cable connection during electrocardiographic recording. Europace. 2007;9:1081–1090. doi: 10.1093/europace/eum198. [DOI] [PubMed] [Google Scholar]
- 9.Harrigan RA, Chan TC, Brady WJ. Electrocardiographic Electrode Misplacement, Misconnection, and Artifact. Journal of Emergency Medicine. 2012;43:1038–1044. doi: 10.1016/j.jemermed.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Karnik AA, Helm RH, Monahan KM. Oversensing of atrial fibrillatory waves in a subcutaneous implantable cardioverter-defibrillator. HeartRhythm Case Rep. 2017;3:e1–e6. doi: 10.1016/j.hrcr.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santini L, Pappalardo A, Schirripa V, Danisi N, Forleo GB, Ammirati F. Oversensing of an unexpected atrial flutter. A new tool to improve detection of supraventricular arrhythmias in subcutaneous implantable cardioverter-defibrillators. HeartRhythm Case Rep. 2017;3:286–288. doi: 10.1016/j.hrcr.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olde Nordkamp LR, Brouwer TF, Barr C, Theuns DA, Boersma LV, Johansen JB, Neuzil P, Wilde AA, Carter N, Husby M, Lambiase PD, Knops RE. Inappropriate shocks in the subcutaneous ICD: Incidence, predictors and management. Int J Cardiol. 2015;195:126–133. doi: 10.1016/j.ijcard.2015.05.135. [DOI] [PubMed] [Google Scholar]
- 13.Afzal MR, Badin A, Weiss R, Augostini R, Hummel JD. T-wave oversensing from postural changes: A rare cause of inappropriate shock from a subcutaneous defibrillator. HeartRhythm Case Rep. 2017;3:380–383. doi: 10.1016/j.hrcr.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstock J, Bader YH, Maron MS, Rowin EJ, Link MS. Subcutaneous Implantable Cardioverter Defibrillator in Patients With Hypertrophic Cardiomyopathy: An Initial Experience. Journal of the American Heart Association. 2016;5 doi: 10.1161/JAHA.115.002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurizi N, Olivotto I, Olde Nordkamp LR, Baldini K, Fumagalli C, Brouwer TF, Knops RE, Cecchi F. Prevalence of subcutaneous implantable cardioverter-defibrillator candidacy based on template ECG screening in patients with hypertrophic cardiomyopathy. Heart rhythm. 2016;13:457–463. doi: 10.1016/j.hrthm.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Kutyifa V, Beck C, Brown MW, Cannom D, Daubert J, Estes M, Greenberg H, Goldenberg I, Hammes S, Huang D, Klein H, Knops R, Kosiborod M, Poole J, Schuger C, Singh JP, Solomon S, Wilber D, Zareba W, Moss AJ, M.S.I.E. Committee Multicenter Automatic Defibrillator Implantation Trial-Subcutaneous Implantable Cardioverter Defibrillator (MADIT S-ICD): Design and clinical protocol. Am Heart J. 2017;189:158–166. doi: 10.1016/j.ahj.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Kors JA, van HG, Sittig AC, van Bemmel JH. Reconstruction of the Frank vectorcardiogram from standard electrocardiographic leads: diagnostic comparison of different methods. Eur Heart J. 1990;11:1083–1092. doi: 10.1093/oxfordjournals.eurheartj.a059647. [DOI] [PubMed] [Google Scholar]
- 18.Cortez D, Sharma N, Devers C, Devers E, Schlegel TT. Visual transform applications for estimating the spatial QRS-T angle from the conventional 12-lead ECG: Kors is still most Frank. J Electrocardiol. 2014;47:12–19. doi: 10.1016/j.jelectrocard.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Man S, Algra AM, Schreurs CA, Borleffs CJ, Scherptong RW, van Erven L, van der Wall EE, Cannegieter SC, Schalij MJ, Swenne CA. Influence of the vectorcardiogram synthesis matrix on the power of the electrocardiogram-derived spatial QRS-T angle to predict arrhythmias in patients with ischemic heart disease and systolic left ventricular dysfunction. J Electrocardiol. 2011;44:410–415. doi: 10.1016/j.jelectrocard.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Boersma L, Barr C, Knops R, Theuns D, Eckardt L, Neuzil P, Scholten M, Hood M, Kuschyk J, Jones P, Duffy E, Husby M, Stein K, Lambiase PD. Implant and Midterm Outcomes of the Subcutaneous Implantable Cardioverter-Defibrillator Registry: The EFFORTLESS Study. J Am Coll Cardiol. 2017;70:830–841. doi: 10.1016/j.jacc.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 21.Chue CD, Kwok CS, Wong CW, Patwala A, Barker D, Zaidi A, Mamas MA, Cunnington C, Ahmed FZ. Efficacy and safety of the subcutaneous implantable cardioverter defibrillator: a systematic review. Heart (British Cardiac Society) 2017;103:1315–1322. doi: 10.1136/heartjnl-2016-310852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


